Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Various Initial Glucose Concentrations on the Growth and Lipid Accumulation of P. tricornutum under Mixotrophic Cultivation
2.2. Effect of Various C/N Ratios on the Growth and Lipid Accumulation of P. tricornutum
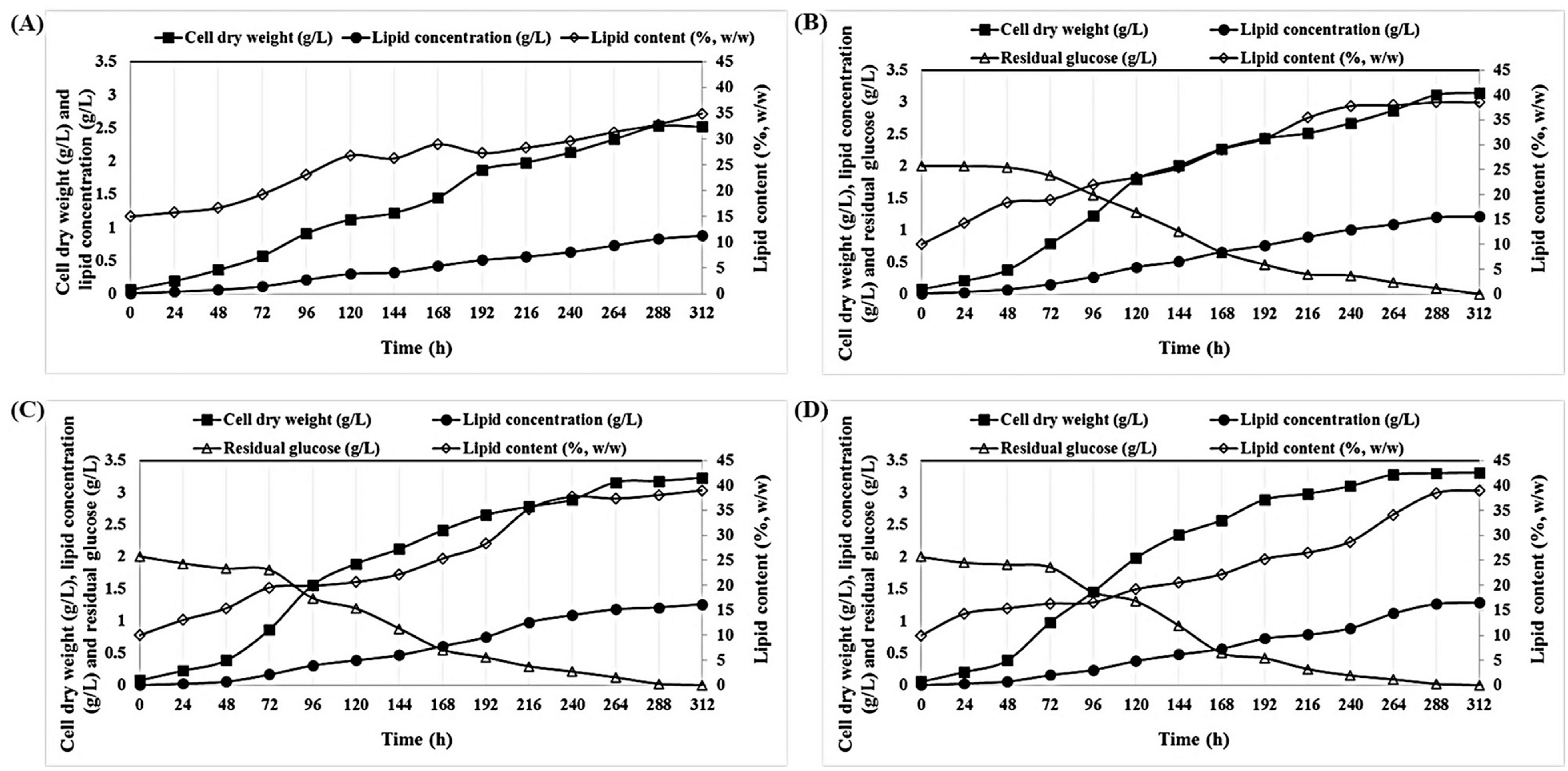
2.3. Mixotrophic Cultivation of P. tricornutum on Wood Hydrolysates
2.4. EPA and DHA Production under Photoautotrophic and Mixotrophic Cultivation
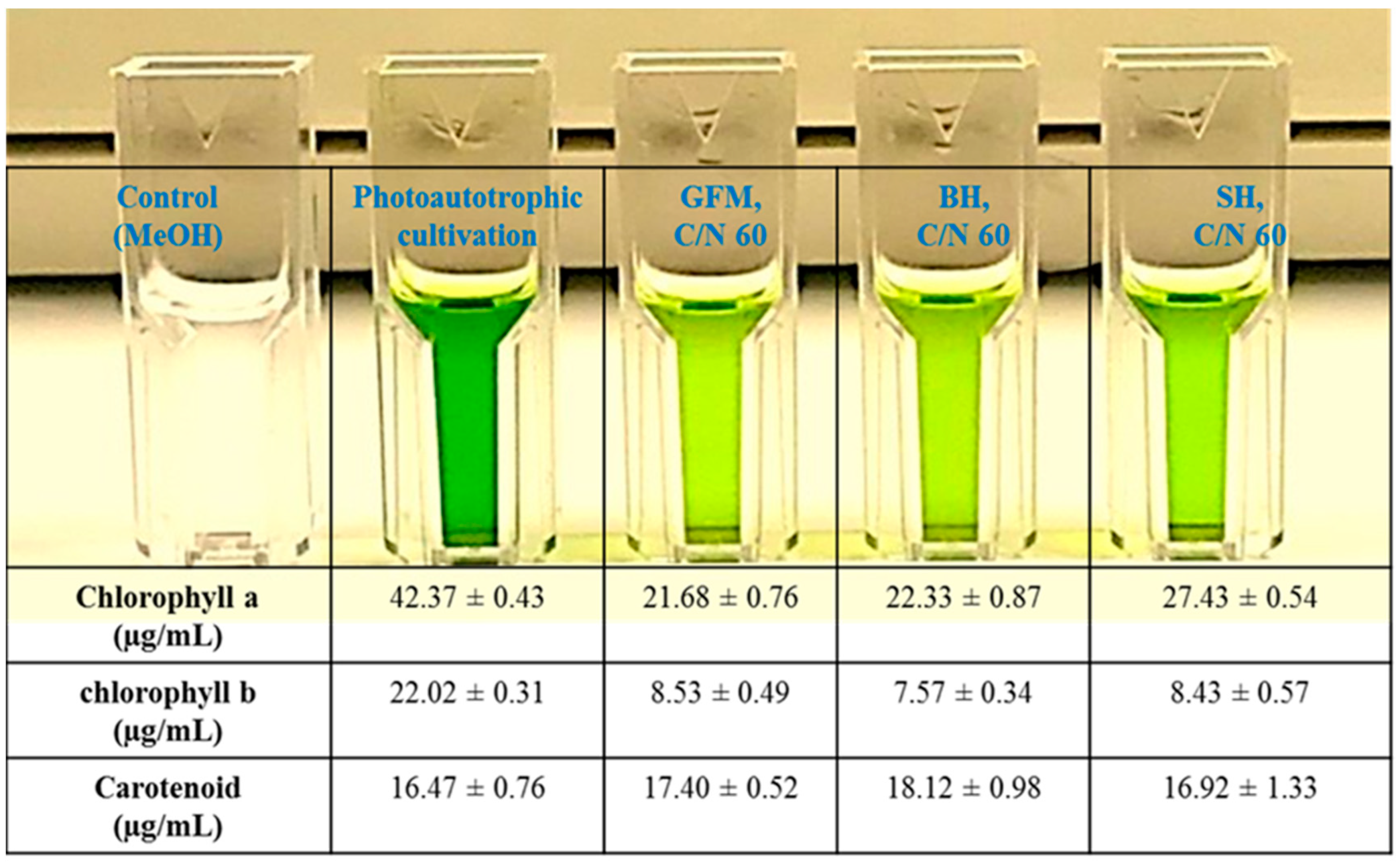
2.5. Pigment Composition in the Lipids Obtained during Mixotrophic Cultivation of P. tricornutum on Wood Hydrolysates
3. Materials and Methods
3.1. Materials
3.2. Medium and Culture Conditions
3.3. Optimization of Biomass and Lipid Production under Mixotrophic Cultivation
3.4. Batch Cultivations of P. tricornutum Using Hydrolysates from Organosolv-Pretreated Birch and Spruce Woodchips
3.5. Analytical Methods
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Lands, B. Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog. Lipid Res. 2014, 55, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Wanasundara, U.N. Omega-3 fatty acid concentrates: Nutritional aspects and production technologies. Trends Food Sci. Technol. 1998, 9, 230–240. [Google Scholar] [CrossRef]
- Shang, T.; Liu, L.; Zhou, J.; Zhang, M.; Hu, Q.; Fang, M.; Wu, Y.; Yao, P.; Gong, Z. Protective effects of various ratios of DHA/EPA supplementation on high-fat diet-induced liver damage in mice. Lipids Health Dis. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; de Diego, S.M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Scheben, A.; Edwards, D. Bottlenecks for genome-edited crops on the road from lab to farm. Genome Biol. 2018, 19, 178. [Google Scholar] [CrossRef]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.-L.; Molina-Jouve, C.; Nicaud, J.-M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.M. Yeast: A new oil producer? OCL - Ol. Corps Gras Lipides 2012, 19, 22–28. [Google Scholar] [CrossRef]
- Ratledge, C. Microbial oils: An introductory overview of current status and future prospects. Ocl 2013, 20, D602. [Google Scholar] [CrossRef]
- Moomaw, W.; Berzin, I.; Tzachor, A. Cutting Out the Middle Fish: Marine Microalgae as the Next Sustainable Omega-3 Fatty Acids and Protein Source. Ind. Biotechnol. 2017, 13, 234–243. [Google Scholar] [CrossRef]
- Adarme-Vega, T.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Lee, J.W.; Chung, C.H. Molecular challenges in microalgae towards cost-effective production of quality biodiesel. Renew. Sustain. Energy Rev. 2017, 74, 139–144. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cerón-García, M.C.; Fernández-Sevilla, J.M.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Mixotrophic growth of Phaeodactylum tricornutum on fructose and glycerol in fed-batch and semi-continuous modes. Bioresour. Technol. 2013, 147, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Quinn, A.H.; Sriram, G. Experimental evidence and isotopomer analysis of mixotrophic glucose metabolism in the marine diatom Phaeodactylum tricornutum. Microb. Cell Fact. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.L.; Powers, S.; Napier, J.A.; Sayanova, O. Heterotrophic production of omega-3 long-chain polyunsaturated fatty acids by trophically converted marine diatom Phaeodactylum tricornutum. Mar. Drugs 2016, 14, 53. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, T.; Rong, J.; He, C.; Wang, Q. Microalgal biofuel revisited: An informatics-based analysis of developments to date and future prospects. Appl. Energy 2015, 155, 585–598. [Google Scholar] [CrossRef]
- Liu, X.; Duan, S.; Li, A.; Xu, N.; Cai, Z.; Hu, Z. Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J. Appl. Phycol. 2009, 21, 239–246. [Google Scholar] [CrossRef]
- Skogsmarkens kolförråd. Forest Statistics 2017, Aktuella Uppgifter om de Svenska Skogarna från Riksskogstaxeringen. Available online: https://www.slu.se/globalassets/ew/org/centrb/rt/dokument/skogsdata/skogsdata_2017.pdf (accessed on 13 February 2019).
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv—Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2019, 273, 521–528. [Google Scholar] [CrossRef]
- Matsakas, L.; Nitsos, C.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. A novel hybrid organosolv—Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Tian, G.; Liu, J. Enhancement of biomass productivity and nutrients removal from pretreated piggery wastewater by mixotrophic cultivation of Desmodesmus sp. CHX1. Desalin. Water Treat. 2013, 51, 7004–7011. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, R.; Pei, G. A study on lipid production of the mixotrophic microalgae Phaeodactylum tricornutum on various carbon sources. Afr. J. Microbiol. Res. 2012, 6, 1041–1047. [Google Scholar] [CrossRef]
- Fábregas, J.; Morales, E.D.; Lamela, T.; Cabezas, B.; Otero, A. Mixotrophic productivity of the marine diatom Phaeodactylum tricornutum cultured with soluble fractions of rye, wheat and potato. World J. Microbiol. Biotechnol. 1997, 13, 349–351. [Google Scholar] [CrossRef]
- Arora, N.; Patel, A.; Pruthi, P.A.; Pruthi, V. Synergistic dynamics of nitrogen and phosphorous influences lipid productivity in Chlorella minutissima for biodiesel production. Bioresour. Technol. 2016, 285530, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Yodsuwan, N.; Sawayama, S.; Sirisansaneeyakul, S. Effect of nitrogen concentration on growth, lipid production and fatty acid profiles of the marine diatom Phaeodactylum tricornutum. Agric. Nat. Resour. 2017, 51, 190–197. [Google Scholar] [CrossRef]
- Ördög, V.; Stirk, W.A.; Bálint, P.; van Staden, J.; Lovász, C. Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J. Appl. Phycol. 2012, 24, 907–914. [Google Scholar] [CrossRef]
- Falkowski, P.G. The Evolution of Modern Eukaryotic Phytoplankton. Science 2004, 305, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; Ward, B.B.; Song, B. Characterization of diatom (Bacillariophyceae) nitrate reductase genes and their detection in marine phytoplankton communities. J. Phycol. 2005, 41, 95–104. [Google Scholar] [CrossRef]
- Bowler, C.; Vardi, A.; Allen, A.E. Oceanographic and Biogeochemical Insights from Diatom Genomes. Ann. Rev. Mar. Sci. 2010, 2, 333–365. [Google Scholar] [CrossRef]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Kopriva, S.; Malin, G. The Response of Diatom Central Carbon Metabolism to Nitrogen Starvation Is Different from That of Green Algae and Higher Plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Cerón García, M.C.; Sánchez Mirón, A.; Fernández Sevilla, J.M.; Molina Grima, E.; García Camacho, F. Mixotrophic growth of the microalga Phaeodactylum tricornutum: Influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem. 2005, 40, 297–305. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab. Eng. 2014, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.G.; Kim, B.H.; Ahn, C.Y.; Oh, H.M. Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii. J. Appl. Phycol. 2011, 23, 1031–1037. [Google Scholar] [CrossRef]
- Hassan, M.; Blanc, P.; Granger, L.-M.; Pareilleux, A.; Goma, G. Lipid production by an unsaturated fatty acid auxotroph of the oleaginous yeast Apiotrichum curvatum grown in single-stage continuous culture. Appl. Microbiol. Biotechnol. 1993, 40, 483–488. [Google Scholar] [CrossRef]
- Julian Davies, R.; Holdsworth, J.E.; Reader, S.L. The effect of low oxygen uptake rate on the fatty acid profile of the oleaginous yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1991, 34, 832–833. [Google Scholar] [CrossRef]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Meesters, P.A.E.P.; Eggink, G. Isolation and characterization of a Δ-9 fatty acid desaturase gene from the oleaginous yeastCryptococcus curvatus CBS 570. Yeast 1996, 12, 723–730. [Google Scholar] [CrossRef]
- Moreton, R.S. Modification of fatty acid composition of lipid accumulating yeasts with cyclopropene fatty acid desaturase inhibitors. Appl. Microbiol. Biotechnol. 1985, 22, 41–45. [Google Scholar] [CrossRef]
- Qiao, H.; Cong, C.; Sun, C.; Li, B.; Wang, J.; Zhang, L. Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum. Aquaculture 2016, 452, 311–317. [Google Scholar] [CrossRef]
- Tressou, J.; Buaud, B.; Simon, N.; Pasteau, S.; Guesnet, P. Very low inadequate dietary intakes of essential n-3 polyunsaturated fatty acids (PUFA) in pregnant and lactating French women: The INCA2 survey. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Brown, R.E.; Zhang, P.-C.; Zhao, Y.-T.; Ju, X.-H.; Song, C. DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Beardall, J.; Heraud, P. Changes in growth, chlorophyll fluorescence and fatty acid composition with culture age in batch cultures of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Bot. Mar. 2006, 49, 165–173. [Google Scholar] [CrossRef]
- Molina Grima, E.; Sánchez Pérez, J.A.; García Camacho, F.; Fernández Sevilla, J.M.; Acién Fernández, F.G.; Urda Cardona, J. Biomass and icosapentaenoic acid productivities from an outdoor batch culture of Phaeodactylum tricornutum UTEX 640 in an airlift tubular photobioreactor. Appl. Microbiol. Biotechnol. 1995, 42, 658–663. [Google Scholar] [CrossRef]
- Alsenani, F.; Ahmed, F.; Schenk, P.M. Nutraceuticals from microalgae. Nutraceuticals Funct. Foods Hum. Heal. Dis. Prev. 2015, 673–684. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Chen, H.; He, C.L.; Wang, Q. Nitrogen Starvation Induced Oxidative Stress in an Oil-Producing Green Alga Chlorella sorokiniana C3. PLoS ONE 2013, 8, e69225. [Google Scholar] [CrossRef]
- Liu, X.J.; Duan, S.-S.; Li, A.-F.; Sun, K.-F. Effects of Glycerol on the Fluorescence Spectra and Chloroplast Ultrastructure of Phaeodactylum tricornutum (Bacillariophyta). J. Integr. Plant Biol. 2009, 51, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, C.; Schmälzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J. 2007, 51, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Toussaint, J.-P.; Cooksey, K.E.; Fields, M.W.; Gerlach, R.; Peyton, B.M.; Carlson, R.P. Physiological and molecular analysis of carbon source supplementation and pH stress-induced lipid accumulation in the marine diatom Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2013, 97, 3625–3642. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
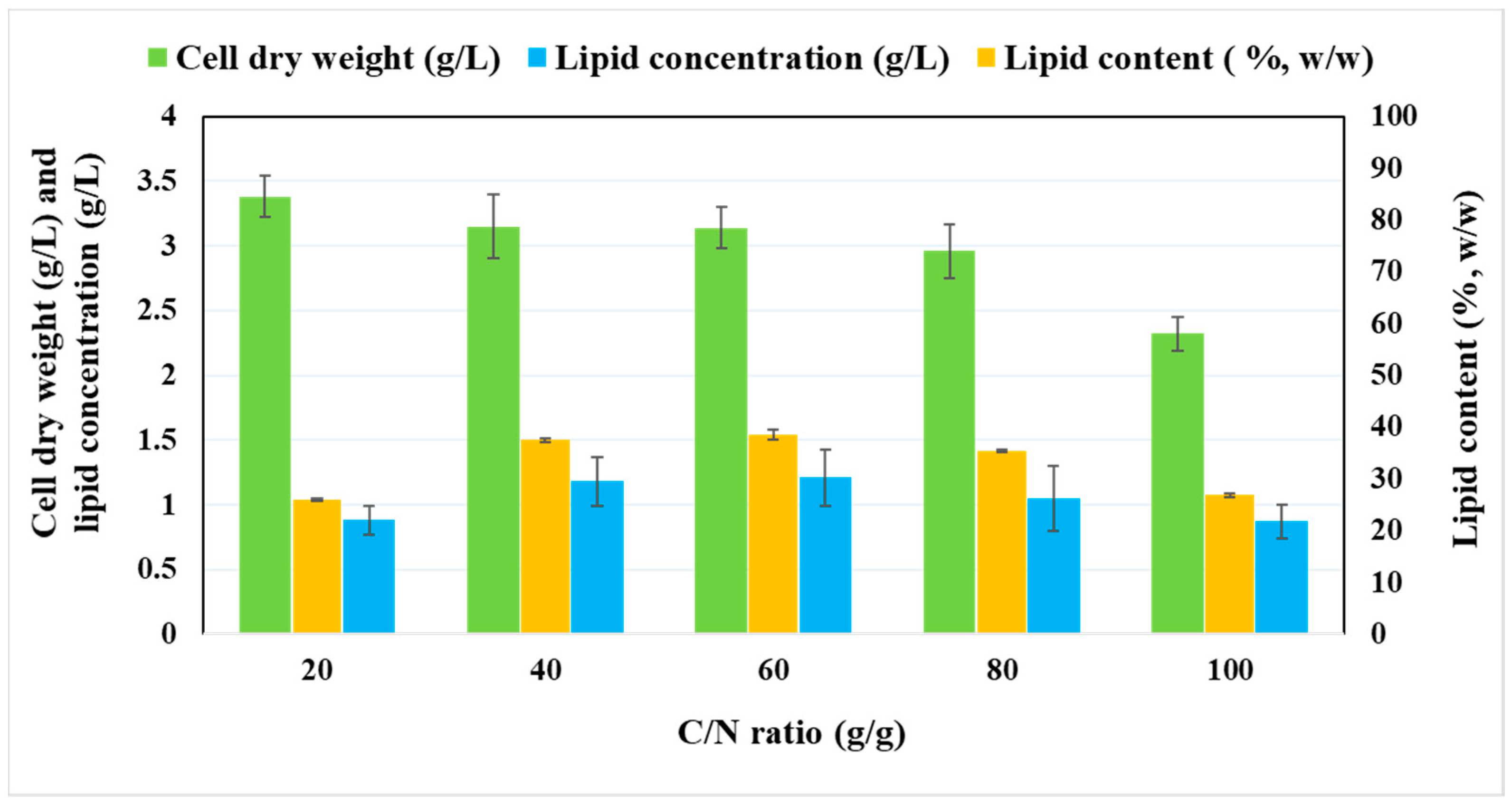
| Initial Glucose Concentration (g/L) in GFM | Cell Dry Weight (g/L) | Biomass Productivity # (g/L/d) | Lipid Concentration (g/L) | Lipid Content (%, w/w) | Lipid Productivity # (g/L/d) | Biomass Yield (g/gsubstrate) | Lipid Yield (g/gsubstrate) | Residual Glucose Concentration (g/L) |
|---|---|---|---|---|---|---|---|---|
| Photoautotrophic cultivation | 0.89 ± 0.11 | 0.081± 0.001 | 0.20 ± 0.06 | 22.47 ± 0.23 | 0.018 ± 0.001 | - | - | - |
| 0 (control) | 2.52 ± 0.14 | 0.193 ± 0.002 | 0.57 ± 0.09 | 22.62 ± 0.28 | 0.043 ± 0.007 | - | - | - |
| 2 | 3.38 ± 0.16 | 0.260 ± 0.003 | 0.88 ± 0.11 | 26.03 ± 0.45 | 0.067 ± 0.009 | 1.69 ± 0.19 | 0.44 ± 0.09 | 0.00 ± 0.00 |
| 4 | 4.10 ± 0.21 | 0.315 ± 0.005 | 1.08 ± 0.12 | 26.34 ± 0.21 | 0.083 ± 0.001 | 1.31 ± 0.21 | 0.34 ± 0.08 | 0.86 ± 0.17 |
| 6 | 4.14 ± 0.31 | 0.318 ± 0.008 | 1.12 ± 0.21 | 27.05 ± 0.71 | 0.086 ± 0.002 | 1.29 ± 0.13 | 0.35 ± 0.07 | 2.80 ± 0.23 |
| 8 | 4.24 ± 0.19 | 0.326 ± 0.004 | 1.15 ± 0.17 | 27.12 ± 0.87 | 0.088 ± 0.001 | 1.19 ± 0.21 | 0.32 ± 0.04 | 4.45 ± 0.41 |
| 10 | 4.32 ± 0.32 | 0.332 ± 0.009 | 1.16 ± 0.23 | 26.85 ± 0.76 | 0.089 ± 0.002 | 1.34 ± 0.19 | 0.36 ± 0.09 | 6.78 ± 0.37 |
| Parameters | Photoautotrophic Cultivation | GFM (C/N, 60) | BH (C/N, 60) | SH (C/N, 60) |
|---|---|---|---|---|
| Cell dry weight (g/L) | 0.89 ± 0.11 | 3.15 ± 0.53 | 3.23 ± 0.32 | 3.31 ± 0.28 |
| Biomass Productivity # (g/L/d) | 0.081± 0.001 | 0.242 ± 0.005 | 0.248 ± 0.004 | 0.254 ± 0.007 |
| Lipids concentration (g/L) | 0.20 ± 0.06 | 1.21 ± 0.19 | 1.26 ± 0.11 | 1.29 ± 0.18 |
| Lipid content (%, w/w) | 22.47 ± 0.23 | 38.41 ± 0.21 | 39.00 ± 0.23 | 38.97 ± 0.43 |
| Lipids productivity # (mg/L/d) | 18.18 ± 0.34 | 93.07 ± 0.68 | 97.00 ± 0.85 | 99.23 ± 1.09 |
| Fatty Acids (%) in Total Lipid | Photoautotrophic Cultivation | Mixotrophic Cultivation Without Glucose | GFM; C/N 20 | GFM; C/N 60 | BH; C/N 60 | SH; C/N 60 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated Fatty Acids (SFAs) | (C14:0) | 8.24 | 30.27 | 7.18 | 25.56 | 2.60 | 24.62 | 2.9 | 26.33 | 3.1 | 27.1 | 2.65 | 26.13 |
| (C16:0) | 15.39 | 13.62 | 12.53 | 11.01 | 13.23 | 12.32 | |||||||
| (C18:0) | 4.32 | 2.96 | 1.72 | 3.8 | 2.89 | 2.65 | |||||||
| (C20:0) | 2.32 | 1.80 | 3.54 | 3.65 | 3.43 | 3.87 | |||||||
| (C24:0) | - | - | 4.23 | 4.97 | 4.45 | 4.64 | |||||||
| Mono Unsaturated Fatty Acids (MUFAs) | (C16:1) | 17.23 | 34.56 | 13.62 | 34.23 | 15.99 | 34.48 | 17.17 | 38.78 | 17.65 | 39.12 | 17.87 | 39.61 |
| (C18:1 n9t) | 15.21 | 18.3 | 15.37 | 16.96 | 16.34 | 16.87 | |||||||
| (C18:1 n9c) | 2.12 | 2.31 | 3.12 | 4.65 | 5.13 | 4.87 | |||||||
| Poly Unsaturated Fatty Acids (PUFAs) | (C18:2 n6c) | 1.32 | 16.40 | 3.20 | 18.91 | 2.54 | 22.12 | 2.8 | 24.74 | 2.56 | 26.68 | 2.71 | 27.47 |
| (C18:3 n3) | - | - | - | - | - | - | |||||||
| (C20:5 n3) EPA | 13.43 | 14.0 | 16.76 | 18.38 | 19.80 | 19.87 | |||||||
| (C22:6 n3) DHA | 1.65 | 1.71 | 2.82 | 3.56 | 4.32 | 4.89 | |||||||
| DHA/EPA | 0.12 | 0.12 | 0.17 | 0.19 | 0.22 | 0.25 | |||||||
| Total fatty acids | 81.23 | 78.70 | 81.22 | 89.85 | 92.9 | 93.21 | |||||||
| Parameters | Photoautotrophic Mode of Cultivation | Mixotrophic Mode of Cultivation | ||||
|---|---|---|---|---|---|---|
| Without Glucose | GSM (Glucose, 2g/L) C/N;20 | GSM (Glucose, 2g/L) C/N;60 | BH (Glucose, 2g/L) C/N;60 | SH (Glucose, 2g/L) C/N;60 | ||
| Total EPA concentration (mg/L) | 26.86 | 79.80 | 147.48 | 222.39 | 249.48 | 256.32 |
| EPA yield (mg/gdry biomass) | 30.17 | 31.66 | 43.63 | 70.60 | 77.23 | 77.43 |
| EPA productivity (mg/L/d) | 2.44 | 6.14 | 11.31 | 17.07 | 19.15 | 19.69 |
| Total DHA concentration (mg/L) | 3.30 | 9.75 | 24.82 | 43.08 | 54.43 | 63.08 |
| DHA yield (mg/gdry biomass) | 3.70 | 3.86 | 7.34 | 13.67 | 16.85 | 19.05 |
| DHA productivity (mg/L/d) | 0.30 | 0.75 | 1.91 | 3.32 | 4.18 | 4.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Matsakas, L.; Hrůzová, K.; Rova, U.; Christakopoulos, P. Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Mar. Drugs 2019, 17, 119. https://doi.org/10.3390/md17020119
Patel A, Matsakas L, Hrůzová K, Rova U, Christakopoulos P. Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Marine Drugs. 2019; 17(2):119. https://doi.org/10.3390/md17020119
Chicago/Turabian StylePatel, Alok, Leonidas Matsakas, Kateřina Hrůzová, Ulrika Rova, and Paul Christakopoulos. 2019. "Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass" Marine Drugs 17, no. 2: 119. https://doi.org/10.3390/md17020119
APA StylePatel, A., Matsakas, L., Hrůzová, K., Rova, U., & Christakopoulos, P. (2019). Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Marine Drugs, 17(2), 119. https://doi.org/10.3390/md17020119