Characterization of the Specific Mode of Action of a Chitin Deacetylase and Separation of the Partially Acetylated Chitosan Oligosaccharides
Abstract
1. Introduction
2. Results and Discussion
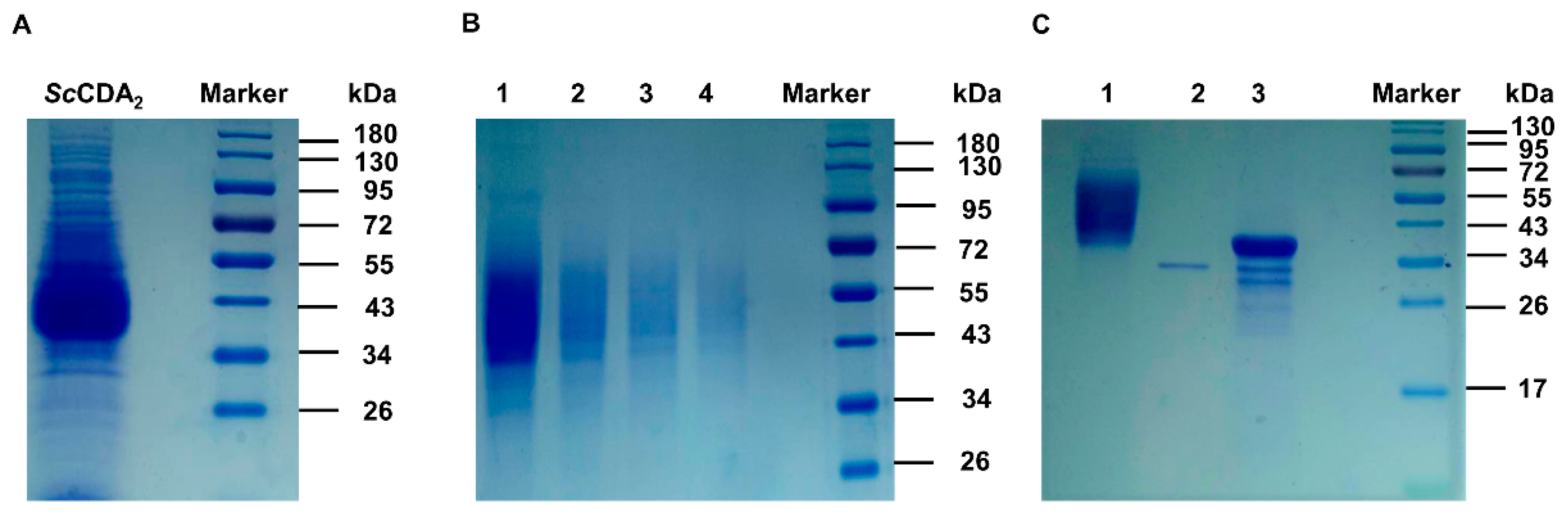
2.1. Bioinformatic Analysis and Expression of ScCDA2
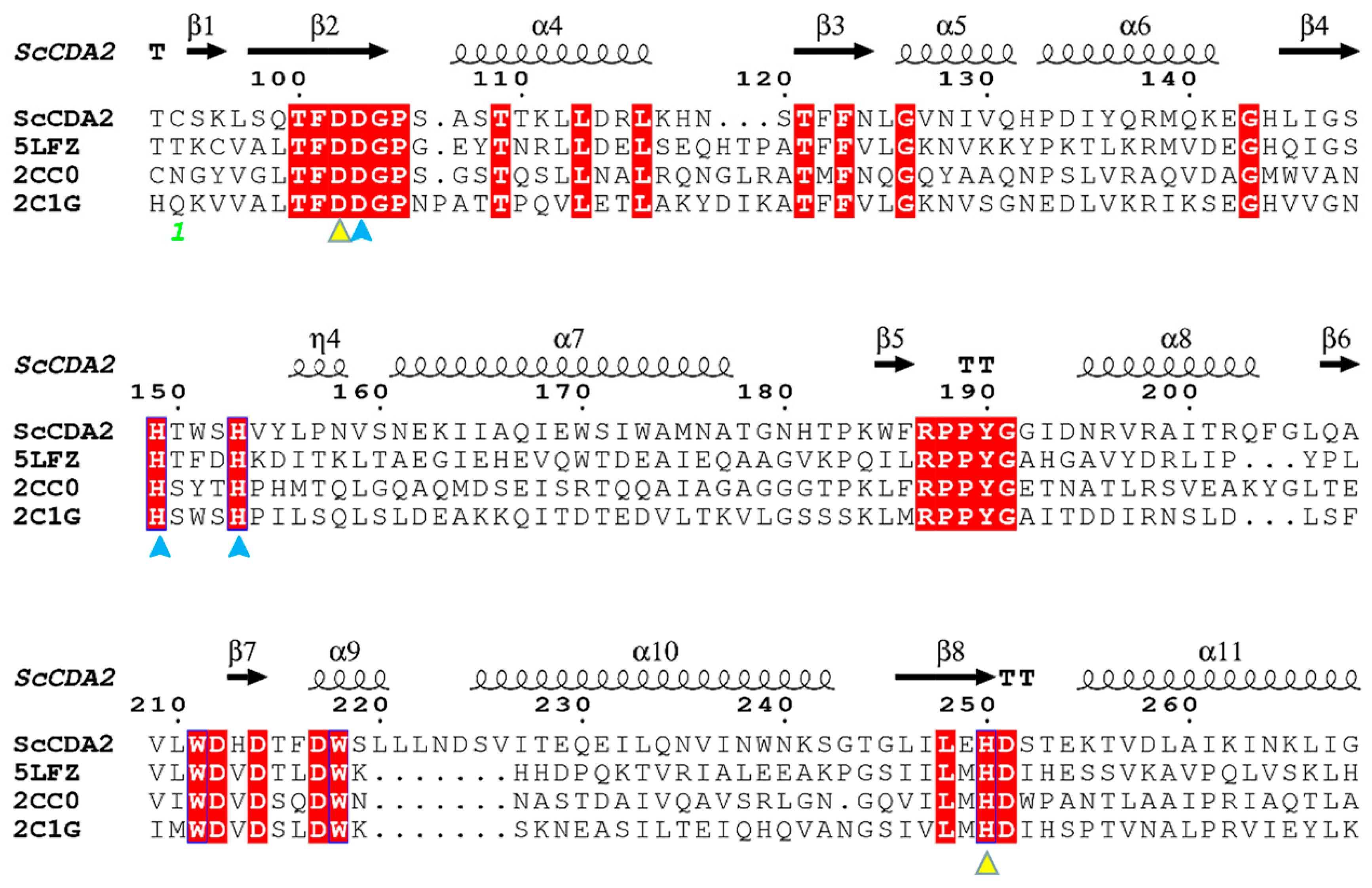
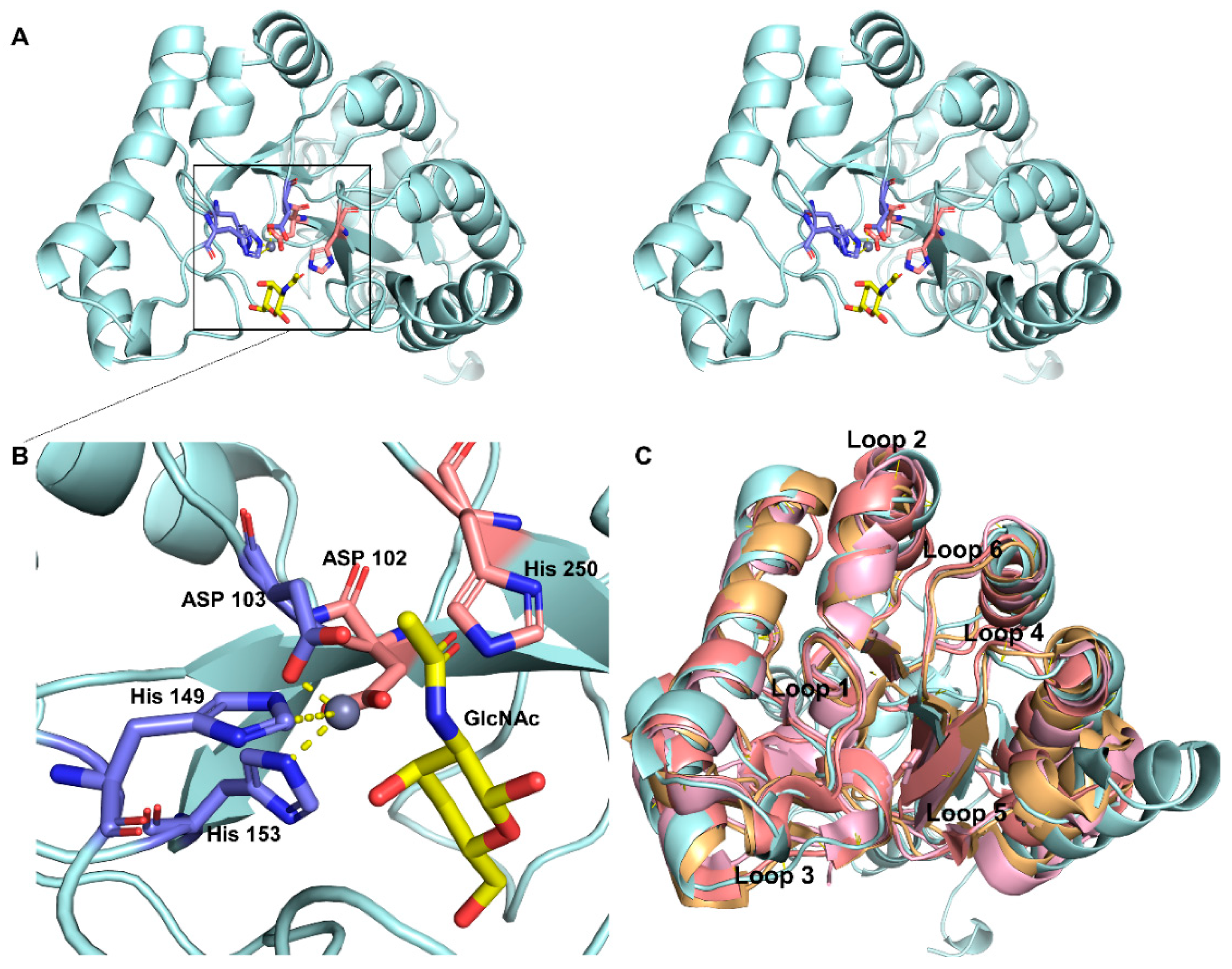
2.2. Homology Modeling and Substrate Binding Specificity of ScCDA2
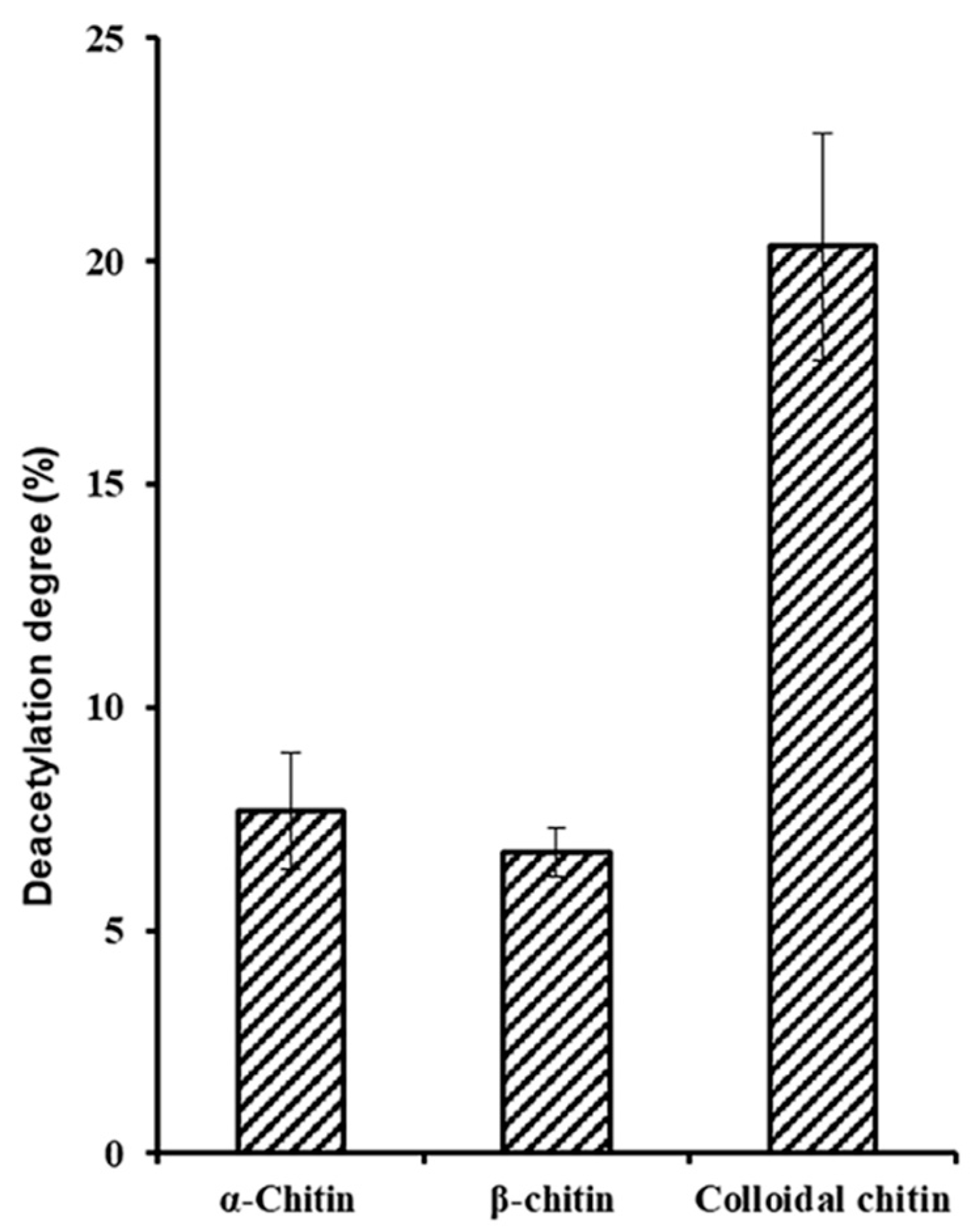
2.3. Biochemical Characterization of ScCDA2
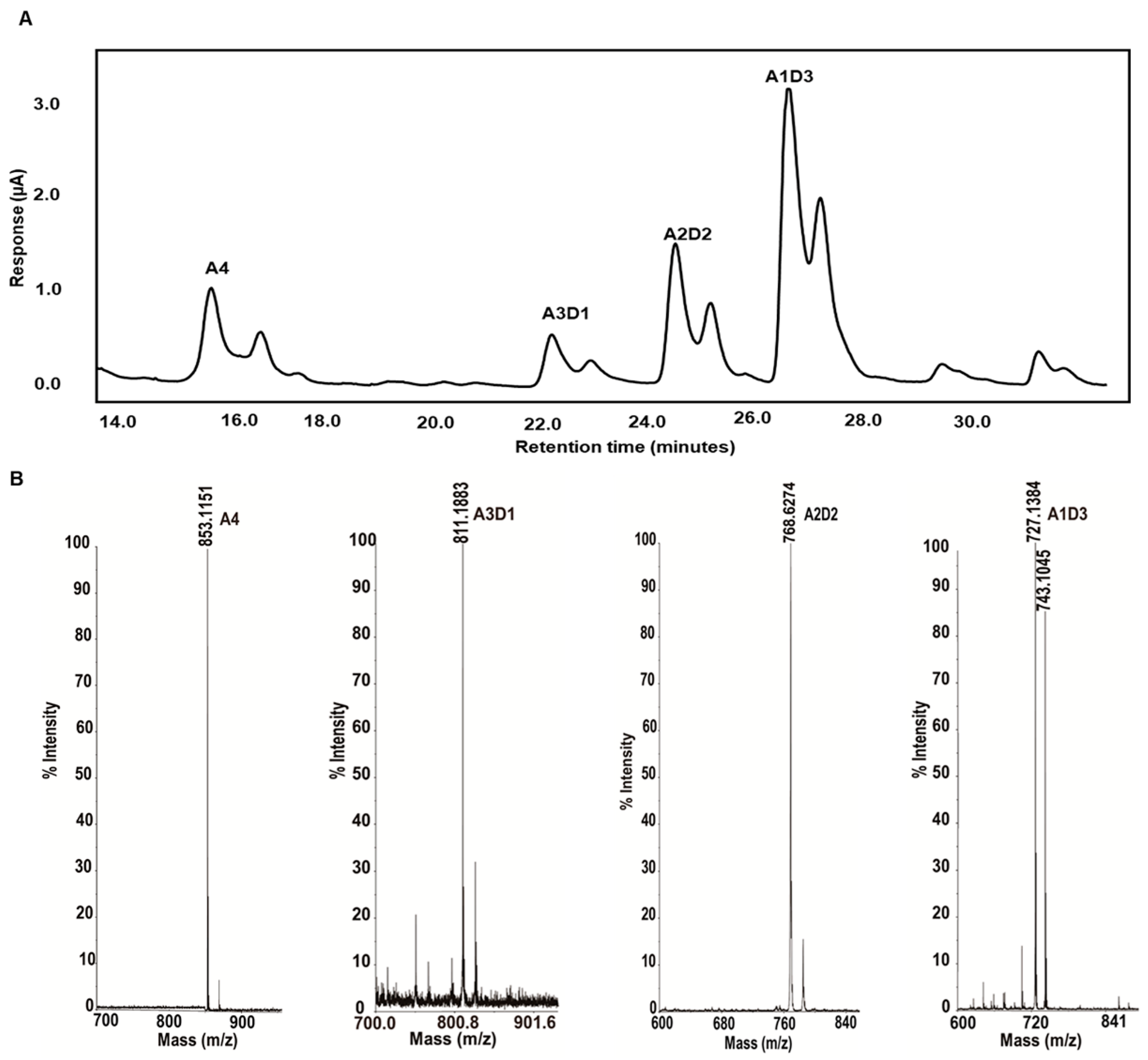
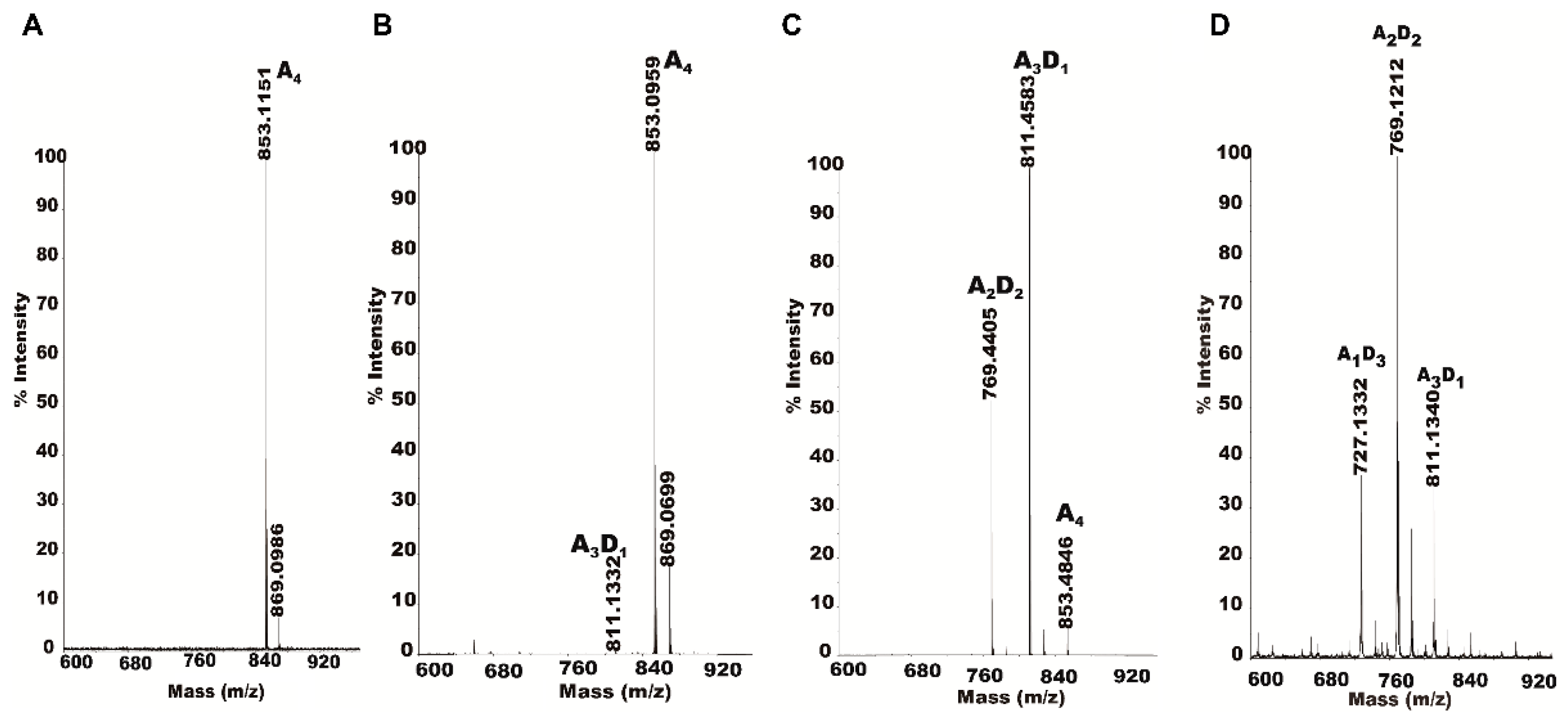
2.4. Isolation and Identification of Partially Acetylated Chitooligosaccharides
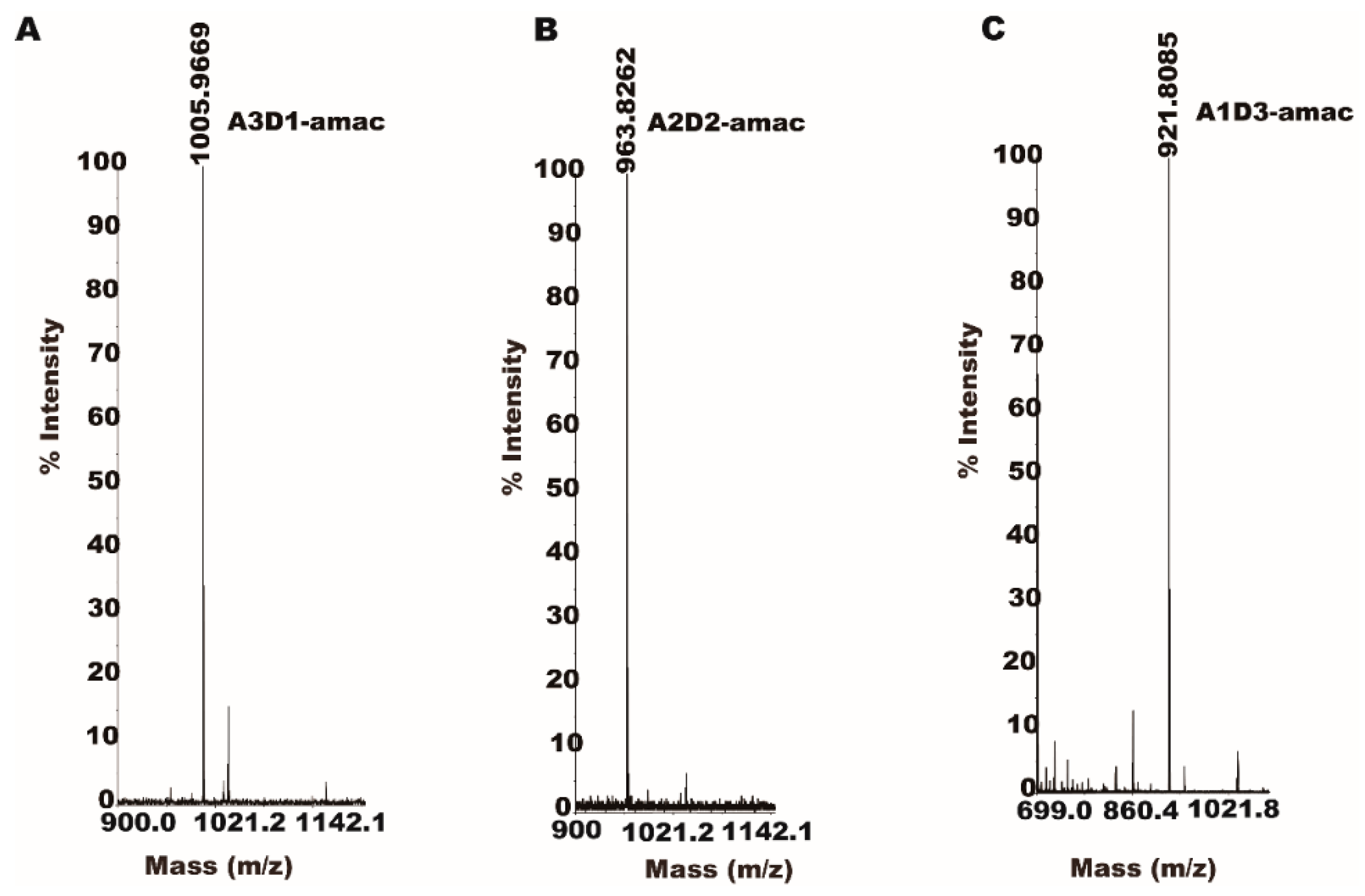
2.5. Partially Acetylated Chitooligosaccharides Production Processes
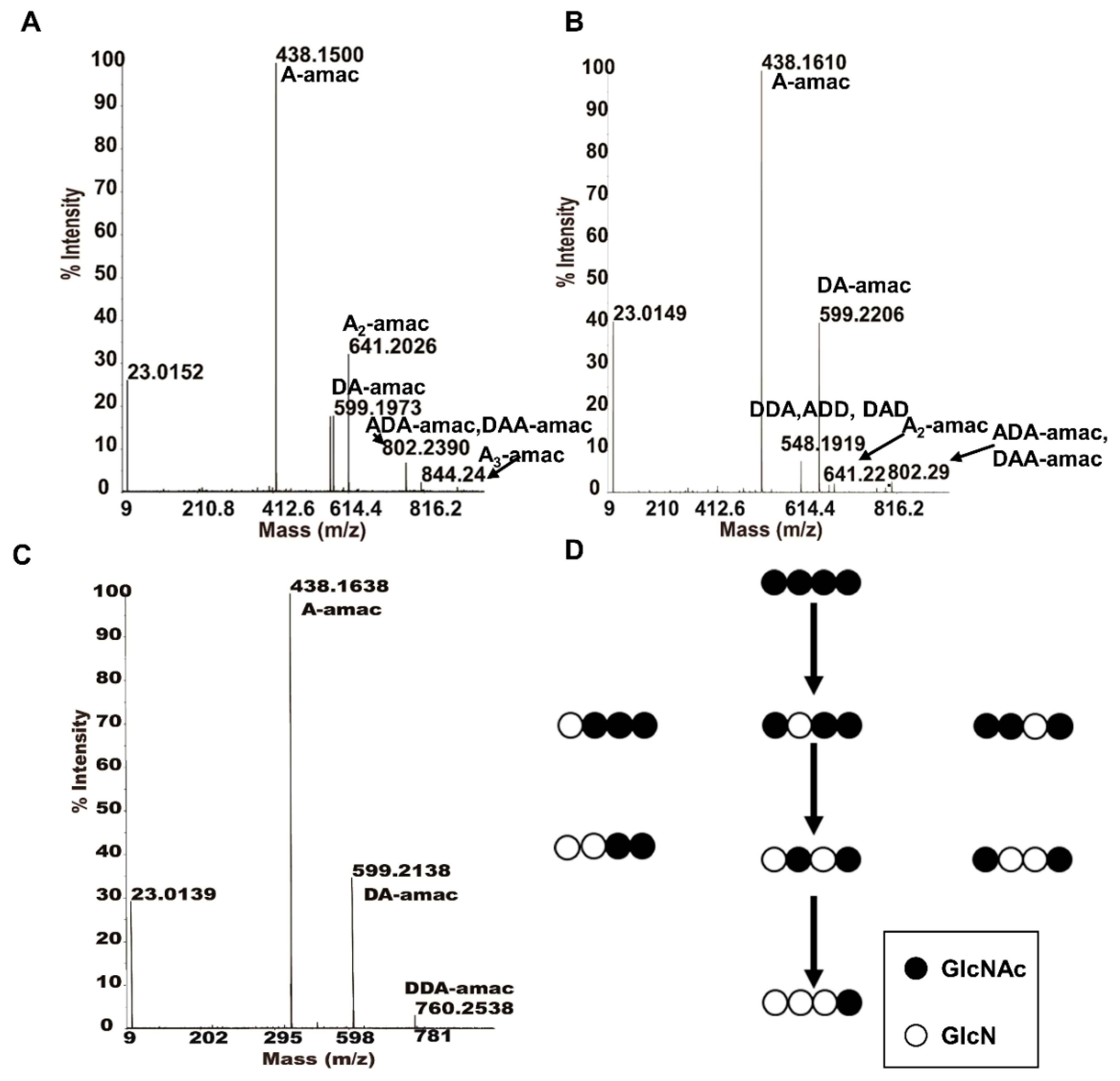
2.6. Specific Mode of Action of ScCDA2 on A4
3. Materials and Methods
3.1. Materials
3.2. Cloning, Expression and Purification of ScCDA2
3.3. ScCDA2 Activity Assay and Biochemistry Properties
3.4. Identification of ScCDA2 Products by MALDI-TOF-MS
3.5. Preparation of Partially Acetylated COS
3.6. Acetylation Pattern Analysis of COS
3.7. Homology Modelling and Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Ozogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Revathi, M.; Saravanan, R.; Shanmugam, A. Production and characterization of chitinase from Vibrio species, a head waste of shrimp Metapenaeus dobsonii (Miers, 1878) and chitin of Sepiella inermis Orbigny, 1848. Adv. Biosci. Biotechnol. 2012, 3, 392–397. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Chambon, R.; Despras, G.; Brossay, A.; Vauzeilles, B.; Urban, D.; Beau, J.M.; Armand, S.; Cottaz, S.; Fort, S. Efficient chemoenzymatic synthesis of lipo-chitin oligosaccharides as plant growth promoters. Green Chem. 2015, 17, 3923–3930. [Google Scholar] [CrossRef]
- Zhang, X.D.; Xiao, G.; Wang, Y.Q.; Zhao, Y.; Su, H.J.; Tan, T.W. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tan, T.W. Insecticidal and fungicidal activities of chitosan and oligo-chitosan. J. Bioact. Compat. Polym. 2003, 18, 391–400. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Wang, Z.; Cui, W.W.; Zhang, Y.; Zhang, Y.; Zheng, S.; Zhang, Y. Sustainable Disposal of Cr(VI): Adsorption-Reduction Strategy for Treating Textile Wastewaters with Amino-Functionalized Boehmite Hazardous Solid Wastes. ACS Sustain. Chem. Eng. 2018, 6, 6811–6819. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.-Q.; Bao, R.-Y.; Liu, Z.; Yang, W.; Xie, B.-H.; Yang, M.-B. Self-Assembled Sponge-like Chitosan/Reduced Graphene Oxide/Montmorillonite Composite Hydrogels without Cross-Linking of Chitosan for Effective Cr(VI) Sorption. ACS Sustain. Chem. Eng. 2017, 5, 1557–1566. [Google Scholar] [CrossRef]
- Xu, G.; Liu, P.; Pranantyo, D.; Neoh, K.-G.; Kang, E.T. Dextran- and Chitosan-Based Antifouling, Antimicrobial Adhesion, and Self-Polishing Multilayer Coatings from pH-Responsive Linkages-Enabled Layer-by-Layer Assembly. ACS Sustain. Chem. Eng. 2018, 6, 3916–3926. [Google Scholar] [CrossRef]
- Duri, S.; Harkins, A.L.; Frazier, A.J.; Tran, C.D. Composites Containing Fullerenes and Polysaccharides: Green and Facile Synthesis, Biocompatibility, and Antimicrobial Activity. ACS Sustain. Chem. Eng. 2017, 5, 5408–5417. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, R.; Duan, B.; Liu, M.; Lu, A.; Zhang, L. Recyclable Universal Solvents for Chitin to Chitosan with Various Degrees of Acetylation and Construction of Robust Hydrogels. ACS Sustain. Chem. Eng. 2017, 5, 2725–2733. [Google Scholar] [CrossRef]
- Naqvi, S.; Cord-Landwehr, S.; Singh, R.; Bernard, F.; Kolkenbrock, S.; El Gueddari, N.E.; Moerschbacher, B.M. A Recombinant Fungal Chitin Deacetylase Produces Fully Defined Chitosan Oligomers with Novel Patterns of Acetylation. Appl. Environ. Microbiol. 2016, 82, 6645–6655. [Google Scholar] [CrossRef]
- Vander, P.; Km, V.R.; Domard, A.; Eddine, E.G.N.; Moerschbacher, B.M. Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol. 1998, 118, 1353. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z.; Song, C.; Hu, Y.; Han, Z.; She, J.; Fan, F.; Wang, J.; Jin, C.; Chang, J.; Zhou, J.-M.; Chai, J. Chitin-Induced Dimerization Activates a Plant Immune Receptor. Science 2012, 336, 1160–1164. [Google Scholar] [CrossRef]
- Lv, Y.M.; Laborda, P.; Huang, K.; Cai, Z.P.; Wang, M.; Lu, A.M.; Doherty, C.; Liu, L.; Flitsch, S.L.; Voglmeir, J. Highly efficient and selective biocatalytic production of glucosamine from chitin. Green Chem. 2017, 19, 527–535. [Google Scholar] [CrossRef]
- Hu, K.; Yang, H.; Liu, G.; Tan, H. Identification and characterization of a polysaccharide deacetylase gene from Bacillus thuringiensis. Can. J. Microbiol. 2006, 52, 935–941. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, H.; He, Y.; He, L. Identification of a chitin deacetylase producing bacteria isolated from soil and its fermentation optimization. Afr. J. Microbiol. Res. 2010, 4, 2597–2603. [Google Scholar]
- Pacheco, N.; Trombotto, S.; David, L.; Shirai, K. Activity of chitin deacetylase from Colletotrichum gloeosporioides on chitinous substrates. Carbohydr. Polym. 2013, 96, 227–232. [Google Scholar] [CrossRef]
- Araki, Y.; Ito, E. Pathway of chitosan formation in mucor-rouxii—Enzymatic deacetylation of chitin. Eur. J. Biochem. 1975, 55, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, C.; Nuero, O.M.; Santamaria, F.; Reyes, F. Purification of a heat-stable chitin deacetylase from aspergillus-nidulans and its role in cell-wall degradation. Curr. Microbiol. 1995, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Martinou, A.; Koutsioulis, D.; Bouriotis, V. Expression, purification, and characterization of a cobalt-activated chitin deacetylase (Cda2p) from Saccharomyces cerevisiae. Protein Expr. Purif. 2002, 24, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-W.; Wang, X.-H.; Tan, X.; Xia, Q.-Y.; Xiang, Z.-H.; Zhao, P. Identification and Molecular Characterization of a Chitin Deacetylase from Bombyx mori Peritrophic Membrane. Int. J. Mol. Sci. 2014, 15, 1946–1961. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Jayaram, S.A.; Hemphala, J.; Senti, K.A.; Tsarouhas, V.; Jin, H.N.; Samakovlis, C. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr. Biol. 2006, 16, 180–185. [Google Scholar] [CrossRef]
- Brosson, D.; Kuhn, L.; Prensier, G.; Vivares, C.P.; Texier, C. The putative chitin deacetylase of Encephalitozoon cuniculi: A surface protein implicated in microsporidian spore-wall formation. FEMS Microbiol. Lett. 2005, 247, 81–90. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.-D.; Muzzarelli, R.A.A. Chitin Deacetylases: Properties and Applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Rohrig, H.; Schmidt, J.; Wieneke, U.; Schell, J. Rhizobium nodb protein involved in nodulation signal synthesis is a chitooligosaccharide deacetylase. Proc. Natl. Acad. Sci. USA 1993, 90, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, O.; Tokuyasu, K.; Withers, S.G. Subsite structure of the endo-type chitin deacetylase from a Deuteromycete, Colletotrichum lindemuthianum: An investigation using steady-state kinetic analysis and MS. Biochem. J. 2003, 374, 369–380. [Google Scholar] [CrossRef]
- Andres, E.; Albesa-Jove, D.; Biarnes, X.; Moerschbacher, B.M.; Guerin, M.E.; Planas, A. Structural basis of chitin oligosaccharide deacetylation. Angew. Chem. Int. Ed. Engl. 2014, 53, 6882–6887. [Google Scholar] [CrossRef] [PubMed]
- Christodoulidou, A.; Bouriotis, V.; Thireos, G. Two sporulation-specific chitin deacetylase-encoding genes are required for the ascospore wall rigidity of Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 31420–31425. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.E.; Hekmat, O.; Schuttelkopf, A.W.; Shrestha, B.; Tokuyasu, K.; Withers, S.G.; van Aalten, D.M.F. Structure and mechanism of chitin deacetylase from the fungal pathogen Colletotrichum lindemuthianum. Biochemistry 2006, 45, 9416–9426. [Google Scholar] [CrossRef]
- Tuveng, T.R.; Rothweiler, U.; Udatha, G.; Vaaje-Kolstad, G.; Smalås, A.; Eijsink, V.G.H. Structure and function of a CE4 deacetylase isolated from a marine environment. PLoS ONE 2017, 12, e0187544. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.J.; Gloster, T.M.; Turkenburg, J.P.; Vincent, F.; Brzozowski, A.M.; Dupont, C.; Shareck, F.; Centeno, M.S.J.; Prates, J.A.M.; Puchart, V.; et al. Structure and activity of two metal ion-dependent acetylxylan esterases involved in plant cell wall degradation reveals a close similarity to peptidoglycan deacetylases. J. Biol. Chem. 2006, 281, 10968–10975. [Google Scholar] [CrossRef] [PubMed]
- Blair, D.E.; Schuttelkopf, A.W.; MacRae, J.I.; van Aalten, D.M.F. Structure and metal-dependent mechanism of peptidoglycan deacetylase, a streptococcal virulence factor. Proc. Natl. Acad. Sci. USA 2005, 102, 15429–15434. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, A.; Aravind, L.; Madden, T.; Shavirin, S.; Spouge, J.; Wolf, Y.; Koonin, E.; Altschul, S. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001, 29, 2994–3005. [Google Scholar] [CrossRef]
- Tarentino, A.L.; Trimble, R.B.; Plummer, T.H., Jr. Enzymatic approaches for studying the structure, synthesis, and processing of glycoproteins. Methods Cell Biol. 1989, 32, 111–139. [Google Scholar] [PubMed]
- Tarentino, A.L.; Plummer, T.H. Enzymatic deglycosylation of asparagine-linked glycans—Purification, properties, and specificity of oligosaccharide-cleaving enzymes from flavobacterium-meningosepticum. In Guide to Techniques in Glycobiology; Lennarz, W.J., Hart, G.W., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 1994; Volume 230, pp. 44–57. [Google Scholar]
- Amore, A.; Knott, B.; Supekar, N.; Shajahan, A.; Azadi, P.; Zhao, P.; Wells, L.; Linger, J.; Hobdey, S.; Vander Wall, T.; et al. Distinct roles of N- and O-glycans in cellulase activity and stability. Proc. Natl. Acad. Sci. USA 2017, 114, 13667–13672. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gay, L.M.; Tuveng, T.R.; Agger, J.W.; Westereng, B.; Mathiesen, G.; Horn, S.J.; Vaaje-Kolstad, G.; van Aalten, D.M.F.; Eijsink, V.G.H. Structure and function of a broad-specificity chitin deacetylase from Aspergillus nidulans FGSC A4. Sci. Rep. 2017, 7, 1746. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Xing, R.G.; Liu, S.; Qin, Y.K.; Li, P.C. Access to N-Acetylated Chitohexaose with Well-Defined Degrees of Acetylation. Biomed. Res. Int. 2017, 2017, 2486515. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Lu, W.P.; Wang, J.N.; Gao, Y.H.; Guo, Y.C. Rapid and Convenient Separation of Chitooligosaccharides by Ion-Exchange Chromatography. In Proceedings of the 5th Annual International Conference on Material Science and Engineering, Xiamen, China, 20–22 October 2017; Aleksandrova, M., Szewczyk, R., Eds.; Iop Publishing Ltd.: Bristol, UK, 2018; Volume 275. [Google Scholar]
- Cao, L.; Wu, J.; Li, X.; Zheng, L.; Wu, M.; Liu, P.; Huang, Q. Validated HPAEC-PAD Method for the Determination of Fully Deacetylated Chitooligosaccharides. Int. J. Mol. Sci. 2016, 17, 1699. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Liu, S.; Xing, R.G.; Qin, Y.K.; Li, P.C. Preparation, characterization and antioxidant activity of two partially N-acetylated chitotrioses. Carbohydr. Polym. 2013, 92, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Xing, R.G.; Liu, S.; Li, R.F.; Qin, Y.K.; Meng, X.T.; Li, P.C. Separation of chito-oligomers with several degrees of polymerization and study of their antioxidant activity. Carbohydr. Polym. 2012, 88, 896–903. [Google Scholar] [CrossRef]
- Aragunde, H.; Biarnes, X.; Planas, A. Substrate Recognition and Specificity of Chitin Deacetylases and Related Family 4 Carbohydrate Esterases. Int. J. Mol. Sci. 2018, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Nagao, N. An Improved Method for the Preparation of Colloidal Chitin by Using Methanesulfonic-Acid. Agric. Biol. Chem. Tokyo 1988, 52, 2111–2112. [Google Scholar]
- Wang, S.; Liu, C.; Liang, T. Fermented and enzymatic production of chitin/chitosan oligosaccharides by extracellular chitinases from Bacillus cereus TKU027. Carbohydr. Polym. 2012, 90, 1305–1313. [Google Scholar] [CrossRef]
- Bahrke, S.; Einarsson, J.M.; Gislason, J.; Haebel, S.; Letzel, M.C.; Peterkatalinić, J.; Peter, M.G. Sequence analysis of chitooligosaccharides by matrix-assisted laser desorption ionization postsource decay mass spectrometry. Biomacromolecules 1900, 3, 696–704. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Li, Z.; Guo, X.; Ling, P. Application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in preparation of chitosan oligosaccharides (COS) with degree of polymerization (DP) 5–12 containing well-distributed acetyl groups. Int. J. Mass Spectrom. 2010, 290, 94–99. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, Y.W.; Jeong, C.S.; Kim, Y.S.; Park, Y. Isolation and tandem mass fragmentations of an anti-inflammatory compound from Aralia elata. Arch. Pharm. Res. 2009, 32, 831–840. [Google Scholar] [CrossRef]
- Krieger, E.; Koraimann, G.; Vriend, G. Increasing the precision of comparative models with YASARA NOVA—A self-parameterizing force field. Proteins-Struct. Funct. Genet. 2002, 47, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Eisenberg, D.; Luthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. In Macromolecular Crystallography, Pt B; Carter, C.W., Sweet, R.M., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 277, pp. 396–404. [Google Scholar]
- Wang, Z.; Sun, H.; Yao, X.; Li, D.; Xu, L.; Li, Y.; Tian, S.; Hou, T. Comprehensive evaluation of ten docking programs on a diverse set of protein-ligand complexes: The prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016, 18, 12964–12975. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.-Y.; Zhao, Y.; Zhang, H.-D.; Wang, W.-X.; Cong, H.-H.; Yin, H. Characterization of the Specific Mode of Action of a Chitin Deacetylase and Separation of the Partially Acetylated Chitosan Oligosaccharides. Mar. Drugs 2019, 17, 74. https://doi.org/10.3390/md17020074
Zhu X-Y, Zhao Y, Zhang H-D, Wang W-X, Cong H-H, Yin H. Characterization of the Specific Mode of Action of a Chitin Deacetylase and Separation of the Partially Acetylated Chitosan Oligosaccharides. Marine Drugs. 2019; 17(2):74. https://doi.org/10.3390/md17020074
Chicago/Turabian StyleZhu, Xian-Yu, Yong Zhao, Huai-Dong Zhang, Wen-Xia Wang, Hai-Hua Cong, and Heng Yin. 2019. "Characterization of the Specific Mode of Action of a Chitin Deacetylase and Separation of the Partially Acetylated Chitosan Oligosaccharides" Marine Drugs 17, no. 2: 74. https://doi.org/10.3390/md17020074
APA StyleZhu, X.-Y., Zhao, Y., Zhang, H.-D., Wang, W.-X., Cong, H.-H., & Yin, H. (2019). Characterization of the Specific Mode of Action of a Chitin Deacetylase and Separation of the Partially Acetylated Chitosan Oligosaccharides. Marine Drugs, 17(2), 74. https://doi.org/10.3390/md17020074




