Rhodoptilometrin, a Crinoid-Derived Anthraquinone, Induces Cell Regeneration by Promoting Wound Healing and Oxidative Phosphorylation in Human Gingival Fibroblast Cells
Abstract
1. Introduction
2. Results
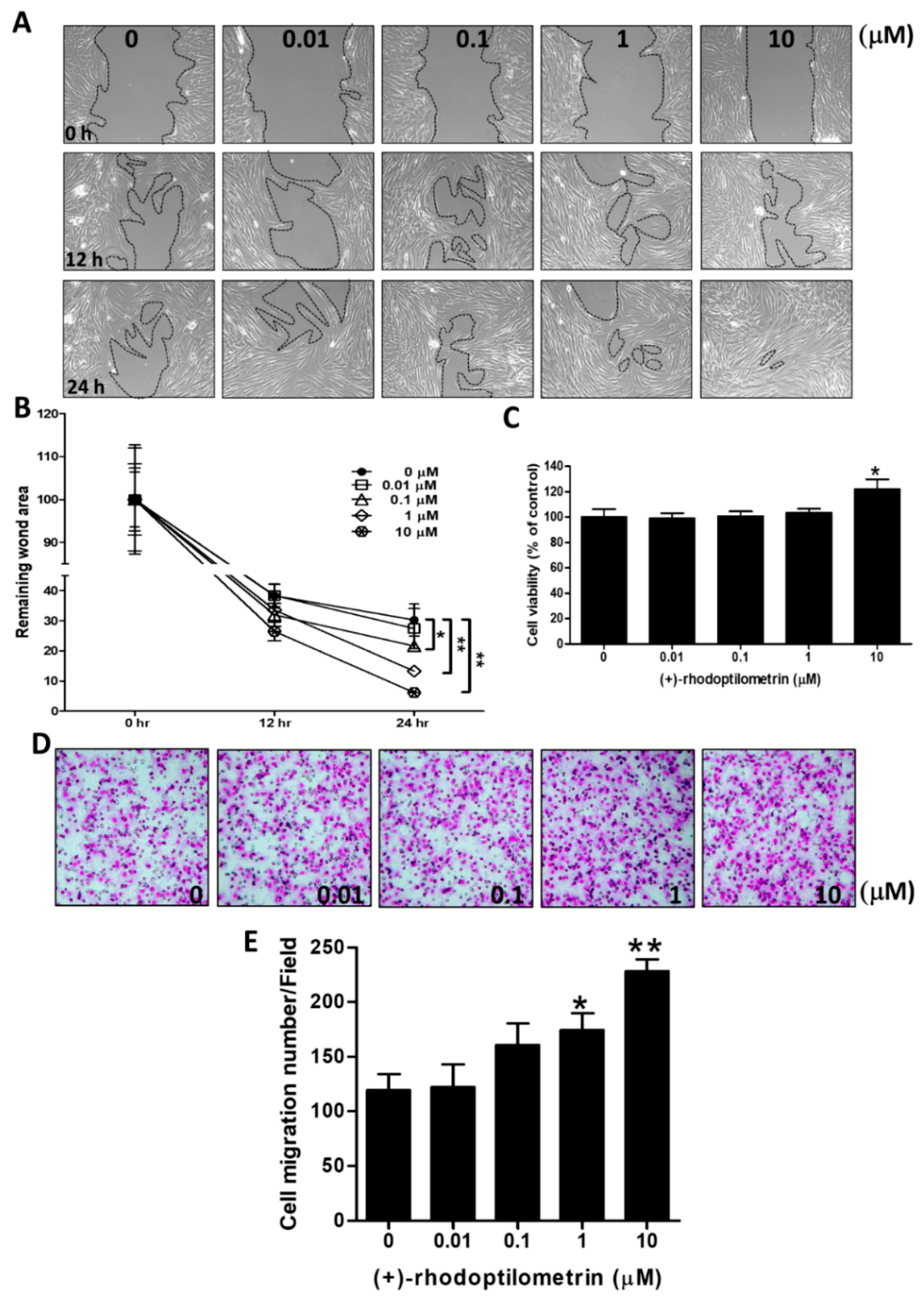
2.1. Effects of (+)-Rhodoptilometrin on Wound Healing, Cell Viability, and Cell Migration in hGF-1 Cells
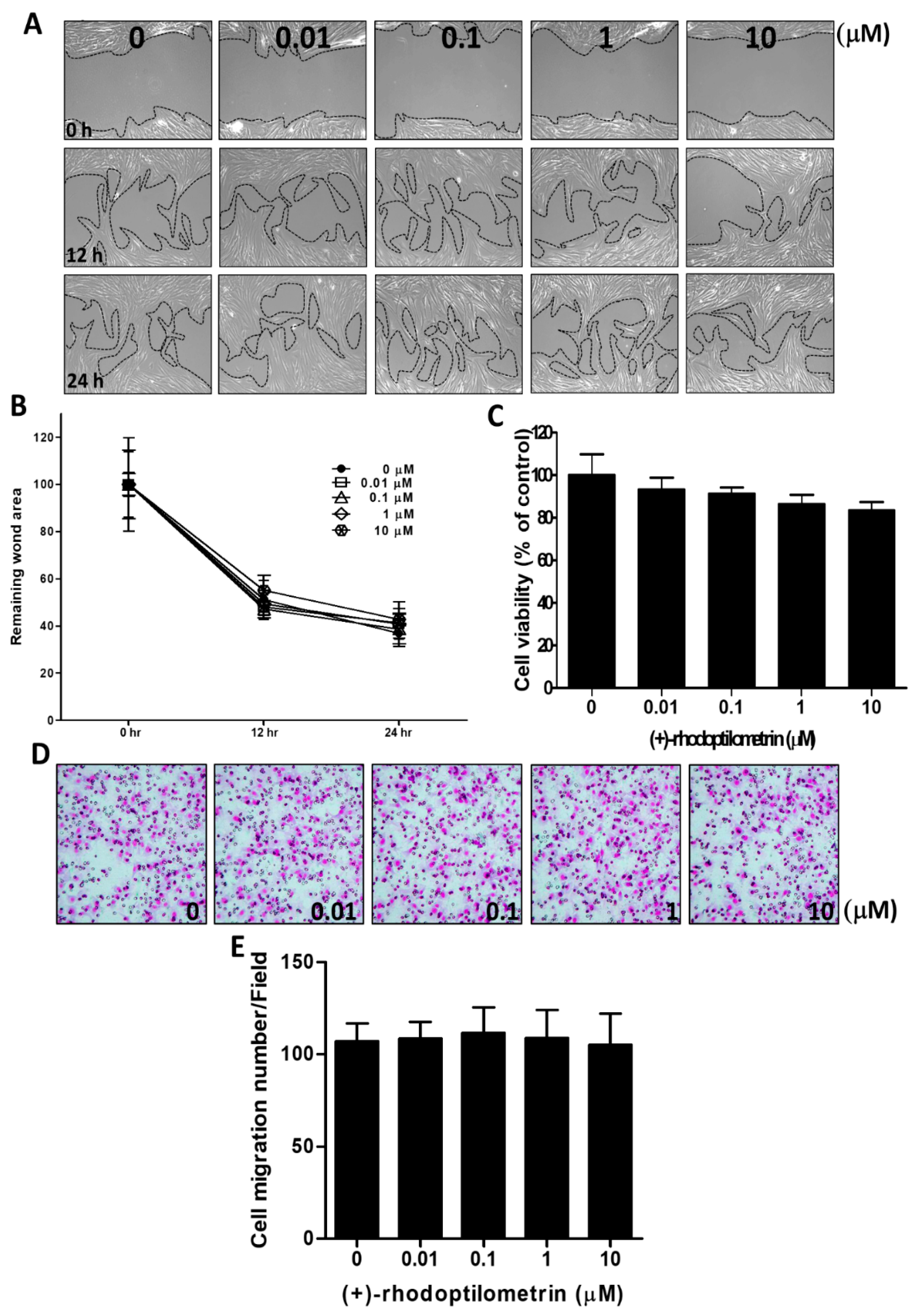
2.2. Effects of (+)-Rhodoptilometrin on Wound Healing, Cell Viability, and Cell Migration in Oral Mucosa Fibroblast (OMF) Cells
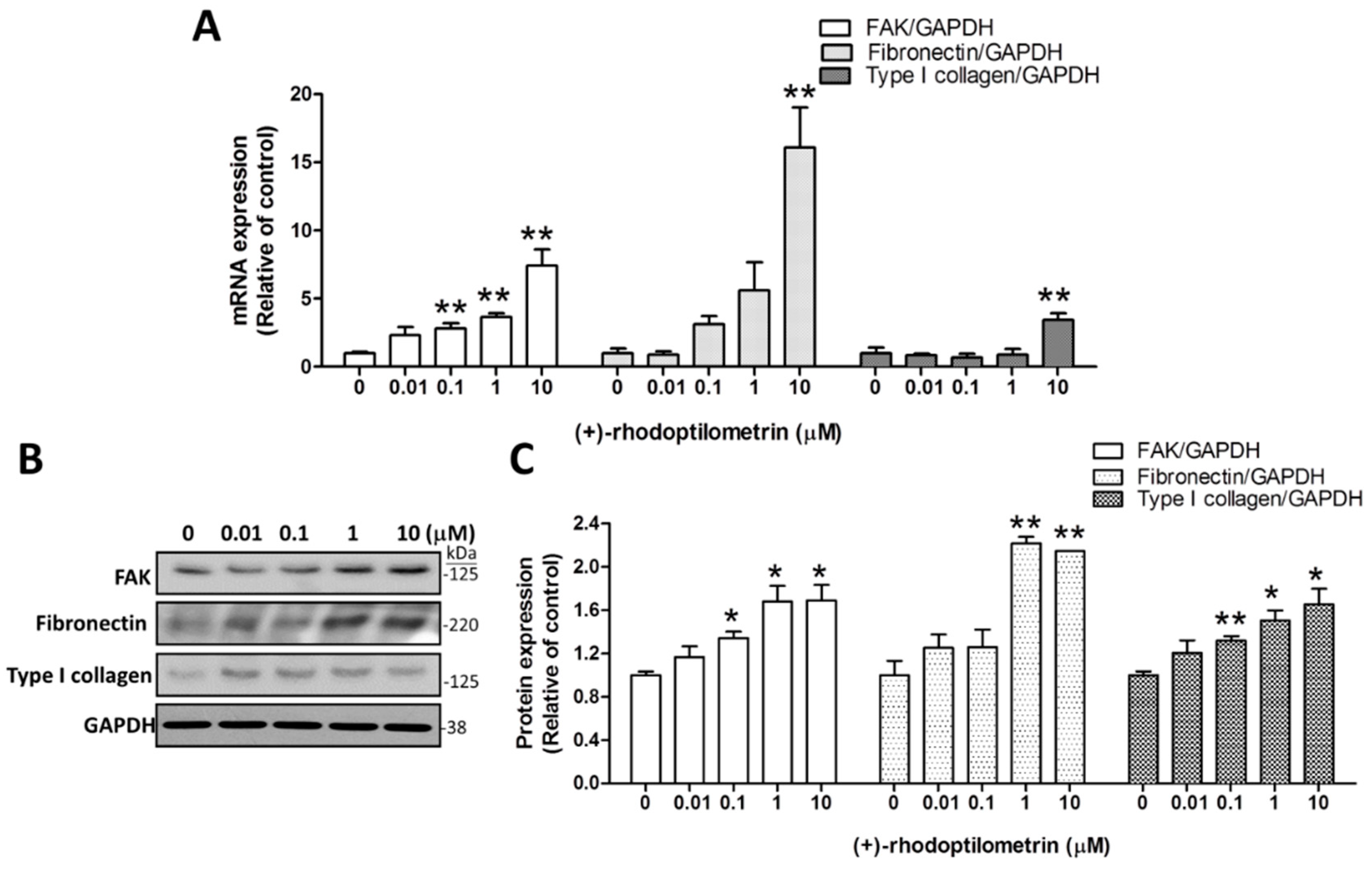
2.3. Effects of (+)-Rhodoptilometrin on the Gene and Protein Expression Levels of FAK, Fibronectin, and Type I Collagen
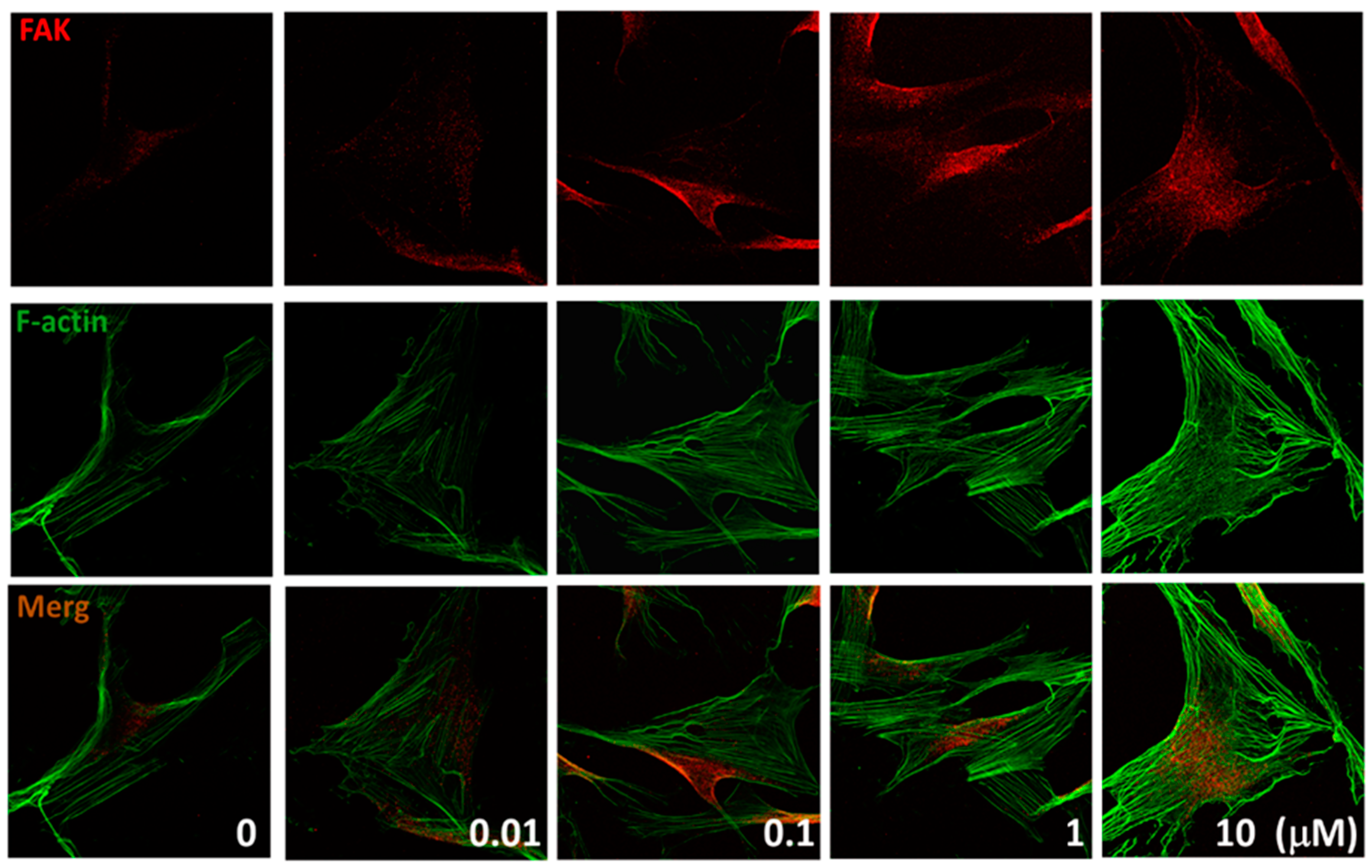
2.4. Effects of (+)-Rhodoptilometrin on the Distribution of FAK, and F-Actin Protein Expression
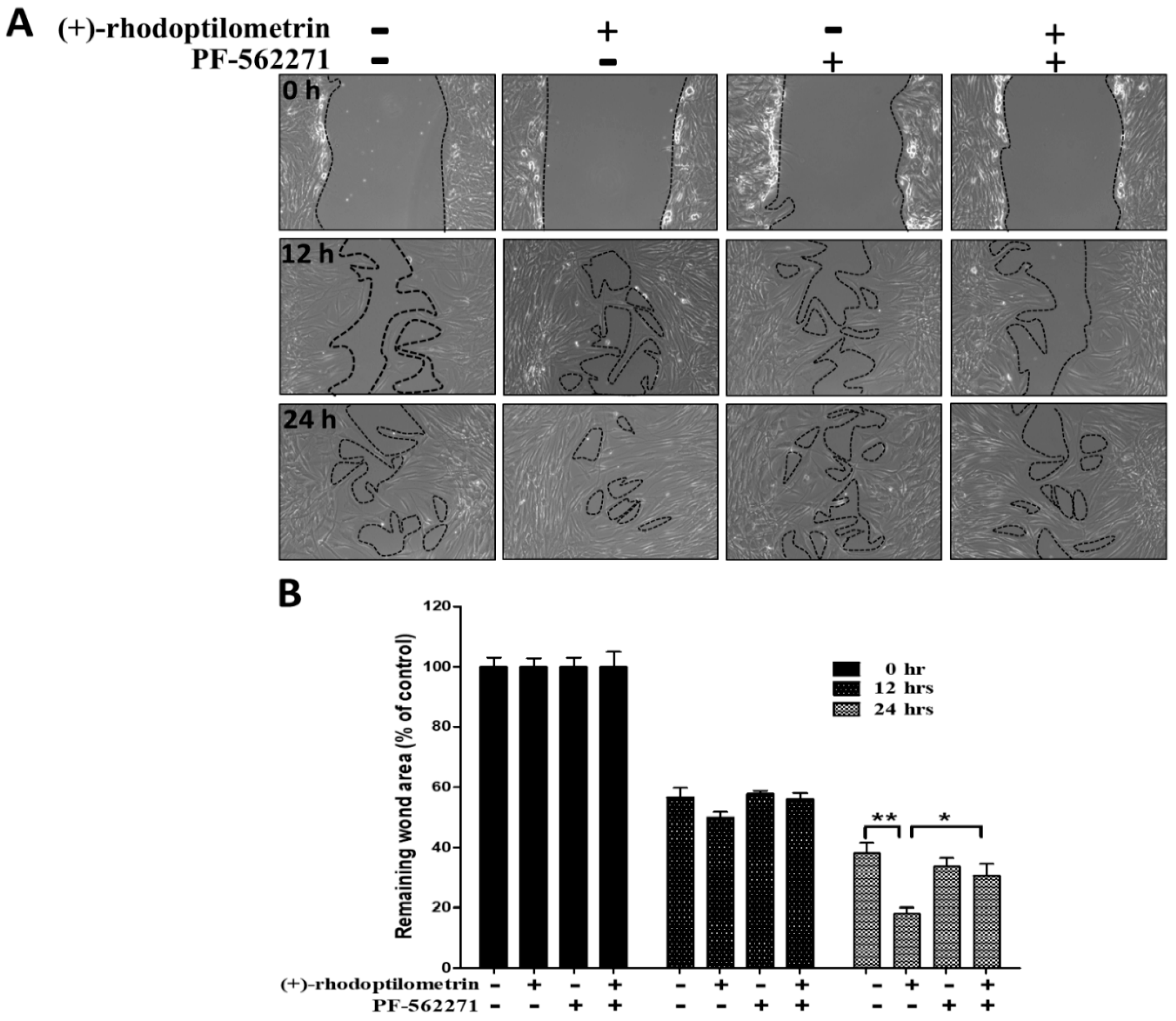
2.5. Effects of the FAK Inhibitor PF-562271 on Reverse Wound Healing in hGF-1 Cells Caused by (+)-Rhodoptilometrin
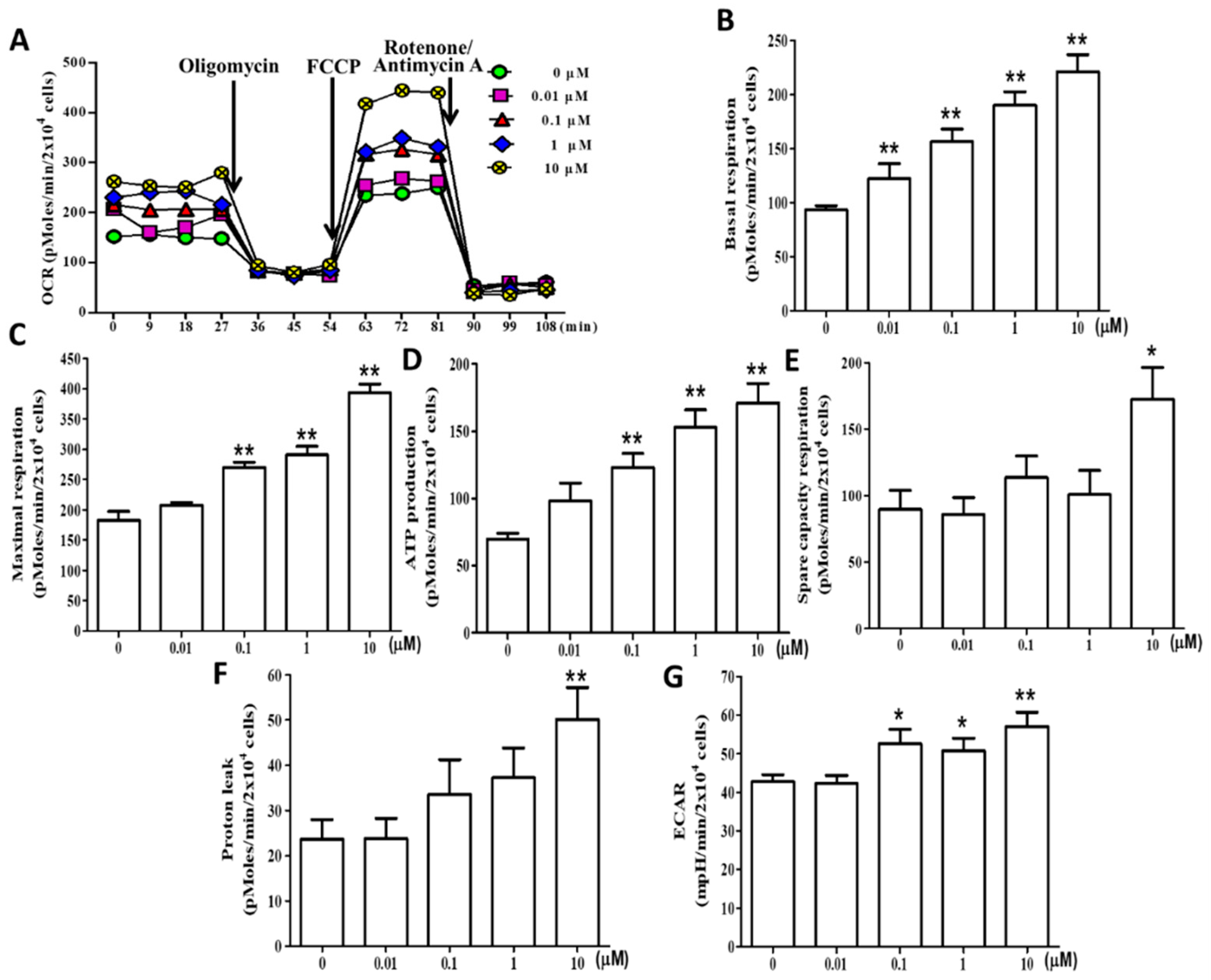
2.6. (+)-Rhodoptilometrin Promotes Mitochondrial Oxidative Phosphorylation (OXPHOS) and Glycolytic Function in hGF-1 Cells
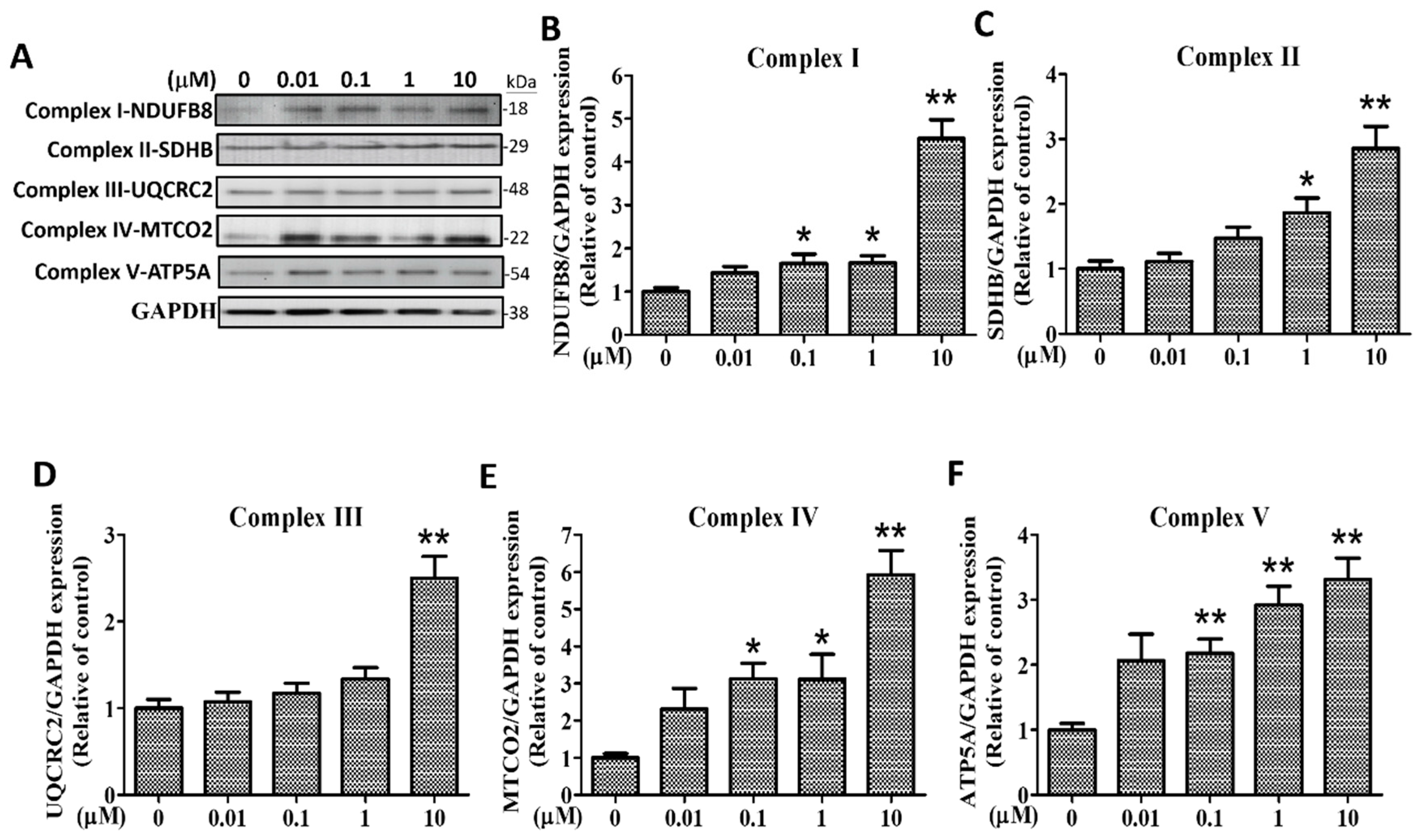
2.7. Effects of (+)-Rhodoptilometrin on the Protein Expression Level of Mitochondrial Complexes I–V in hGF-1 Cells
3. Discussion
4. Materials and Methods
4.1. Chemical
4.2. Cell Culture
4.3. Cell Viability Experiment (MTT Assay)
4.4. Wound-Healing Measurement (Scratch-Test Assay)
4.5. Transwell Migration Assay
4.6. Quantitative Real-Time PCR Analysis
4.7. Cell Protein Analysis
4.8. Immunofluorescence Chemical Staining of Cells
4.9. Measurement of Mitochondrial Function
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hellyer, P.H.; Beighton, D.; Heath, M.R.; Lynch, E.J. Root caries in older people attending a general dental practice in East Sussex. Br. Dent. J. 1990, 169, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahadni, A.; Linden, G.J. Dentine hypersensitivity in Jordanian dental attenders. A case control study. J. Clin. Periodontol. 2002, 29, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Kassab, M.M.; Cohen, R.E. The etiology and prevalence of gingival recession. J. Am. Dent. Assoc. (1939) 2003, 134, 220–225. [Google Scholar] [CrossRef]
- Kapferer-Seebacher, I.; Pepin, M.; Werner, R.; Aitman, T.J.; Nordgren, A.; Stoiber, H.; Thielens, N.; Gaboriaud, C.; Amberger, A.; Schossig, A.; et al. Periodontal Ehlers-Danlos Syndrome Is Caused by Mutations in C1R and C1S, which Encode Subcomponents C1r and C1s of Complement. Am. J. Hum. Genet. 2016, 99, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Kapferer-Seebacher, I.; Lundberg, P. Periodontal manifestations of Ehlers-Danlos syndromes: A systematic review. J. Periodontol. 2017, 44, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Jati, A.S.; Furquim, L.Z.; Consolaro, A. Gingival recession: Its causes and types, and the importance of orthodontic treatment. Dent. Press J. Orthod. 2016, 21, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Gorman, W.J. Prevalence and etiology of gingival recession. J. Periodontol. 1967, 38, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Lost, C. Depth of alveolar bone dehiscences in relation to gingival recessions. J. Clin. Periodontol. 1984, 11, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Chrysanthakopoulos, N.A. Aetiology and Severity of Gingival Recession in an Adult Population Sample in Greece. Dent. Res. J. 2011, 8, 64–70. [Google Scholar]
- Pradeep, K.; Rajababu, P.; Satyanarayana, D.; Sagar, V. Gingival Recession: Review and Strategies in Treatment of Recession. Case Rep. Dent. 2012, 2012, 563421. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.A.; Mathias, I.F.; da Silva Neves, F.L.; Castro Dos Santos, N.C.; Araujo, C.F.; Neves Jardini, M.A.; Bresciani, E.; Santamaria, M.P. Connective tissue graft and crown-resin composite restoration for the treatment of gingival recession associated with noncarious cervical lesions: Case series. Int. J. Periodontics Restor. Dent. 2017, 37, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin Hypersensitivity: Etiology, Diagnosis and Treatment; A Literature Review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- Ab Rahman, M.R.; Abdul Razak, F.; Mohd Bakri, M. Evaluation of Wound Closure Activity of Nigella sativa, Melastoma malabathricum, Pluchea indica, and Piper sarmentosum Extracts on Scratched Monolayer of Human Gingival Fibroblasts. Evid.-Based Complement. Altern. Med. 2014, 2014, 190342. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.C.; Everts, V.; Leethanakul, C.; Pavasant, P.; Ampornaramveth, R.S. Rinsing with Saline Promotes Human Gingival Fibroblast Wound Healing In Vitro. PLoS ONE 2016, 11, e0159843. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Sato, M.J.; Yanagida, T.; Ueda, M. Functional analysis of spontaneous cell movement under different physiological conditions. PLoS ONE 2008, 3, e2648. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.Q.; Landman, K.A.; Hughes, B.D. Modelling directional guidance and motility regulation in cell migration. Bull. Math. Biol. 2006, 68, 25–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Kim, C.; Wood, L.; Neal, D.; Kamm, R.D.; Asada, H.H. Integrating focal adhesion dynamics, cytoskeleton remodeling, and actin motor activity for predicting cell migration on 3D curved surfaces of the extracellular matrix. Integr. Biol. 2012, 4, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Mitra, S.K.; Ilic, D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim. Biophys. Acta 2004, 1692, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Martinez, J. Differential uPA expression by TGF-beta1 in gingival fibroblasts. J. Dent. Res. 2006, 85, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Caceres, M.; Oyarzun, A.; Smith, P.C. Defective Wound-healing in Aging Gingival Tissue. J. Dent. Res. 2014, 93, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.A. Neandertal genetics. Science 1997, 277, 1024–1025. [Google Scholar] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123 Pt 24, 4195–4200. [Google Scholar] [CrossRef]
- Goldenthal, M.J.; Marin-Garcia, J. Mitochondrial signaling pathways: A receiver/integrator organelle. Mol. Cell. Biochem. 2004, 262, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zamurs, L.K.; Idoate, M.A.; Hanssen, E.; Gomez-Ibanez, A.; Pastor, P.; Lamande, S.R. Aberrant mitochondria in a Bethlem myopathy patient with a homozygous amino acid substitution that destabilizes the collagen VI alpha2(VI) chain. J. Biol. Chem. 2015, 290, 4272–4281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, Q.; Zhou, Y.; Dier, U.; Hempel, N.; Hochwald, S.N. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene 2016, 35, 1926–1942. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Keasey, M.P.; Razskazovskiy, V.; Banerjee, K.; Jia, C.; Lovins, C.; Wright, G.L.; Hagg, T. Integrin-FAK signaling rapidly and potently promotes mitochondrial function through STAT3. Cell Commun. Signal. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Erba, E.; Bergamaschi, D.; Bassano, L.; Damia, G.; Ronzoni, S.; Faircloth, G.T.; D’Incalci, M. Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur. J. Cancer 2001, 37, 97–105. [Google Scholar] [CrossRef]
- Sharma, G.M.; Burkholder, P.R. Studies on the antimicrobial substances of sponges. II. Structure and synthesis of a bromine-containing antibacterial compound from a marine sponge. Tetrahedron Lett. 1967, 42, 4147–4150. [Google Scholar] [CrossRef]
- Taamma, A.; Misset, J.L.; Riofrio, M.; Guzman, C.; Brain, E.; Lopez Lazaro, L.; Rosing, H.; Jimeno, J.M.; Cvitkovic, E. Phase I and pharmacokinetic study of ecteinascidin-743, a new marine compound, administered as a 24-h continuous infusion in patients with solid tumors. J. Clin. Oncol. 2001, 19, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Powell, V.H.; Sutherland, M.D. Pigments of marine animals. VI. Anthraquinoid pigments of the crinoids Ptilometra australis Wilton and Tropiometra afra Hartlaub. Aust. J. Chem. 1967, 20, 541–553. [Google Scholar] [CrossRef]
- Wright, A.D.; Nielson, J.L.; Tapiolas, D.M.; Motti, C.A.; Ovenden, S.P.; Kearns, P.S.; Liptrot, C.H. Detailed NMR, including 1,1-ADEQUATE, and anticancer studies of compounds from the echinoderm Colobometra perspinosa. Mar. Drugs 2009, 7, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Tsai, S.J.; Chiang, M.Y.; Wen, Z.H.; Su, J.H. Anti-inflammatory anthraquinones from the crinoid Himerometra magnipinna. Nat. Prod. Commun. 2015, 10, 317–318. [Google Scholar] [PubMed]
- Vien, L.T.; Hanh, T.T.H.; Huong, P.T.T.; Dang, N.H.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Anthraquinone and Butenolide Constituents from the Crinoid Capillaster multiradiatus. Chem. Pharm. Bull. 2018, 66, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.M.; Weng, S.W.; Chang, A.Y.; Huang, H.T.; Lin, H.Y.; Chuang, J.H.; Lin, T.K.; Liou, C.W.; Tai, M.H.; Lin, C.Y.; et al. Altered mitochondrial dynamics and response to insulin in cybrid cells harboring a diabetes-susceptible mitochondrial DNA haplogroup. Free Radic. Biol. Med. 2016, 96, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, I.; Francetti, L.; Del Fabbro, M.; Testori, T.; Weinstein, R.L. Enamel matrix proteins (Emdogain) in combination with coronally advanced flap or subepithelial connective tissue graft in the treatment of shallow gingival recessions. Int. J. Periodontics Restor. Dent. 2002, 22, 583–593. [Google Scholar]
- Nemcovsky, C.E.; Artzi, Z.; Tal, H.; Kozlovsky, A.; Moses, O. A multicenter comparative study of two root coverage procedures: Coronally advanced flap with addition of enamel matrix proteins and subpedicle connective tissue graft. J. Periodontol. 2004, 75, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Cueva, M.A.; Boltchi, F.E.; Hallmon, W.W.; Nunn, M.E.; Rivera-Hidalgo, F.; Rees, T. A comparative study of coronally advanced flaps with and without the addition of enamel matrix derivative in the treatment of marginal tissue recession. J. Periodontol. 2004, 75, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 87–105. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Choi, H.; Lee, H.; Lee, J.W.; Weber, H.P.; Pae, A. Cellular viability and genetic expression of human gingival fibroblasts to zirconia with enamel matrix derivative (Emdogain®). J. Adv. Prosthodont. 2014, 6, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Furuta, Y.; Kanazawa, S.; Takeda, N.; Sobue, K.; Nakatsuji, N.; Nomura, S.; Fujimoto, J.; Okada, M.; Yamamoto, T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995, 377, 539–544. [Google Scholar] [PubMed]
- Gilmore, A.P.; Romer, L.H. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell 1996, 7, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Cary, L.A.; Chang, J.F.; Guan, J.L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 1996, 109 Pt 7, 1787–1794. [Google Scholar]
- Owen, J.D.; Ruest, P.J.; Fry, D.W.; Hanks, S.K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999, 19, 4806–4818. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.D.; Kiosses, W.B.; Sieg, D.J.; Otey, C.A.; Schlaepfer, D.D.; Schwartz, M.A. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 2000, 113 Pt 20, 3673–3678. [Google Scholar]
- Chen, B.H.; Tzen, J.T.; Bresnick, A.R.; Chen, H.C. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J. Biol. Chem. 2002, 277, 33857–33863. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, B.; McKenzie, A.J.; Heintz, N.H.; Howe, A.K. AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol. Biol. Cell 2016, 27, 2662–2674. [Google Scholar] [CrossRef] [PubMed]
- Caino, M.C.; Ghosh, J.C.; Chae, Y.C.; Vaira, V.; Rivadeneira, D.B.; Faversani, A.; Rampini, P.; Kossenkov, A.V.; Aird, K.M.; Zhang, R.; et al. PI3K therapy reprograms mitochondrial trafficking to fuel tumor cell invasion. Proc. Natl. Acad. Sci. USA 2015, 112, 8638–8643. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.P.; Bhatia, S.N.; Toner, M.; Irimia, D. Mitochondrial localization and the persistent migration of epithelial cancer cells. Biophys. J. 2013, 104, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Yu, M.; Xie, Y.; Huang, Y.; Wolff, D.W.; Abel, P.W.; Tu, Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 2013, 32, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Genova, M.L.; Bianchi, C.; Lenaz, G. Structural organization of the mitochondrial respiratory chain. Ital. J. Biochem. 2003, 52, 58–61. [Google Scholar] [PubMed]
- Kiebish, M.A.; Han, X.; Cheng, H.; Chuang, J.H.; Seyfried, T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 2008, 49, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Yizhak, K.; Le Devedec, S.E.; Rogkoti, V.M.; Baenke, F.; de Boer, V.C.; Frezza, C.; Schulze, A.; van de Water, B.; Ruppin, E. A computational study of the Warburg effect identifies metabolic targets inhibiting cancer migration. Mol. Syst. Biol. 2014, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
| Name | Gene No. | Gene Length (bps) | Primer Sequence 5′–3′ | Amplicon (bps) | Annealing Temperature |
|---|---|---|---|---|---|
| COL1 | NM_000088.3 | 4393 | F: GTGAACCCGGACCCACTG | 203 | 60 |
| R: CAGACCCTTGGCACCAGG | |||||
| FAK | NM_005607.4 | 3225 | F: GAAGCCTTGCCAGCCTCA | 183 | 60 |
| R: GTGGGGCTGGCTGGATTT | |||||
| Fn | NM_002026.2 | 7068 | F: GTCAGCCCAACTCCCACC | 209 | 60 |
| R: TTGGTGGCCGTACTGCTG | |||||
| GAPDH | NM_002046 | 1401 | F: CAATGCCTCCTGCACCACCA | 175 | 60 |
| R: GATGTTCTGGAGAGCCCCGC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-C.; Lai, Y.-C.; Kuo, T.-J.; Su, J.-H.; Sung, P.-J.; Feng, C.-W.; Lin, Y.-Y.; Chen, P.-C.; Tai, M.-H.; Cheng, S.-Y.; et al. Rhodoptilometrin, a Crinoid-Derived Anthraquinone, Induces Cell Regeneration by Promoting Wound Healing and Oxidative Phosphorylation in Human Gingival Fibroblast Cells. Mar. Drugs 2019, 17, 138. https://doi.org/10.3390/md17030138
Tseng C-C, Lai Y-C, Kuo T-J, Su J-H, Sung P-J, Feng C-W, Lin Y-Y, Chen P-C, Tai M-H, Cheng S-Y, et al. Rhodoptilometrin, a Crinoid-Derived Anthraquinone, Induces Cell Regeneration by Promoting Wound Healing and Oxidative Phosphorylation in Human Gingival Fibroblast Cells. Marine Drugs. 2019; 17(3):138. https://doi.org/10.3390/md17030138
Chicago/Turabian StyleTseng, Chung-Chih, Yu-Cheng Lai, Tsu-Jen Kuo, Jui-Hsin Su, Ping-Jyun Sung, Chien-Wei Feng, Yen-You Lin, Pei-Chin Chen, Ming-Hong Tai, Shu-Yu Cheng, and et al. 2019. "Rhodoptilometrin, a Crinoid-Derived Anthraquinone, Induces Cell Regeneration by Promoting Wound Healing and Oxidative Phosphorylation in Human Gingival Fibroblast Cells" Marine Drugs 17, no. 3: 138. https://doi.org/10.3390/md17030138
APA StyleTseng, C.-C., Lai, Y.-C., Kuo, T.-J., Su, J.-H., Sung, P.-J., Feng, C.-W., Lin, Y.-Y., Chen, P.-C., Tai, M.-H., Cheng, S.-Y., Kuo, H.-M., & Wen, Z.-H. (2019). Rhodoptilometrin, a Crinoid-Derived Anthraquinone, Induces Cell Regeneration by Promoting Wound Healing and Oxidative Phosphorylation in Human Gingival Fibroblast Cells. Marine Drugs, 17(3), 138. https://doi.org/10.3390/md17030138






