Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactivity-Guided Isolation and Dereplication of the Active Antiviral Fraction
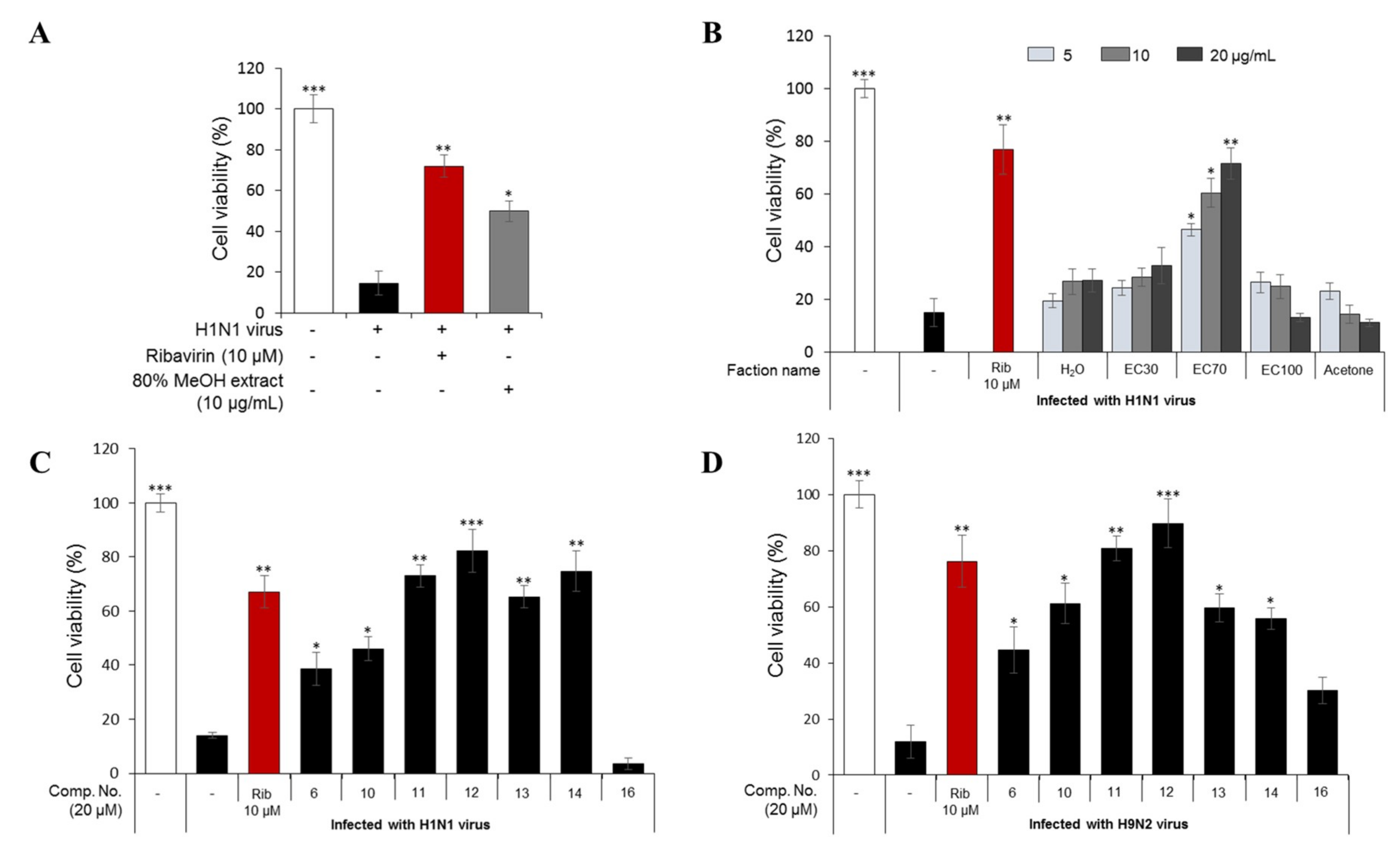
2.1.1. Bioactivity-Guided Fractionation Based on Antiviral Activity Against Human Influenza H1N1 A/PR/8/34 Virus
2.1.2. Dereplication and Isolation of the Phlorotannins from the EC70 Fraction Using HPLC-qTOFMS and RMD
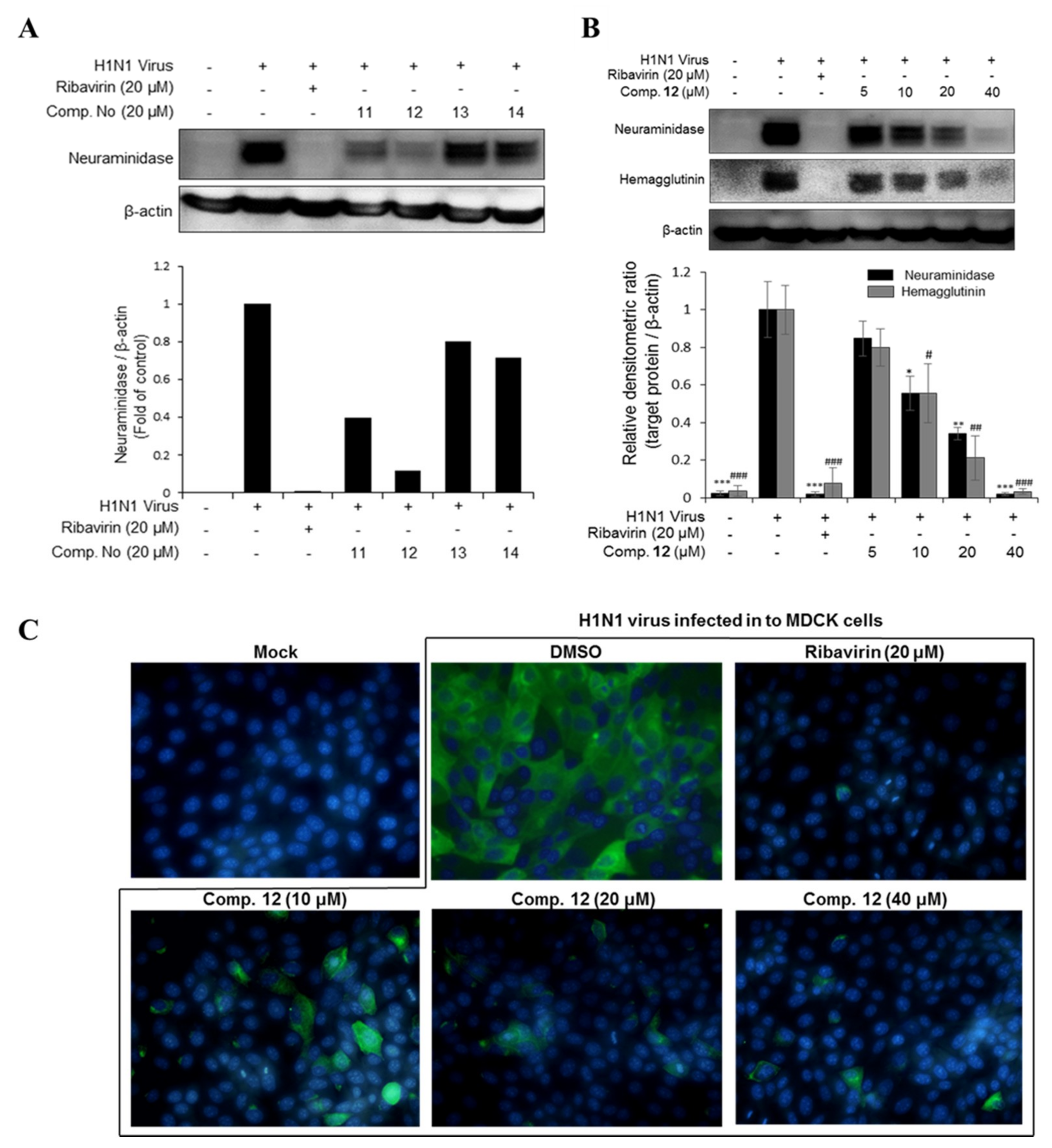
2.1.3. Antiviral Activities of the Major Phlorotannins in the EC70 Active Fraction against H1N1 and H9N2
2.2. Identification of Two New Phlorotannins from the EC30 Fraction of E. cava using Dereplication and RMD Rules
2.3. Discussion of the Potential Applications of the Developed Dereplication Strategy and Phlorotannins
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.3.1. Dibenzodioxin-fucodiphloroethol (DFD) (1)
4.3.2. Dibenzodioxin-fucodiphloroeckol (DFE) (2)
4.4. HPLC-qTOFMS Measurement
4.5. Cell Cultures and Viruses
4.6. Cytotoxicity Assay
4.7. Cytopathic Effect (CPE) Assay
4.8. Western Blot Analysis
4.9. Immunofluorescence Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Park, J.Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorganic. Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Tanaka, R.; Miyake, H.; Tamaru, Y.; Ueda, M.; Shibata, T. Evaluation for antioxidative properties of phlorotannins isolated from the brown alga Eisenia bicyclis, by the H-ORAC method. Food. Nutr. Sci. 2013, 4, 78–82. [Google Scholar]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a New Anti-Allergic Phlorotannin, Phlorofucofuroeckol-B, from an Edible Brown Alga, Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Kido, M.; Mori, H.; Nakayama, Y.; Takahashi, M. Structure of an anti-plasmin inhibitor, eckol, isolated from the brown alga Ecklonia kurome Okamura and inhibitory activities of its derivatives on plasma plasmin inhibitors. Chem. Pharm. Bull. 1989, 37, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.J.; Kwon, T.H.; Park, G.H.; Song, H.M.; Lee, S.J.; Park, N.H.; Jeong, J.B. In vitro anticancer activity of phlorofucofuroeckol a via upregulation of activating transcription factor 3 against human colorectal cancer cells. Mar. Drugs 2016, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Son, B.S.; Kim, B.K.; Chee, H.Y.; Song, K.S.; Lee, B.H.; Shin, H.C.; Lee, K.B. Antihypertensive effect of Ecklonia cava extract. Korean J. Pharmacogn. 2006, 37, 200–205. [Google Scholar]
- Bu, H.J.; Ham, Y.M.; Kim, J.M.; Lee, S.J.; Hyun, J.W.; Lee, N.H. Elastase and hyaluronidase inhibition activities of phlorotannins isolated from Ecklonia cava. Korean J. Pharmacogn. 2006, 37, 92–96. [Google Scholar]
- Kwon, H.J.; Ryu, Y.B.; Kim, Y.M.; Song, N.; Kim, C.Y.; Rho, M.C.; Jeong, J.H.; Cho, K.O.; Lee, W.S.; Park, S.J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorganic Med. Chem. 2013, 21, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Moghadami, M. A narrative review of influenza: A seasonal and pandemic disease. Iran. J. Med. Sci. 2017, 42, 2–13. [Google Scholar] [PubMed]
- Masters, B.R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; ISBN 13-978-1-4557-4801-3. [Google Scholar]
- Rao, S.; Nyquist, A.C.; Stillwell, P.C. Influenza. In Kendig’s Disorders of the Respiratory Tract in Children, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 460–465.e2. [Google Scholar]
- Pagano, M.; Castagnolo, D.; Bernardini, M.; Fallacara, A.L.; Laurenzana, I.; Deodato, D.; Kessler, U.; Pilger, B.; Stergiou, L.; Strunze, S.; et al. The fight against the influenza A virus H1N1: Synthesis, molecular modeling, and biological evaluation of benzofurazan derivatives as viral RNA polymerase inhibitors. Chem. Med. Chem. 2014, 9, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, Y.J.; Guo, B.L.; Cen, S. Anti-influenza triterpenoid saponins (saikosaponins) from the roots of Bupleurum marginatum var. stenophyllum. Bioorg. Med. Chem. Lett. 2017, 27, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.J.; Subbarao, K. Global Epidemiology of Influenza: Past and Present. Annu. Rev. Med. 2000, 51, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Korteweg, C.; Gu, J. Pandemic influenza A (H1N1) virus infection and avian influenza A (H5N1) virus infection: A comparative analysis. Biochem. Cell Biol. 2010, 88, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, B. Pandemic and Avian Influenza A Viruses in Humans: Epidemiology, Virology, Clinical Characteristics, and Treatment Strategy. Clin. Chest Med. 2017, 38, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, Y.; Noda, T.; Kawakami, E.; Akkina, R.; Kawaoka, Y. Identification of novel influenza a virus proteins translated from PA mRNA. J. Virol. 2013, 87, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Batra, J.; Lal, S.K. Interplay between influenza A virus and host factors: Targets for antiviral intervention. Arch. Virol. 2015, 160, 1877–1891. [Google Scholar] [CrossRef] [PubMed]
- FDA Influenza (flu) Antiviral Drugs and Related Information. Available online: https://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm100228.htm (accessed on 1 March 2019).
- Kildgaard, S.; Subko, K.; Phillips, E.; Goidts, V.; De La Cruz, M.; Díaz, C.; Gotfredsen, C.H.; Andersen, B.; Frisvad, J.C.; Nielsen, K.F.; et al. A dereplication and bioguided discovery approach to reveal new compounds from a marine-derived fungus Stilbella fimetaria. Mar. Drugs 2017, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Sleno, L. The use of mass defect in modern mass spectrometry. J. Mass Spectrom. 2012, 47, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, E.A.P.; Celiz, M.D.; Jones, A.D. Relative Mass Defect Filtering of Mass Spectra: A path to discovery of plant specialized metabolites. Plant Physiol. 2015, 167, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Stagliano, M.C.; DeKeyser, J.G.; Omiecinski, C.J.; Jones, D.D. Bioassay-directed fractionation for discovery of bioactive neutral lipids guided by relative mass defect filtering and multiplexed collision-induced dissociation. Rapid Commun. Mass Spectrom. 2010, 24, 3578–3584. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Fontaine, F.; Cooper, M.A. Natural product libraries: Assembly, maintenance, and screening. Planta Med. 2014, 80, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R.A. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins a and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and structural determination of two novel phlorotannins from the brown alga Ecklonia kurome Okamura, and their radical scavenging activities. Mar. Drugs 2013, 11, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Eom, S.H.; Kim, Y.M. Protective effect of phlorotannins from Eisenia bicyclis against lipopolysaccharide-stimulated inflammation in HepG2 cells. Environ. Toxicol. Pharmacol. 2013, 35, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, S.H.; Le, Q.T.; Kim, M.M.; Kim, S.K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, Z.J.; Ryu, B.M.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Jung, M.H.; Avunje, S.; Nikapitiya, C.; Kang, S.Y.; Ryu, Y.B.; Lee, W.S.; Jung, S.J. Efficacy of algal Ecklonia cava extract against viral hemorrhagic septicemia virus (VHSV). Fish Shellfish Immunol. 2018, 72, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.B.; Kwak, J.H. Antiviral phlorotannin from Eisenia bicyclis against human papilloma virus in vitro. Planta Med. 2015, 81, 22. [Google Scholar] [CrossRef]
- Eom, S.H.; Moon, S.Y.; Lee, D.S.; Kim, H.J.; Park, K.; Lee, E.W.; Kim, T.H.; Chung, Y.H.; Lee, M.S.; Kim, Y.M. In vitro antiviral activity of dieckol and phlorofucofuroeckol-A isolated from edible brown alga Eisenia bicyclis against murine norovirus. Algae 2015, 30, 241–246. [Google Scholar] [CrossRef]

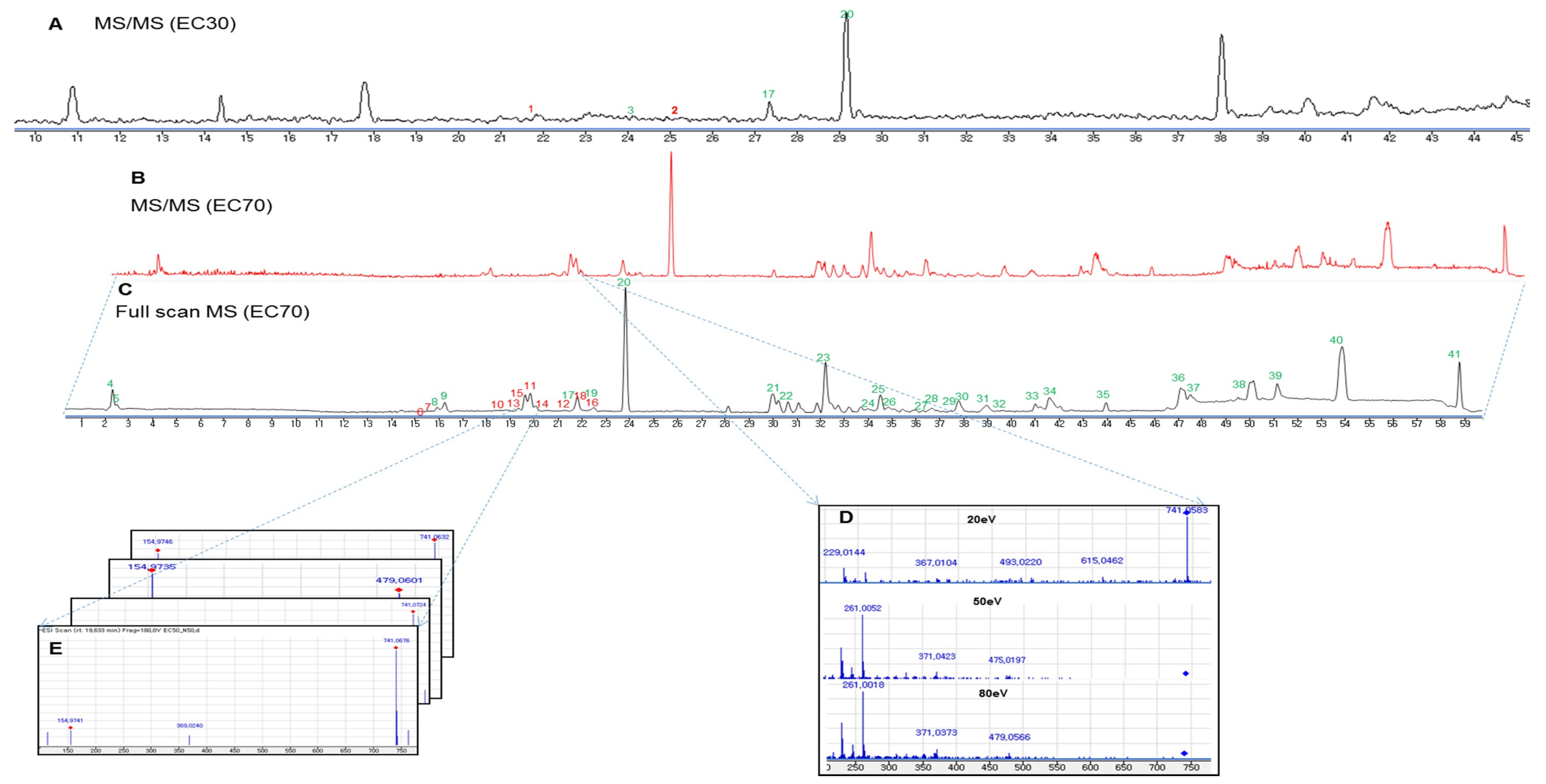
| No | Compound Name | RT (min) | MS 1 | MS 2 | [M − H]− | Chemical Formula | Calcd. Mass | Dif. | RMD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Compound 1 | 21.793 | 195, 247, 389, 479, 583, 743 | 139, 231, 285, 353, 447, 493, 643, 743 | 743.0881 | C36H23O18 | 743.0884 | −1.73 | 119 |
| 2 | Compound 2 | 25.279 | 195, 359, 479, 556, 663, 982, 1113 | 231, 353, 461, 705, 925, 1104, 1113 | 1113.1146 | C54H33O27 | 1113.1209 | −7.94 | 103 |
| 3 | Benzo[1,2-b:3,4-b′]bis[1,4] benzodioxin-1,3,6,9,11-pentol | 24.015 | 195, 211, 246, 292, 369 | 193, 230, 267, 285, 310, 369 | 369.0224 | C18H9O9 | 369.0247 | −4.80 | 61 |
| 4 | Unknown | 2.268 | 162, 197 | 163, 821 | 162.8392 | - | 5154 | ||
| 5 | Unknown | 2.631 | 122, 139, 168, 195 | 111, 139, 171 | 195.9504 | - | 4850 | ||
| 6 | Eckol | 15.543 | 154, 371 | 217, 246, 282, 371 | 371.0446 | C18H11O9 | 371.0403 | 6.56 | 120 |
| 7 | 7-Phloroeckol | 15.845 | 154, 495 | 263, 297, 387, 488, 495, 616 | 495.0511 | C24H15O12 | 495.0564 | −7.28 | 103 |
| 8 | 2-Phloroeckol | 16.003 | 154, 263, 495 | 201, 229, 283, 346, 495 | 495.0528 | C24H15O12 | 495.0564 | −6.00 | 107 |
| 9 | Dibenzo[b,e][1,4]dioxin-1,2,4,7,9-pentol | 16.209 | 137, 155, 263 | 207, 218, 263 | 263.0157 | C12H7O7 | 263.0192 | −5.92 | 60 |
| 10 | 6,6′-Bieckol | 18.854 | 154, 741 | 229, 261, 371, 479, 585, 666, 741 | 741.0647 | C36H21O18 | 741.0728 | −9.00 | 87 |
| 13 | Dibenzo[1,4]dioxine-2,4,7,9-tetraol | 19.274 | 123, 155, 247 | 141, 195, 247, 385, 479 | 247.0243 | C12H7O6 | 247.0243 | 0.00 | 98 |
| 15 | 6,8′-Bieckol | 19.569 | 741 | 260, 371, 479, 615, 741 | 741.0737 | C36H21O18 | 741.0728 | 3.00 | 99 |
| 11 | Dieckol | 19.917 | 321, 741 | 229, 261, 371, 430, 545, 714, 741 | 741.0723 | C36H21O18 | 741.0728 | −2.24 | 98 |
| 14 | Dioxinodehydroeckol | 20.370 | 123, 196, 325, 369 | 123, 161, 173, 199, 261, 369 | 369.0269 | C18H9O9 | 369.0247 | 4.69 | 73 |
| 12 | Phlorofucofuroeckol A | 21.796 | 155, 601 | 245, 385, 447, 493, 601 | 601.0640 | C30H17O14 | 601.0618 | 4.69 | 106 |
| 17 | 2,7″-phloroglucinol 6,6′-bieckol (PHB) | 22.018 | 155, 973 | 229, 353, 427, 493, 707, 806, 973 | 973.1153 | C48H29O23 | 973.1100 | 7.28 | 118 |
| 18 | 974-A | 22.050 | 113, 601, 973 | 229, 353, 393, 605, 707, 805, 941 | 973.1038 | C48H29O23 | 973.1100 | −7.87 | 107 |
| 19 | Unknown | 22.44 | 155, 369, 551 | 223, 304, 551, 583, 710 | 551.1816 | - | 329 | ||
| 16 | Fucofuroeckol A | 22.897 | 155, 477, | 2551, 352, 477, 545 | 477.0425 | C24H13O11 | 477.0457 | −5.66 | 89 |
| 20 | Unknown | 23.799 | 242, 310 | 201, 271, 348 | 242.1758 | - | 726 | ||
| 21 | 6,9,12-Octadecatrienoic acid, (6Z,9Z,12Z) | 29.966 | 277, 527 | 264, 353, 481, | 277.2125 | C18H29O2 | 277.2168 | −6.56 | 767 |
| 22 | 9,12-Octadecadienoic acid (9Z,12Z) | 30.496 | 279 | 218, 248, 279, 346, 380 | 279.2168 | C18H31O2 | 279.2324 | −12.49 | 776 |
| 23 | Ecklonialactone B (R/S) | 32.281 | 265, 291 | 201, 291 | 291.2020 | C18H27O3 | 291.1960 | 7.75 | 694 |
| 24 | Eicosanoic acid | 34.206 | 311 | 225, 311, 349 | 311.2848 | C20H39O2 | 311.2950 | −10.10 | 915 |
| 25 | Ecklonialactone B (S/R) | 34.499 | 291, 555, 623 | 251, 291, 411,651 | 291.1938 | C18H27O3 | 291.1960 | −4.96 | 666 |
| 26 | 11,14,17-Eicosatrienoic acid, (11Z,14Z,17Z)- | 34.771 | 305 | 211, 284, 305, 248, 583, 804 | 305.2410 | C20H33O2 | 305.2481 | −8.43 | 790 |
| 27 | Tricosanoic acid | 36.294 | 353 | 257, 333, 353, 529 | 353.3407 | C23H45O2 | 353.3420 | −3.61 | 964 |
| 28 | Heneicosanoic acid | 36.667 | 325 | 225, 239, 248, 267, 282, 325 | 325.3131 | C21H41O2 | 325.3107 | 4.90 | 962 |
| 29 | Unknown | 37.806 | 293, 325 | 281, 325, 386, 449, 674 | 325.1812 | - | 557 | ||
| 30 | Unknown | 37.869 | 239, 293 | 207, 239, 243, 383 | 239.0709 | - | 297 | ||
| 31 | Unknown | 38.947 | 321 | 248, 321, 399, 572, 815 | 321.2178 | - | 678 | ||
| 32 | Docosanoic acid | 39.599 | 339 | 226, 339, 433, 660, 809 | 339.3292 | C22H43O2 | 339.3263 | 5.39 | 970 |
| 33 | Unknown | 41.028 | 346 | 206, 254, 330, 346, 642 | 346.1092 | - | 316 | ||
| 34 | Unknown | 41.59 | 346, 485 | 280, 421, 485 | 485.2671 | - | 550 | ||
| 35 | 5,8,11,14,17-Eicosapentaenoic acid, (5Z,8Z,11Z,14Z,17Z) | 43.93 | 301, 369 | 205, 269, 301, 440 | 301.2099 | C20H29O2 | 301.2168 | −8.31 | 697 |
| 36 | Triacontanoic acid | 47.116 | 346, 451, 535, 691, 775 | 295, 387, 451, 456 | 451.4499 | C30H59O2 | 451.4515 | −4.00 | 997 |
| 37 | Dotricontanoic acid | 47.575 | 346, 479, 609, 691, 775 | 461, 479, 648, 866 | 479.2903 | C32H63O2 | 479.4828 | −5.00 | 606 |
| 38 | Unknown | 49.963 | 223, 297, 441, 535, 701, 849 | 254, 333, 441, 673 | 441.2074 | - | 470 | ||
| 39 | Unknown | 51.118 | 149, 223 | 149, 448 | 149.0021 | - | 14 | ||
| 40 | Unknown | 53.856 | 149, 223, 297 | 149, 221, 350, 630.7708 | 149.0086 | - | 58 | ||
| 41 | Unknown | 58.796 | 135 | 135.9698, 287.5036 | 135.9751 | - | 7171 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.M.; Doan, T.P.; Ha, T.K.Q.; Kim, H.W.; Lee, B.W.; Pham, H.T.T.; Cho, T.O.; Oh, W.K. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Mar. Drugs 2019, 17, 149. https://doi.org/10.3390/md17030149
Cho HM, Doan TP, Ha TKQ, Kim HW, Lee BW, Pham HTT, Cho TO, Oh WK. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Marine Drugs. 2019; 17(3):149. https://doi.org/10.3390/md17030149
Chicago/Turabian StyleCho, Hyo Moon, Thi Phuong Doan, Thi Kim Quy Ha, Hyun Woo Kim, Ba Wool Lee, Ha Thanh Tung Pham, Tae Oh Cho, and Won Keun Oh. 2019. "Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava" Marine Drugs 17, no. 3: 149. https://doi.org/10.3390/md17030149
APA StyleCho, H. M., Doan, T. P., Ha, T. K. Q., Kim, H. W., Lee, B. W., Pham, H. T. T., Cho, T. O., & Oh, W. K. (2019). Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (qTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia cava. Marine Drugs, 17(3), 149. https://doi.org/10.3390/md17030149





