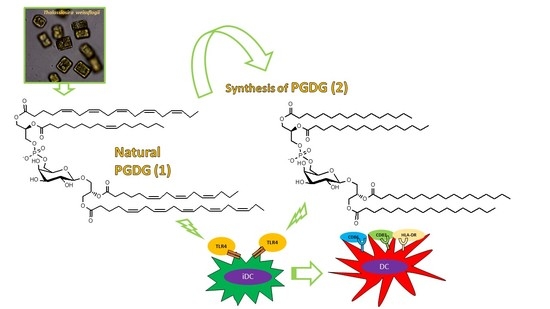
Immunostimulatory Phosphatidylmonogalactosyldiacylglycerols (PGDG) from the Marine Diatom Thalassiosira weissflogii: Inspiration for a Novel Synthetic Toll-Like Receptor 4 Agonist
Abstract
:1. Introduction
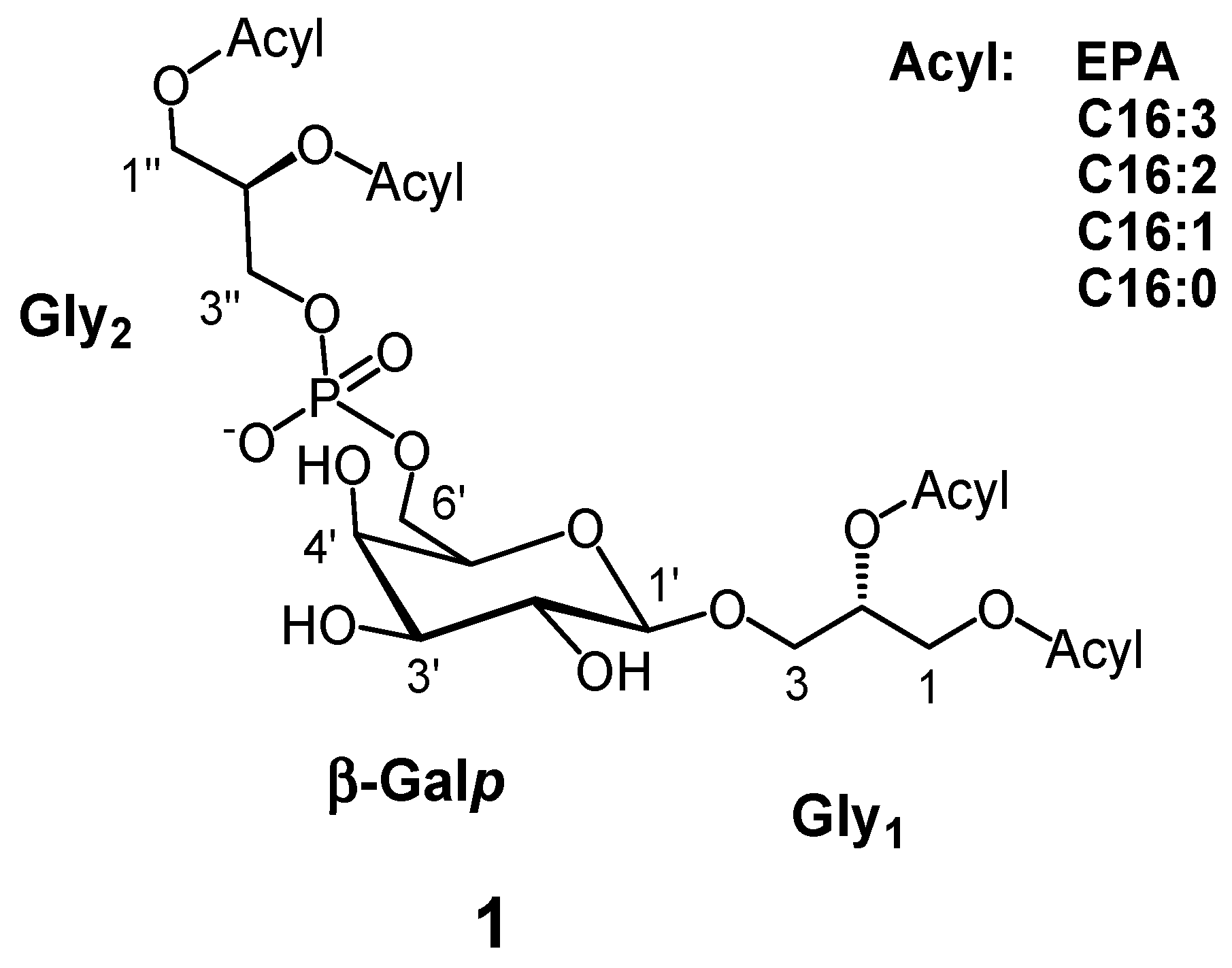
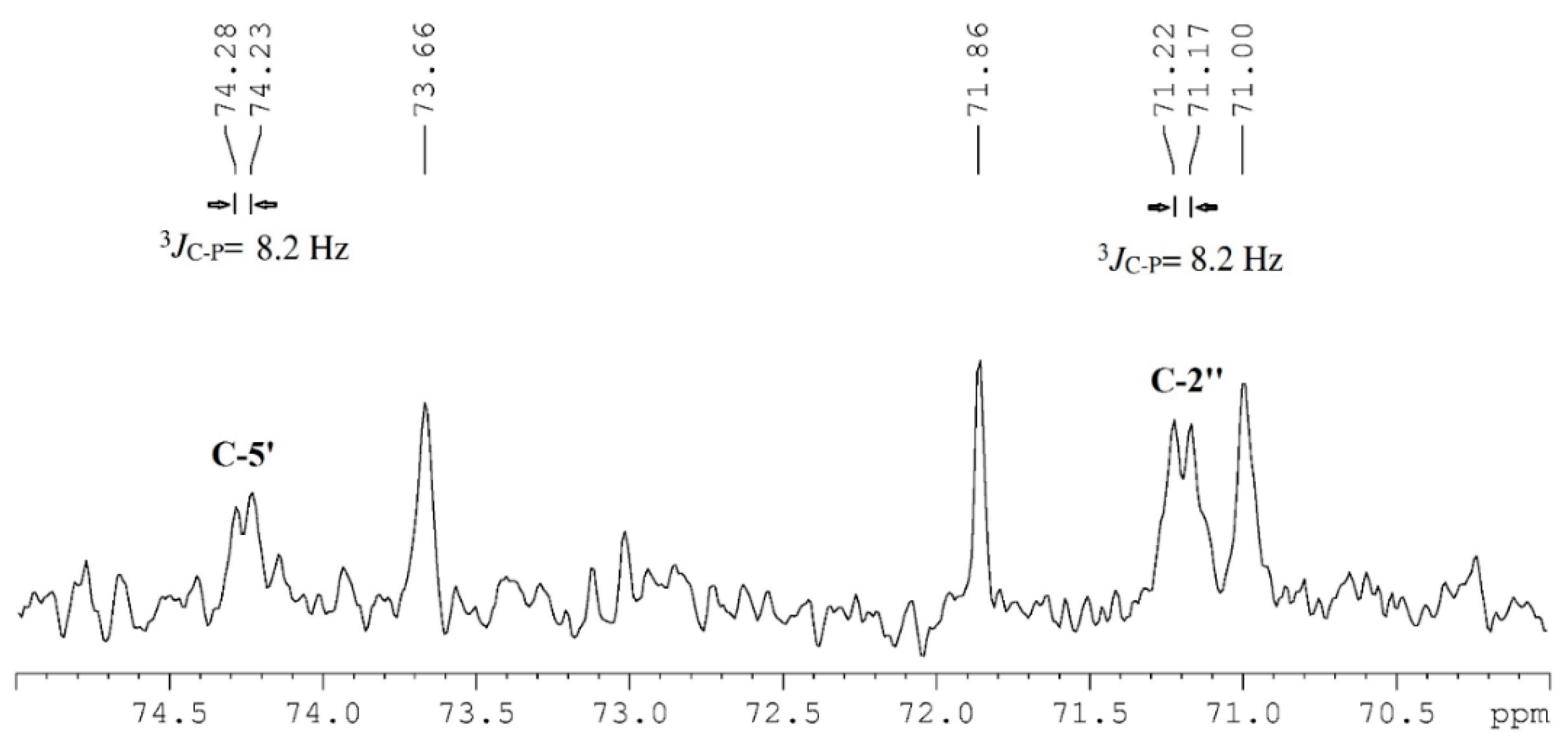
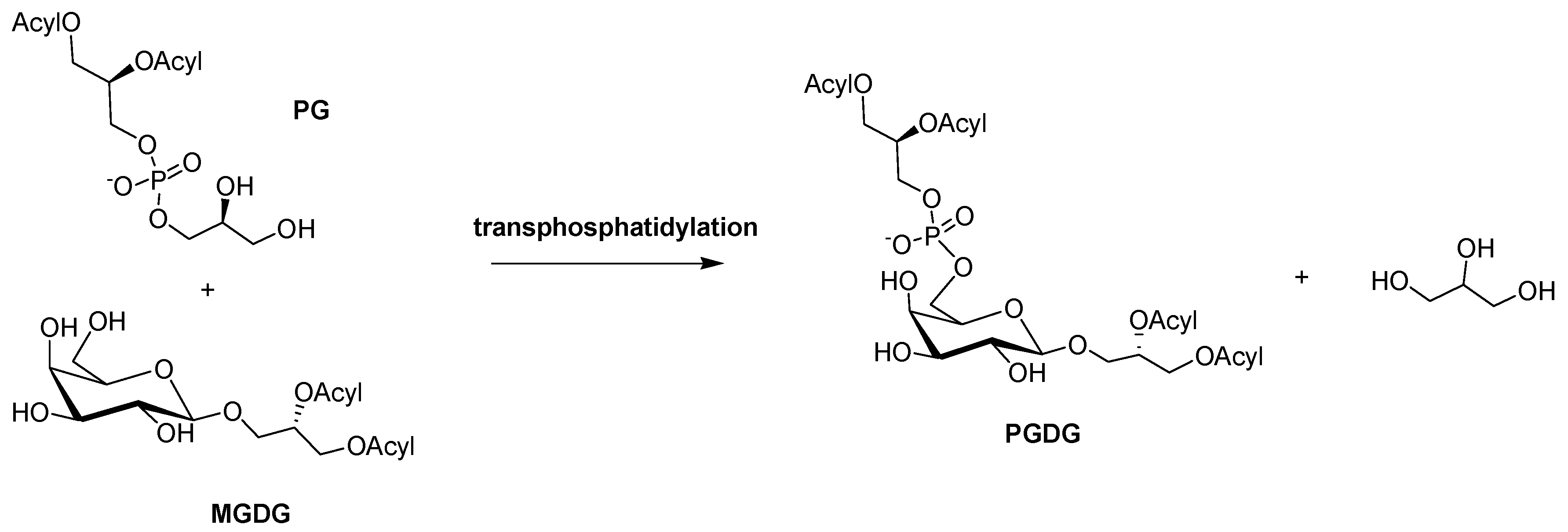
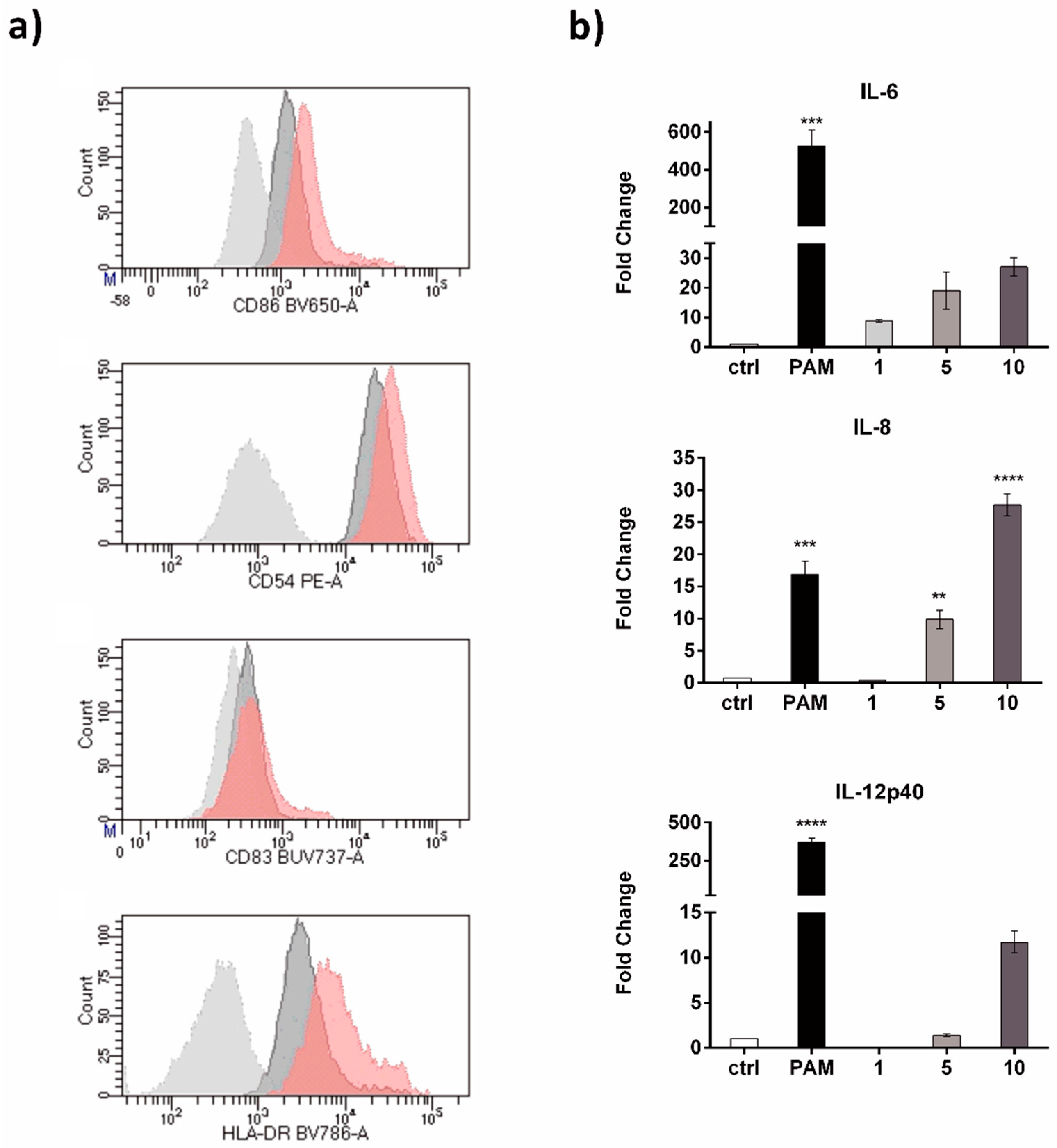
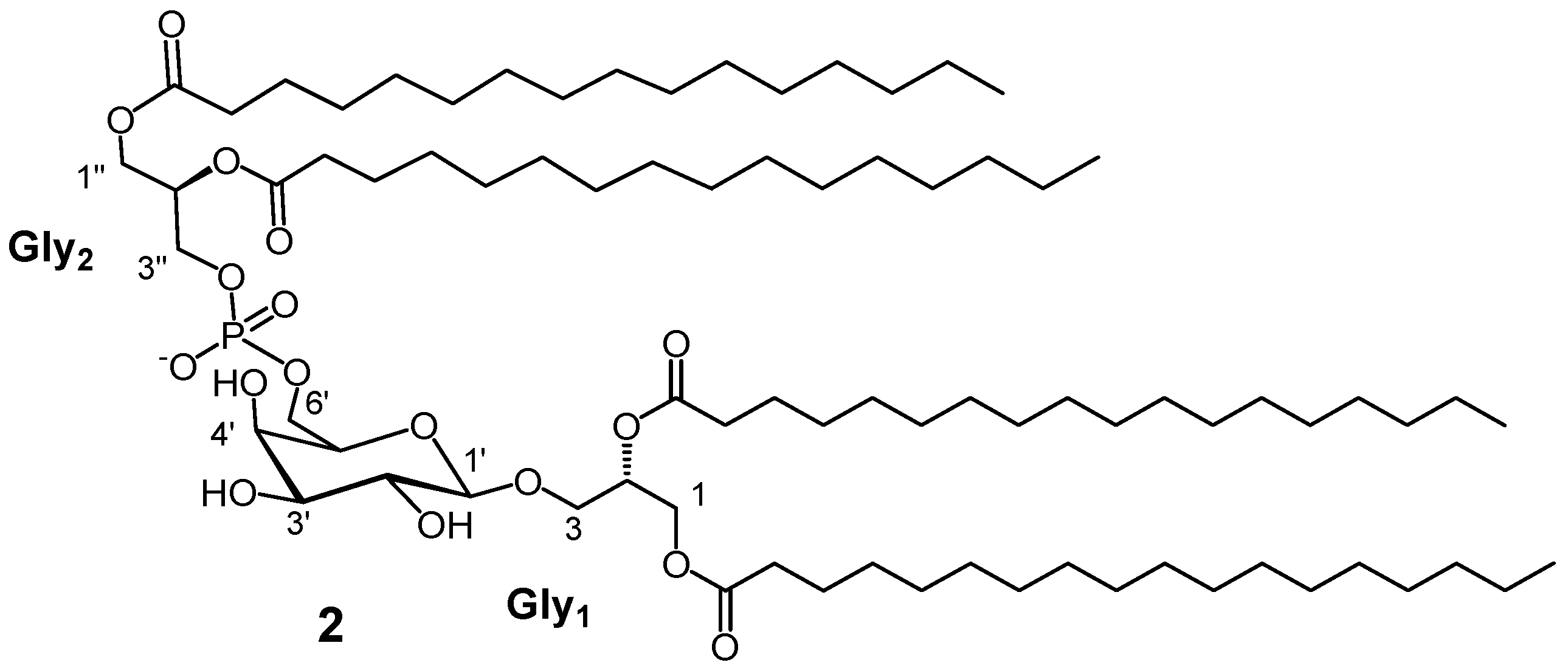
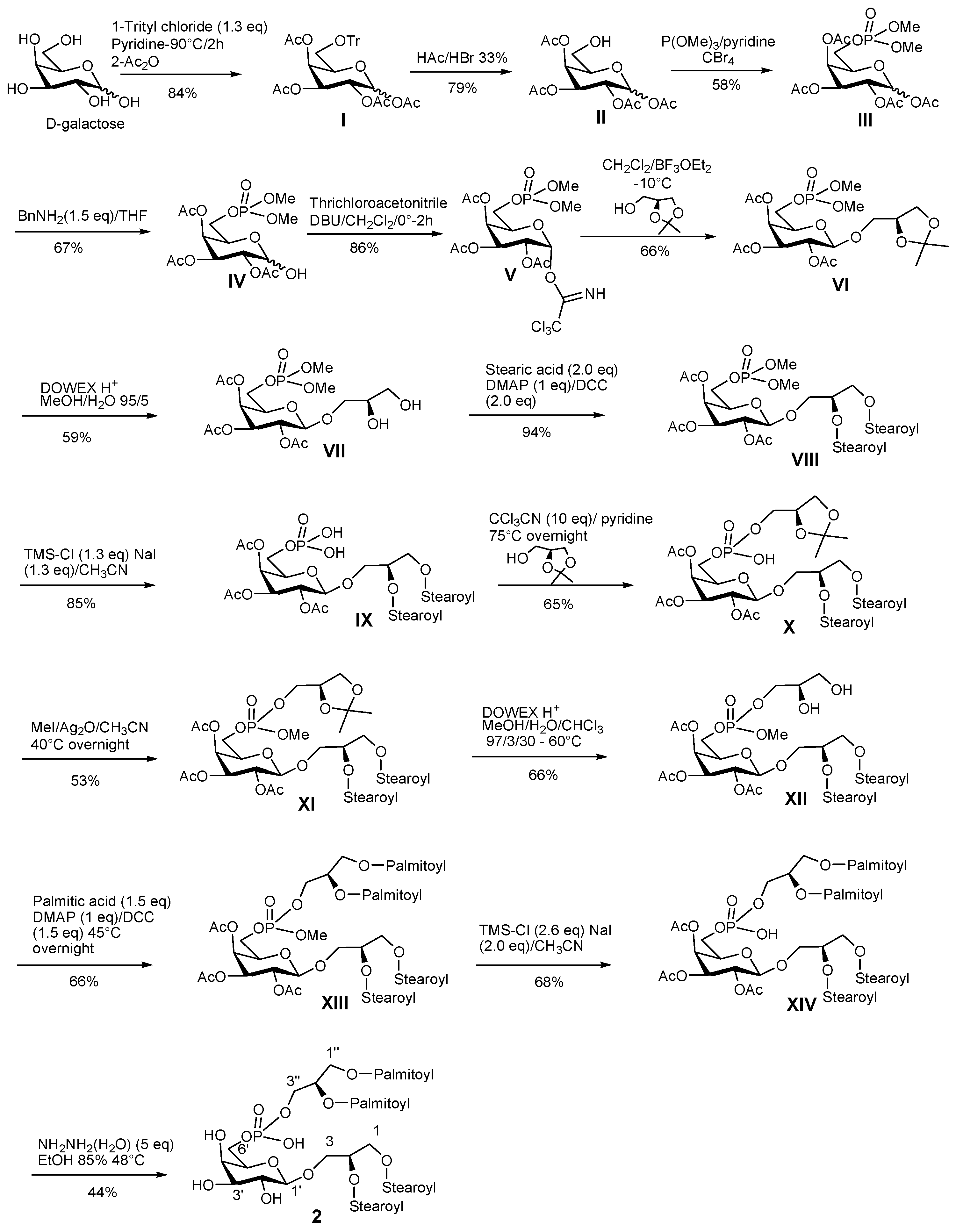
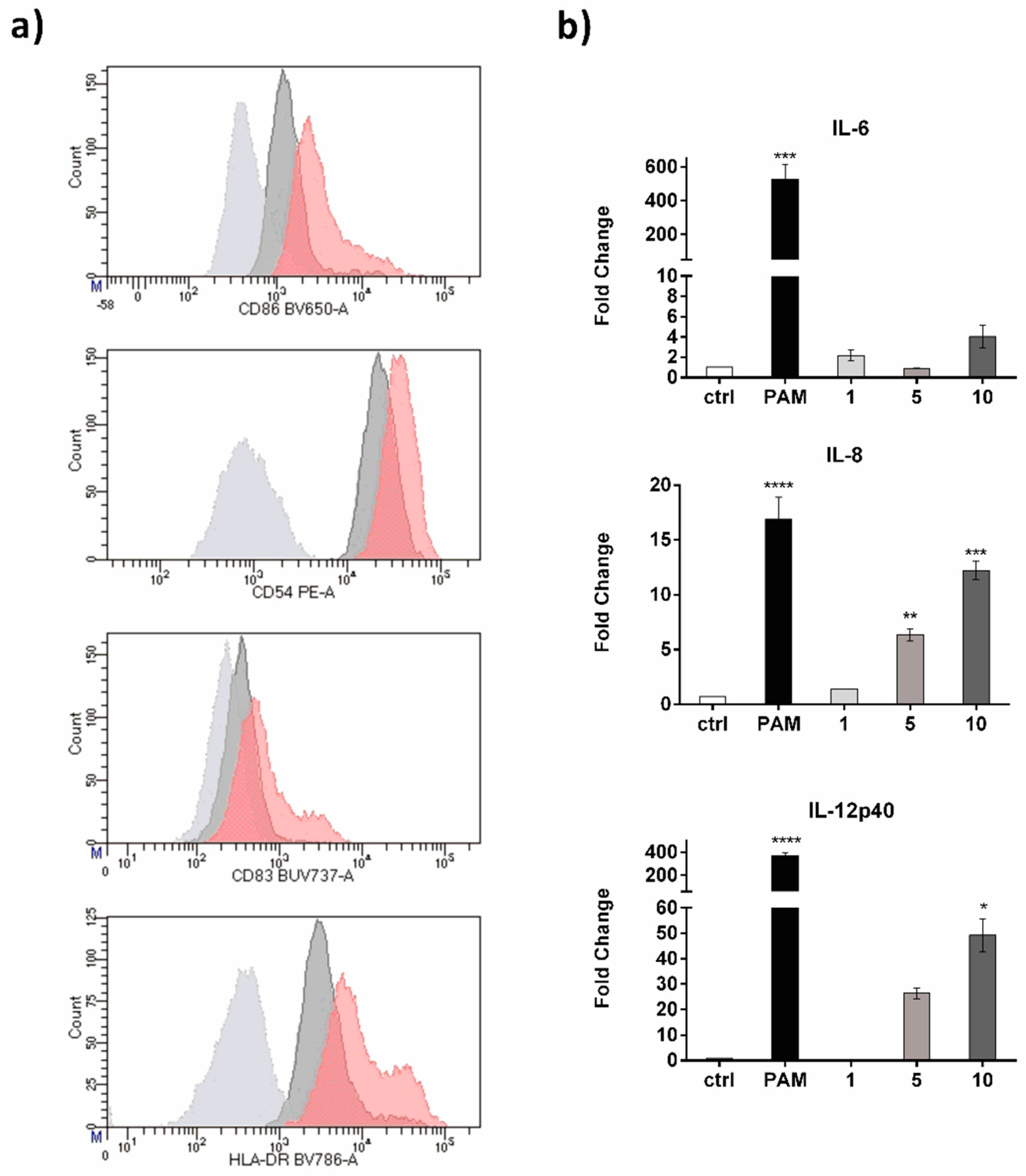
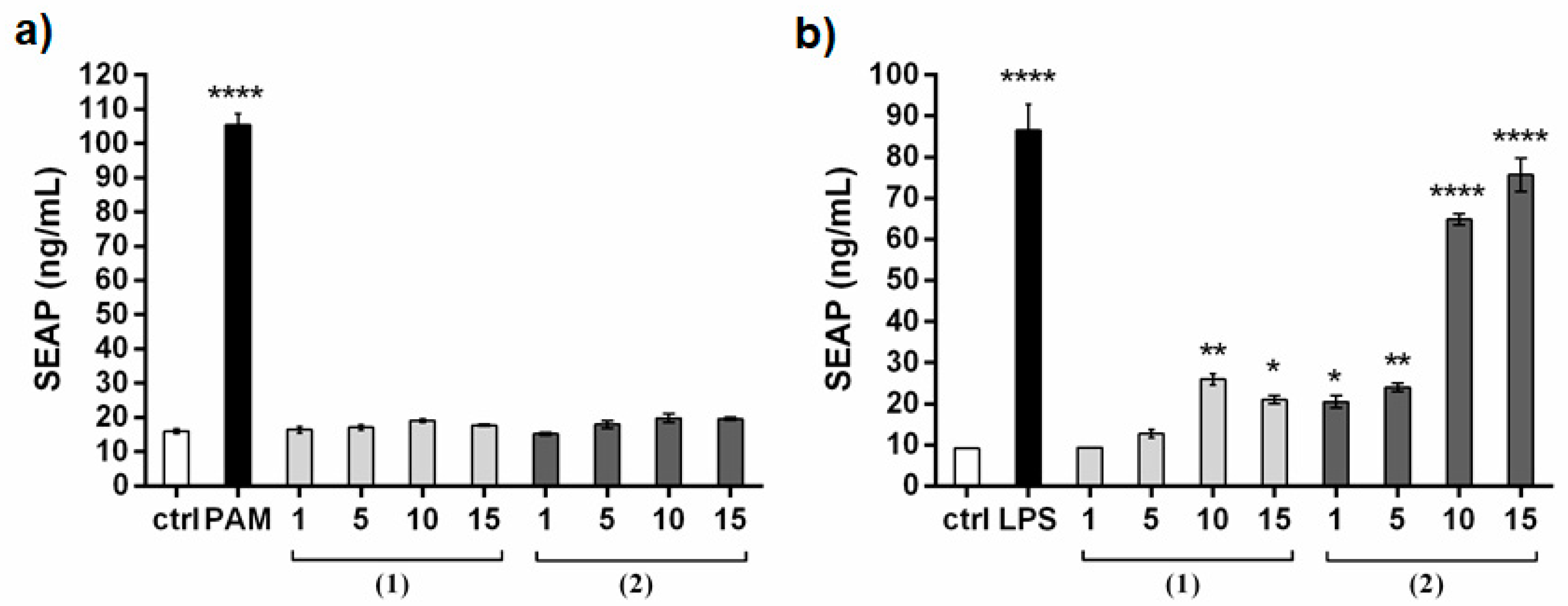
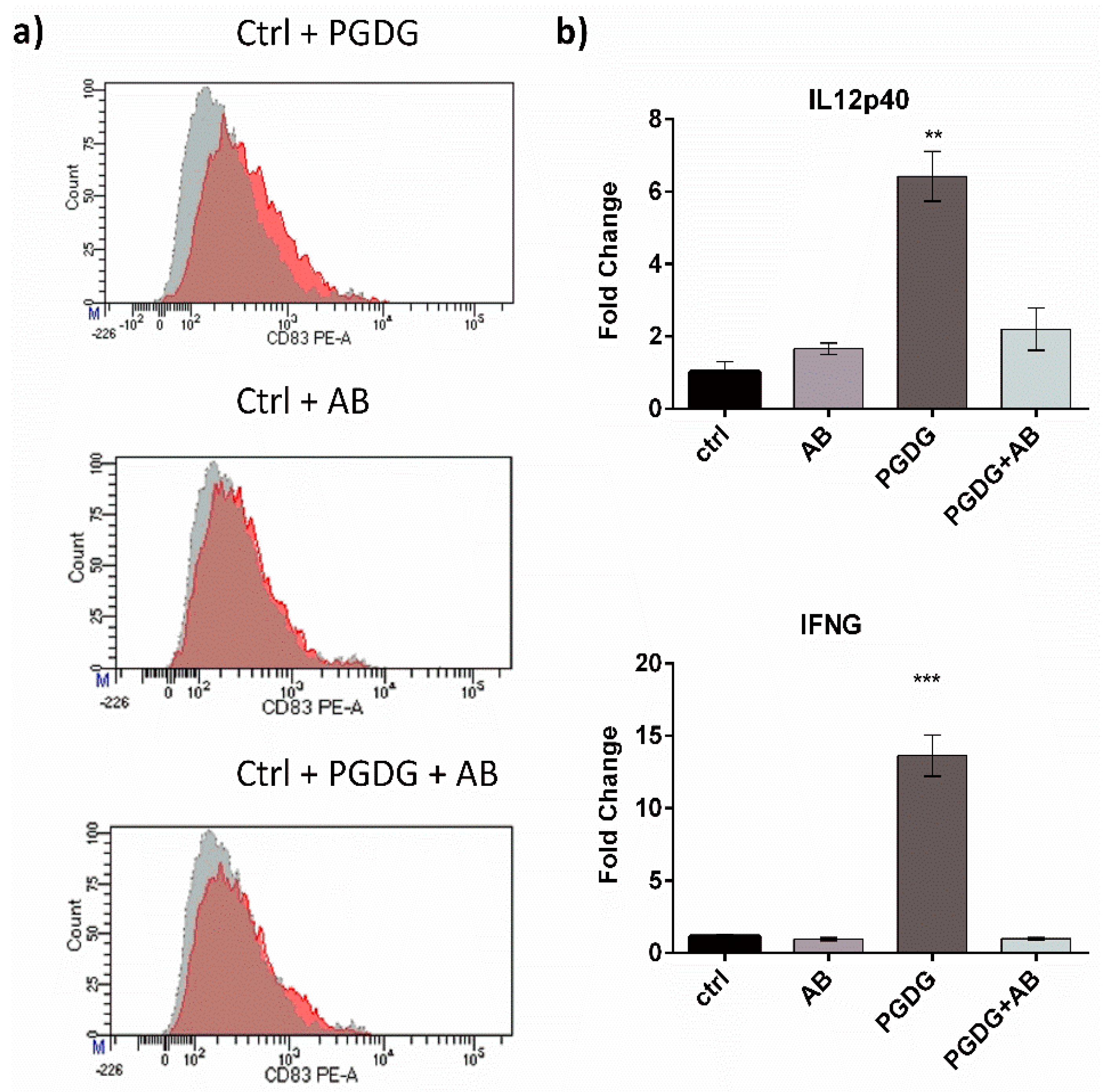
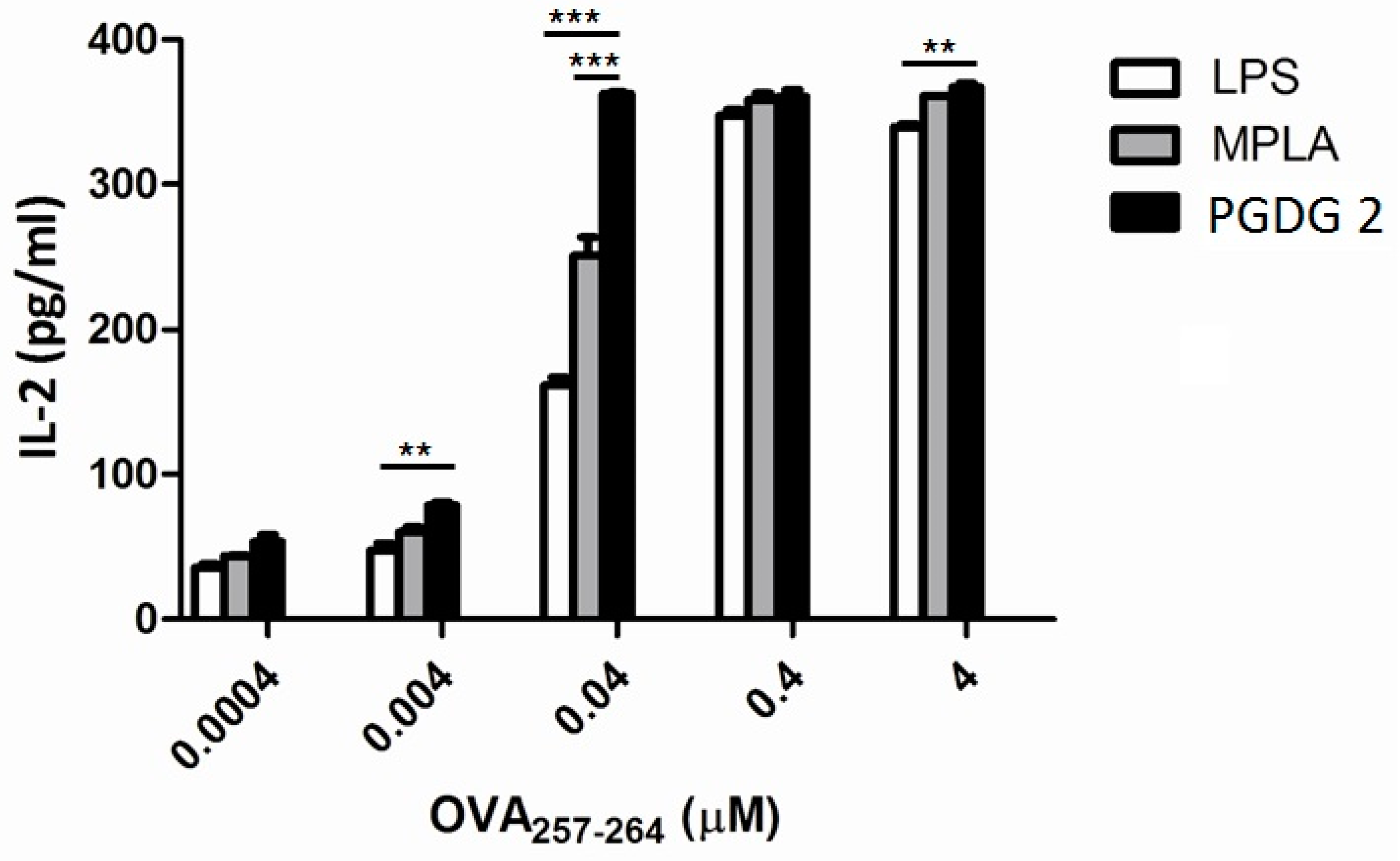
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Culture Condition
3.3. Extraction, Fractionation and Identification of PGDG (1)
3.4. Fatty Acid Analysis
3.5. LC-ESI-/MS/MS Analysis
3.6. Monosaccharide Analysis
3.7. Synthetic Procedure for PGDG 2
3.8. Immunological Assays
3.8.1. moDCs Generation
3.8.2. Cells Staining and Stimulation
3.8.3. Real Time PCR Analysis
3.8.4. TLR-2 and TLR-4 Assays
3.8.5. BM-DCs Generation
3.8.6. B3Z CD8+ T Cell Activation Assay
3.8.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Short, S.A.; White, D.C. Metabolism of the glucosyl diglycerides and phosphatidylglucose of Staphylococcus aureus. J. Bacteriol. 1970, 104, 126–132. [Google Scholar] [PubMed]
- Nagatsuka, Y.; Kasama, T.; Ohashi, Y.; Uzawa, J.; Ono, Y.; Shimizu, K.; Hirabayashi, Y. A new phosphoglycerolipid, ‘phosphatidylglucose’, found in human cord red cells by multi-reactive monoclonal anti-i cold agglutinin, mAb GL-1/GL-2. FEBS Lett. 2001, 497, 141–147. [Google Scholar] [CrossRef]
- Nagatsuka, Y.; Hirabayashi, Y. Phosphatidylglucoside: A new marker for lipid rafts. Biochim. Biophys. Acta 2008, 1780, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Hayakawa, T.; Murate, M.; Greimel, P.; Nagatsuda, Y.; Kobayashi, T.; Hirabayashi, Y. Phosphatidylglucoside: Its structure, thermal behavior, and domain formation in plasma membranes. Chem. Phys. Lipids 2012, 165, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Henrickson, C.V. Glucose-containing phospholipids in Mycoplasma laidlawii, strain B. J. Lip. Res. 1965, 6, 106–111. [Google Scholar]
- Wilkinson, S.G. Lipids of Pseudomonas diminuta. Biochim. Biophys. Acta 1969, 187, 492–500. [Google Scholar] [CrossRef]
- Wilkinson, S.G.; Bell, M.E. The phosphoglucolipid from Pseudomonas diminuta. Biochim. Biophys. Acta 1971, 248, 293–299. [Google Scholar] [CrossRef]
- Shaw, N.; Smith, P.F.; Verheij, H.M. The structure of ‘phosphatidylglucose’. Biochem. J. 1970, 120, 439–441. [Google Scholar] [CrossRef]
- Fischer, W.; Ishizuka, I.; Landgraft, H.R.; Herrmann, J. Glycerophosphoryl diglucosyl diglyceride, a new phosphoglycolipid from Streptococci. Biochim. Biophys. Acta 1973, 296, 527–545. [Google Scholar] [CrossRef]
- Veerkamp, J.H.; Van Schaik, F.W. Biochim. Biochemical changes in Bifidobacterium bifidum var. Pennsylvanicus after cell wall inhibition VII. Structure of the phosphogalactolipids. Biochim. Biophys. Acta 1974, 348, 370–387. [Google Scholar] [CrossRef]
- Fischer, W.; Landgraft, H.R. Glycerophosphoryl phosphatidyl kojibiosyl diacylglycerol, a novel phosphoglucolipid from Streptococcus faecalis. Biochim. Biophys. Acta 1975, 380, 227–244. [Google Scholar] [CrossRef]
- Abraham, W.; Meyer, H.; Lindholst, S.; Vancanneyt, M.; Smit, J. Phospho- and sulfolipids as biomarkers of Caulobacter sensu lato, Brevundimonas and Hyphomonas. J. Appl. Microbiol. 1997, 20, 522–539. [Google Scholar] [CrossRef]
- Wicken, A.J.; Knox, K.W. Lipoteichoic Acids: A new class of bacterial antigen. Science 1975, 187, 1161–1162. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.; Hammon, K.; Poulton, L.; Smyth, M.; Baxtera, A. NKT cell: Facts functions and fallacies. Immunol. Today 2000, 21, 573–583. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Mitsunobe, K.; Fujiwara, S.; Mori, M.; Hashimoto, M.; Suda, Y.; Kusumoto, S.; Fukase, K. Synthesis and biological activity of phosphoglycolipids from Thermus thermophilus. Org. Biomol. Chem. 2013, 11, 5034–5041. [Google Scholar] [CrossRef] [PubMed]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; Fontana, A. Development and application of a novel SPE-method for bioassay-guided fractionation of marine extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef] [PubMed]
- Cutignano, A.; Luongo, E.; Nuzzo, G.; Pagano, D.; Manzo, E.; Sardo, A.; Fontana, A. Profiling of complex lipids in marine microalgae by UHPLC/tandem mass spectrometry. Algal Res. 2016, 17, 348–358. [Google Scholar] [CrossRef]
- Cutignano, A.; d’Ippolito, G.; Romano, G.; Lamari, N.; Cimino, G.; Febbraio, F.; Nucci, R.; Fontana, A. Chloroplastic glycolipids fuel aldehyde biosynthesis in the marine diatom Thalassiosira rotula. ChemBioChem 2006, 7, 450–456. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Tucci, S.; Cutignano, A.; Romano, R.; Cimino, G.; Miralto, A.; Fontana, A. The role of complex lipids in the synthesis of bioactive aldehydes of the marine diatom Skeletonema costatum. Biochim. Biophys. Acta/Mol. Cell Biol. Lipids 2004, 1686, 100–107. [Google Scholar]
- Murari, R.; Abd El-Rahman, M.M.A.; Wedmir, Y.; Parthasarathy, S.; Baumann, W.J. Carbon-13 nuclear magnetic resonance of phospholipids in solution. Spectral and stereochemical assignments based on 13C-31P and 13C-14N couplings. J. Org. Chem. 1982, 47, 2158–2163. [Google Scholar] [CrossRef]
- Williamson, M.P.; Griffin, C.E. Three- and four-bond 31P-1H coupling constants and germinal protons nonequivalence in ethyl esters of phosphorus acids. J. Phys. Chem. 1968, 72, 4043–4047. [Google Scholar] [CrossRef]
- Pieringer, R.A.; Ganfield, M.W. Phosphatidylkojibiosyl diglyceride: Metabolism and function as an anchor in bacterial cell membranes. Lipids 1975, 10, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.M.; Pieringer, R.A. Phosphatidylmonoglucosyl diacylglycerol of Pseudomonas diminuta ATCC 11568. J. Biol. Chem. 1977, 252, 4395–4401. [Google Scholar] [PubMed]
- Nuzzo, G.; Gallo, C.; d’Ippolito, G.; Cutignano, A.; Fontana, A. Composition and quantitation of microalgal lipids by ERETIC 1H NMR method. Mar. Drugs 2013, 11, 3742–3753. [Google Scholar] [CrossRef] [PubMed]
- Norikatsu, M.; Jimin, G.; Hironori, M.; Yasushi, O.; Hiroaki, T.; Akira, T. Discovery of novel immunostimulants by dendritic-cell–based functional screening. Blood 2005, 106, 3082–3089. [Google Scholar]
- Rossi, M.; Young, J.W. Human dendritic cells: Potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005, 175, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.M.; Martin, S.; Garg, A.D.; Agostinis, P. Immature, semi-mature, and fully mature dendritic cells: Toward a DC-cancer cells interface, that augments anticancer immunity. Front Immunol. 2013, 4, 438. [Google Scholar] [CrossRef]
- Rovati, B.; Mariucci, S.; Manzoni, M.; Bencardino, K.; Danova, M. Flow cytometric detection of circulating dendritic cells in healthy subjects. Eur. J. Histochem. 2008, 52, 45–52. [Google Scholar] [CrossRef]
- Cummings, R.D. The repertoire of glycan determinants in the human glycome. Mol. Biosys. 2009, 5, 1087–1104. [Google Scholar] [CrossRef]
- Vartabedian, V.F.; Savage, P.B.; Teyton, L. The processing and presentation of lipids and glycolipids to the immune system. Immun. Rev. 2016, 272, 109–119. [Google Scholar] [CrossRef]
- Dowling, J.K.; Mansell, A. Toll-like receptors: The swiss army knife of immunity and vaccine development. Clin. Trasl. Immunol. 2016, 5, e85. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; et al. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci. Rep. 2017, 7, 6286. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, C.A.A.; Visser, G.M.; Van Boom, J.H. Synthesis of phosphatidyl-β-glucosyl glycerol containing a dioleoyl diglyceride moiety: Application of the tetraisopropyldisiloxane-1,3-diyl (tips) protecting group in sugar chemistry. part IV. Tetrahedron 1985, 41, 4557–4565. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Michel, J. Facile synthesis of α- and β-O-glycosyl imidates; preparation of glycosides and disaccharides. Angew. Chem. Int. Ed. 1980, 19, 731–732. [Google Scholar] [CrossRef]
- Schmidt, R.R. New methods for the synthesis of glycosides and oligosaccharides-Are there alternatives to the Koenigs-Knorr method? Angew. Chem. Int. Ed. 1986, 25, 212–235. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Pagano, D.; Fontana, A. An efficient and versatile chemical synthesis of bioactive glyco-glycerolipids. Tetrahedron Lett. 2012, 53, 879–881. [Google Scholar] [CrossRef]
- Sallusto, F.; Palermo, B.; Lenig, D.; Miettinen, M.; Matikainen, S.; Julkunen, I.; Forster, R.; Burgstahler, R.; Lipp, M.; Lanzavecchia, A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999, 29, 1617–1625. [Google Scholar] [CrossRef]
- Hellman, P.; Eriksson, H. Early activation markers of human peripheral dendritic cells. Hum. Immunol. 2007, 68, 324–333. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Hussain, S.; Zheng, Y.; Sanjabi, S.; Ouaaz, F.; Beg, A. Distinct roles of different NF-kB subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J. Immunol. 2007, 178, 6777–6788. [Google Scholar] [CrossRef]
- Steinhagen, F.; Kino, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 17, 3341–3355. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; GolenBock, D.T.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Hua, K.F.; Yang, Y.L.; Zou, W.; Chen, Y.P.; Liang, S.M.; Hsu, H.Y.; Wu, S.H. TLR-independent induction of human monocyte IL-1 by phosphoglycolipids from thermophilic bacteria. Glycoconj. J. 2008, 25, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I. Role of lipoteichoic acid in infection and inflammation. Lancet 2002, 2, 171–179. [Google Scholar] [CrossRef]
- Burger-Kentischer, A.; Abele, I.S.; Finkelmeier, D.; Wiesmüller, K.H.; Rupp, S. A new cell-based innate immune receptor assay for the examination of receptor activity, ligand specificity, signaling pathways and the detection of pyrogens. J. Immunol. Methods 2010, 358, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Fullerton, D.A.; Ao, L.; Zheng, D.; Zhao, K.S.; Meng, X. BMP-2 and TGF-β1 mediate biglycan-induced pro-osteogenic reprogramming in aortic valve interstitial cells. J. Mol. Med. [CrossRef] [PubMed]
- Hirano, N.; Butler, M.O.; Xia, Z.; Ansén, S.; von Bergwelt-Baildon, M.S.; Neuberg, D.; Freeman, G.J.; Nadler, L.M. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood 2006, 107, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.C.; Day, S.; Wright, M.D.; Stojanovska, L.; Apostolopoulos, Y. Enhanced dendritic cell-mediated antigen-specific CD4+ T cell responses: IFN-gamma aids TLR stimulation. J. Drug Deliv. 2013, 2013, 516749. [Google Scholar] [CrossRef] [PubMed]
- Karttunen, J.; Sanderson, S.; Shastri, N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. USA 1992, 89, 6020–6024. [Google Scholar] [CrossRef] [PubMed]
- Stöver, A.G.; Da Silva Correia, J.; Evans, J.T.; Cluff, C.W.; Elliott, M.W.; Jeffery, E.W.; Johnson, D.A.; Lacy, M.J.; Baldridge, J.R.; Probst, P.; et al. Structure-activity relationship of synthetic Toll-like receptor 4 agonists. J. Biol. Chem. 2004, 279, 4440–4449. [Google Scholar]
| 1 | 2 | 1 | 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 13C, ppm | 1H, δ, m, J (Hz) | 13C, ppm | 1H, δ, m, J (Hz) | 13C, ppm | 1H, δ, m, J (Hz) | 13C, ppm | 1H, δ, m, J (Hz) | ||
| Gal | Fatty acyl | ||||||||
| 1′ | 104.5 | 4.23, d, 7.2 | 104.5 | 4.23, d, 7.1 | C=O | 174.1–174.4 | 174.2, 174.6 | ||
| 2′ | 71.8 | 3.53, dd, 9.7, 7.2 | 71.9 | 3.53, dd, 9.8, 7.1 | α-CH2 | 34.0–34.8 | 2.34, m | 34.6, 34.8 | 2.33, m |
| 3′ | 73.7 | 3.51, dd, 9.7, 3.3 | 73.7 | 3.51, dd, 9.8, 3.2 | β-CH2 | 25.1–25.5 | 1.62–1.69, m | 25.5 | 1.61, m |
| 4′ | 68.3 | 3.99, m | 68.0 | 3.99, brd, 3.2 | CH2 (chain) | 29.6–30.3 | 1.27–1.32, m | 29.7–30.3 | 1.27, m |
| 5′ | 74.3 | 3.63, dd, 8.4, 5.7 | 74.3 | 3.64, dd, 8.4, 5.8 | HC=CH | 128.4–130.6 | 5.36, m | ||
| 6′a | 63.5 | 4.09, m | 63.3 | 4.08, m | =CHCH2CH2- | 27.1–27.7 | 2.02–2.11, m | ||
| 6′b | 3.92, m | 3.93, m | |||||||
| Gly1 | =CHCH2CH= | 26.2 | 2.82–2.85, m | ||||||
| 1a | 63.3 | 4.40, brd, 12.1 | 63.2 | 4.39, dd, 12.2. 2.9 | -CH2CH3 | 23.2–23.3 | 1.31, m | 23.2 | 1.30, m |
| 1b | 4.23, m | 4.24, m | |||||||
| 2 | 71.0 | 5.25, m | 71.1 | 5.25, m | =CHCH2CH2CH3 | 23.5 | 1.38, m | ||
| 3a | 68.1 | 3.99, m | 68.2 | 3.96, dd, 10.7, 5.5 | =CHCH2CH3 | 20.9 | 2.07, m | ||
| 3b | 3.68, dd, 10.5, 5.6 | 3.68, dd, 10.7, 5.3 | |||||||
| Gly2 | -(CH2)nCH3 | 14.0–14.4 | 0.88, t, 7.1 | 14.3 | 0.89, t, 7.1 | ||||
| 1″a | 63.4 | 4.44, dd, 12.1, 3.0 | 63.3 | 4.44, dd, 12.1, 3.2 | =CHCH2CH2CH3 | 14.0–14.4 | 0.92, t, 7.2 | ||
| 1″b | 4.20, m | 4.21, m | |||||||
| 2″ | 71.2 | 5.23, m | 71.0 | 5.25, m | =CHCH2CH3 | 14.0–14.4 | 0.97, t, 7.5 | ||
| 3″ | 64.1 | 4.00, m | 64.3 | 3.99, m | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo, E.; Gallo, C.; Sartorius, R.; Nuzzo, G.; Sardo, A.; De Berardinis, P.; Fontana, A.; Cutignano, A. Immunostimulatory Phosphatidylmonogalactosyldiacylglycerols (PGDG) from the Marine Diatom Thalassiosira weissflogii: Inspiration for a Novel Synthetic Toll-Like Receptor 4 Agonist. Mar. Drugs 2019, 17, 103. https://doi.org/10.3390/md17020103
Manzo E, Gallo C, Sartorius R, Nuzzo G, Sardo A, De Berardinis P, Fontana A, Cutignano A. Immunostimulatory Phosphatidylmonogalactosyldiacylglycerols (PGDG) from the Marine Diatom Thalassiosira weissflogii: Inspiration for a Novel Synthetic Toll-Like Receptor 4 Agonist. Marine Drugs. 2019; 17(2):103. https://doi.org/10.3390/md17020103
Chicago/Turabian StyleManzo, Emiliano, Carmela Gallo, Rossella Sartorius, Genoveffa Nuzzo, Angela Sardo, Piergiuseppe De Berardinis, Angelo Fontana, and Adele Cutignano. 2019. "Immunostimulatory Phosphatidylmonogalactosyldiacylglycerols (PGDG) from the Marine Diatom Thalassiosira weissflogii: Inspiration for a Novel Synthetic Toll-Like Receptor 4 Agonist" Marine Drugs 17, no. 2: 103. https://doi.org/10.3390/md17020103
APA StyleManzo, E., Gallo, C., Sartorius, R., Nuzzo, G., Sardo, A., De Berardinis, P., Fontana, A., & Cutignano, A. (2019). Immunostimulatory Phosphatidylmonogalactosyldiacylglycerols (PGDG) from the Marine Diatom Thalassiosira weissflogii: Inspiration for a Novel Synthetic Toll-Like Receptor 4 Agonist. Marine Drugs, 17(2), 103. https://doi.org/10.3390/md17020103