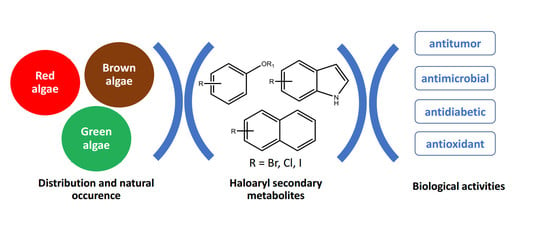
Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae
Abstract
:1. Introduction
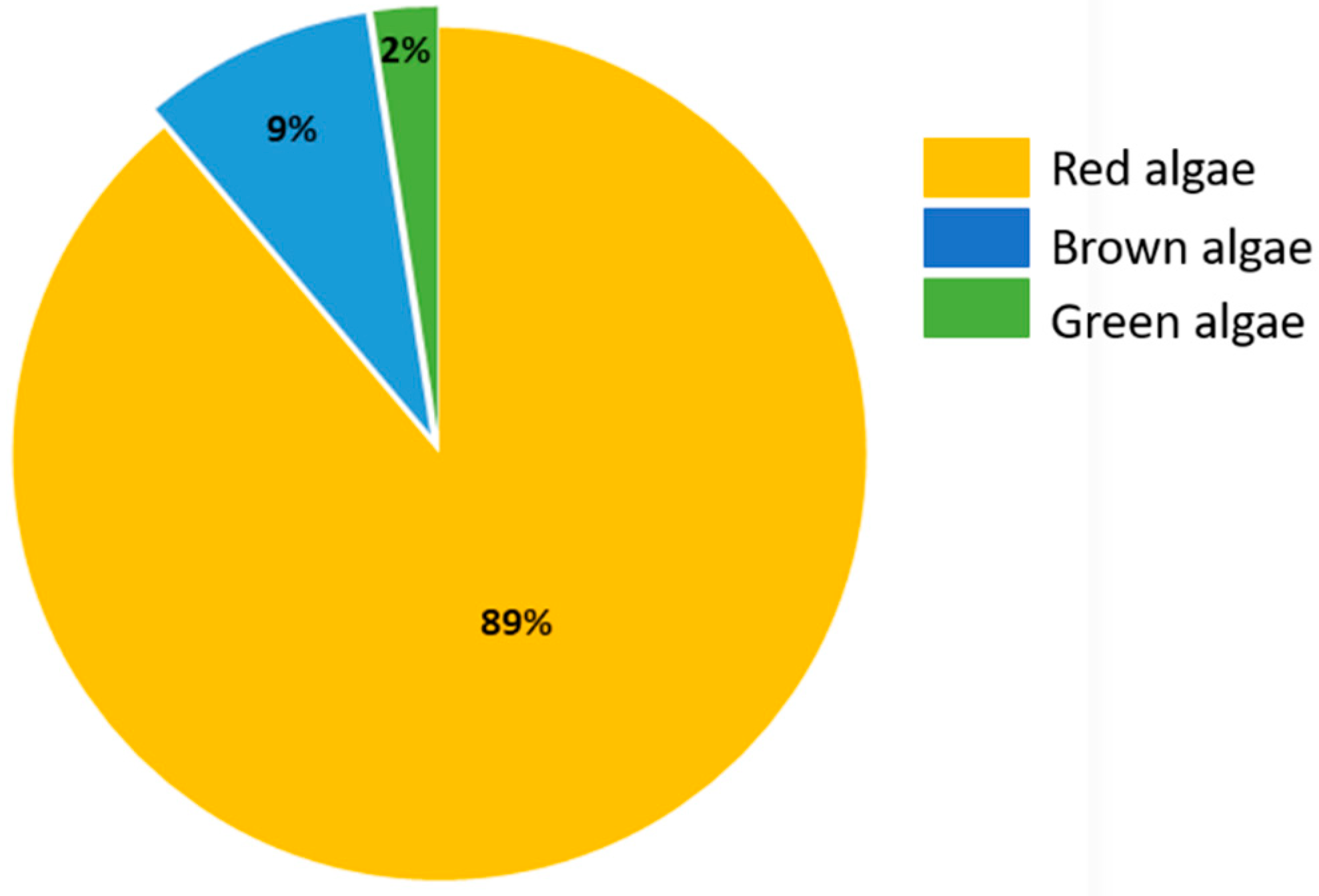
2. Haloaryl Secondary Metabolites Isolated from Macroalgae
2.1. Haloaryl Secondary Metabolites Isolated from Red Algae
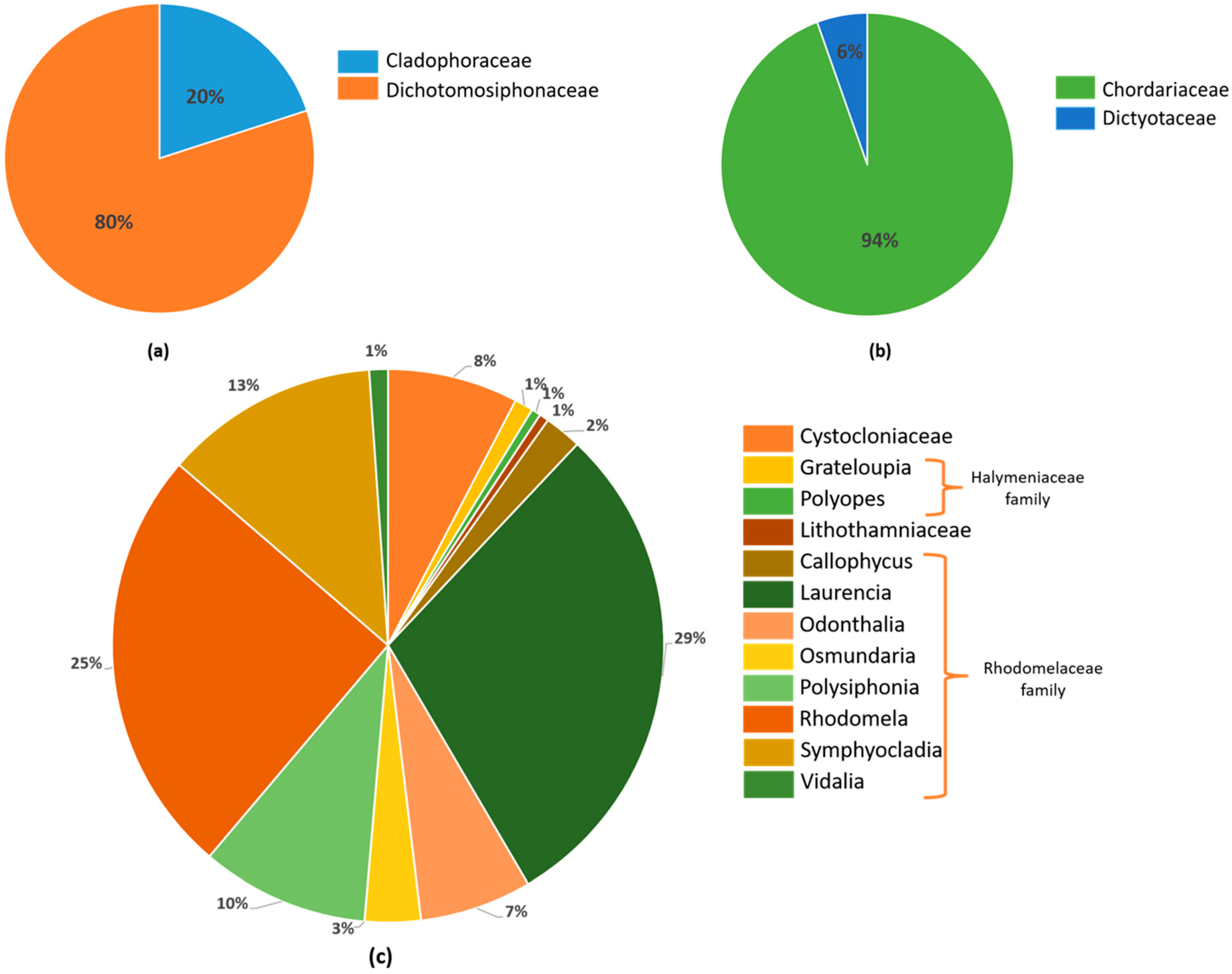
2.1.1. Cystocloniaceae Family
2.1.2. Halymeniaceae Family
2.1.3. Lithothamniaceae Family
2.1.4. Rhodomelaceae Family
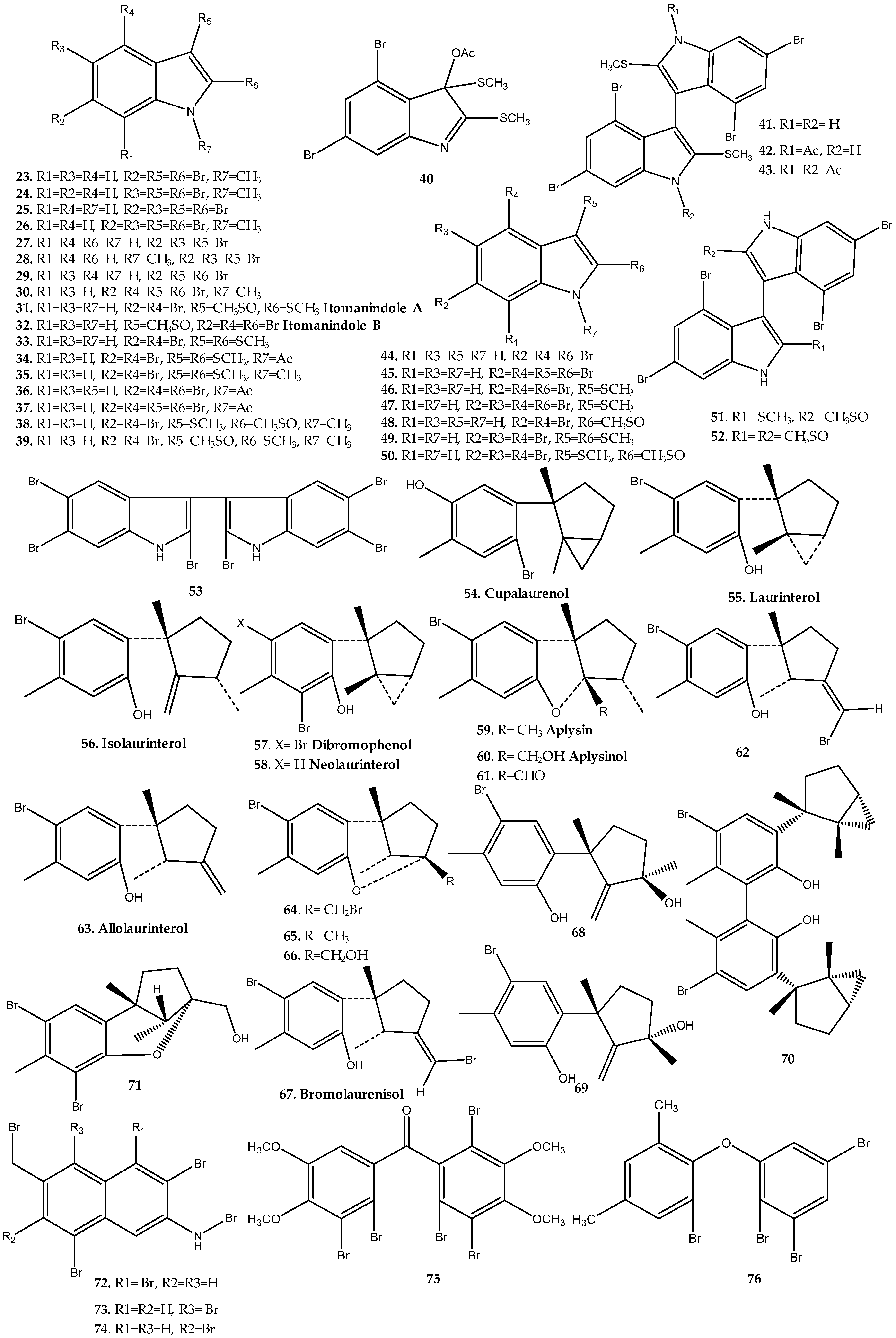
Callophycus Genus
Laurencia Genus
Odonthalia Genus
Osmundaria Genus
Polysiphonia Genus
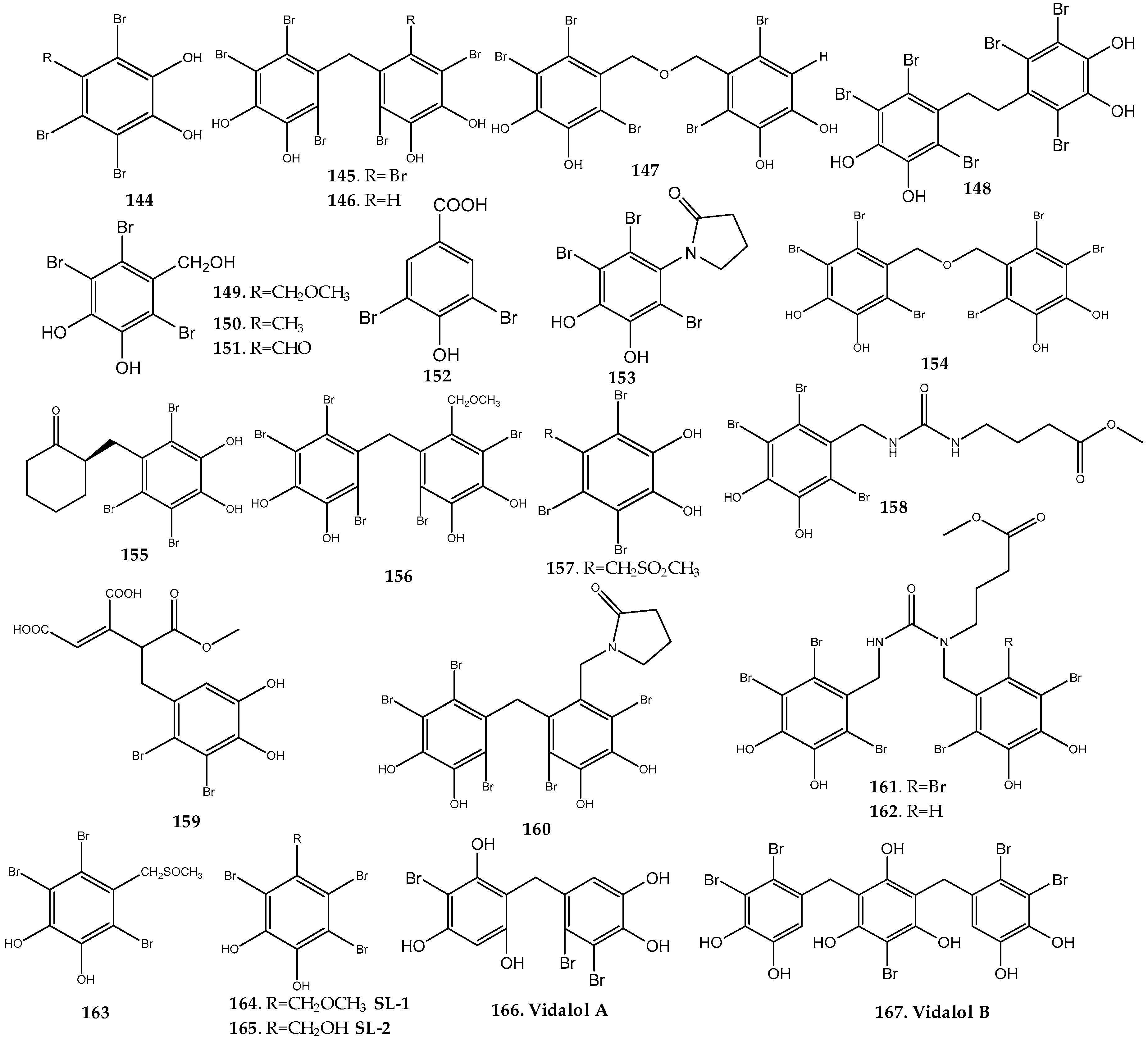
Rhodomela Genus
Symphyocladia Genus
Vidalia Genus
2.2. Haloaryl Secondary Metabolites Isolated from Brown Algae
2.2.1. Chordariaceae Family
2.2.2. Dictyotaceae Family
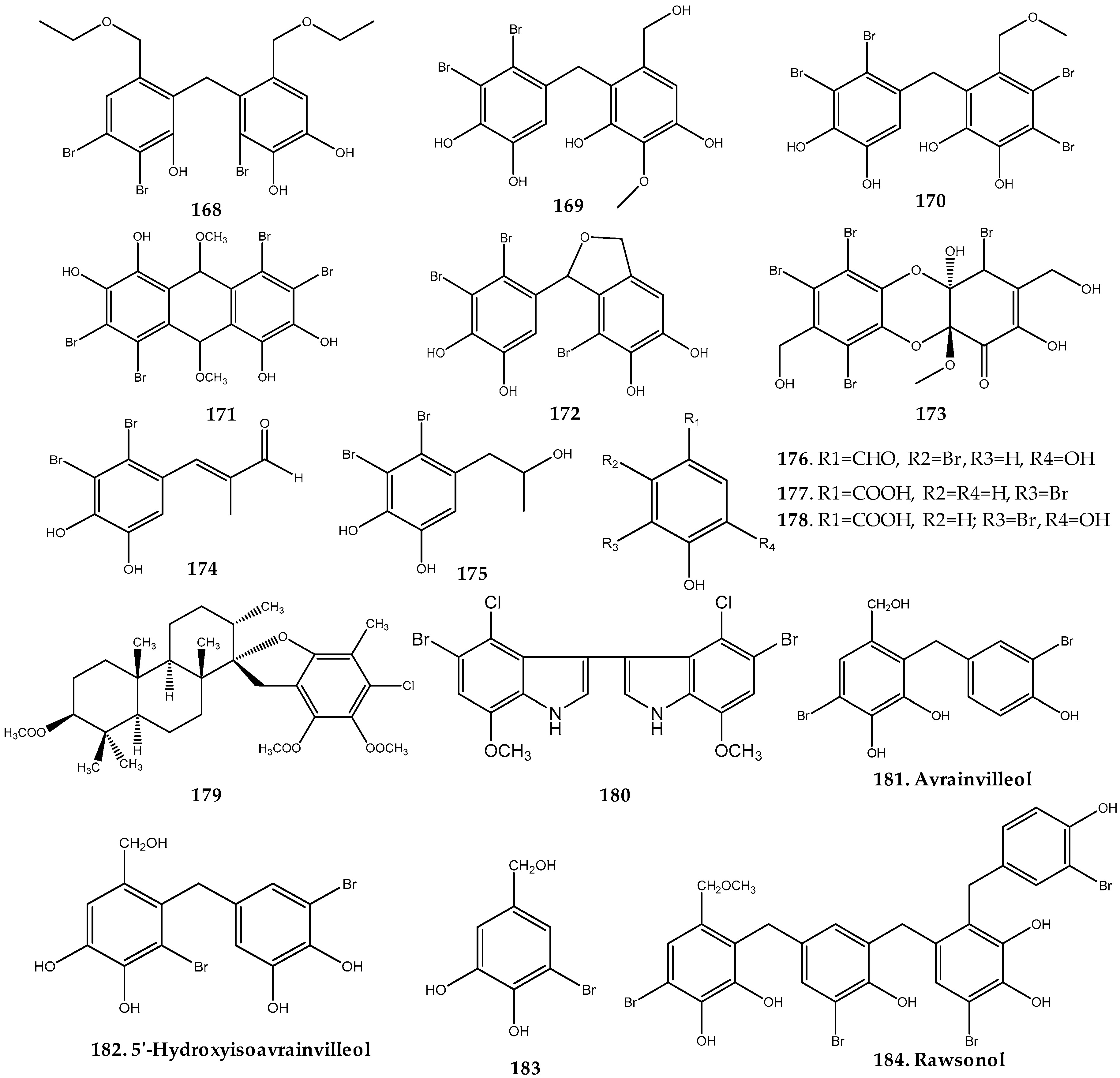
2.3. Haloaryl Secondary Metabolites Isolated from Green Algae
2.3.1. Cladophoraceae Family
2.3.2. Dichotomosiphonaceae Family
3. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Sandy, M. Mechanistic considerations of halogenating enzymes. Nature 2009, 460, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Brumley, D.; Alexander, T.S.; Jasmin, C.; Carranza, F.A.; Nelson, K.; Quave, C.L.; Kubanek, J. Iodinated meroditerpenes from a red alga Callophycus sp. J. Org. Chem. 2017, 82, 4160–4169. [Google Scholar] [CrossRef] [PubMed]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of seaweeds. In Seaweed in Health and Disease Prevention; Elsevier Inc.: London, UK, 2016; pp. 41–106. [Google Scholar]
- Woolner, V.H.; Jones, C.M.; Field, J.J.; Fadzilah, N.H.; Munkacsi, A.B.; Miller, J.H.; Keyzers, R.A.; Northcote, P.T. Polyhalogenated indoles from the red alga rhodophyllis membranacea: The first isolation of bromo-chloro-iodo secondary metabolites. J. Nat. Prod. 2016, 79, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Nam, K.A.; Kurihara, H.; Kim, S.M. Potent alpha-glucosidase inhibitors purified from the red alga grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. Alpha-glucosidase inhibitory activity of bromophenol purified from the red alga polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Two new bromophenols from the red alga odonthalia corymbifera. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, J. Dibenzyl bromophenols with diverse dimerization patterns from the brown alga leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New nitrogen-containing bromophenols from the marine red alga rhodomela confervoides and their radical scavenging activity. Food Chem. 2012, 135, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, F.; He, J.; Li, J.; Fan, X.; Han, L. Inhibition of bromophenols against ptp1b and anti-hyperglycemic effect of rhodomela confervoides extract in diabetic rats. Sci. Bull. 2008, 53, 2476–2479. [Google Scholar] [CrossRef]
- Qi, X.; Liu, G.; Qiu, L.; Lin, X.; Liu, M. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, represses angiogenesis in huvec cells and in zebrafish embryos via inhibiting the vegf signal systems. Biomed. Pharmacother. 2015, 75, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, W.; Wei, J.; Qiu, L.; Lin, X. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in k562 cells and inhibits topoisomerase i in vitro. Toxicol. Lett. 2012, 211, 126–134. [Google Scholar] [CrossRef]
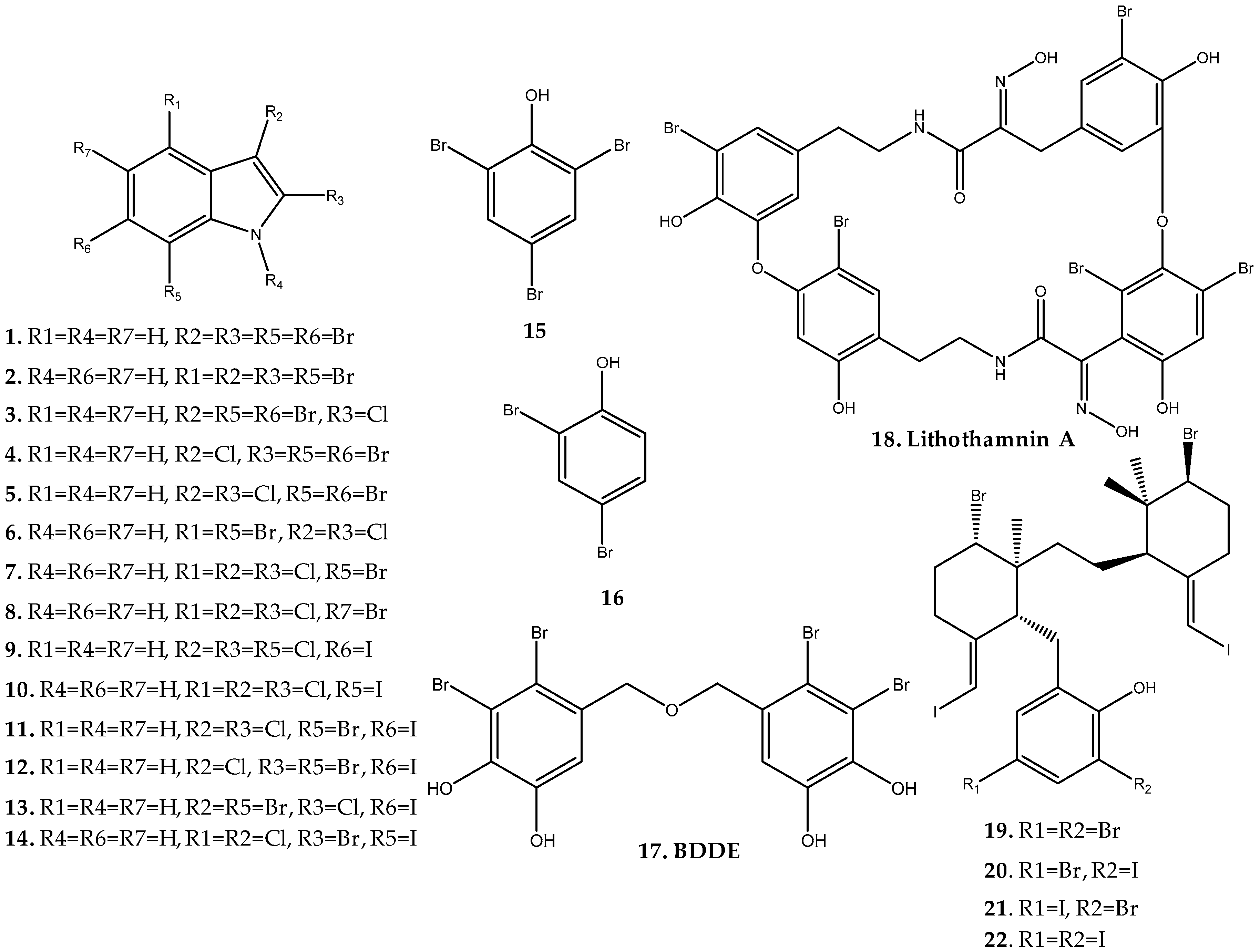
- Van Wyk, A.W.W.; Zuck, K.M.; McKee, T.C. Lithothamnin a, the first bastadin-like metabolite from the red alga lithothamnion fragilissimum. J. Nat. Prod. 2011, 74, 1275–1280. [Google Scholar] [CrossRef]
- Carter, G.T.; Rinehart, K.L.; Li, L.H.; Kuentzel, S.L.; Connor, J.L. Brominated indoles from laurencia brongniartii. Tetrahedron Lett. 1978, 19, 4479–4482. [Google Scholar] [CrossRef]
- Ji, N.Y.; Li, X.M.; Ding, L.P.; Wang, B.G. Aristolane sesquiterpenes and highly brominated indoles from the marine red alga laurencia similis (rhodomelaceae). Helv. Chim. Acta 2007, 90, 385–391. [Google Scholar] [CrossRef]
- Ji, N.; Li, X.; Cui, C.; Wang, B. Terpenes and polybromoindoles from the marine red alga laurencia decumbens (rhodomelaceae). Helv. Chim. Acta 2007, 90, 1731–1736. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Gruner, M.; Andriamanantoanina, H.; Andriamihaja, B.; Bauer, I.; Knölker, H.J. Red algae (rhodophyta) from the coast of madagascar: Preliminary bioactivity studies and isolation of natural products. Mar. Drugs 2015, 13, 4197–4216. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Ishii, T.; Lee, T.K.; Suzuki, M.; Zhaoqi, Z. Antibacterial activities of a new brominated diterpene from Borneon laurencia spp. Mar. Drugs 2010, 8, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Higa, T.; Bernardinelli, G.; Jefford, C.W. Sulfur-containing polybromoindoles from the red alga laurencia brongniartii. Tetrahedron 1989, 45, 7301–7310. [Google Scholar] [CrossRef]
- El-Gamal, A.A.; Wang, W.L.; Duh, C.Y. Sulfur-containing polybromoindoles from the formosan red alga laurencia brongniartii. J. Nat. Prod. 2005, 68, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yuan, Z.H.; Li, J.; Guo, S.J.; Deng, L.P.; Han, L.J.; Zhu, X.B.; Shi, D.Y. Two new bromoindoles from red alga laurencia similis. Chin. Chem. Lett. 2009, 20, 456–458. [Google Scholar] [CrossRef]
- Suzuki, M.; Kurosawa, E. Halogenated and non-halogenated aromatic sesquiterpenes from the red algae laurencia okamurai yamada. Bull. Chem. Soc. Jpn. 1979, 52, 3352–3354. [Google Scholar] [CrossRef]
- Kladi, M.; Xenaki, H.; Vagias, C.; Papazafiri, P.; Roussis, V. New cytotoxic sesquiterpenes from the red algae laurencia obtusa and laurencia microcladia. Tetrahedron 2006, 62, 182–189. [Google Scholar] [CrossRef]
- Ji, N.Y.; Li, X.M.; Li, K.; Ding, L.P.; Wang, B.G. Laurane-derived sesquiterpenes from the marine red alga laurencia tristicha (rhodomelaceae). Nat. Prod. Res. 2008, 22, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Sun, W.S.; Wang, B.; Cheng, W.; Liang, H.; Zhao, Y.Y.; Zhang, Q.Y.; Wu, J. A novel brominated cuparene-derived sesquiterpene ether from the red alga Laurencia sp. J. Asian Nat. Prod. Res. 2010, 12, 916–920. [Google Scholar] [CrossRef]
- Qin, J.; Su, H.; Zhang, Y.; Gao, J.; Zhu, L.; Wu, X.; Pan, H.; Li, X. Highly brominated metabolites from marine red alga laurencia similis inhibit protein tyrosine phosphatase 1b. Bioorg. Med. Chem. Lett. 2010, 20, 7152–7154. [Google Scholar] [CrossRef]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, K. Inhibitory potencies of bromophenols from rhodomelaceae algae against α-glucosidase activity. Fish. Sci. 1999, 65, 300–303. [Google Scholar] [CrossRef]
- Lee, H.; Lee, T.; Lee, J.; Chae, C.; Chung, S.; Shin, D.; Shin, J.; Oh, K. Inhibition of the pathogenicity of magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga odonthalia corymbifera. J. Agric. Food Chem. 2007, 55, 6923–6928. [Google Scholar] [CrossRef]
- Islam, M.R.; Mikami, D.; Kurihara, H. Two new algal bromophenols from odonthalia corymbifera. Tetrahedron Lett. 2017, 58, 4119–4121. [Google Scholar] [CrossRef]
- Popplewell, W.L.; Northcote, P.T. Colensolide a: A new nitrogenous bromophenol from the new zealand marine red alga osmundaria colensoi. Tetrahedron Lett. 2009, 50, 6814–6817. [Google Scholar] [CrossRef]
- Shoeib, N.A.; Bibby, M.C.; Blunden, G.; Linley, P.A.; Swaine, D.J.; Wheelhouse, R.T.; Wright, C.W. In-vitro cytotoxic activities of the major bromophenols of the red alga polysiphonia lanosa and some novel synthetic isomers. J. Nat. Prod. 2004, 67, 1445–1449. [Google Scholar] [CrossRef]
- Li, K.; Li, X.M.; Ji, N.Y.; Wang, B.G. Natural bromophenols from the marine red alga polysiphonia urceolata (rhodomelaceae): Structural elucidation and dpph radical-scavenging activity. Bioorg. Med. Chem. Lett. 2007, 15, 6627–6631. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.M.; Ji, N.Y.; Wang, B.G. Bromophenols from the marine red alga polysiphonia urceolata with dpph radical scavenging activity. J. Nat. Prod. 2008, 71, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.M.; Ji, N.Y.; Gloer, J.B.; Wang, B.G. Urceolatin, a structurally unique bromophenol from polysiphonia urceolata. Org. Lett. 2008, 10, 1429–1432. [Google Scholar] [CrossRef]
- Aknin, M.; Samb, A.; Mirailles, J.; Costantino, V.; Fattorusso, E.; Mangoni, A. Polysiphenol, a new brominated 9,10-dihydrophenanthrene from the senegalese red alga polysyphonia ferulacea. Tetrahedron Lett. 1992, 33, 555–558. [Google Scholar] [CrossRef]
- Barrett, T.N.; Braddock, D.C.; Monta, A.; Webb, M.R.; White, A.J.P. Total synthesis of the marine metabolite (±)-polysiphenol via highly regioselective intramolecular oxidative coupling. J. Nat. Prod. 2011, 74, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In vitro antiviral activity of red alga, polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef]
- Han, L.; Xu, N.; Shi, J.; Yan, X.; Zheng, C. Isolation and pharmacological activities of bromophenols fromrhodomela confervoides. Chin. J. Oceanol. Limnol. 2005, 23, 226–229. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, J.; Wang, S.; Li, S.; Yang, Y.; Shi, J.; Fan, X.; He, L. Bromophenols coupled with methyl γ-ureidobutyrate and bromophenol sulfates from the red alga rhodomela confervoides. J. Nat. Prod. 2006, 69, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Luo, J.; Jiang, B.; Wang, L.; Wang, S.; Wang, C.; Fu, C.; Li, J.; Shi, D. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxy-phenyl)-methane inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via modulating β1-integrin/fak signaling. Mar. Drugs 2015, 13, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. Isolation, characterization, and antioxidant activity of bromophenols of the marine red alga rhodomela confervoides. J. Agric. Food Chem. 2011, 59, 9916–9921. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, L.; Gao, L.; Gao, J.; Chen, J.; Li, J.; Song, F. Two new bromophenols with radical scavenging activity from marine red alga symphyocladia latiuscula. Mar. Drugs 2013, 11, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Okada, Y.; Shi, H.; Wang, Y.; Okuyama, T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Gao, L.; Cui, C.; Li, C.; Li, J.; Wang, B. Extraction and ptp1b inhibitory activity of bromophenols from the marine red alga symphyocladia latiuscula. Chin. J. Oceanol. Limnol. 2011, 29, 686–690. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, X.; Wang, B. Highly brominated mono- and bis-phenols from the marine red alga symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, L.; Gao, J.; Gao, L.; Song, F. Antifungal bromophenols from marine red alga symphyocladia latiuscula. Chem. Biodivers. 2014, 11, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, L.; Wang, Y.; Wang, S.; Song, F. A new bromobenzyl methyl sulphoxide from marine red alga symphyocladia latiuscula. Nat. Prod. Res. 2013, 27, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Oh, M.Y.; Jin, D.H.; Hong, Y.K. Identification of a taq DNA polymerase inhibitor from the red seaweed symphyocladia latiuscula. J. Environ. Biol. 2008, 29, 475–478. [Google Scholar] [PubMed]
- Wiemer, D.F.; Idler, D.D.; Fenical, W. Vidalols a and b, new anti-inflammatory bromophenols from the caribbean marine red algavidalia obtusaloba. Experientia 1991, 47, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.E. The ecology and evolution of seaweed-herbivore interactions on coral reefs. Coral Reefs 1997, 16, S67–S76. [Google Scholar] [CrossRef]
- Shi, D.; Li, J.; Guo, S.; Su, H.; Fan, X. The antitumor effect of bromophenol derivatives in vitro and leathesia nana extract in vivo. Chin. J. Oceanol. Limnol. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Areche, C.; San-Martín, A.; Rovirosa, J.; Soto-Delgado, J.; Contreras, R. An unusual halogenated meroditerpenoid from stypopodium flabelliforme: Studies by nmr spectroscopic and computational methods. Phytochemistry 2009, 70, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.Y.; Han, L.J.; Sun, J.; Li, S.; Wang, S.J.; Yang, Y.C.; Fan, X.; Shi, J.G. A new halogenated biindole and a new apo-carotenone from green alga chaetomorpha basiretorsa setchell. Chin. Chem. Lett. 2005, 16, 777–780. [Google Scholar]
- Carte, B.K.; Troupe, N.; Chan, J.A.; Westley, J.W.; Faulkner, D.J. Rawsonol, an inhibitor of hmg-coa reductase from the tropical green alga avrainvillea rawsoni. Phytochemistry 1989, 28, 2917–2919. [Google Scholar] [CrossRef]
- Colon, M.; Guevara, P.; Gerwick, W.H.; Ballantine, D. 5’-hydroxyisoavrainvilleol, a new diphenylmethane derivative from the tropical green alga avrainvillea nigricans. J. Nat. Prod. 1987, 50, 368–374. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Cheney, D.P. Bioprocess engineering of cell and tissue cultures for marine seaweeds. Aquacult. Eng. 2004, 32, 11–41. [Google Scholar] [CrossRef]
- Barahona, L.F.; Rorrer, G.L. Isolation of halogenated monoterpenes from bioreactor-cultured microplantlets of the macrophytic red algae ochtodes secundiramea and portieria hornemannii. J. Nat. Prod. 2003, 66, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Polzin, J.J.; Rorrer, G.L.; Cheney, D.P. Metabolic flux analysis of halogenated monoterpene biosynthesis in microplantlets of the macrophytic red alga ochtodes secundiramea. Biomol. Eng. 2003, 20, 205–215. [Google Scholar] [CrossRef]


| Number of Halogens | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromo | Chloro | Iodo | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Halophenols | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - monoaryl halophenols | 23 | 35 | 12 | - | - | - | 1 | - | - | - | - | - | 2 | 1 | - | - | - | - |
| - dimers | 1 | 7 | 15 | 6 | 6 | 4 | - | - | - | - | - | - | - | - | - | - | - | - |
| - trimers | - | - | - | 1 | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - tetramers | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Indoles | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - monoaryl | 4 | 11 | 13 | 8 | - | - | 3 | 5 | 4 | - | - | - | 6 | - | - | - | - | - |
| - dimers | - | 1 | - | 5 | - | 1 | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Sesquiterpenes | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - monoaryl | 15 | 2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| - dimers | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Naphthalene derivatives | - | - | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Total of haloaryl derivatives | 43 | 57 | 43 | 21 | 8 | 5 | 5 | 5 | 4 | - | - | - | 8 | 1 | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae. Mar. Drugs 2019, 17, 73. https://doi.org/10.3390/md17020073
Jesus A, Correia-da-Silva M, Afonso C, Pinto M, Cidade H. Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae. Marine Drugs. 2019; 17(2):73. https://doi.org/10.3390/md17020073
Chicago/Turabian StyleJesus, Ana, Marta Correia-da-Silva, Carlos Afonso, Madalena Pinto, and Honorina Cidade. 2019. "Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae" Marine Drugs 17, no. 2: 73. https://doi.org/10.3390/md17020073
APA StyleJesus, A., Correia-da-Silva, M., Afonso, C., Pinto, M., & Cidade, H. (2019). Isolation and Potential Biological Applications of Haloaryl Secondary Metabolites from Macroalgae. Marine Drugs, 17(2), 73. https://doi.org/10.3390/md17020073