Pepsin-Soluble Collagen from the Skin of Lophius litulo: A Preliminary Study Evaluating Physicochemical, Antioxidant, and Wound Healing Properties
Abstract
1. Introduction
2. Results and Discussion
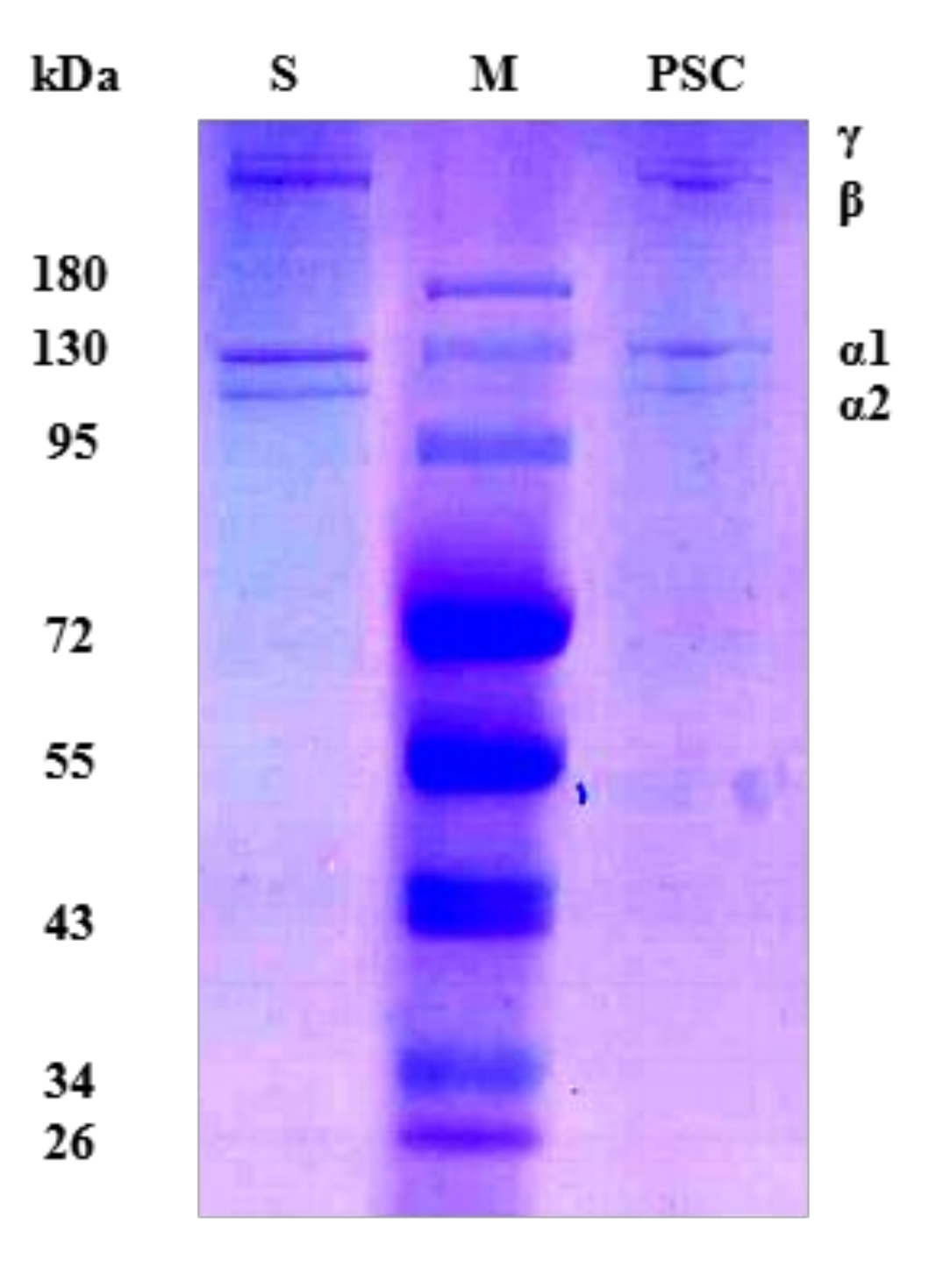
2.1. Sodium Dodecylsulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.2. Amino Acid Composition
2.3. Relative Solubility
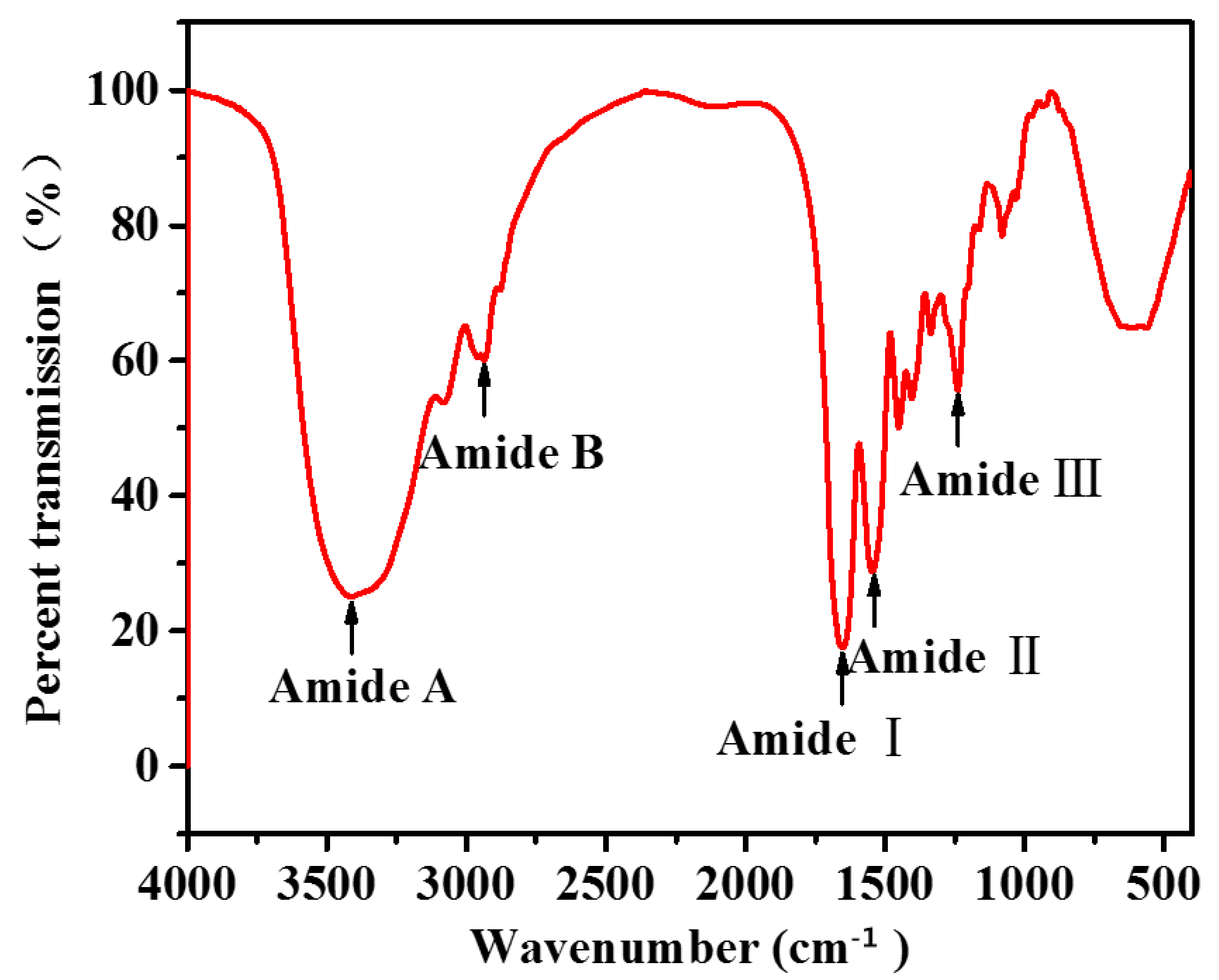
2.4. Fourier Transform Infrared (FTIR) Spectroscopy
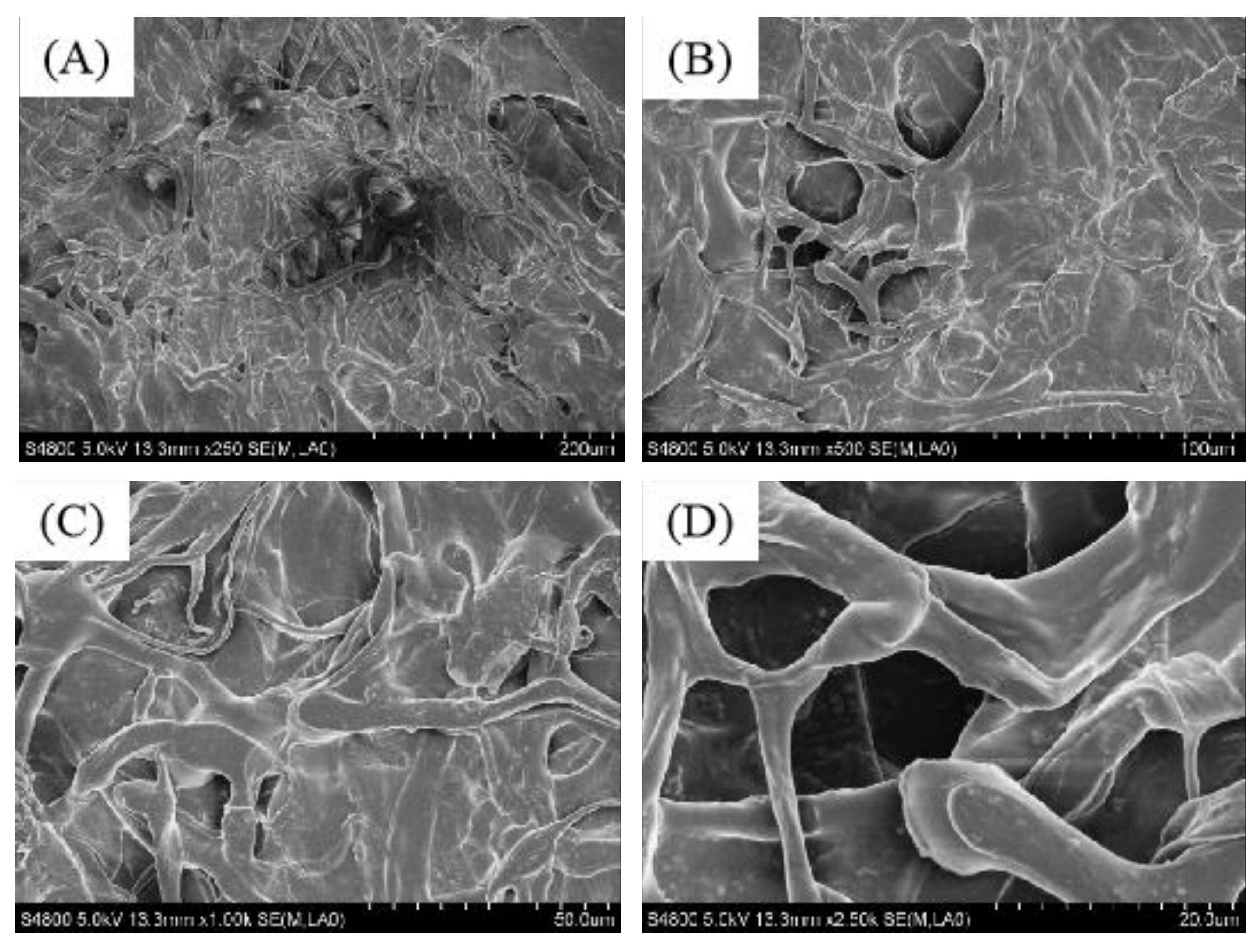
2.5. Scanning Electron Microscope (SEM) Analysis
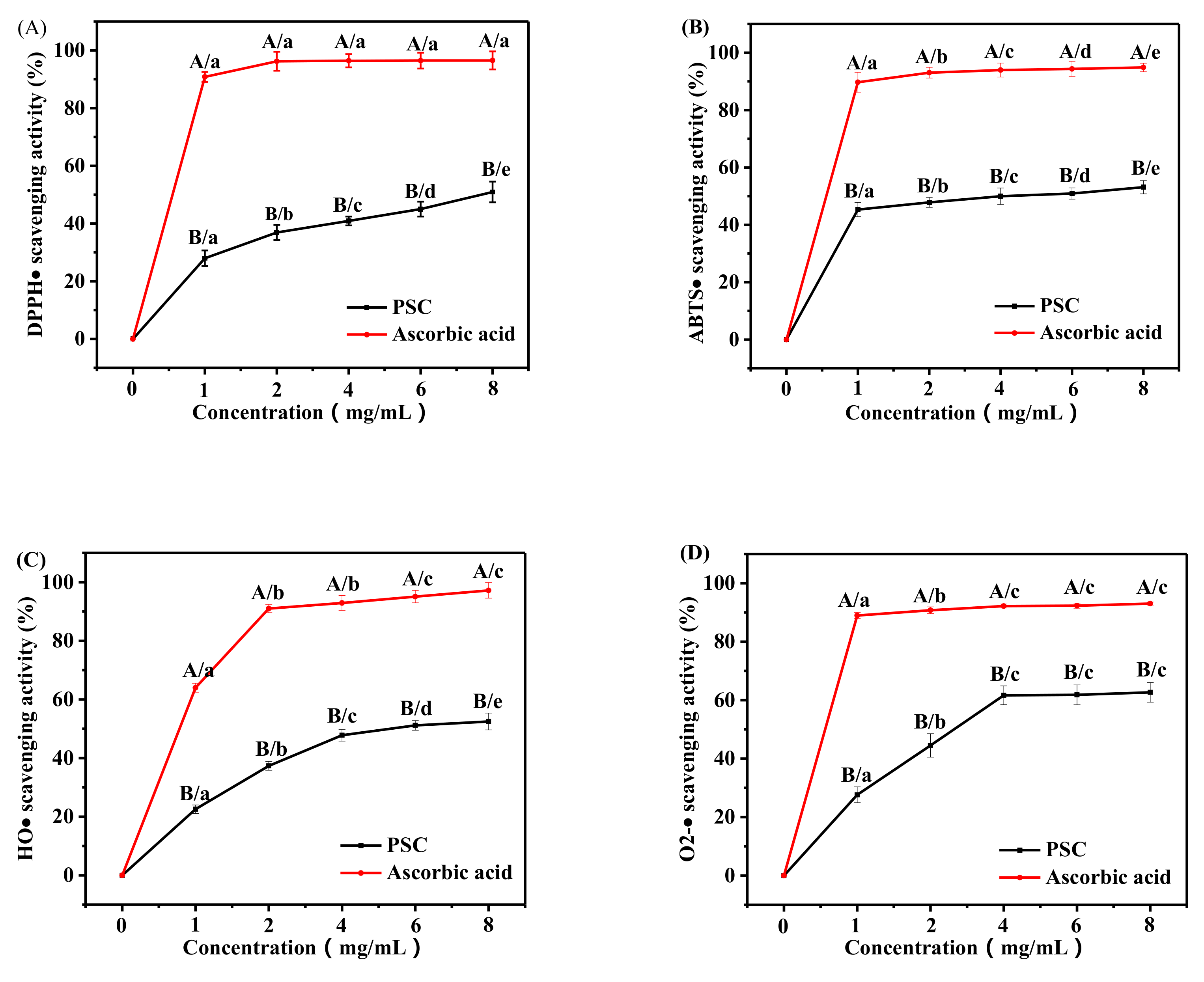
2.6. Antioxidant Activity
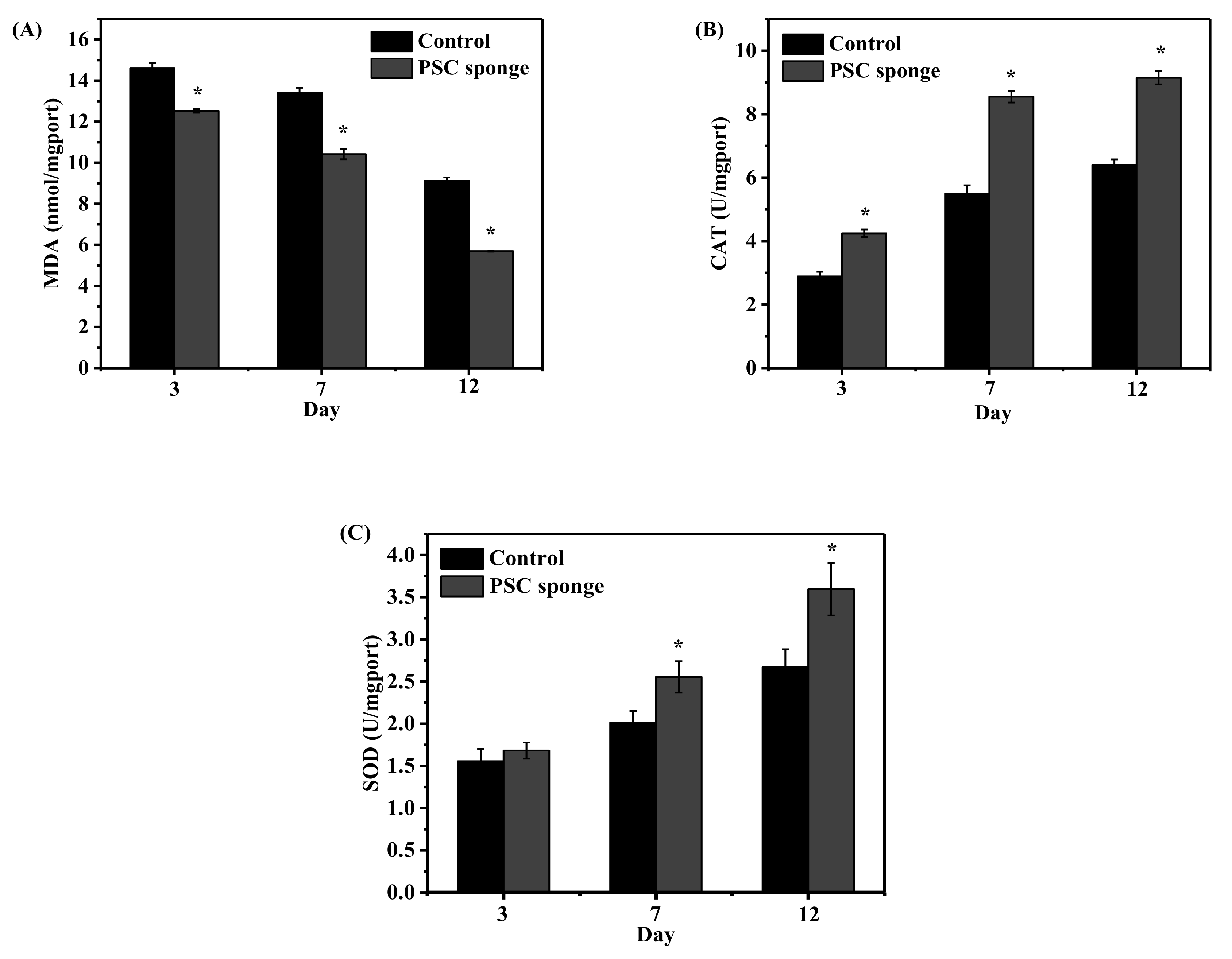
2.7. Estimation of Antioxidant Status in Animal Serum
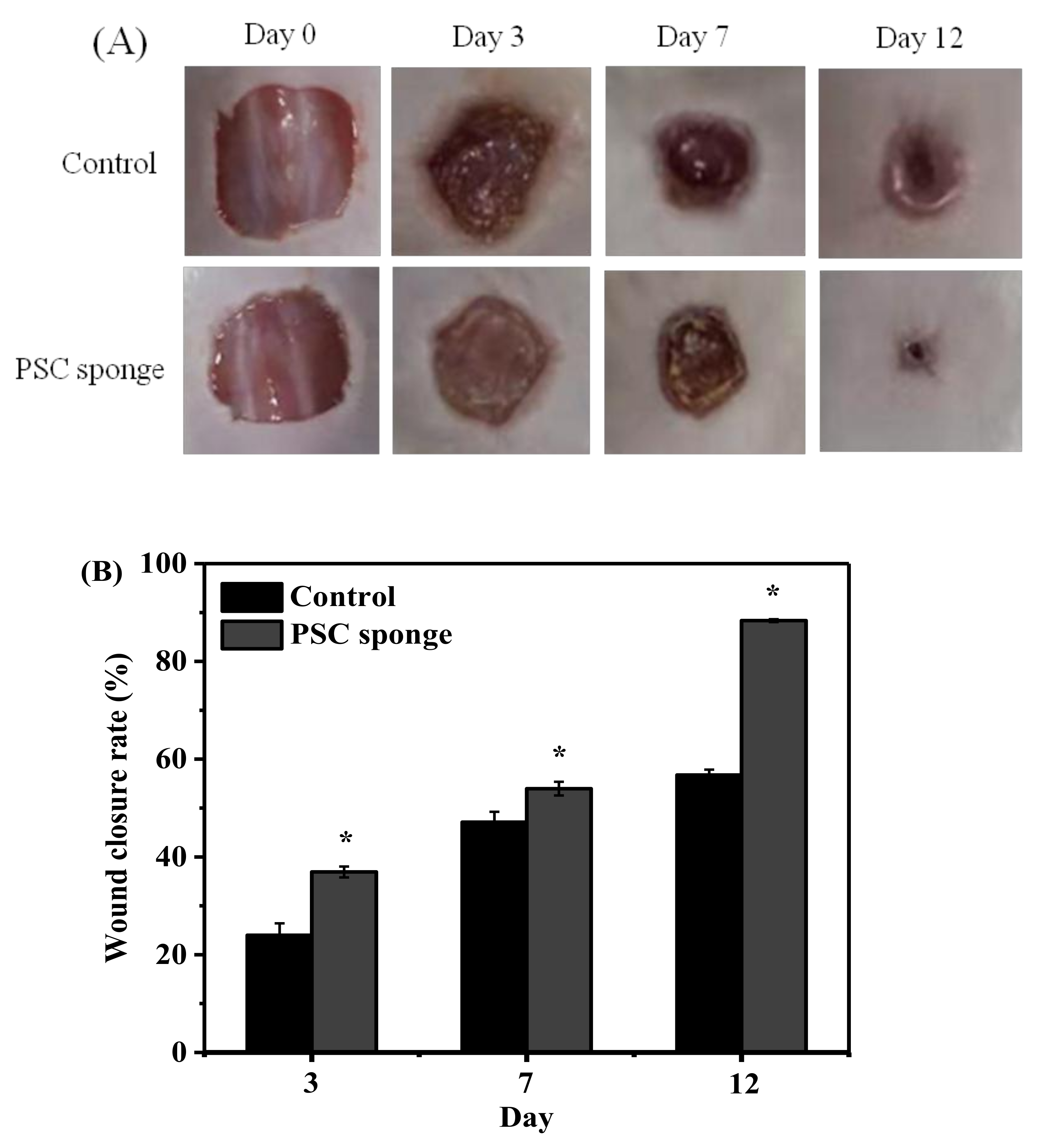
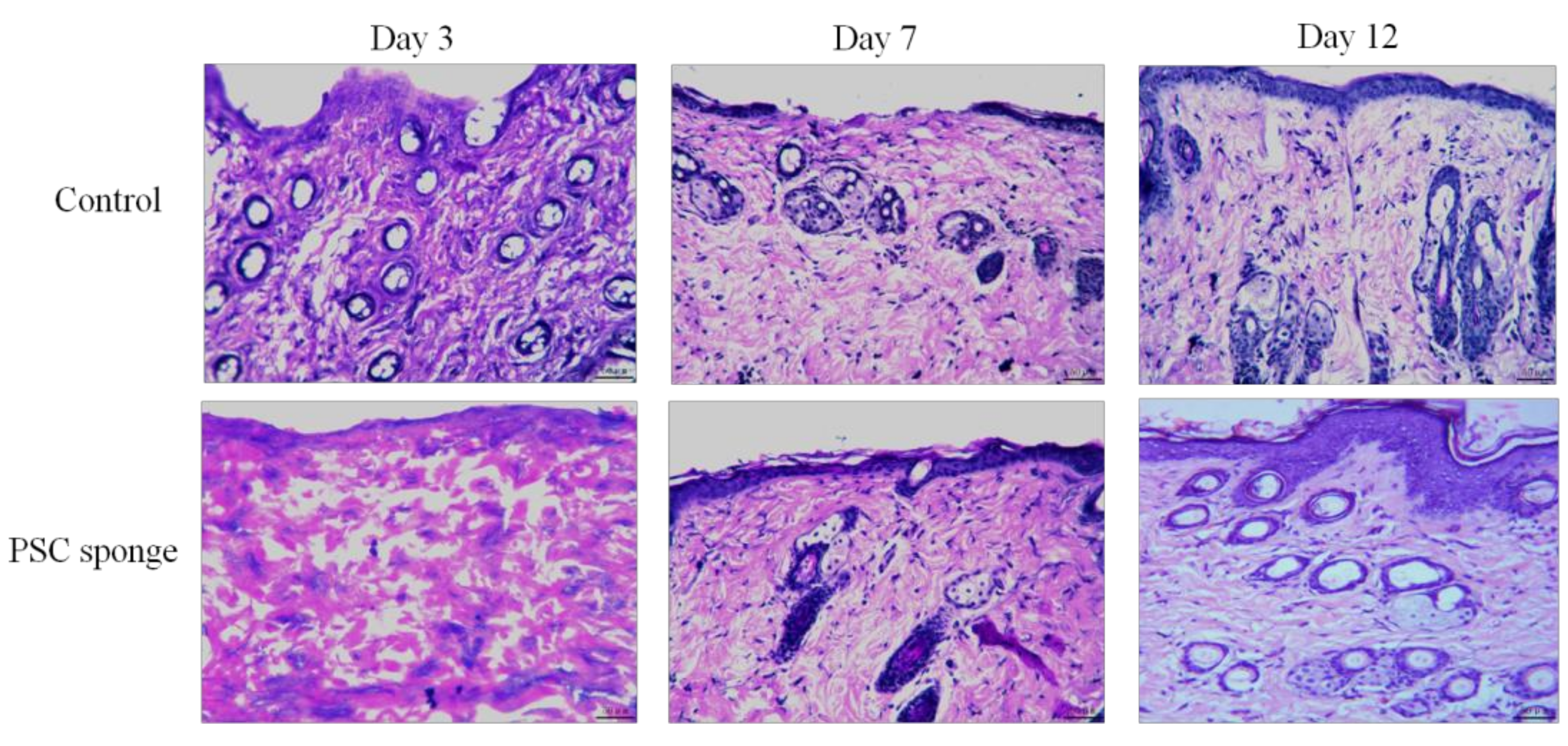
2.8. Evaluation of Biocompatibility in the Skin Wound Model
3. Materials and Methods
3.1. Materials and Chemical Reagents
3.2. SDS-PAGE Analysis
3.3. Amino Acid Analysis
3.4. Relative Solubility
3.5. FTIR Spectroscopic Analysis
3.6. SEM Analysis
3.7. Antioxidant Activity
3.7.1. DPPH Radical Scavenging Activity
3.7.2. ABTS Radical Scavenging Activity
3.7.3. Hydroxyl Radical Scavenging Activity
3.7.4. Superoxide Anion Radical Scavenging Activity
3.8. Animals Grouping and Wound Creation
3.9. Assessment of Serum Antioxidant Levels
3.10. Histomorphological Observation
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Azizur Rahman, M. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef]
- Aravinthan, A.; Park, J.K.; Hossain, M.A.; Sharmila, J.; Kim, H.J.; Kang, C.W.; Kim, N.S.; Kim, J.H. Collagen-based sponge hastens wound healing via decrease of inflammatory cytokines. 3 Biotech 2018, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.O.; Lee, Y.; Hwang, J.W.; Kim, H.; Kim, S.M.; Chang, S.W.; Lee, H.S.; Choi, Y.S. Wound healing properties of a 3-D scaffold comprising soluble silkworm gland hydrolysate and human collagen. Colloids Surf. B 2014, 116, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maity, P.P.; Moulik, D. Accelerating full thickness wound healing using collagen sponge of mrigal fish (Cirrhinus cirrhosus) scale origin. Int. J. Biol. Macromol. 2016, 93, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Kikuchi, M.; Takakuda, K.; Koyama, Y.; Matsumoto, H.N.; Ichinose, S.; Tanaka, J.; Kawauchi, T.; Shinomiya, K. The biocompatibility and osteoconductive activity of a novel hydroxyapatite/collagen composite biomaterial, and its function as a carrier of rhbmp-2. J. Biomed. Mater. Res. 2001, 54, 445–453. [Google Scholar] [CrossRef]
- Lukasiewicz, A.; Skopinska-Wisniewska, J.; Marszalek, A.; Molski, S.; Drewa, T. Collagen/polypropylene composite mesh biocompatibility in abdominal wall reconstruction. Plast. Reconstr. Surg. 2013, 131, 731e–740e. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Jin, H.X.; Yang, F.; Jin, S.J.; Liu, C.J.; Zhang, L.K.; Huang, J.; Wang, S.G.; Yan, Z.Y.; Cai, X.W.; et al. Physicochemical, antioxidant properties of giantcroaker (Nibeajaponica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef]
- Janda, J.; Nfonsam, V.; Calienes, F.; Sligh, J.E.; Jandova, J. Modulation of ROS levels in fibroblasts by altering mitochondria regulates the process of wound healing. Arch. Dermatol. Res. 2016, 308, 239–248. [Google Scholar] [CrossRef]
- Shao, Y.; Dang, M.Y.; Lin, Y.K.; Xue, F. Evaluation of wound healing activity of plumbagin in diabetic rats. Life Sci. 2019, 231, 116422. [Google Scholar] [CrossRef]
- Abood, W.N.; Al-Henhena, N.A.; Abood, A.N.; Al-Obaidi, M.M.J.; Bartan, R.A. Wound-healing potential of the fruit extract of Phaleria macrocarpa. Bosn. J. Basic Med. Sci. 2015, 15, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, Z.; Chen, H.X.; Guo, N.; Li, L. Anisakid and raphidascaridid nematodes (Ascaridoidea) infection in the important marine food-fish Lophius litulon (Jordan) (Lophiiformes: Lophiidae). Int. J. Food Microbiol. 2018, 284, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Wang, B.; Deng, Y.Y.; Wang, Y.M.; Deng, S.G.; Ma, J.Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Ma, H.W.; Yang, H.C.; Fu, W.D.; Liao, M.F.; Zhou, Y.F.; Xiang, X.W.; Chen, M.; Zheng, B. Antioxidant activity of collagen peptides from Lophius litulon skin. Food Sci. 2014, 35, 80–84. [Google Scholar]
- Noitup, P.; Garnjanagoonchorn, W.; Morrissey, M.T. Fish skin type I collagen. J. Aquat. Food Prod. Technol. 2005, 14, 17–28. [Google Scholar] [CrossRef]
- Lin, X.H.; Chen, Y.Y.; Jin, H.X.; Zhao, Q.L.; Liu, C.J.; Li, R.W.; Yu, F.M.; Chen, Y.; Huang, F.F.; Yang, Z.S.; et al. Collagen Extracted from Bigeye Tuna (Thunnus obesus) Skin by Isoelectric Precipitation: Physicochemical Properties, Proliferation, and Migration Activities. Mar. Drugs 2019, 17, 261. [Google Scholar] [CrossRef]
- Tang, Y.P.; Jin, S.J.; Li, X.; Li, X.Y.; Hu, X.Y.; Chen, Y.; Huang, F.F.; Yang, Z.S.; Yu, F.M.; Ding, G.F. Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 222. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.X.; Li, S.D.; Hong, P.Z. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Alemán, A.; Giménez, B.; Montero, P.; Gómez-Guillén, M.C. Antioxidant activity of several marine skin gelatins. LWT-Food Sci. Technol. 2011, 44, 407–413. [Google Scholar] [CrossRef]
- Mcgavin, M.H.; Krajewskapietrasik, D.; Rydén, C.; Höök, M. Identification of a staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect. Immun. 1993, 61, 2479–2485. [Google Scholar] [PubMed]
- Hofmann, M.; Winzer, M.; Weber, C.; Gieseler, H. Low-volume solubility assessment during high-concentration protein formulation development. J. Pharm. Pharmacol. 2016, 70, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Savre, M. Maillard-Induced Glycation of Whey Protein Using Maltodextrin and Effect on Solubility, Thermal Stability, and Emulsification Properties. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2016. [Google Scholar]
- Jeevithan, E.; Bao, B.; Bu, Y.; Zhou, Y.; Zhao, Q.; Wu, W. TypeⅡcollagen and gelatin from silvertip shark (Carcharhinus albimarginatus) cartilage: Isolation, purification, physicochemical and antioxidant properties. Mar. Drugs 2014, 12, 3852–3873. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.B.; Chi, C.F.; Yang, F.; Zhao, Y.Q.; Wang, B. Physicochemical properties of acid- and pepsin-soluble collagens from the cartilage of siberian sturgeon. Environ. Sci. Pollut. Res. 2018, 25, 31427–31438. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Li, M.; Yi, R.Z.; Bai, K.K.; Wang, G.Y.; Tan, R.; Sun, S.S.; Xu, N.H. Electrodialysis Extraction of Pufferfish Skin (Takifugu flavidus): A Promising Source of Collagen. Mar. Drugs 2019, 17, 25. [Google Scholar] [CrossRef]
- Abe, Y.; Krimm, S. Normal vibrations of crystalline polyglycine I. Biopolymers 1972, 11, 1817–1839. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Cai, P.P.; Li, P.P.; Zhang, M.H.; Sun, Z.L.; Sun, C.; Xu, W.M.; Wang, D.Y. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2017, 105, 1602–1610. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer). Food Chem. 2013, 138, 2435–2441. [Google Scholar] [CrossRef]
- Yu, F.M.; Zong, C.H.; Jin, S.J.; Zheng, J.W.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.F.; Yang, Z.S.; Tang, Y.P.; et al. Optimization of extraction conditions and characterization of pepsin-solubilised collagen from skin of giant croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Gad, A.; El-Hay, G.; Abu-Hussein, A.; Habib, S.I.; Badr, N.A.; Hashem, A.A. Chemical and biological evaluation of egyptian nile tilapia (Oreochromis niloticas) fish scale collagen. Int. J. Biol. Macromol. 2015, 79, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, optimization and characterization of collagen from sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Sun, L.; Li, B.; Song, W.; Si, L.; Hou, H. Characterization of pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process. Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Diren Sigirci, B.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In Vitro Evaluation of Antioxidant, Anti-Inflammatory, Antimicrobial and Wound Healing Potential of Thymus Sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas. Mar. Drugs 2019, 24, 3353. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.P.; Chen, Y.F.; Zhang, J.L.; You, M.M.; Wang, K.; Hu, F.L. Mechanisms underlying the wound healing potential of propolis based on its, in vitro, antioxidant activity. Phytomedicine 2017, 34, 76–84. [Google Scholar] [CrossRef]
- Vittorazzi, C.; Endringer, D.C.; Andrade, T.U.; Scherer, R.; Fronza, M. Antioxidant, antimicrobial and wound healing properties of Struthanthus vulgaris. Pharm. Biol. 2016, 54, 331–337. [Google Scholar] [CrossRef]
- Ren, S.; Li, J.; Guan, H. The antioxidant effects of complexes of tilapia fish skin collagen and different marine oligosaccharides. J. Ocean Univ. China 2010, 9, 399–407. [Google Scholar] [CrossRef]
- Inal, M.E.; Kanbak, G.; Sunal, E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. 2001, 305, 75–80. [Google Scholar] [CrossRef]
- Yang, Q.M.; Pan, X.H.; Kong, W.B.; Yang, H.; Su, Y.D.; Zhang, L.; Zhang, Y.N.; Yang, Y.L.; Ding, L.; Liu, G.A. Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food. Chem. 2010, 118, 84–89. [Google Scholar]
- Ashcroft, G.S.; Mills, S.J.; Ashworth, J.J. Ageing and wound healing. Biogerontology 2002, 3, 337–345. [Google Scholar] [CrossRef]
- Majumder, P.; Paridhavi, M. A novel polyherbal formulation hastens diabetic wound healing with potent antioxidant potential: A comprehensive pharmacological investigation. Nat. Prod. Chem. Res. 2018, 6, 4. [Google Scholar] [CrossRef]

| Amino Acids | Content (g/100 g) |
|---|---|
| Aspartic acid (Asp) | 2.529 |
| Threonine (Thr) | 1.310 |
| Serine (Ser) | 2.522 |
| Glutamic (Glu) | 4.708 |
| Priline (Pro) | 4.720 |
| Glycine (Gly) | 9.684 |
| Alanine (Ala) * | 4.009 |
| Valine (Val) * | 1.640 |
| Methionine (Met) * | 1.044 |
| Isoleucine (Ile) | 0.262 |
| Leucine (Leu) * | 1.312 |
| Tyrosine (Tyr) * | 0.000 |
| Phenylalanine (Phe) * | 0.991 |
| Lysine (Lys) | 1.462 |
| Histidine (His) | 0.931 |
| Arginine (Arg) | 3.484 |
| Hydroxyproline (Hyp) | 1.554 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zheng, J.; Tian, X.; Tang, Y.; Ding, G.; Yang, Z.; Jin, H. Pepsin-Soluble Collagen from the Skin of Lophius litulo: A Preliminary Study Evaluating Physicochemical, Antioxidant, and Wound Healing Properties. Mar. Drugs 2019, 17, 708. https://doi.org/10.3390/md17120708
Zhang W, Zheng J, Tian X, Tang Y, Ding G, Yang Z, Jin H. Pepsin-Soluble Collagen from the Skin of Lophius litulo: A Preliminary Study Evaluating Physicochemical, Antioxidant, and Wound Healing Properties. Marine Drugs. 2019; 17(12):708. https://doi.org/10.3390/md17120708
Chicago/Turabian StyleZhang, Wen, Jiawen Zheng, Xiaoxiao Tian, Yunping Tang, Guofang Ding, Zuisu Yang, and Huoxi Jin. 2019. "Pepsin-Soluble Collagen from the Skin of Lophius litulo: A Preliminary Study Evaluating Physicochemical, Antioxidant, and Wound Healing Properties" Marine Drugs 17, no. 12: 708. https://doi.org/10.3390/md17120708
APA StyleZhang, W., Zheng, J., Tian, X., Tang, Y., Ding, G., Yang, Z., & Jin, H. (2019). Pepsin-Soluble Collagen from the Skin of Lophius litulo: A Preliminary Study Evaluating Physicochemical, Antioxidant, and Wound Healing Properties. Marine Drugs, 17(12), 708. https://doi.org/10.3390/md17120708




