The Incidence of Tetrodotoxin and Its Analogs in the Indian Ocean and the Red Sea
Abstract
1. Introduction
2. Tetrodotoxin
3. TTX Detection Methods
4. Geographic Occurrence and Incidence of TTXs in the Indian Ocean and the Red Sea
5. Final Considerations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Onuma, Y.; Satake, M.; Ukena, T.; Roux, J.; Chanteau, S.; Rasolofonirina, N.; Ratsimaloto, M.; Naoki, H.; Yasumoto, T. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon 1999, 37, 55–65. [Google Scholar] [CrossRef]
- Mbaé, S.B.A.; Mlindassé, M.; Mihidjaé, S.; Seyler, T. Food-poisoning outbreak and fatality following ingestion of sea turtle meat in the rural community of Ndrondroni, Mohéli Island, Comoros, December 2012. Toxicon 2016, 120, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Ranaivoson, G.; de Ribes Champetier, G.; Mamy, E.R.; Jeannerod, G.; Razafinjato, P.; Chanteau, S. Mass food poisoning after eating sea turtle in the Antalaha district. Arch. Inst. Pasteur Madagascar 1994, 61, 84–86. [Google Scholar] [PubMed]
- Boisier, P.; Ranaivoson, G.; Rasolofonirina, N.; Roux, J.; Chanteau, S.; Takeshi, Y. Fatal mass poisoning in Madagascar following ingestion of a shark (Carcharhinus leucas): Clinical and epidemiological aspects and isolation of toxins. Toxicon 1995, 33, 1359–1364. [Google Scholar] [CrossRef]
- Auawithoothij, W.; Noomhorm, A. Shewanella putrefaciens, a major microbial species related to tetrodotoxin (TTX)-accumulation of puffer fish Lagocephalus lunaris. J. Appl. Microbiol. 2012, 113, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.A.; Hwang, D.F.; Tsai, Y.H.; Chen, H.C.; Jeng, S.S.; Noguchi, T.; Ohwada, K.; Hasimoto, K. Microflora and tetrodotoxin-producing bacteria in a gastropod, Niotha clathrata. Food Chem Toxicol. 1995, 33, 929–934. [Google Scholar] [CrossRef]
- Hwang, D.F.; Arakawa, O.; Saito, T.; Noguchi, T.; Simidu, U.; Tsukamoto, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus. Mar Biol. 1989, 100, 327–332. [Google Scholar] [CrossRef]
- Lee, M.J.; Jeong, D.Y.; Kim, W.S.; Kim, H.D.; Kim, C.H.; Park, W.W.; Park, Y.H.; Kim, K.S.; Kim, H.M.; Kim, D.S. A tetrodotoxin-producing Vibrio strain, LM-1, from the puffer fish Fugu vermicularis radiatus. Appl. Environ. Microbiol. 2000, 66, 1698–1701. [Google Scholar] [CrossRef]
- Ritchie, K.B.; Nagelkerken, I.; James, S.; Smith, G.W. Environmental microbiology: A tetrodotoxin-producing marine pathogen. Nature 2000, 404, 354. [Google Scholar] [CrossRef]
- Silva, M.; Pratheepa, V.K.; Botana, L.M.; Vasconcelos, V. Emergent toxins in North Atlantic temperate waters: A challenge for monitoring programs and legislation. Toxins 2015, 7, 859–885. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-F.; Yu, P.H.-F.; Chan, P.-L.; Yan, Q.; Wong, P.-K. Two novel species of tetrodotoxin-producing bacteria isolated from toxic marine puffer fishes. Toxicon 2004, 44, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Yotsu, M.; Yamazaki, T.; Meguro, Y.; Endo, A.; Murata, M.; Naoki, H.; Yasumoto, T. Production of tetrodotoxin and its derivatives by Pseudomonas sp. isolated from the skin of a pufferfish. Toxicon 1987, 25, 225–228. [Google Scholar] [PubMed]
- Yotsu-Yamashita, M.; Mebs, D.; Kwet, A.; Schneider, M. Tetrodotoxin and its analogue 6-epitetrodotoxin in newts (Triturus spp.; Urodela, Salamandridae) from southern Germany. Toxicon 2007, 50, 306–309. [Google Scholar] [PubMed]
- Ahmed, S. Puffer fish tragedy in Bangladesh: An incident of Takifugu oblongus poisoning in Degholia, Khulna. Afr. J. Mar. Sci. 2006, 28, 457–458. [Google Scholar] [CrossRef]
- Chopra, S.A. A case of fatal puffer-fish poisoning in a Zanzibari fisherman. East. Afr. Med. J. 1967, 44, 493–496. [Google Scholar]
- Rafiqui Islam, M.; Chowdhury, F.R.; Das, S.K.; Rahman, S.; Mahmudur, M.D.; Amin, M.D.R. Outbreak of Puffer Fish Poisoning in Dhaka City. J. Med. 2018, 19, 30–34. [Google Scholar] [CrossRef]
- Ravaonindrina, N.; Andriamaso, T.H.; Rasolofonirina, N. Puffer fish poisoning in Madagascar: Four case reports. Arch. Inst. Pasteur Madagascar 2001, 67, 61–64. [Google Scholar]
- Yong, Y.S.; Quek, L.S.; Lim, E.K.; Ngo, A. A case report of puffer fish poisoning in Singapore. Case Rep. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Zaki, M.A.; Mossa, A.E.W. Red Sea puffer fish poisoning: Emergency diagnosis and management of human intoxication. Egypt. J. Aquat. Res. 2005, 31, 370–378. [Google Scholar]
- Chowdhury, F.R.; Ahasan, H.A.M.N.; Al Mamun, A.; Rashid, A.K.M.M.; Al Mahboob, A. Puffer fish (Tetrodotoxin) poisoning: An analysis and outcome of six cases. Trop. Dr. 2007, 37, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Jelinek, G.A. Never eat an ugly fish: Three cases of tetrodotoxin poisoning from Western Australia. Emerg. Med. 1997, 9, 136–142. [Google Scholar] [CrossRef]
- Field, J. Puffer fish poisoning. Emerg. Med. J. 1998, 15, 334–336. [Google Scholar] [CrossRef]
- Ghose, A.; Ahmed, H.; Basher, A.; Amin, M.R.; Sayeed, A.A.; Faiz, M.A. Tetrodotoxin poisoning in Blangadesh: A case study. J. Med. Toxicol. 2008, 4, 216. [Google Scholar]
- Homaira, N.; Rahman, M.; Luby, S.P.; Rahman, M.; Haider, M.S.; Faruque, L.I.; Khan, D.; Parveen, S.; Gurley, E.S. Multiple outbreaks of puffer fish intoxication in Bangladesh, 2008. Am. J. Trop. Med. Hyg. 2010, 83, 440–444. [Google Scholar] [CrossRef]
- NàzmuíAhêsan, H.A.M.; AbdutfàhAíMâmun, C.H.R. Puffer fish poisoning: A clinical analysis. Pak. J. Med. Sci. 2003, 19, 29–32. [Google Scholar]
- Nazmul, A.; Al Mamun, A.; Rasul, C.H.; Roy, P.K. Puffer fish poisoning (tetrodotoxin) in Bangladesh: Clinical profile and role of anticholinesterase drugs. Trop. Dr. 2005, 35, 235–236. [Google Scholar] [CrossRef]
- Puech, B.; Batsalle, B.; Roget, P.; Turquet, J.; Quod, J.P.; Allyn, J.; Idoumbin, J.P.; Chane-Ming, J.; Villefranque, J.; Mougin-Damour, K.; et al. Family tetrodotoxin poisoning in Reunion Island (Southwest Indian Ocean) following the consumption of Lagocephalus sceleratus (Pufferfish). Bull. Soc. Pathol. Exot. 2014, 107, 79–84. [Google Scholar] [CrossRef]
- Taylor, A.D.; Vaisocherová, H.; Deeds, J.; DeGrasse, S.; Jiang, S. Tetrodotoxin detection by a surface plasmon resonance sensor in pufferfish matrices and urine. J. Sens. 2011, 2011. [Google Scholar] [CrossRef]
- Brillantes, S.; Samosorn, W.; Faknoi, S.; Oshima, Y. Toxicity of puffers landed and marketed in Thailand. Fish. Sci. 2003, 69, 1224–1230. [Google Scholar] [CrossRef]
- Islam, Q.T.; Razzak, M.A.; Islam, M.A.; Bari, M.I.; Basher, A.; Chowdhury, F.R.; Sayeduzzaman, A.B.M.; Ahasan, H.A.M.N.; Faiz, M.A.; Arakawa, O.; et al. Puffer fish poisoning in Bangladesh: Clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.; Christian, B.; Ahmed, M.S.; Luckas, B. Determination of tetrodotoxin and its analogs in the puffer fish Takifugu oblongus from Bangladesh by hydrophilic interaction chromatography and mass-spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1997. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; Yacout, G.A.; El-Samra, M.; Ali, A.; Kotb, S.M. Toxicity of the Red Sea pufferfish Pleuranacanthus sceleratus “El-Karad”. Ecotoxicol. Environ. Saf. 2003, 56, 367–372. [Google Scholar] [CrossRef]
- Ghosh, S.; Hazra, A.K.; Banerjee, S.; Mukherjee, B. The Seasonal Toxicological Profile of Four Puffer Fish Species Collected Along Bengal Coast, India. Indian J. Mar. Sci. 2004, 33, 276–280. [Google Scholar]
- Chulanetra, M.; Sookrung, N.; Srimanote, P.; Indrawattana, N.; Thanongsaksrikul, J.; Sakolvaree, Y.; Chongsa-Nguan, M.; Kurazono, H.; Chaicumpa, W. Toxic marine puffer fish in Thailand seas and tetrodotoxin they contained. Toxins 2011, 3, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Sabrah, M.M.; El-Ganainy, A.A.; Zaky, M.A. Biology and toxicity of the pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the Gulf of Suez. Egypt. J. Aquat. Res. 2006, 32, 283–297. [Google Scholar]
- Haque, M.A.; Islam, Q.T.; Ekram, A.R.M.S. Puffer fish poisoning. TAJ J. Teach. Assoc. 2008, 21, 199–202. [Google Scholar] [CrossRef]
- Vasconcelos, V.; Azevedo, J.; Silva, M.; Ramos, V. Effects of marine toxins on the reproduction and early stages development of aquatic organisms. Mar. Drugs 2010, 8, 59–79. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef]
- Noguchi, T.; Jeon, J.K.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. Occurrence of tetrodotoxin and anhydrotetrodotoxin in Vibrio sp. isolated from the intestines of a xanthid crab, Atergatis floridus. J. Biochem. 1986, 99, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapongkul, J.; Krittayapoositpot, P. An epidemic of tetrodotoxin poisoning following ingestion of the horseshoe crab Carcinoscorpius rotundicauda. Vertigo 1995, 30, 42. [Google Scholar]
- Kungsuwan, A.; Suvapeepan, S.; Suwansakornkul, P. Tetrodotoxin in the horseshoe crab Carcinoscorpius rotundicauda inhabiting Thailand. Nippon Suisan Gakkaishi 1987, 53, 261–266. [Google Scholar] [CrossRef]
- Ngya, L.; Yu, C.-F.; Takatani, T.; Arakawa, O. Toxicity assessment for the horseshoe crab Carcinoscorpius rotundicauda collected from Cambodia. Toxicon 2007, 49, 843–847. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barreg ard, L.; Bignami, M.; Br€uschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Roudo 2017. Scientific opinion on the risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, 4752–4817. [Google Scholar]
- Hanifin, C.T.; Yotsu-Yamashita, M.; Yasumoto, T.; Brodie, E.D. Toxicity of dangerous prey: Variation of tetrodotoxin levels within and among populations of the newt Taricha granulosa. J. Chem. Ecol. 1999, 25, 2161–2175. [Google Scholar] [CrossRef]
- Kudo, Y.; Yasumoto, T.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Isolation and structural determination of the first 8-epi-type tetrodotoxin analogs from the newt, Cynops ensicauda popei, and comparison of tetrodotoxin analogs profiles of this newt and the puffer fish, Fugu poecilonotus. Mar. Drugs 2012, 10, 655–667. [Google Scholar] [CrossRef]
- Kim, Y.H.; Brown, G.B.; Mosher, F.A. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science 2001, 189, 151–152. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Tsuruda, K.; Arakawa, O.; Noguchi, T. Occurrence of tetrodotoxin in the skin of a rhacophoridid frog Polypedates sp. from Bangladesh. Toxicon 2001, 39, 937–941. [Google Scholar] [CrossRef]
- Cliff, J.; Nicala, D.; Saute, F.; Givragy, R.; Azambuja, G.; Taela, A.; Chavane, L.; Howarth, J. Konzo associated with war in Mozambique. Trop. Med. Int. Heal. 1997, 2, 1068–1074. [Google Scholar] [CrossRef]
- Yan, Q.; Yu, P.H.-F.; Li, H.-Z. Detection of tetrodotoxin and bacterial production by Serratia marcescens. World J. Microbiol. Biotechnol. 2005, 21, 1255–1258. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Liang, S.; Ren, D.; Yan, X.; Bao, B. A novel TTX-producing Aeromonas isolated from the ovary of Takifugu obscurus. Toxicon 2010, 56, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Lee, J.-S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Finn, J.; Fukushima, K.; Sakugawa, S.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Isolation of 6-deoxytetrodotoxin from the pufferfish, Takifugu pardalis, and a comparison of the effects of the C-6 and C-11 hydroxy groups of tetrodotoxin on its activity. J. Nat. Prod. 2014, 77, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Abe, Y.; Kudo, Y.; Ritson-Williams, R.; Paul, V.J.; Konoki, K.; Cho, Y.; Adachi, M.; Imazu, T.; Nishikawa, T. First identification of 5, 11-dideoxytetrodotoxin in marine animals, and characterization of major fragment ions of tetrodotoxin and its analogs by high resolution ESI-MS/MS. Mar. Drugs 2013, 11, 2799–2813. [Google Scholar] [CrossRef]
- Satake, Y.; Adachi, M.; Tokoro, S.; Yotsu-Yamashita, M.; Isobe, M.; Nishikawa, T. Synthesis of 5-and 8-Deoxytetrodotoxin. Chem. Asian J. 2014, 9, 1922–1932. [Google Scholar] [CrossRef]
- Jang, J.-H.; Yotsu-Yamashita, M. 6, 11-Dideoxytetrodotoxin from the puffer fish, Fugu pardalis. Toxicon 2007, 50, 947–951. [Google Scholar] [CrossRef]
- Jan, L.Y.; Jan, Y.N. Tracing the roots of ion channels. Cell 1992, 69, 715–718. [Google Scholar] [CrossRef]
- Ghosh, S.; Hazra, A.K.; Banerjee, S.; Mukherjee, B. Ecological monitoring for ascertaining the bio-safety of liver lipids from some Indian marine puffer fishes. Fish. Sci. 2005, 71, 29–37. [Google Scholar] [CrossRef]
- Indumathi, S.M.; Khora, S.S. Toxicity assessment and screening of tetrodotoxin in the oblong blowfish (Takifugu oblongus) from the Tamil Nadu Coast of Bay of Bengal, India. Asian Pac. J. Trop. Med. 2017, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nor Azman, A.; Samsur, M.; Mohammed, M.; Shabdin, M.L.; Fasihuddin, B.A. Assessment of proximate composition and tetrodotoxin content in the muscle of Yellow puffer fish, Xenopterus naritus (Richardson 1848) from Sarawak, Malaysia. Int. Food Res. J. 2015, 22, 2280–2287. [Google Scholar]
- Veeruraj, A.; Pugazhvendan, S.R.; Ajithkumar, T.T.; Arumugam, M. Isolation and Identification of Cytotoxic and Biological Active Toxin from the Puffer Fish Arothron stellatus. Toxicol. Res. 2016, 32, 215. [Google Scholar] [CrossRef]
- Bragadeeswaran, S.; Therasa, D.; Prabhu, K.; Kathiresan, K. Biomedical and pharmacological potential of tetrodotoxin-producing bacteria isolated from marine pufferfish Arothron hispidus (Muller, 1841). J. Venom. Anim Toxins Incl Trop Dis. 2010, 16, 421–431. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- How, C.-K.; Chern, C.-H.; Huang, Y.-C.; Wang, L.-M.; Lee, C.-H. Tetrodotoxin poisoning. Am. J. Emerg. Med. 2003, 21, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.H.; Chew, L.P. Puffer fish poisoning: A family affair. Med. J. Malaysia 2009, 64, 181–182. [Google Scholar]
- Yooko, A. Chemical studies on tetrodotoxin. Report III. Isolation of spheroidine. J. Chem. Soc. Jpn. 1950, 71, 591–592. [Google Scholar]
- Nagashima, Y.; Maruyama, N.; Noguchi, T.; Hashimoto, K. Analysis of Paralytic Shellfish Poison and Tetrodotoxin by Ion-Pairing High Performance Liquid Chromatography. Nippon suisan Gakkaishi 1987, 53, 819–823. [Google Scholar] [CrossRef]
- Noguch, T.; Arakawa, O. Tetrodotoxin–distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef]
- Nagashima, Y.; Nishio, S.; Noguchi, T.; Arakawa, O.; Kanoh, S.; Hashimoto, K. Detection of tetrodotoxin by thin-layer chromatography/fast atom bombardment mass spectrometry. Anal. Biochem. 1988, 175, 258–262. [Google Scholar] [CrossRef]
- Mahmud, Y.; Arakawa, O.; Ichinose, A.; Tanu, M.B.; Takatani, T.; Tsuruda, K.; Kawatsu, K.; Hamano, Y.; Noguchi, T. Intracellular visualization of tetrodotoxin (TTX) in the skin of a puffer Tetraodon nigroviridis by immunoenzymatic technique. Toxicon 2003, 41, 605–611. [Google Scholar] [CrossRef]
- Hwang, D.F.; Cheng, C.A.; Tsai, H.T.; Shih, D.Y.C.; Ko, H.C.; Yang, R.Z.; Jeng, S.S. Identification of tetrodotoxin and paralytic shellfish toxins in marine gastropods implicated in food poisoning. Fish. Sci. 1995, 61, 675–679. [Google Scholar] [CrossRef]
- Jeon, J.; Narita, H.; Nara, M.; Noguchi, T.; Maruyama, J.; Hashimoto, K. Occurrence of tetrodotoxin in a gastropod mollusk,” araregai” Niotha clathrata. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 2099–2102. [Google Scholar] [CrossRef]
- Man, C.N.; Noor, N.M.; Harn, G.L.; Lajis, R.; Mohamad, S. Screening of tetrodotoxin in puffers using gas chromatography–mass spectrometry. J. Chromatogr. A 2010, 1217, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, K.; Arakawa, O.; Kawatsu, K.; Hamano, Y.; Takatani, T.; Noguchi, T. Secretory glands of tetrodotoxin in the skin of the Japanese newt Cynops pyrrhogaster. Toxicon 2002, 40, 131–136. [Google Scholar] [CrossRef]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar] [CrossRef]
- Thuesen, E.V.; Kogure, K.; Hashimoto, K.; Nemoto, T. Poison arrowworms: A tetrodotoxin venom in the marine phylum Chaetognatha. J. Exp. Mar. Biol. Ecol. 1988, 116, 249–256. [Google Scholar] [CrossRef]
- O’leary, M.A.; Schneider, J.J.; Isbister, G.K. Use of high performance liquid chromatography to measure tetrodotoxin in serum and urine of poisoned patients. Toxicon 2004, 44, 549–553. [Google Scholar] [CrossRef]
- Hwang, D.F.; Chueh, C.H.; Jeng, S.S. Occurrence of tetrodotoxin in the gastropod mollusk Natica lineata (lined moon shell). Toxicon 1990, 28, 21–27. [Google Scholar] [CrossRef]
- Mahmud, Y.; Okada, K.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Intra-tissue distribution of tetrodotoxin in two marine puffers Takifugu vermicularis and Chelonodon patoca. Toxicon 2003, 41, 13–18. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Ho, P.-H.; Hwang, C.-C.; Hwang, P.-A.; Cheng, C.-A.; Hwang, D.-F. Tetrodotoxin in several species of xanthid crabs in southern Taiwan. Food Chem. 2006, 95, 205–212. [Google Scholar] [CrossRef]
- Shiu, Y.-C.; Lu, Y.-H.; Tsai, Y.-H.; Chen, S.-K.; Hwang, D.-F. Occurrence of tetrodotoxin in the causative gastropod Polinices didyma and another gastropod Natica lineata collected from western Taiwan. J. Food Drug Anal. 2003, 11, 159–163. [Google Scholar]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5, 6, 11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Harada, T. Occurrence of tetrodotoxin in the Japanese ivory shell Babylonia japonica. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 909–914. [Google Scholar] [CrossRef]
- Hwang, D.-F.; Shiu, Y.-C.; Hwang, P.-A.; Lu, Y.-H. Tetrodotoxin in gastropods (snails) implicated in food poisoning in Northern Taiwan. J. Food Prot. 2002, 65, 1341–1344. [Google Scholar] [CrossRef]
- Suleiman, M.; Muhammad, J.; Jelip, J.; William, T.; Chua, T.H. AN OUTBREAK OF TETRODOTOXIN POISONING FROM CONSUMING HORSESHOE CRABS IN SABAH. Southeast Asian J. Trop. Med. Public Health. 2017, 48, 197–203. [Google Scholar]
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi, A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54, 50–55. [Google Scholar] [CrossRef]
- Doucette, G.J.; Powell, C.L.; Do, E.U.; Byon, C.Y.; Cleves, F.; McClain, S.G. Evaluation of 11-[3H]-tetrodotoxin use in a heterologous receptor binding assay for PSP toxins. Toxicon 2000, 38, 1465–1474. [Google Scholar] [CrossRef]
- Bignami, G.S.; Raybould, T.J.G.; Sachinvala, N.D.; Grothaus, P.G.; Simpson, S.B.; Lazo, C.B.; Byrnes, J.B.; Moore, R.E.; Vann, D.C. Monoclonal antibody-based enzyme-linked immunoassays for the measurement of palytoxin in biological samples. Toxicon 1992, 30, 687–700. [Google Scholar] [CrossRef]
- Kawatsu, K.; Shibata, T.; Hamano, Y. Application of immunoaffinity chromatography for detection of tetrodotoxin from urine samples of poisoned patients. Toxicon 1999, 37, 325–333. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Localization of tetrodotoxin in the skin of a brackishwater puffer Tetraodon steindachneri on the basis of immunohistological study. Toxicon 2002, 40, 103–106. [Google Scholar] [CrossRef]
- Luo, X.; Yu, R.-C.; Wang, X.-J.; Zhou, M.-J. Toxin composition and toxicity dynamics of marine gastropod Nassarius spp. collected from Lianyungang, China. Food Addit. Contam. Part A. 2012, 29, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Tsai, Y.-H.; Lu, Y.-H.; Hwang, D.-F. Paralytic toxins in three new gastropod (Olividae) species implicated in food poisoning in southern Taiwan. Toxicon 2003, 41, 529–533. [Google Scholar] [CrossRef]
- Chen, X.W.; Liu, H.X.; Jin, Y.B.; Li, S.F.; Bi, X.; Chung, S.; Zhang, S.S.; Jiang, Y.Y. Separation, identification and quantification of tetrodotoxin and its analogs by LC–MS without calibration of individual analogs. Toxicon 2011, 57, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Nzoughet, J.K.; Campbell, K.; Barnes, P.; Cooper, K.M.; Chevallier, O.P.; Elliott, C.T. Comparison of sample preparation methods, validation of an UPLC–MS/MS procedure for the quantification of tetrodotoxin present in marine gastropods and analysis of pufferfish. Food Chem. 2013, 136, 1584–1589. [Google Scholar] [CrossRef]
- Rodríguez, P.; Alfonso, A.; Otero, P.; Katikou, P.; Georgantelis, D.; Botana, L.M. Liquid chromatography–mass spectrometry method to detect Tetrodotoxin and Its analogues in the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from European waters. Food Chem. 2012, 132, 1103–1111. [Google Scholar] [CrossRef]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography–electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar] [CrossRef]
- Isbister, G.K.; Son, J.; Wang, F.; Maclean, C.J.; Lin, C.S.; Ujma, J.; Balit, C.R.; Smith, B.; Milder, D.G.; Kiernan, M.C. Puffer fish poisoning: A potentially life-threatening condition. Med. J. Aust. 2002, 177, 650–653. [Google Scholar]
- Cheng, C.A. Paralytic toxins of the gastropod Natica lineata in Pingtung Prefecture. Food Sci. 1996, 23, 845–853. [Google Scholar]
- Narita, T.; Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Watanabe, Y. Occurrence of tetrodotoxin in a trumpet shell,” boshubora” Charonia sauliae. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 935–941. [Google Scholar] [CrossRef]
- Noguchi, T.; Maruyama, J.; Narita, H.; Kanehisa, H. Occurrence of tetrodotoxin in the gastropod mollusk Tutufa lissostoma (frog shell). Toxicon 1984, 22, 219–226. [Google Scholar] [CrossRef]
- Noguchi, T.; Uzu, A.; Koyama, K.; Hashimoto, K. Occurrence of tetrodotoxin as the major toxin in a xanthid crab Atergatis floridus. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 1887–1892. [Google Scholar] [CrossRef]
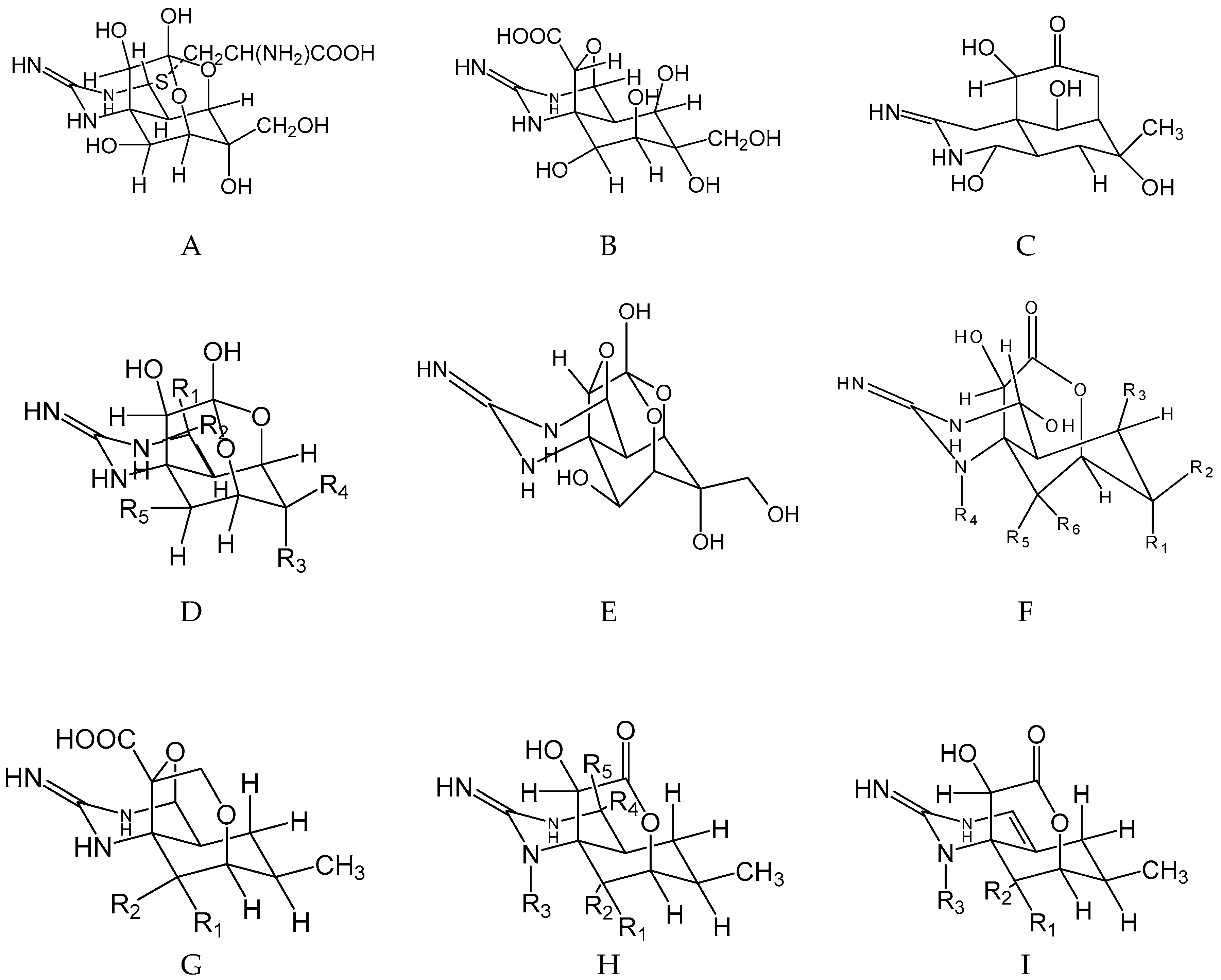
| E | R1 | R2 | R3 | R4 | R5 | |
| TTX (*) | H | OH | OH | CH2OH | OH | |
| 4-epiTTX (*) | OH | H | OH | CH2OH | OH | |
| 6-epiTTX (*) | H | OH | CH2OH | OH | OH | |
| 11-deoxyTTX (*) | H | OH | OH | CH3 | OH | |
| 6,11-dideoxyTTX | H | OH | H | CH3 | OH | |
| 8,11-dideoxyTTX | H | OH | OH | CH3 | H | |
| 11-oxoTTX (*) | H | OH | OH | CH(OH)2 | OH | |
| 11-norTTX-6,6-diol | H | OH | OH | OH | OH | |
| 11-norTTX-6(R)-ol (*) | H | OH | H | OH | OH | |
| 11-norTTX-6(S)-ol (*) | H | OH | OH | H | OH | |
| Chiriquitoxin | H | OH | OH | CH(OH)CH(NH3+)COO− | OH | |
| TTX-8-O-hemisuccinate | H | OH | OH | CH2OH | OOC(CH2)2COO− | |
| TTX-11-carboxylic acid | H | OH | OH | COO− | OH | |
| TTX (*) | H | OH | OH | CH2OH | OH | |
| F | R1 | R2 | R3 | R4 | R5 | R6 |
| 5-deoxyTTX(*) | OH | CH2OH | H | H | OH | H |
| 5,11-dideoxyTTX (*) | OH | CH3 | H | H | OH | H |
| 5,6,11-trideoxyTTX (*) | H | CH3 | H | H | OH | H |
| 8-epi-5,6,11-trideoxyTTX | H | CH3 | H | H | H | OH |
| G | R1 | R2 | ||||
| 4,9-anhydro-5,6,11-trideoxyTTX | H | OH | ||||
| 4.9-anhydro-8-epi-5,6,11-trideoxyTTX | OH | H | ||||
| H | R1 | R2 | R3 | R4 | R5 | |
| 1-hydroxy-8-epi-5,6,11-trideoxyTTX | OH | H | OH | OH | H | |
| 4-epi-5,6,11-trideoxyTTX | H | OH | H | H | OH | |
| I | R1 | R2 | R3 | |||
| 4,4a-anhydro-5,6,11-trideoxyTTX | H | OH | H | |||
| 1-hydroxy-4,4a-anhydro-8-epi-5,5,11-trideooxyTTX | OH | H | OH |
| TTX Analogs | TEF | CAS Number |
|---|---|---|
| TTX | 1.0 | 4368-28-9 |
| 11-oxoTTX | 0.75 | 123665-88-3 |
| 11-deoxyTTX | 0.14 | - |
| 11-norTTX-6(R)-ol | 0.17 | - |
| 11-norTTX-6(S)-ol | 0.19 | - |
| 4-epiTTX | 0.16 | 98242-82-1 |
| 4,9-anhydroTTX | 0.02 | 13072-89-4 |
| 6,11-dideoxyTTX | 0.02 | - |
| 5-deoxyTTX | 0.01 | - |
| 5,6,11-trideoxyTTX | 0.01 | - |
| 4-CysTTX | - | - |
| 5,11-dideoxyTTX | - | - |
| Level | Affected System | Specific Symptoms |
|---|---|---|
| 1 | Neuromuscular | Paresthesia of lips, tongue, and pharynx, taste disturbance, dizziness, headache, diaphoresis, pupillary constriction |
| Gastrointestinal | Salivation, hypersalivation, nausea, vomiting, hyperemesis, hematemesis, hypermotility, diarrhea, abdominal pain | |
| 2 | Neuromuscular | Advanced general paresthesia, paralysis of phalanges and extremities, pupillary dilatation, reflex changes |
| 3 | Neuromuscular | Dysarthria, dysphagia, aphagia, lethargy, incoordination, ataxia, floating sensation, cranial nerve palsies, muscular fasciculation |
| Cardiovascular/pulmonary | Hypotension or hypertension, vasomotor blockade, cardiac arrhythmias, atrioventricular node conduction abnormalities, cyanosis, pallor, dyspnea | |
| Dermatologic | Exfoliative dermatitis, petechiae, and blistering | |
| 4 | Respiratory failure, impaired mental faculties, extreme hypotension, seizures, loss of deep tendon and spinal reflexes | |
| Analysis Method | LOD | LOQ |
|---|---|---|
| MBA [12,36,52,89] | 1.1 μg·g−1 [89] | - |
| Enzymatic assays [31,36,52,73,77,82,89,91,92,93] | 2 ng·mL−1 [92] | - |
| TLC–MS [13,72] | 0.1 μg [72] | - |
| HPLC–FLD [84,94,95] | 1.27 μg·g−1 [94] | |
| GC–MS [76,84,95] | 0.5 μg·g−1 [76] | 1.0 μg·g−1 [76] |
| LC–MS/MS/UPLC–MS/MS [33,40,96,97,98] | 0.09–16 ng·mL−1 [33,40,96,97,98] | 5–63 ng·mL−1 [40] |
| SPR [30] | 0.3–20 ng·mL−1 [30] | - |
| HPLC–FLD [15,32,99] | 40-100 ng·g−1 [15] | - |
| Producing Species | Vector | Sample Tissue | Location | Country | Poisoning Date | TTX | Detection | Maximum Concentration | Poisoning Victims | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Australia | ||||||||||
| Unknown | Puffer fish Lagocephalus scleratus | Close to Fremantle Hospital | Australia | 13 May 1996 | TTX | Symptomatology | - | 3 people | [23] | |
| Unknown | Puffer fish Lagocephalus scleratus | Port Hedland | Australia | 1998 | TTX | Symptomatology | - | 1 person | [24] | |
| Unknown | Toad fish Tetractenos hamiltoni | New South Wales | Australia | 1 January 2001 to 13 April 2002 | TTX | Symptomatology | - | 11 people | [100] | |
| Unknown | Toad fish Tetractenos hamiltoni | Urine | Australia | 2004 | TTX | HPLC–UVD | 5 ng/mL | 7 people | [80] | |
| Serum | 20 ng/mL | |||||||||
| Asian countries | ||||||||||
| Unknown | Puffer fish | Khulna | Bangladesh | April 18 2002 | TTX | Symptomatology | - | 45 people | [27] | |
| Unknown | Puffer fish Takifugu oblongus | Skin | Khulna | Bangladesh | 18 May 2002 | TTX | MBA | 18.9 MU/g | 36 people, 7 deaths | [16] |
| Muscle | 4.4 MU | |||||||||
| Liver | 4.9 MU/g | |||||||||
| Gonads | 132.0 MU/g | |||||||||
| Viscera categories | 37.0 MU/g | |||||||||
| Natore | - | |||||||||
| Dhaka | ||||||||||
| Unknown | Puffer fish | Liver | Khulna | Bangladesh | 24 July 2005 | TTX | Symptomatology | - | 6 people | [22] |
| Unknown | Skin | Khulna | Bangladesh | 25 March 2006 | TTX | LC–MS/MS | 25.35 μg·g−1 | NPI | [33] | |
| Anhydro | 7.71 μg·g−1 | |||||||||
| 11-Deoxy | 1.12 μg·g−1 | |||||||||
| Trideoxy | 15.31 μg·g−1 | |||||||||
| Muscle | TTX | 1.64 μg·g−1 | ||||||||
| Anhydro | - | |||||||||
| 11-Deoxy | - | |||||||||
| Trideoxy | - | |||||||||
| Liver | TTX | 45.71 μg·g−1 | ||||||||
| Anhydro | 29.17 μg·g−1 | |||||||||
| 11-Deoxy | - | |||||||||
| Trideoxy | 9.09 μg·g−1 | |||||||||
| Ovary | TTX | 356.00 μg·g−1 | ||||||||
| Anhydro | 85.87 μg·g−1 | |||||||||
| 11-Deoxy | 26.00 μg·g−1 | |||||||||
| Trideoxy | 2,929.70 μg·g−1 | |||||||||
| Unknown | Puffer fish | Dhaka | Bangladesh | 2008 | TTX | Symptomatology | - | 11 people | [25] | |
| Unknown | Puffer Fish | Narshingdi | Bangladesh | April and June 2008 | TTX | Symptomatology | - | 95 people, 14 deaths | [26] | |
| Natore | ||||||||||
| Dhaka | ||||||||||
| Unknown | Puffer Fish | Dhaka City | Bangladesh | October 2014 | TTX | Symptomatology | - | 11 people, 4 deaths | [18] | |
| Unknown | Puffer fish | - | Khulna | Bangladesh | - | TTX | Symptomatology | - | 37 people, 8 deaths | [28] |
| Unknown | Puffer fish Chelonodon patoca | Liver | Bay of Bengal | India | June 1998 to March 2001 | TTX | MBA | 25.9 MU/g | NPI | [61] |
| Ovary | 183 MU/g | |||||||||
| Sphaeroides oblongus | Liver | 16 MU/g | ||||||||
| Ovary | 7.9 MU/g | |||||||||
| Lagocephalus inermis | Liver | 5.5 MU/g | ||||||||
| Ovary | 28.9 MU/g | |||||||||
| Lagocephalus lunaris | Liver | 5.9 MU/g | ||||||||
| Ovary | 16.6 MU/g | |||||||||
| Unknown | Puffer fish Chelenodon potoca | Liver | Bengal coast | India | June 2000–March 2001 | TTX | MBA | 27.8 MU/g | NPI | [35] |
| Ovary | 156.7 MU/g | |||||||||
| Takifugu oblongus | Liver | 11.75 MU/g | ||||||||
| Ovary | 29.1 MU/g | |||||||||
| Lagocephalus lunaris | Liver | 9 MU/g | ||||||||
| Ovary | 30.1 MU/g | |||||||||
| Lagocephalus inermis | Liver | 5.7 MU/g | ||||||||
| Ovary | 9.64 MU/g | |||||||||
| Kytococcus sedentarius | Puffer fish Arothron hispidus | Skin | Annankil fish landings at Parangipettai | India | 2010 | TTX | MBA | - | NPI | [65] |
| Intestine | - | |||||||||
| Liver | - | |||||||||
| Cellulomonas fimi | Muscle | 4.4 MU | ||||||||
| Liver | 4.9 MU/g | |||||||||
| Gonads | 132.0 MU/g | |||||||||
| Bacillus lentimorbus | Viscera categories | 37.0 MU/g | ||||||||
| Natore | - | |||||||||
| Dhaka | - | |||||||||
| Unknown | Puffer fish Arothron stellatus | Muscles | Parangipettai | India | 2016 | TTX | HPLC–FLD, TLC–UVD | Qualitative | NPI | [64] |
| Gonads | 4-epi | |||||||||
| Liver | anhydro | |||||||||
| Unknown | Puffer fish Takifugu oblongus | Skin | Kasimedu fishing harbor, Chennai, Tamil Nadu | India | 2016 | TTX | MBA | 75.88 MU/g | NPI | [62] |
| GC–MS | 16.5 MU/g | |||||||||
| HPLC | 18 MU/g | |||||||||
| Liver | MBA | 143.33 MU/g | ||||||||
| GC–MS | 32.5 MU/g | |||||||||
| HPLC | 48 MU/g | |||||||||
| Ovary | MBA | 163 MU/g | ||||||||
| GC–MS | 34.5 μg | |||||||||
| HPLC | 51 μg | |||||||||
| Unknown | Puffer fish | - | Johor | Malaysia | May 2008 | TTX | Symptomatology | - | 34 people | [68] |
| Unknown | Carcinoscorpius rotundicauda | Urine | Kota Marudu | Malaysia | June–August 2011 | TTX | GC–MS | 1.3–602 ng/mL | 30 people | [88] |
| Unknown | Puffer fish Xenopterus naritus | Muscle | Manggut | Malaysia | February and July 2013 | TTX | LC–MS/MS | 27.19 μg/g | NPI | [63] |
| Kaong | 16.09 μg/g | |||||||||
| Unknown | Puffer fish Lageocephalus scitalleratus | Alexandra Hospital | Singapore | 2013 | TTX | Symptomatology | 1 person | [20] | ||
| Unknown | Tetraodon nigroviridis | Reproduc tive tissue | Satun | Thailand | April to July 2010 | TTX | LC–MS/MS, MBA | 63.57 MU/g | NPI | [36] |
| Liver | 97.08 MU/g | |||||||||
| Digestive tissue | 43.33 MU/g | |||||||||
| Muscle | 22.12 MU/g | |||||||||
| Arothron reticularis | Reproductive tissue | - | ||||||||
| Liver | 2.08 MU/g | |||||||||
| Digestive tissue | 3.16 MU/g | |||||||||
| Muscle | 4.02 MU/g | |||||||||
| African countries | ||||||||||
| Unknown | Puffer fish Lagocephalus lunaris | Gonads | National Research Center, Dokki, Cairo, | Egypt | September 1990 through May 1991 | TTX | TLC–UVD, MBA | 752 MU/g | NPI | [34] |
| Liver | 246 MU/g | |||||||||
| Muscles | 127 MU/g | |||||||||
| Digestive tract | 221 MU/g | |||||||||
| Skin | 119 MU/g | |||||||||
| Unknown | Puffer fish Lagocephalus sceleratus | Gonads | Attaka fishing harbor | Egypt | October 2002 and June 2003 | TTX | MBA | 3950 MU/g | NPI | [37] |
| Unknown | Puffer fish Lagocephulus scleratus | Muscle | Suez Gulf | Egypt | 23 December 2004 | TTX | 7 people | [21] | ||
| Unknown | Puffer fish | Nosy Be Island | Madagascar | July 1998 | TTX | MBA | 16 UM/g | 3 people, 1 death | [19] | |
| Unknown | Puffer fish Lagocephalus sceleratus | Liver | Reunion Island | Reunion Island | 10 September 2013 | TTX | MBA, LC–MS/MS | 95 MU/g | 10 people | [29] |
| Flesh | 5 MU/g | |||||||||
| Unknown | Puffer fish, Tetraodontidae family | Zanzibar | Tanzania | TTX | Symptomatology | - | 1 death | [17] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamele, I.J.; Silva, M.; Vasconcelos, V. The Incidence of Tetrodotoxin and Its Analogs in the Indian Ocean and the Red Sea. Mar. Drugs 2019, 17, 28. https://doi.org/10.3390/md17010028
Tamele IJ, Silva M, Vasconcelos V. The Incidence of Tetrodotoxin and Its Analogs in the Indian Ocean and the Red Sea. Marine Drugs. 2019; 17(1):28. https://doi.org/10.3390/md17010028
Chicago/Turabian StyleTamele, Isidro José, Marisa Silva, and Vitor Vasconcelos. 2019. "The Incidence of Tetrodotoxin and Its Analogs in the Indian Ocean and the Red Sea" Marine Drugs 17, no. 1: 28. https://doi.org/10.3390/md17010028
APA StyleTamele, I. J., Silva, M., & Vasconcelos, V. (2019). The Incidence of Tetrodotoxin and Its Analogs in the Indian Ocean and the Red Sea. Marine Drugs, 17(1), 28. https://doi.org/10.3390/md17010028






