Abstract
The aim of this study is to investigate the physicochemical properties, biosafety, and biocompatibility of the collagen extract from the skin of Nile tilapia, and evaluate its use as a potential material for biomedical applications. Two extraction methods were used to obtain acid-soluble collagen (ASC) and pepsin-soluble collagen (PSC) from tilapia skin. Amino acid composition, FTIR, and SDS-PAGE results showed that ASC and PSC were type I collagen. The molecular form of ASC and PSC is (α1)2α2. The FTIR spectra of ASC and PSC were similar, and the characteristic peaks corresponding to amide A, amide B, amide I, amide II, and amide III were 3323 cm−1, 2931 cm−1, 1677 cm−1, 1546 cm−1, and 1242 cm−1, respectively. Denaturation temperatures (Td) were 36.1 °C and 34.4 °C, respectively. SEM images showed the loose and porous structure of collagen, indicting its physical foundation for use in applications of biomedical materials. Negative results were obtained in an endotoxin test. Proliferation rates of osteoblastic (MC3T3E1) cells and fibroblast (L929) cells from mouse and human umbilical vein endothelial cells (HUVEC) were increased in the collagen-treated group compared with the controls. Furthermore, the acute systemic toxicity test showed no acute systemic toxicity of the ASC and PSC collagen sponges. These findings indicated that the collagen from Nile tilapia skin is highly biocompatible in nature and could be used as a suitable biomedical material.
1. Introduction
Collagen is the main structural protein in the extracellular matrix (ECM), constituting approximately 30% of the whole body protein content in animals []. More than 29 different types of collagen have been identified and described. In the human body, most of the collagen is type I []. The collagen protein contains triple-helix structures that consist of three almost identical polypeptide chains []. Type I collagen is present in bone, skin, dentin, cornea, blood vessels, fibrocartilage, and tendon; it has the unique ability to form fibrils that have high tensile strength and important functions [,]. In the past decades, it has been widely used in food manufacturing and the cosmetics industry [].
Recently, the interest in collagen has become widespread among medicine and tissue engineering because of its predominance in the ECM, excellent biocompatibility, low antigenicity and available methods of isolation from a variety of sources [,]. Currently, the major sources of collagen are the tendon or skin of bovine and porcine. However, the prevalence of transferring diseases including foot-and-mouth disease (FMD), bovine spongiform encephalopathy (BSE), and transmissible spongiform encephalopathy (TSE), and the religious barriers of Muslims and Jews [,,], have limited its application []. Therefore, it is essential to find a safety source of collagen for human application. Marine collagen has been isolated and characterized from various marine sources, and can generally be categorized according to source: vertebrates or invertebrates. Vertebrates sources include cat fish [], silvertip shark [], salmon [], yellow tuna [], and marine mammals such as minke whale []. Invertebrates source include jellyfish [,,], squid [], and sponges [,,,]. Researchers have demonstrated that similar characteristics exist between marine collagen and mammalian [,,]. However, some differences exist between collagen extracted from marine sources and collagen extracted from mammals. Compared with the mammalian collagen, marine collagen has lower gelling and melting temperatures, but relatively higher viscosities than equivalent bovine forms []. Fish collagens show a similar amino acid distribution to mammalian collagen, with decreased amounts of proline and hydroxyproline, and increased serine, threonine, and in some cases, methionine and hydroxylysine []. Compared with mammalian collagen, the difference in the amino acid distribution of fish collagen causes labile cross-links and heat sensitivity []. In recent years, marine collagen has been widely used in medicine and tissue engineering fields [], such as cartilage [], corneal [], ligament [], muscle [], skin [], tracheal [], and vascular [].
The Nile tilapia (Oreochromis niloticus) is a worldwide cultured fish that possesses an important position in China’s aquaculture and exports industry []. Tilapia skin is a main by-product of its processing, which contains approximately 30% collagen []. Our previous study revealed that both acid-soluble collagen (ASC) and pepsin-soluble collagen (PSC) extracted from Nile tilapia skin can be used as raw materials in food and cosmetic preparation []. Further, we want to explore whether either is suitable for use in biomedical applications. However, the biocompatibilities of pure collagen extracted from Nile tilapia need be addressed, since the biocompatibilities of fish collagen are profoundly influenced by the molecular composition and arrangement, which is thought to be varied by different extraction methods. In this work, we extract acid-soluble (ASC) and pepsin-soluble (PSC) collagen from Nile tilapia skin, and then describe their physical properties, chemical properties, and biocompatibilities, in order to explore the possibility for applications in biomedical fields.
2. Results and Discussion
2.1. Preparation of ASC and PSC
The yields (dry weight basis, 19.07% and 19.61% respectively) of ASC and PSC were in highly agreement with former reports [,]. Comparison of the collagens extract from different fish have been reported, including the skin of paper nautilus (55.2%) [], bigeye snapper (1.59%) [], deep-sea redfish (10.3%) [], Japanese sea bass (40.7%) [], yellow sea bream (40.1%) [], and grass carp (45.3%) []. In contrast, the yield of collagen from tilapia was different from these reported species. The results suggest that some discrepancies might exist among these fish species. In addition, the yield of ASC was a little bit lower than the yield of PSC, this might be due to the disadvantage of the solubility of cross-links formed through the reaction of aldehyde with lysine and hydroxylysine at telopeptide helical sites [], which also can be explained by the results of Fourier transform infrared (FTIR) analysis. Besides the effects on solubility, the pepsin that is used in the extraction of PSC might bring other changes, such as the stability and biocompatibility of the resultant collagens.
2.2. Amino Acid Composition
Table 1 shows the amino acid composition of ASC and PSC, which is expressed as residues per 1000 total amino acid residues. According to Table 1, glycine is the most important component in ASC and PSC, with 322 and 343 residues, accounting for about one-third of the total amino acid residues. It is slightly higher than common aquatic organisms carp skin (311 residues), cod skin (308 residues), squid skin (269 residues) [,], and very similar to land mammals’ bovine skin (320 residues), bovine skin (334 residues), and porcine skin (326 residues) [,]. Glycine is the most important amino acid in collagen. All members of the collagen family have a tripeptide (Gly-X-Y) repetitive structure, which plays an important role in the formation of the triple-helix structure. The tripeptides (Gly-X-Y) are repeatedly arranged on each chain of collagen, accounting for about 20–30% of all tripeptide structures. The X position of Gly-X-Y is often occupied by proline, which is consistent with the result in Table 1 (115 and 106 proline residues in ASC and PSC).

Table 1.
The amino acid composition of acid-soluble collagen (ASC) and pepsin-soluble collagen (PSC) from Nile tilapia skin.
The existence of the triple-helix structure is the most direct evidence to distinguish collagen from gelatin. []. As is known, electrospinning is a method of stretching a polymer solution to fibers that have a diameter of about several hundred nanometers by electrostatic force. Due to its wide applicability, high efficiency, and simplicity, electrospinning is widely used in the field of tissue engineering scaffold materials.
In addition, it is worth noting that the content of hydroxyproline in tilapia skin is ASC (70 residues) and PSC (86 residues). As a characteristic component of collagen, the content of collagen in raw materials can be measured by the ratio of hydroxyproline. No cystine and tryptophan were detected in both the ASC and PSC of tilapia skin collagen, which was consistent with the characteristics of type I collagen.
2.3. FTIR Analysis
The FTIR spectra of ASC and PSC are exhibited in Figure 1. Each peak in the FTIR spectrums corresponds to the vibration of functional groups in the molecule []. The secondary structure of collagen is closely related to different types of hydrogen bonds []. By analyzing the FTIR spectrum of ASC and PSC, the different effects of the two extraction methods on the secondary structure of collagen were obtained. At room temperature, ASC and PSC mainly exhibited five absorption peaks at 3323 cm−1, 2931 cm−1, 1677 cm−1, 1546 cm−1, and 1242 cm−1, corresponding to amide A, amide B, amide I, amide II, and amide III, respectively.
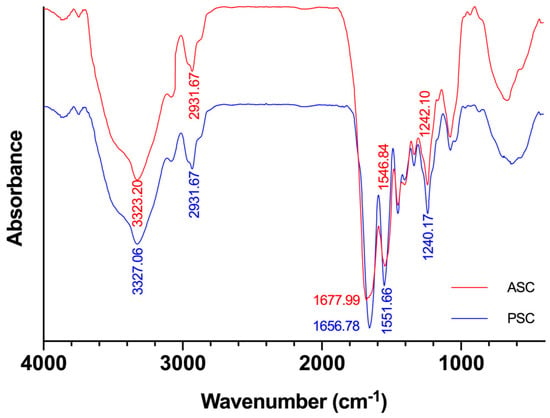
Figure 1.
Fourier transform infrared spectroscopy (FTIR) of ASC and PSC from Nile tilapia skin.
The wavenumber of the free N–H stretching vibration was located in the range of 3400–3440 cm−1, and the wavenumber of amide A was measured at 3323 cm−1, indicating that the N–H stretching vibration and the hydrogen bonding are combined []. The amide A absorption peak of PSC at 3323.20 cm−1 is slightly higher than ASC at the wavenumber of 3327.06 cm−1, indicating that more N–H groups in the ASC are hydrogen-bonded, which suggested that the PSC is slightly weaker than ASC in structural stability. Both ASC and PSC have a weak absorption peak at the amide B band at 2931.67 cm−1, indicating the asymmetric stretching vibration of –CH2 []. Studies have shown that the amide I, amide II, and amide III bands are related to the triple-helix structure of collagen []. The amide I bands were attributed to C=O stretching vibration, and the amide I absorption bands of ASC and PSC appeared at 1677.99 cm−1 and 1654.78 cm−1, respectively. The red shift of C=O stretching vibration may be caused by the use of the pepsin-degraded part of the telopeptides during the preparation process. The telopeptides plays an important role in the triple-helix structure of collagen, which was attributed to the covalent aldol cross-linking and the collagen fiber formation. Excision of the telopeptides does not completely destroy the natural collagen structure [], but it leads to an incomplete collagen protein structure and increased solubility [], which explained why the PSC yields are higher than those of the ASC. From the consideration of biomedical materials applications, much attention should be paid to the effects of the telopeptides on the immunogenicity of collagen. It has been reported that the immunogenicity of collagen’s telopeptides was considered the most important factor of the collagen-induced immune response []. Previous studies have also suggested that the peptides located at the center of the triple helix of pepsin-treated collagen (from skin of bovine) are the major antigenic sites that cause human immune responses [].
Amide II bands produced by N–H bending vibrations and C–N stretching vibrations are usually located in the range of 1550 to 1600 cm−1. Research has shown that the red shift of the amide II peak is related to the hydrogen bond increase of the N–H group []. The amide II absorption bands of ASC and PSC were detected at wavenumbers of 1546.84 cm−1 and 1551.66 cm−1, respectively. The result indicates that there are more hydrogen bonds between the peptide chains in ASC than PSC.
The amide III bands represent the combination of the C–N stretching vibration and the N–H bending vibration []. The amide III absorption bands of ASC and PSC appeared at 1242.10 cm−1 and 1240.17 cm−1 respectively, which is consistent with previous studies [].
2.4. Thermal Denaturation Temperatures of ASC and PSC
The tripeptide chains are bound by non-covalent bonds such as hydrogen bonds, which is the basis of the stability of collagen. When the collagen molecules absorbed enough heat from the outside, these non-covalent bonds were destroyed, causing the triple-helix structure to become a random coil structure and destroying the biological properties of collagen. The thermal stability of ASC and PSC were studied by viscosity measurement []. According to Figure 2, ASC and PSC have similar curves, and their denaturation temperatures (Td) were 36.1 °C and 34.4 °C, respectively. The Td values can be regarded as the temperature at which the triple-helix structure of collagen is deformed into a random coil structure. The Td values of ASC and PSC from tilapia skin are similar to the collagen extracted from fish living in warm tropical climates such as salmon (29.3 °C) and bigeye snapper (30.4 °C) [], and higher than the cold-water fish, such as Baltic herring (15.0 °C) and Argentine salmon (10.0 °C) [], but lower than terrestrial animals such as bovine (39.7 °C) or porcine (37 °C) []. The difference in amino acid composition was the primary cause of the different thermostability of collagen. The loss of the PSC telopeptides has a certain influence on the stability of the triple-helix structure, resulting in lower thermal stability than ASC, which is consistent with the results of amino acid composition analysis and FTIR analysis. Although the thermal stability of tilapia skin collagen is lower than that of terrestrial organisms, it is higher than that of common aquatic organisms, which is an advantage for its application in the field of biomedical materials.
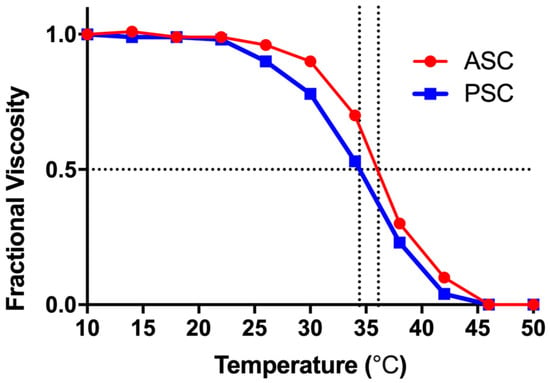
Figure 2.
Thermal denaturation curves of ASC and PSC from Nile tilapia skin. The denaturation temperature was determined as the mid-point temperature where viscosity changes reach 0.5.
2.5. SDS-PAGE
SDS-PAGE results present the subunit composition and type of collagen instinctively. As shown in Figure 3, ASC and PSC have very similar protein bands, which have been identified as trimers (γ chains), dimers (β chains), and two alpha chains (α1 and α2). Up to now, it has been reported that there are two different collagen trimers in tilapia skin, (α1)2α2 and α1α2α3, and that the two chains of α1 and α2 have the same molecular weight, which cannot be distinguished by electrophoresis []. According to Sun [], the structure of Nile tilapia collagen is (α1)2α2 type. The band intensity of α1 is twice that of α2, indicating that both ASC and PSC are type I collagen. In addition, the electrophoresis bands are clear, and have no low molecular weight bands, showing that the molecular structure of collagen was well preserved during the extraction process. Although some telopeptides were lost after the treatment of PSC, the banding pattern of PSC was similar to ASC, indicating that the PSC extraction process does not affect the integrity of the triple-helix structure.
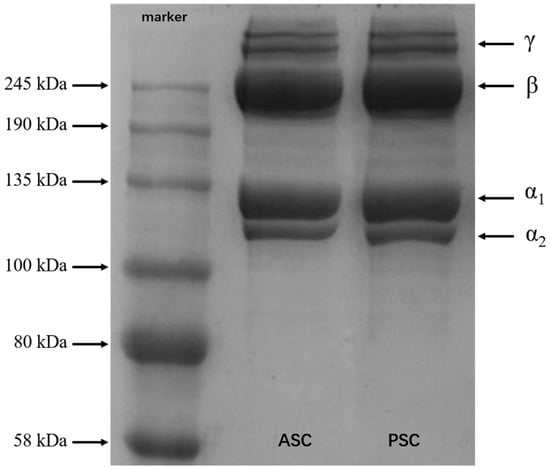
Figure 3.
SDS-PAGE patterns of ASC and PSC from Nile tilapia skin.
2.6. Morphology
Figure 4 shows the ultrastructure of the cross-section of the lyophilized collagen sponge. The graphs showed that ASC and PSC exhibit slightly different microscopic morphology. From the 50× magnified image, ASC and PSC display a loose porous network structure, but the pores of ASC show a more uniform and less fiber structure pattern than PSC. As shown in the 400× magnified image, ASC is a dense sheet-like film with uniform alignment, and PSC exhibits irregularly arranged curls.
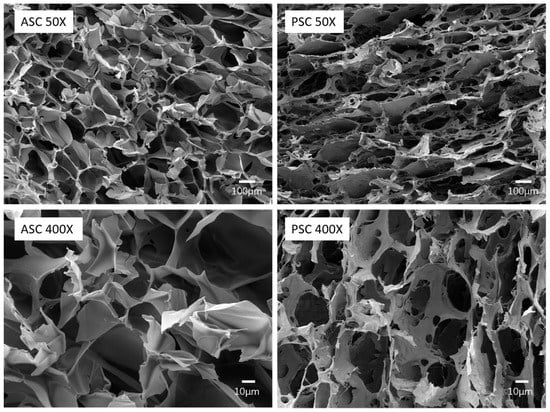
Figure 4.
Morphological features of Nile tilapia skin collagen using SEM.
The microstructures determine the physicochemical properties and biofunctionality of the materials; they have a significant value to the application of collagen in biomedical materials. The SEM results of ASC present typical characteristics of aquatic collagen, such as miiuy croaker [] and Acipenser schrenckii [], while the microstructures of PSC exhibit a fibrous structure similar to that of terrestrial collagen, such as bovine []. This difference may be caused by the structure of PSC being changed under the influence of pepsin, forming more collagen fibers in a non-crosslinked state, which is consistent with the results of FTIR. In the field of biomedical materials, greater porosity facilitates the migration of cells into the interior of the scaffolds, which has certain advantages for promoting wound healing. The lower degree of cross-linking is beneficial to the dissolution and re-processing of collagen, and is suitable for many processes such as electrospinning. Therefore, the microstructures of ASC and PSC indicate that they are appropriate for use in different biomedical material fields.
2.7. Endotoxin Test
Endotoxin, which is also as known as lipopolysaccharide, is found in the outer membrane of Gram-negative bacteria. When endotoxin invades the body, it can cause shock, fever, a fall in blood pressure, and death [,]. Endotoxin must be eliminated as much as possible from biomedically used materials. We used an endotoxin denial test to investigate the biosafety of ASC and PSC. The results show that negative results were obtained in endotoxin (below 0.01 EU/mL) in leach liquor of ASC and PSC.
2.8. Cell Proliferation
In order to fully reflect the application potential of ASC and PSC in biomedical materials, MC3T3E1, L929, and human umbilical vein endothelial cells (HUVEC) cells were selected to test its in vitro cytotoxicity. Porcine collagen (PC) was used as a comparison group. According to Figure 5, the addition of collagen significantly promoted cell proliferation compared to the control group. MC3T3E1 and L929 cells had higher proliferation rates in the ASC treatment groups. However, HUVEC had a higher proliferation rate after PSC treatment. Besides, the ASC and PSC treatment groups had higher proliferation rates than the PC group in all three kinds of cells. It has been reported that Nile tilapia collagen contains an antibacterial tilapia piscidin (tp4) that has the ability to stimulate cell proliferation and activate epidermal growth factor (EGF), transforming growth factor (TGF), and vascular endothelial growth factor (VEGF) []. In the study of blue shark skin collagen, PSC had a higher proliferation rate for differentiated mouse bone marrow-mesenchymal stem (dMBMS) cells than ASC, while ASC and PSC had no significant difference regarding the proliferation rate of MC3T3E1 cells []. The results indicate that both ASC and PSC have potential application in fields requiring wound dressing. The osteogenic properties of ASC make it suitable for applications in the field of bone and cartilage repair, while PSC could be used in areas such as the promotion of angiogenesis or artificial blood vessels.
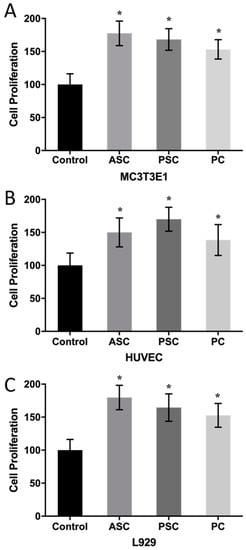
Figure 5.
The effect of ASC, PSC, and porcine collagen (PC) on cell proliferation. (A): MC3T3E1, (B): L929, (C): HUVEC. Values with * show significant differences (p < 0.05) between groups, as determined by one-way ANOVA.
2.9. Biocompatibility Evaluation
The acute systemic toxicity assay was usually selected to measure the adverse effect of biomedical materials that result either from a single exposure or from multiple exposures in a short period of time. It is an important indicator of biosafety assessment []. To evaluate the biosafety of aquatic collagen, porcine collagen was designated as a comparison group. As shown in Figure 6, no significant difference in body weight was observed between the experimental groups and the control group at four hours, 24 h, 48 h, and 72 h after the intraperitoneal injection of the collagen leach liquor. Moreover, the weight of the control group and the experimental groups increased significantly over time, and no dead samples appeared. Furthermore, there were no significant differences between the ASC, PSC, and PC groups. The results showed that ASC and PSC collagen sponges were produced by our process without acute systemic toxicity, and there was not a significant difference from the acute toxicity from commercially available porcine collagen products.
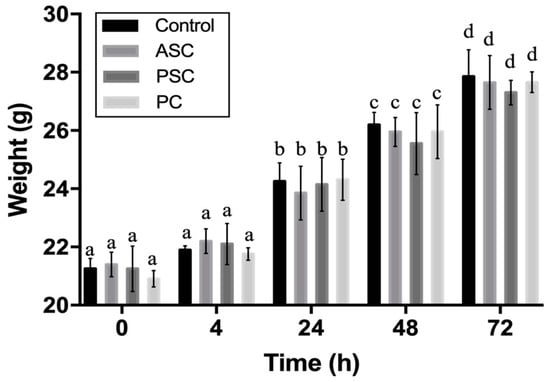
Figure 6.
Weight changes of mice after intraperitoneal injection. The different letters in the same group (same type of the bar) represent significant difference (p < 0.05).
3. Materials and Methods
3.1. Raw Materials
Nile tilapia skins were procured from Zhenhua Aquatic Product Company (Guangzhou, Guangdong province of China). Frozen skins were thawed with running water and removed from the residuals manually. They were chopped into small pieces and stored at −20 °C. Porcine collagen (PC) was obtained from Kele Biotech (Chengdu, China). All of the other reagents used were of analytical grade.
3.2. Preparation of ASC
All the procedures were carried out at 4 °C to minimize collagen denaturation. An acellular environment was used in the extraction process to reduce the exogenous pyrogen. The pieces of Nile tilapia skins were soaked with 0.1 M of NaOH for 24 h with continuous stirring to remove the non-collagenous proteins. Washing the fish skins repeatedly with cooled deionized water ensured that they were neutralized. Adding 0.5 M of acetic acid in a ratio of 1:50 (w/v) started extraction for two days. Then, they were centrifuged at 10,000 rpm for 30 min at 4 °C. NaCl was added to the collected supernatants until a concentration of 0.9 M was reached to salt out the collagen. The precipitated collagen was separated by centrifugation at 10,000 rpm for 30 min at 4 °C and dissolved in 0.5 M of acetic acid. Then, the solution was dialyzed for 24 h against 0.1 M of acetic acid in a dialysis membrane with a molecular weight cut-off of 50 kDa, and then for 48 h against ultra-pure water; the water was changed every eight hours. The resulting collagen was freeze-dried for three days and sealed in polythene bags until further use.
3.3. Preparation of PSC
The extraction process of PSC was basically identical to the extraction of ASC except for slightly differences. The tilapia skins were extracted by 0.5 M of acetic acid containing 0.1% (w/v) pepsin for 48 h with stirring. Then, the supernatant was dialyzed against 0.02 M of Na2HPO4 for 24 h with solution changed every eight hours before being dialyzed against 0.1 M of acetic acid.
3.4. Extraction Yield of ASC and PSC
The calculation of ASC and PSC yield referred to previous reports [] and the equation was as follows:
yield (%) = weight of dried collagen (g) × 100/weight of dried skins (g)
3.5. Amino Acid Composition
The ASC and PSC samples were hydrolyzed by dissolving in 6 M of HCl at 110 °C for 24 h. The solution was analyzed with an amino acid analyzer (835-50, Hitachi, Tokyo, Japan).
3.6. Denaturation Temperature (Td)
The denaturation temperature of collagen from tilapia skin was determined by differential scanning calorimetry (DSC) (Netzsch DSC 200PC, Selb, Bavaria, Germany). A collagen sample with a concentration of 5 mg/mL was dissolved in 0.05 M of acetic acid and sealed in an aluminum pan for scanning. Then, the endothermal curve from 10 °C to 50 °C was obtained at a rate of 5 °C/min in a nitrogen atmosphere.
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
The infrared absorption characteristics of collagen were obtained by an FTIR spectrometer (Tensor 27, BRUKER, Bremen, Germany). First, one-mg collagen samples were mixed with potassium bromide (KBr) at a ratio of 1:100 and pressed into pellets with a manual mechanical squeezing device. The spectra were recorded with a wavenumber range from 4000 to 400 cm−1 at the resolution of 2 cm−1.
3.8. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The protein molecular mass analysis was studied by SDS-PAGE according to Laemmli [] with 7.5% resolving gel and 5% stacking gel and 120 voltage using a Bio-Rad electrophoresis. The protein bands were stained with Coomassie Blue R250 and destained with 30% (v/v) methanol and 10% (v/v) acetic acid. The molecular mass of the subunits was analyzed by the location of the bands.
3.9. Scanning Electron Microscopy (SEM)
The morphology of ASC and PSC was observed using a scanning electron microscope (JSM-840, JEOL, Tokyo, Japan) operated at 5 kV. The samples were pasted on a blade and sputter-coated with gold at 30 mA for 15 min. The SEM images were obtained at 50× and 400× magnification.
3.10. Endotoxin Denial Test
An endotoxin denial test was undertaken using a chromogenic end-point tachypleus amebocyte lysate (CE TAL) assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Test samples and standards were prepared according to manufacturer’s guidelines. Absorbance was measured at 405 nm using a microplate spectrophotometer (SynergyTM 2, BioTek, Winooski, VT, USA). Data were corrected to exclude background readings. A negative result was defined as a level below 0.01 EU/mL [].
3.11. Cell Culture
MC3T3E1 and L929 cells were obtained from a cell bank (Procell Life Science & Technology Co., Ltd., Wuhan, China) and HUVEC were received from an institute of zoology (Chinese academy of sciences, Beijing, China). MC3T3E1 was grown as monolayer at 37 °C in a 5% CO2 incubator and cultured in Dulbecco’s modified eagle medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS) and 1% (w/v) penicillin-streptomycin. L929 and HUVEC cells were cultured in MEM and DMEM/F12 (1:1), respectively.
3.12. Cytotoxicity In Vitro
The collagen samples were cut into disks sized for a 48-well plate and sterilized by 60Co for 18 h with an accumulative dose of 20 kGy before using. The cells were seeded a concentration of 1 × 104 cells/well and cultured with a specific medium. After seeding for 48 h, 50 μL of thiazolyl blue tetrazolium bromide (MTT) solution was added to the wells and incubated for four hours at 37 °C/5% CO2. Wells with collagen were used as negative controls of cytotoxicity. We dissolved the formazan salts using DMSO with shaking for 10 min and replaced the solution with blank wells. The absorbance was measured using a 96-well microplate reader at 490 nm [].
3.13. Acute Systemic Toxicity Assay
All animal procedures were approved by the ethical committee of animal research in the Ocean University of China, and complied with the requirements of the National Act on the use of experimental animals (China). The acute systemic toxicity of tilapia collagen was investigated according to ISO 10993-11:2009. The collagen samples were cut into 1×1 cm sizes with 0.5-mm thickness and sterilized by 60Co before using. The leach liquor was prepared at a ratio of three cm2/mL collagen into saline between the total surface area of the materials for 72 h at 37 °C. Then, 20 Kunming (KM) mice (Licensed ID: SCXK 2014-0007) were divided into four groups randomly. Three experimental groups were intraperitoneally injected the leach liquor of ASC, PSC, and PC with a dose of 50 mL/kg respectively, and the blank control group was injected saline with a dose of 50 mL. The weight of mouse was weighed and recorded immediately after injection and at four hours, 24 h, 48 h, and 72 h after injection. The criteria that were used to assess acute systemic toxicity are shown in Table 2.

Table 2.
The criteria used to assess acute systemic toxicity.
3.14. Statistical Analysis
All of the experiments were replicated in triplicate and the values were expressed as means ± standard deviation (SD). The analyses of multiple groups by one-way ANOVA were using Macintosh GraphPad Prism Version 7, and we considered p-values of less than 0.05 to measure the significant differences.
4. Conclusions
This study showed that ASC and PSC extracted from Nile tilapia skin have typical type I collagen characteristics. No significant differences were found in the amino acid composition and physicochemical properties of ASC and PSC. Both ASC and PSC have a complete triple-helix structure. The thermal stability of PSC was slightly lower than that of ASC, similar to mammals, and higher than cold-water fish. ASC has the effect of promoting osteogenesis and fibroblastation, while PSC is beneficial to the formation of vascular endothelial cells by comparison, and neither ASC nor PSC cause acute systemic toxicity. In summary, collagen extracted from Nile tilapia skin is a biocompatible type I collagen with potential as a biomedical material.
Author Contributions
Conceptualization, W.-K.S.; validation, L.-L.S.; formal analysis, W.-K.S.; resources, D.L. and H.H.; data curation, W.-K.S.; writing—original draft preparation, W.-K.S.; writing—review and editing, H.H.; supervision, B.-F.L.; project administration, H.H.; funding acquisition, B.-F.L.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 31772046 and 31471606), Key Research & Development Plan of Shandong Province (Nos. 2017YYSP015, 2016YYSP005 and 2016YYSP017), and National Key R&D Program of China (2018YFC0311200).
Acknowledgments
The authors acknowledge Yuan-Yuan Wang (Marine Biomedical Research Institute of Qingdao) for the kind contribution of cell cultures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, L.; Li, B.; Song, W.; Si, L.; Hou, H. Characterization of Pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]
- Birk, D.E.; Bruckner, P. Collagen Suprastructures. In Collagen; Brinckmann, J., Notbohm, H., Müller, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247, pp. 185–205. ISBN 978-3-540-23272-8. [Google Scholar]
- Ogawa, M.; Portier, R.J.; Moody, M.W.; Bell, J.; Schexnayder, M.A.; Losso, J.N. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocephalus). Food Chem. 2004, 88, 495–501. [Google Scholar] [CrossRef]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.-P.; Horejs, C.-M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.M.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Tatara, A.M.; Kontoyiannis, D.P.; Mikos, A.G. Drug delivery and tissue engineering to promote wound healing in the immunocompromised host: Current challenges and future directions. Adv. Drug Deliv. Rev. 2018, 129, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kolacna, L.; Bakesova, J.; Varga, F.; Kostakova, E.; Planka, L.; Necas, A.; Lukas, D.; Amler, E.; Pelouch, V. Biochemical and Biophysical Aspects of Collagen Nanostructure in the Extracellular Matrix. Physiol. Res. 2007, 56, S51. [Google Scholar] [PubMed]
- Chuaychan, S.; Benjakul, S.; Kishimura, H. Characteristics of acid- and pepsin-soluble collagens from scale of seabass (Lates calcarifer). Lwt Food Sci. Technol. 2015, 63, 71–76. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Extraction and characterisation of pepsin-solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chem. 2010, 120, 817–824. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Mizuta, S.; Yokoyama, Y.; Yoshinaka, R. Purification and characterization of molecular species of collagen in the skin of skate (Raja kenojei). Food Chem. 2007, 100, 921–925. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Bama, P.; Vijayalakshimi, M.; Jayasimman, R.; Kalaichelvan, P.T.; Deccaraman, M. Extraction of collagen from cat fish (Tachysurus maculatus) by pepsin digestion and preparation and characterization of collagen chitosan sheet. Int. J. Pharm. Pharm. Sci. 2010, 2, 5. [Google Scholar]
- Jeevithan, E.; Bao, B.; Bu, Y.; Zhou, Y.; Zhao, Q.; Wu, W. Type II Collagen and Gelatin from Silvertip Shark (Carcharhinus albimarginatus) Cartilage: Isolation, Purification, Physicochemical and Antioxidant Properties. Mar. Drugs 2014, 12, 3852–3873. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Marques, A.; Martins, E.; Silva, T.; Reis, R. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Woo, J.-W.; Yu, S.-J.; Cho, S.-M.; Lee, Y.-B.; Kim, S.-B. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocoll. 2008, 22, 879–887. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N.; Nagashima, T. Collagen from common minke whale (Balaenoptera acutorostrata) unesu. Food Chem. 2008, 111, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [PubMed]
- Bermueller, C.; Schwarz, S.; Elsaesser, A.F.; Sewing, J.; Baur, N.; von Bomhard, A.; Scheithauer, M.; Notbohm, H.; Rotter, N. Marine Collagen Scaffolds for Nasal Cartilage Repair: Prevention of Nasal Septal Perforations in a New Orthotopic Rat Model Using Tissue Engineering Techniques. Tissue Eng. Part A 2013, 19, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish collagen and alginate: Combined marine materials for superior chondrogenesis of hMSC. Mater. Sci. Eng. C 2016, 64, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Wichuda, J.; Sunthorn, C.; Busarakum, P. Comparison of the properties of collagen extracted from dried jellyfish and dried squid. Afr. J. Biotechnol. 2016, 15, 642–648. [Google Scholar] [CrossRef]
- Pozzolini, M.; Bruzzone, F.; Berilli, V.; Mussino, F.; Cerrano, C.; Benatti, U.; Giovine, M. Molecular Characterization of a Nonfibrillar Collagen from the Marine Sponge Chondrosia reniformis Nardo 1847 and Positive Effects of Soluble Silicates on Its Expression. Mar. Biotechnol. 2012, 14, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural Studies on the Collagen of the Marine Sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Preparation and characterization of marine sponge collagen nanoparticles and employment for the transdermal delivery of 17β-estradiol-hemihydrate. Drug Dev. Ind. Pharm. 2009, 35, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, C.; Lin, H.; Yang, P.; Hong, P.; Jiang, Z. Isolation and characterisation of acid-solubilised collagen from the skin of Nile tilapia (Oreochromis niloticus). Food Chem. 2009, 116, 879–883. [Google Scholar] [CrossRef]
- Exposito, J.-Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The Fibrillar Collagen Family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Eastoe, J.E. The amino acid composition of fish collagen and gelatin. Biochem. J. 1957, 65, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, B.H. Investigation of viscosity and gelation properties of different mammalian and fish gelatins. Food Hydrocoll. 1991, 5, 353–361. [Google Scholar] [CrossRef]
- Berillis, P. Marine collagen: Extraction and applications. In Research Trends in Biochemistry, Molecular Biology and Microbiology; Madhukar, S., Ed.; SM Group: Dover, DE, USA, 2015; pp. 1–13. [Google Scholar]
- Nagai, N.; Yunoki, S.; Suzuki, T.; Sakata, M.; Tajima, K.; Munekata, M. Application of cross-linked salmon atelocollagen to the scaffold of human periodontal ligament cells. J. Biosci. Bioeng. 2004, 97, 389–394. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Tsai, W.-C.; Chang, S.-H. Collagen-PVA aligned nanofiber on collagen sponge as bi-layered scaffold for surface cartilage repair. J. Biomater. Sci. Polym. Ed. 2017, 28, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.S.; Verbridge, S.S.; Dahlgren, L.A.; Kakar, S.; Guelcher, S.A.; Goldstein, A.S. Fiber/collagen composites for ligament tissue engineering: Influence of elastic moduli of sparse aligned fibers on mesenchymal stem cells: Stiffness of Sparse Aligned Fibers on MSC Differentiation. J. Biomed. Mater. Res. Part A 2016, 104, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Tamura, K.; Mineguchi, R.; Ishikawa, Y.; Haraguchi, Y.; Shimizu, T.; Hara, Y. In situ cross-linked electrospun fiber scaffold of collagen for fabricating cell-dense muscle tissue. J. Artif. Organs 2016, 19, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Rother, S.; Zimmermann, H.; Lee, P.S.; Moeller, S.; Schnabelrauch, M.; Koul, V.; Jordan, R.; Hintze, V.; Scharnweber, D. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application—The role of chondroitin sulfate and sulfated hyaluronan. Mater. Sci. Eng. C 2017, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zheng, H.; Chen, J.; Wang, Y.; Sun, B.; Morsi, Y.; El-Hamshary, H.; Al-Deyab, S.S.; Chen, C.; Mo, X. Application of a bilayer tubular scaffold based on electrospun poly (L-lactide-co-caprolactone) collagen fibers and yarns for tracheal tissue engineering. J. Mater. Chem. B 2017, 5, 139–150. [Google Scholar] [CrossRef]
- Haghjooy Javanmard, S.; Anari, J.; Zargar Kharazi, A.; Vatankhah, E. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. Biomater. Appl. 2016, 31, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyan, Y.E.; Zeng, S.; Wenguo, Y.U.; Wenlong, W.U.; Zeng, X.; Huang, L. Study on nutrient components and the extracting condition of the skin gelatin of tilapia. South China Fish. Sci. 2008, 4, 55–60. [Google Scholar]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.; Qiao, Y.; Tian, Y.; Liu, J.; Qin, S.; Wu, W. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Wang, H.; Zhang, H.; Wang, M.; Liu, L. Physical-Chemical Properties of Collagens from Skin, Scale, and Bone of Grass Carp (Ctenopharyngodon idella). J. Aquat. Food Prod. Technol. 2014, 23, 264–277. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N. Preparation and partial characterization of collagen from paper nautilus (Argonauta argo, Linnaeus) outer skin. Food Chem. 2002, 76, 149–153. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008, 108, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T. Isolation of collagen from fish waste material—Skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N. Preparation and characterization of several fish bone collagens. J. Food Biochem. 2000, 24, 427–436. [Google Scholar] [CrossRef]
- Knott, L.; Bailey, A.J. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone 1998, 22, 181–187. [Google Scholar] [CrossRef]
- Ikoma, T.; Kobayashi, H.; Tanaka, J.; Walsh, D.; Mann, S. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol. 2003, 32, 199–204. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wang, B.; Chi, C.; Zhang, Q.-H.; Gong, Y.; Tang, J.-J.; Luo, H.; Ding, G. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Cui, F.; Xue, C.; Li, Z.; Zhang, Y.; Dong, P.; Fu, X.; Gao, X. Characterization and subunit composition of collagen from the body wall of sea cucumber Stichopus japonicus. Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Telemeco, T.A.; Ayres, C.; Bowlin, G.L.; Wnek, G.E.; Boland, E.D.; Cohen, N.; Baumgarten, C.M.; Mathews, J.; Simpson, D.G. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005, 1, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Noreen, R.; Moenner, M.; Hwu, Y.; Petibois, C. FTIR spectro-imaging of collagens for characterization and grading of gliomas. Biotechnol. Adv. 2012, 30, 1432–1446. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Zhao, Y.-Q.; He, Y.; Chi, C.-F.; Wang, B. Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Scales of Miiuy Croaker (Miichthys Miiuy). Mar. Drugs 2018, 16, 394. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.; Cole, C.G.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Heu, M.S.; Lee, J.H.; Kim, H.J.; Jee, S.J.; Lee, J.S.; Jeon, Y.-J.; Shahidi, F.; Kim, J.-S. Characterization of acid- and pepsin-soluble collagens from flatfish skin. Food Sci. Biotechnol. 2010, 19, 27–33. [Google Scholar] [CrossRef]
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater. Sci. Eng. C 2017, 77, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Beil, W.; Timpl, R.; Furthmayr, H. Conformation dependence of antigenic determinants on the collagen molecule. Immunology 1973, 24, 13–24. [Google Scholar] [PubMed]
- Ellingsworth, L.R.; Delustro, F.; Brennan, J.E.; Sawamura, S.; Mcpherson, J. The human immune response to reconstituted bovine collagen. J. Immunol. 1986, 136, 877–882. [Google Scholar] [PubMed]
- Wu, Q.-Q.; Li, T.; Wang, B.; Ding, G.-F. Preparation and characterization of acid and pepsin-soluble collagens from scales of croceine and redlip croakers. Food Sci. Biotechnol. 2015, 24, 2003–2010. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Kimura, S.; Ohno, Y.; Miyauchi, Y.; Uchida, N. Fish skin type I collagen: Wide distribution of an α3 subunit in teleosts. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 88, 27–34. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Q.; Chen, T.; Wang, Z.; Xu, J.; Ma, H. Characterization of collagen from the skin of Amur sturgeon (Acipenser schrenckii). Food Hydrocoll. 2014, 38, 104–109. [Google Scholar] [CrossRef]
- León-Mancilla, B.H.; Araiza-Téllez, M.A.; Flores-Flores, J.O.; Piña-Barba, M.C. Physico-chemical characterization of collagen scaffolds for tissue engineering. J. Appl. Res. Technol. 2016, 14, 77–85. [Google Scholar] [CrossRef]
- Brock-Utne, J.G.; Gaffin, S.L. Endotoxins and Anti-endotoxins (Their Relevance to the Anaesthetist and the Intensive Care Specialist). Anaesth. Intensive Care 1989, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.J.; McCutchan, J.A.; Fierer, J.; Glauser, M.P.; Sadoff, J.C.; Douglas, H.; Braude, A.I. Treatment of Gram-Negative Bacteremia and Shock with Human Antiserum to a Mutant Escherichia coli. New Engl. J. Med. 1982, 307, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-N.; Chan, Y.-L.; Wu, C.-J.; Chen, J.-Y. Tilapia Piscidin 4 (TP4) Stimulates Cell Proliferation and Wound Closure in MRSA-Infected Wounds in Mice. Mar. Drugs 2015, 13, 2813–2833. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Lee, J.; Wang, S.; Henrotin, Y.; de Val, J.; Regenstein, J.M.; Lim, S.; Bao, B.; Wu, W. Evaluation of Differentiated Bone Cells Proliferation by Blue Shark Skin Collagen via Biochemical for Bone Tissue Engineering. Mar. Drugs 2018, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Liao, N.; Cui, F.; Park, M.; Kim, H.-Y. Fabrication and durable antibacterial properties of electrospun chitosan nanofibers with silver nanoparticles. Int. J. Biol. Macromol. 2015, 79, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pei, X.; Liu, H.; Zhou, D. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus). Int. J. Biol. Macromol. 2018, 106, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Igawa, K.; Sugimoto, K.; Yoshizawa, Y.; Yanagiguchi, K.; Ikeda, T.; Yamada, S.; Hayashi, Y. Biological Safety of Fish (Tilapia) Collagen. Biomed Res. Int. 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Marques, A.; Silva, T.; Reis, R. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).