Design and Synthesis of a Chitodisaccharide-Based Affinity Resin for Chitosanases Purification
Abstract
1. Introduction
2. Results and Discussion
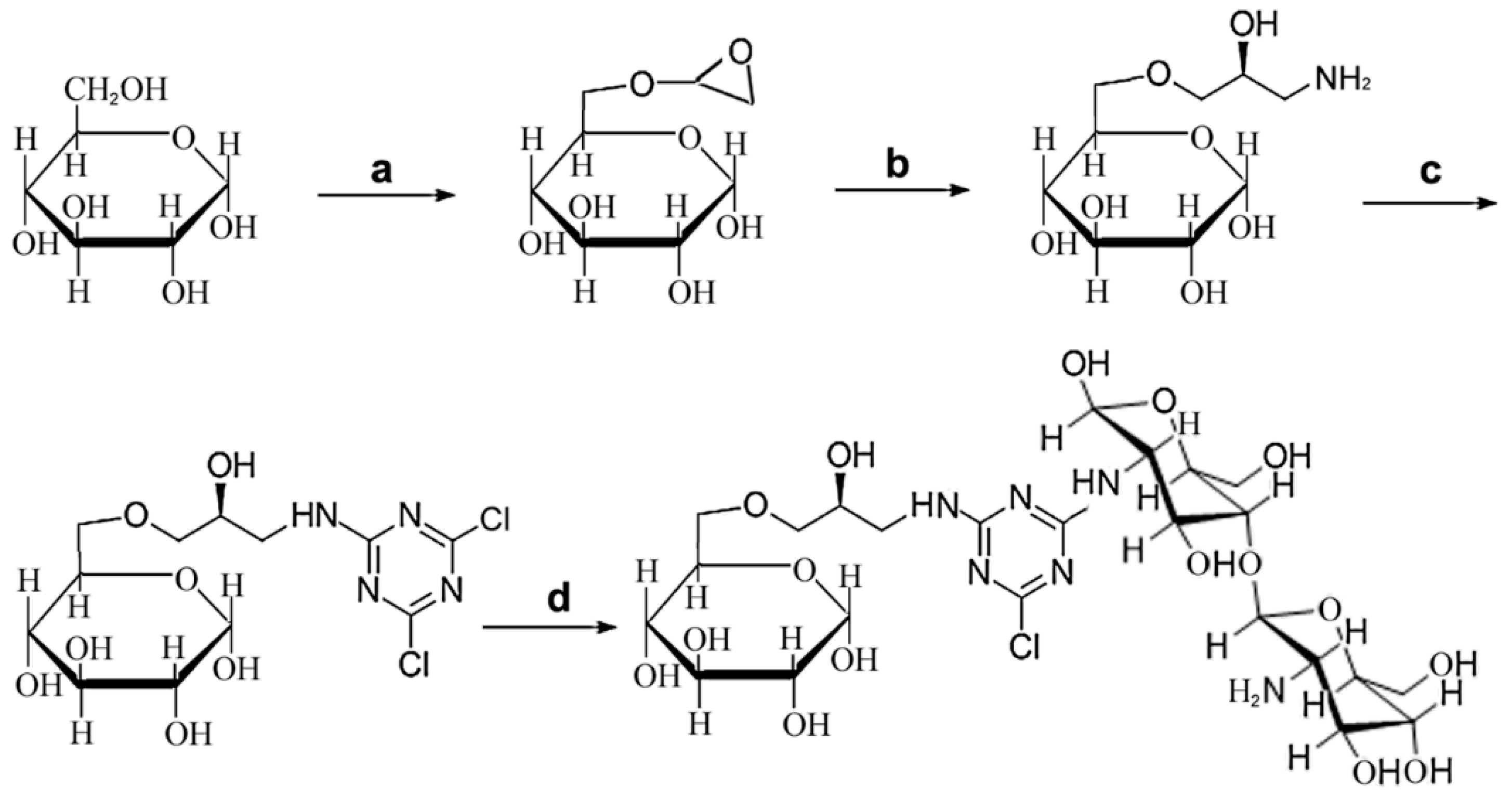
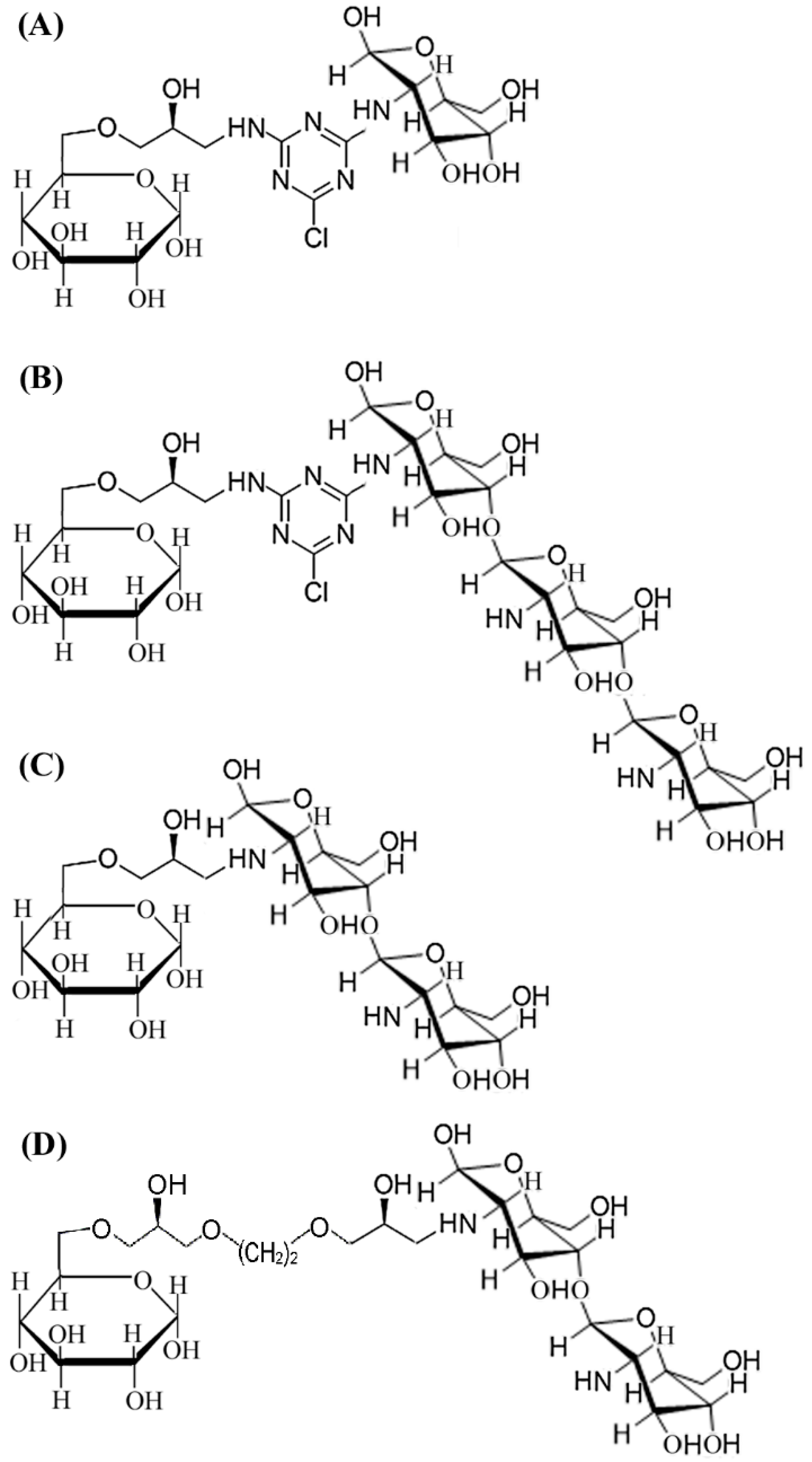
2.1. Design and Synthesis of CHOS-based Affinity Resin
2.2. Affinity Purification of Chitosanases from Different GH Families
2.3. Direct Purification of Chitosanases from Bacterial Culture Medium
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Affinity Resins
3.3. Expression and Purification of Three Typical Chitosanase
3.4. Calculation of Desorption Constant of Chitosanase
3.5. Affinity Purification of Chitosanases Using CHDS-Based Resin
3.6. Direct Affinity Purification of Chitosanases from Marine Bacteria
3.7. Assay of Enzyme Activity
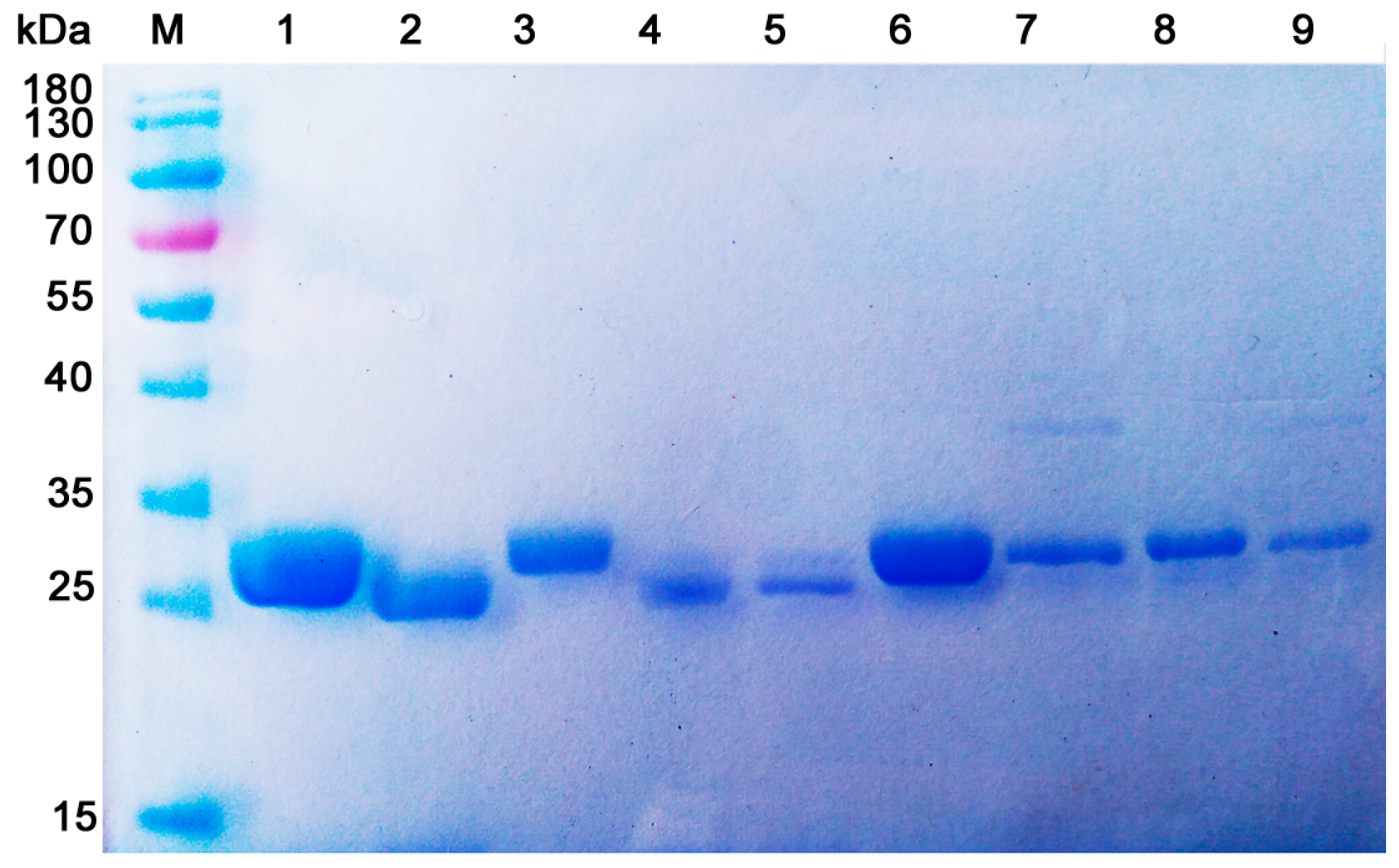
3.8. Analysis of Protein Purity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Shinya, S.; Fukamizo, T. Interaction between chitosan and its related enzymes: A review. Int. J. Biol. Macromol. 2017, 104, 1422–1435. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Chen, Y.J.; Yen, Y.H.; Wang, S.L. The antitumor activity of the hydrolysates of chitinous materials hydrolyzed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem. 2007, 42, 527–534. [Google Scholar] [CrossRef]
- Shen, K.T.; Chen, M.H.; Chan, H.Y.; Jeng, J.H.; Wang, Y.J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Das, S.N.; Madhuprakash, J.; Sarma, P.V.; Purushotham, P.; Suma, K.; Manjeet, K.; Rambabu, S.; Gueddari, N.E.; Moerschbacher, B.M.; Podile, A.R. Biotechnological approaches for field applications of chitooligosaccharides (COS) to induce innate immunity in plants. Crit. Rev. Biotechnol. 2015, 35, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.N., Jr.; El Gueddari, N.E.; Moerschbacher, B.M.; Peter, M.G.; Franco, T.T. Growth of phytopathogenic fungi in the presence of partially acetylated chitooligosaccharides. Mycopathologia 2008, 166, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Park, P.J.; Kim, S.K. Antimicrobial effect of chitooligosacchardies produced by bioreactor. Carbohydr. Polym. 2001, 44, 71–76. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.S.; Choi, J.W.; Yi, S.Y.; Shin, W.S. Antidiabetic effects of chitosan oligosaccharides in neonatal streptozotocin-induced noninsulin-dependent diabetes mellitus in rats. Biol. Pharm. Bull. 2003, 26, 1100–1103. [Google Scholar] [CrossRef]
- Jung, W.J.; Park, R.D. Bioproduction of chitooligosaccharides: Present and perspectives. Mar. Drugs. 2014, 12, 5328–5356. [Google Scholar] [CrossRef]
- Chavan, S.B.; Deshpande, M.V. Chitinolytic enzymes: An appraisal as a product of commercial potential. Biotechnol. Prog. 2013, 29, 833–846. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucl. Acids Res. 2014, 42, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Shimono, K.; Ochiai, N.; Shigeru, K.; Kurita, M.; Ohta, Y.; Tanaka, K.; Matsuda, H.; Kawamukai, M. Purification, characterization, and gene analysis of a chitosanase (ChoA) from matsuebacter chitosanotabidus 3001. J. Bacteriol. 1999, 181, 6642–6649. [Google Scholar] [PubMed]
- Shehata, A.N.; Abd El Aty, A.A.; Darwish, D.A.; Abdel Wahab, W.A.; Mostafa, F.A. Purification, physicochemical and thermodynamic studies of antifungal chitinase with production of bioactive chitosan-oligosaccharide from newly isolated Aspergillus Griseoaurantiacus KX010988. Int. J. Biol. Macromol. 2018, 107, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Amakata, D.; Matsuo, Y.; Matsuda, H.; Kawamukai, M. New chitosan-degrading strains that produce chitosanases similar to ChoA of mitsuaria chitosanitabida. Appl. Environ. Microbiol. 2005, 71, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Chen, S.J.; Wang, C.L. Purification and characterization of chitinases and chitosanases from a new species strain Pseudomonas sp. TKU015 using shrimp shells as a substrate. Carbohydr. Res. 2008, 343, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Labrou, N.E. Design and selection of ligands for affinity chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 790, 67–78. [Google Scholar] [CrossRef]
- Mountford, S.J.; Daly, R.; Robinson, A.J.; Hearn, M.T. Design, synthesis and evaluation of pyridine-based chromatographic adsorbents for antibody purification. J. Chromatogr. A 2014, 1355, 15–25. [Google Scholar] [CrossRef]
- Fasoli, E.; Reyes, Y.R.; Guzman, O.M.; Rosado, A.; Cruz, V.R.; Borges, A.; Martinez, E.; Bansal, V. Para-aminobenzamidine linked regenerated cellulose membranes for plasminogen activator purification: Effect of spacer arm length and ligand density. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 930, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Xu, X.; Lin, S.; Wang, Y.; Hao, J.; Sun, M. Structure-based design and synthesis of a new phenylboronic-modified affinity medium for metalloprotease purification. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Yang, J.; Bao, J.; Liu, J.; Lin, S.; Hao, J.; Sun, M. Affinity purification of metalloprotease from marine bacterium using immobilized metal affinity chromatography. J. Sep. Sci. 2016, 39, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yang, H.; Xiao, X.; Zhang, L.; Zhang, Y.; Tong, Y.; Chen, Y.; Wang, W. Affinity purification of urinary trypsin inhibitor from human urine. J. Sep. Sci. 2012, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Bhushan, R. Amino acids as chiral auxiliaries in cyanuric chloride-based chiral derivatizing agents for enantioseparation by liquid chromatography. Biomed. Chromatogr. 2014, 28, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Wang, S.; Xu, W.; Han, B.; Liu, W.; Jones, D.N.; Liu, W. Structural insights into the substrate-binding mechanism for a novel chitosanase. Biochem. J. 2014, 461, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Shi, Y.; Wang, S.; Yang, Y.; Han, B.; Liu, W.; Jones, D.N.; Liu, W. Structural and biochemical insights into the degradation mechanism of chitosan by chitosanase OU01. Biochim. Biophys. Acta 2015, 1850, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Tan, H.Q.; Zhu, C.; Liao, L.; Zhang, X.Q.; Wu, M. Cloning and overexpression of a new chitosanase gene from Penicillium sp. D-1. AMB Express 2012, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Lin, S.; Yang, J.; Ma, Z.; Wang, Y.; Liu, J.; Hao, J.; Sun, M. Rapid and efficient one-step purification of a serralysin family protease by using a p-aminobenzamidine-modified affinity medium. J. Sep. Sci. 2017, 40, 1960–1965. [Google Scholar] [CrossRef] [PubMed]
- Viens, P.; Lacombe-Harvey, M.E.; Brzezinski, R. Chitosanases from family 46 of glycoside hydrolases: From proteins to phenotypes. Mar. Drugs. 2015, 13, 6566–6587. [Google Scholar] [CrossRef]
- Belenguer-Sapina, C.; Pellicer-Castell, E.; El Haskouri, J.; Guillem, C.; Simo-Alfonso, E.F.; Amoros, P.; Mauri-Aucejo, A. Design, characterization and comparison of materials based on beta and gamma cyclodextrin covalently connected to microporous silica for environmental analysis. J. Chromatogr. A 2018, 1563, 10–19. [Google Scholar] [CrossRef]
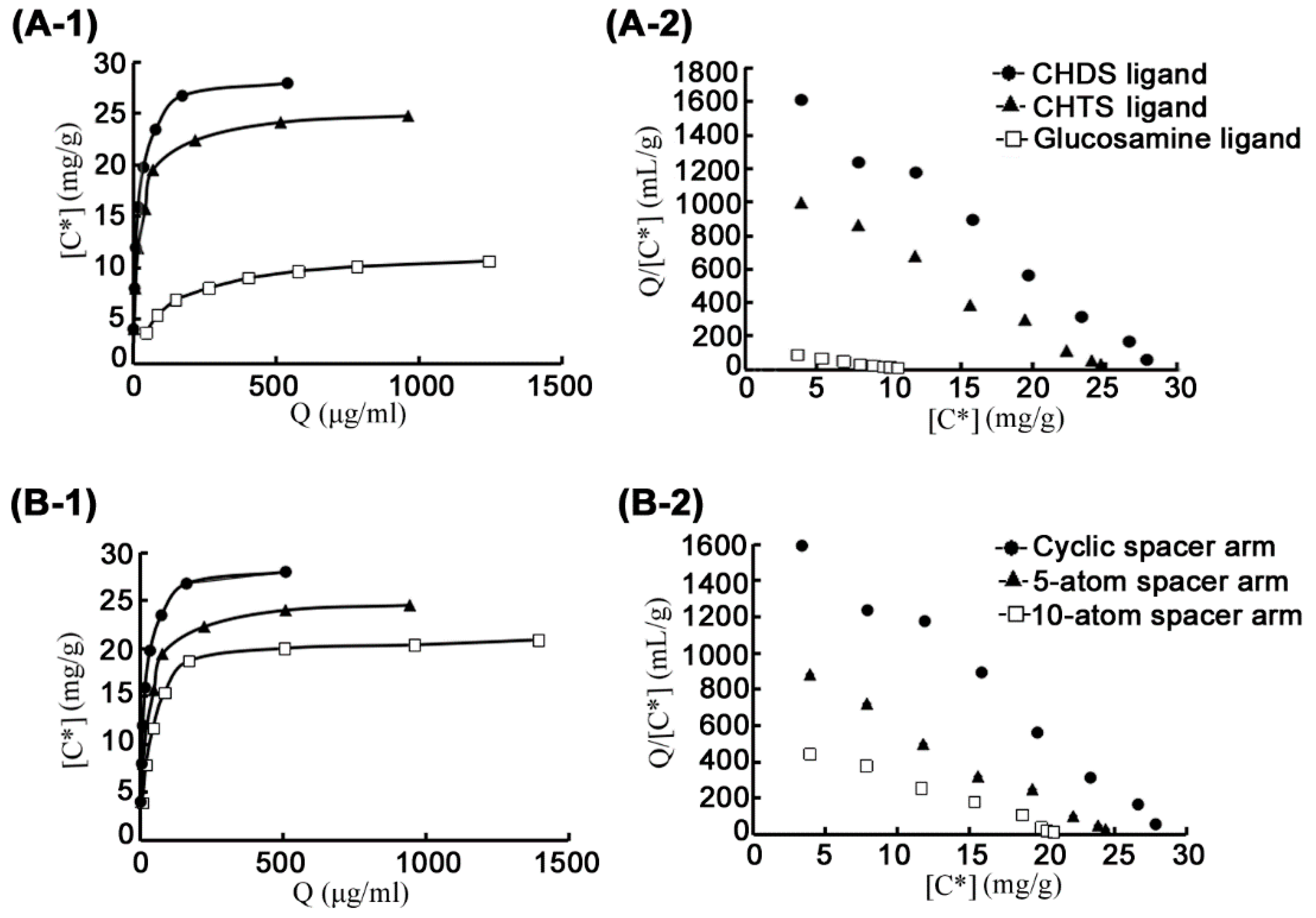

| Ligands | Spacer Arms | Ligand Density (μmol/mL) | Kd (μg/mL) | Qmax (mg/g) |
|---|---|---|---|---|
| Glucoamine | Cyanuric chloride | 20.9 | 88.5 | 10.6 |
| CHTS a | Cyanuric chloride | 20.9 | 20.7 | 24.7 |
| CHDS b | Cyanuric chloride | 20.9 | 16.4 | 30.9 |
| CHDS | 5-atom spacer | 41.8 | 24.2 | 24.4 |
| CHDS | 10-atom spacer | 27.8 | 38.8 | 20.8 |
| Enzymes | Purification Method | Activity Recovery (%) | Protein Purity (%) | Specific Activity (U/mg) |
|---|---|---|---|---|
| CsnOU01 (GH46) | CHDS-based protocol a | 64.1 | 97.8 | 356.8 |
| Traditional protocol c | 28.2 | 94.5 | 358.5 | |
| IMAC protocol b | 71.8 | 95.6 | 306.7 | |
| Csn (GH75) | CHDS-based protocol | 45.2 | 96.3 | 664.6 |
| Traditional protocol d | 10.5 | 93.2 | 682.7 | |
| IMAC protocol | 60.7 | 96.4 | 592.3 | |
| ChoA (GH80) | CHDS-based protocol | 40.8 | 97.1 | 851.4 |
| Traditional protocol e | 9.2 | 89.6 | 847.6 | |
| IMAC protocol | 63.4 | 90.3 | 727.8 |
| Number | Bacterium | Activity Recovery (%) | Protein Purity (%) |
|---|---|---|---|
| 1 | Bacillus sp. QD08 | 49.2 | 95.4 |
| 2 | Bacillus sp. QD102 | 40.6 | 96.2 |
| 3 | Bacillus sp. QD72 | 41.1 | 98.1 |
| 4 | Paenibacillus sp. QD03 | 10.5 | 92.2 |
| 5 | Mitsuaria sp. QD129 | 10.7 | 90.5 |
| 6 | Mitsuaria sp. QD130 | 39.5 | 96.7 |
| 7 | Renibacterium sp. QD52 | 20.3 | 90.1 |
| 8 | Serratia sp. QD07 | 12.7 | 97.8 |
| 9 | Flavobacterium sp. QD28 | 11.6 | 91.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, L.; Chen, X.; Sun, M.; Han, Y. Design and Synthesis of a Chitodisaccharide-Based Affinity Resin for Chitosanases Purification. Mar. Drugs 2019, 17, 68. https://doi.org/10.3390/md17010068
Li S, Wang L, Chen X, Sun M, Han Y. Design and Synthesis of a Chitodisaccharide-Based Affinity Resin for Chitosanases Purification. Marine Drugs. 2019; 17(1):68. https://doi.org/10.3390/md17010068
Chicago/Turabian StyleLi, Shangyong, Linna Wang, Xuehong Chen, Mi Sun, and Yantao Han. 2019. "Design and Synthesis of a Chitodisaccharide-Based Affinity Resin for Chitosanases Purification" Marine Drugs 17, no. 1: 68. https://doi.org/10.3390/md17010068
APA StyleLi, S., Wang, L., Chen, X., Sun, M., & Han, Y. (2019). Design and Synthesis of a Chitodisaccharide-Based Affinity Resin for Chitosanases Purification. Marine Drugs, 17(1), 68. https://doi.org/10.3390/md17010068




