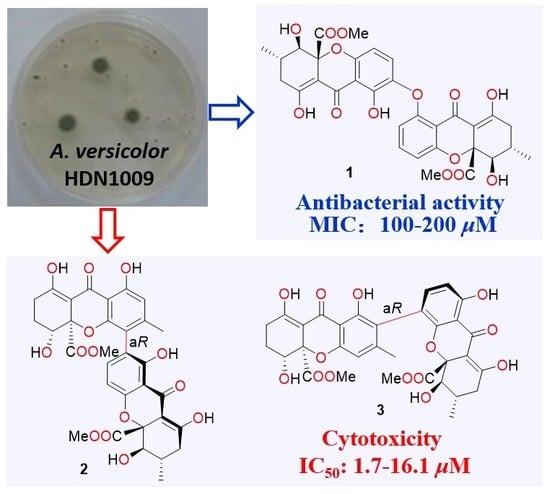
Cytotoxic Tetrahydroxanthone Dimers from the Mangrove-Associated Fungus Aspergillus versicolor HDN1009
Abstract
:1. Introduction
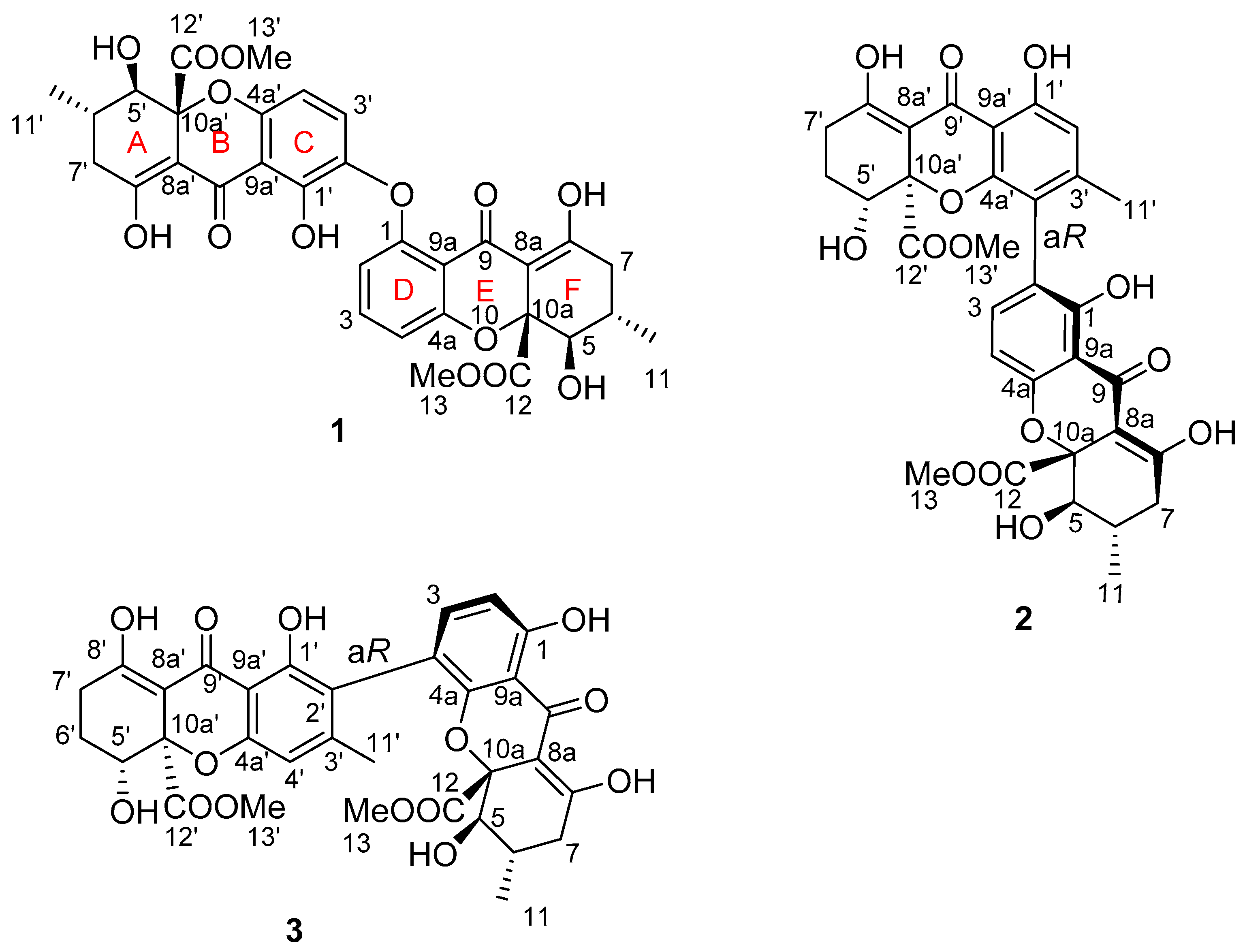
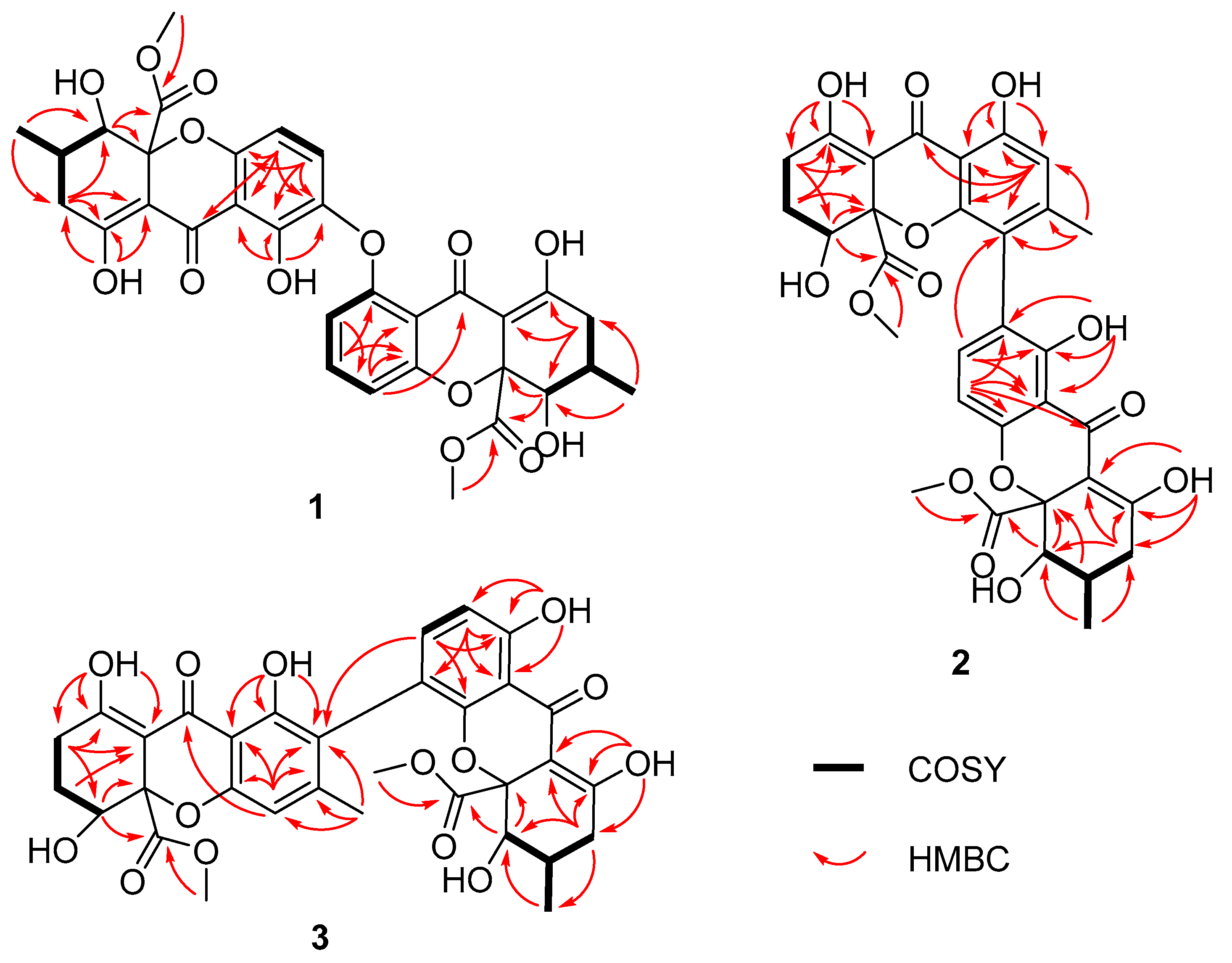
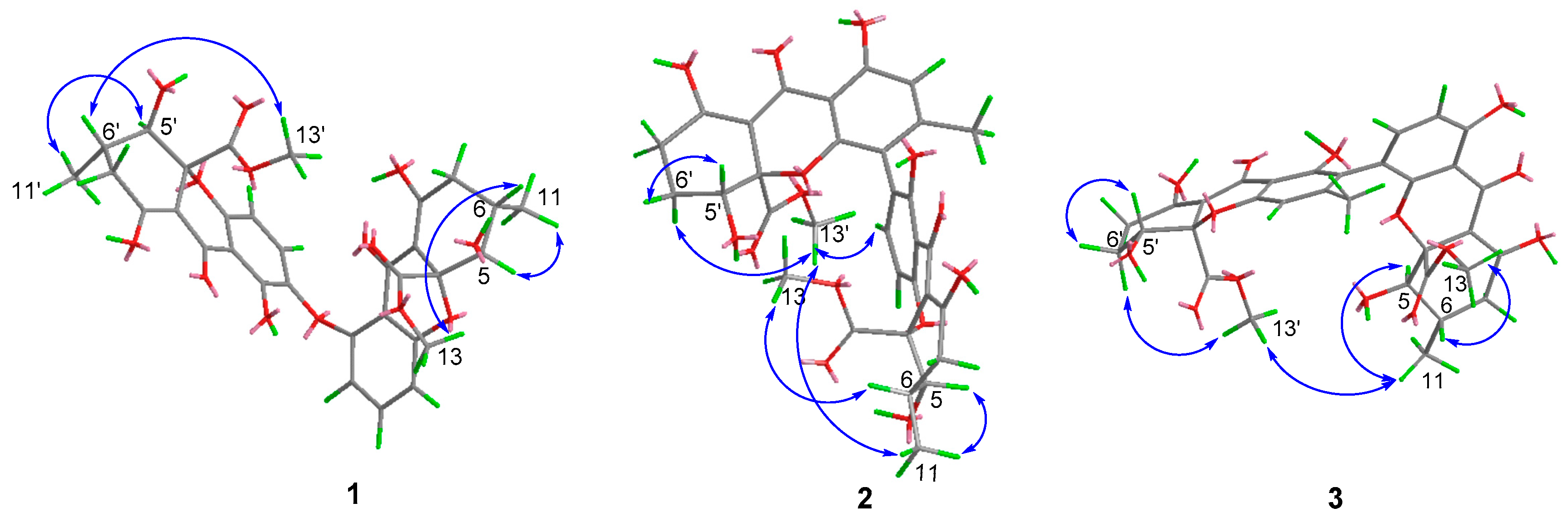
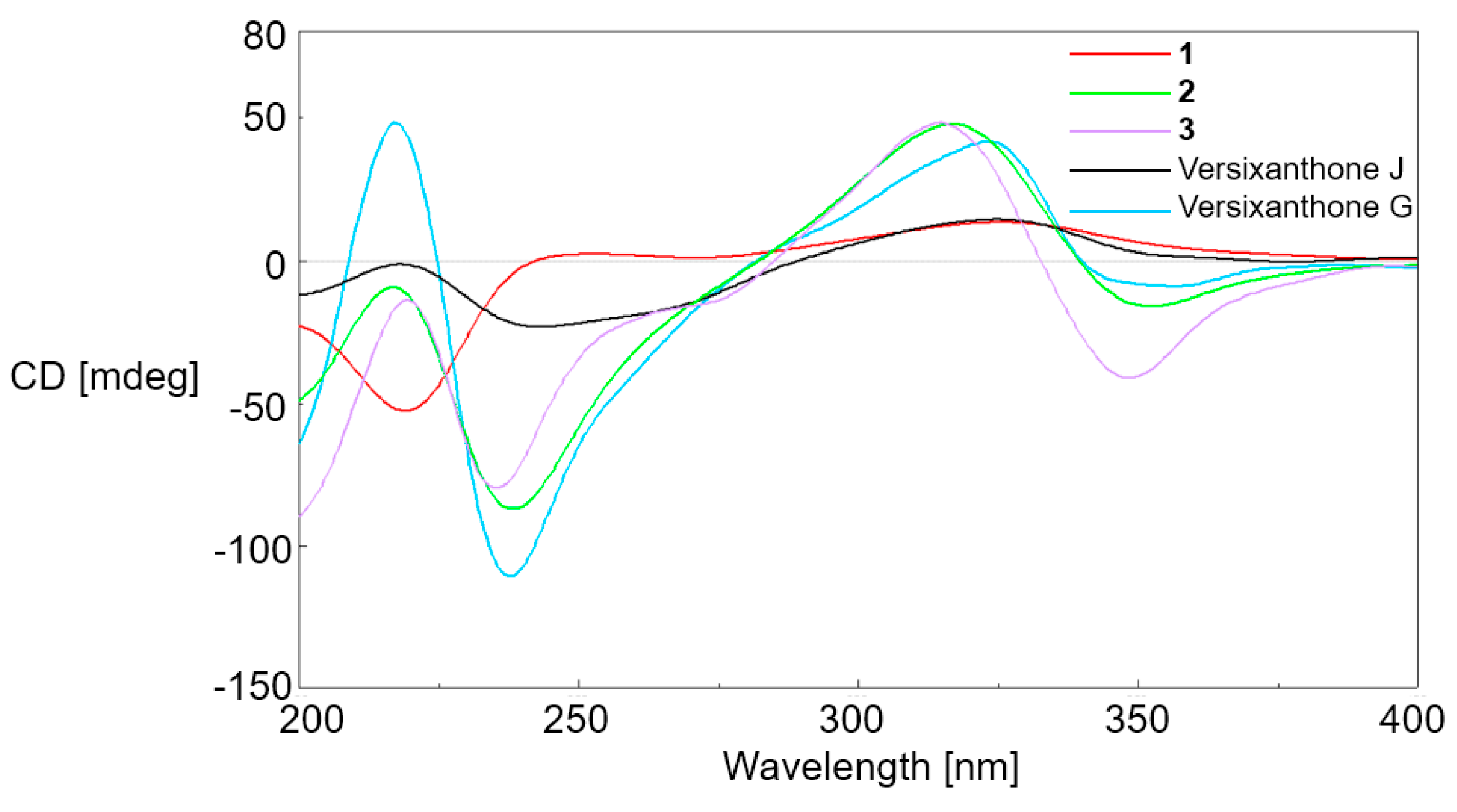
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation
3.5. Assay of Cytotoxicity
3.6. Assay of Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Masters, K.S.; Brase, S. Xanthones from Fungi, Lichens, and Bacteria: The Natural Products and Their Synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Qi, X.; Mo, X.; Yu, G.; Wang, Q.; Zhu, T.; Gu, Q.; Liu, M.; Li, J.; Li, D. Structure-based discovery of cytotoxic dimeric tetrahydroxanthones as potential topoisomerase I inhibitors from a marine-derived fungus. Eur. J. Med. Chem. 2018, 148, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Palasarn, S.; Kocharin, K.; Saenboonreung, J. A Cytotoxic Xanthone Dimer from the Entomopathogenic Fungus Aschersonia sp. BCC 8401. J. Nat. Prod. 2005, 68, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Chutrakul, C.; Boonruangprapal, T.; Suvannakad, R.; Isaka, M.; Sirithunya, P.; Toojinda, T.; Kirtikara, K. Ascherxanthone B from Aschersonia luteola, a new antifungal compound active against rice blast pathogen Magnaporthe grisea. J. Appl. Microbiol. 2009, 107, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ukai, K.; Namikoshi, M. Asperdichrome, an unusual dimer of tetrahydroxanthone through an ether bond, with protein tyrosine phosphatase 1B inhibitory activity, from the Okinawan freshwater Aspergillus sp. TPU1343. Tetrahedron Lett. 2016, 57, 732–735. [Google Scholar] [CrossRef]
- Wu, G.; Yu, G.; Kurtán, T.; Mándi, A.; Peng, J.; Mo, X.; Liu, M.; Li, H.; Sun, X.; Li, J.; et al. Versixanthones A–F, Cytotoxic Xanthone-Chromanone Dimers from the Marine-Derived Fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 2015, 78, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, M.; Wang, Y.; Wang, X.; Luo, J.; Luo, J.; Kong, L. Unusual dimeric tetrahydroxanthone derivatives from Aspergillus lentulus and the determination of their axial chiralities. Sci. Rep. 2016, 6, 38958. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, J.; Wang, J.; Shi, L.; Li, K.; Lin, X.; Min, Y.; Yang, B.; Tang, L.; Liu, Y.; et al. Cytotoxic and Antibacterial Eremophilane Sesquiterpenes from the Marine-Derived Fungus Cochliobolus lunatus SCSIO41401. J. Nat. Prod. 2018, 81, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Lenta, B.N.; Ngatchou, J.; Frese, M.; Ladoh-Yemeda, F.; Voundi, S.; Nardella, F.; Michalek, C.; Wibberg, D.; Ngouela, S.; Tsamo, E.; et al. Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum. Z. Naturforsch. B J. Chem. Sci. 2016, 71, 1159–1167. [Google Scholar] [CrossRef]
- Yu, G.; Wang, S.; Wang, L.; Che, Q.; Zhu, T.; Zhang, G.; Gu, Q.; Guo, P.; Li, D. Lipid-Lowering Polyketides from the Fungus Penicillium steckii HDN13-279. Mar. Drugs 2018, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhu, T.J.; Liu, H.B.; Fang, Y.C.; Zhu, W.M.; Gu, Q.Q. Cytotoxic polyketides from a marine-derived fungus, Aspergillus glaucus. J. Nat. Prod. 2008, 71, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
| No. | 1 | Asperdichrome | 2 | 3 |
|---|---|---|---|---|
| 2 | 6.36 d (8.4) | 6.39, d (8.2) | -- | 6.62 d (8.4) |
| 3 | 7.30 t (8.3) | 7.28, t (8.2) | 7.69 d (8.4) | 7.18 d (8.4) |
| 4 | 6.80 d (8.4) | 6.76, d (8.2) | 6.69 d (8.4) | -- |
| 5 | 3.95 d (11.3) | 4.12 d (1.4) | 3.94 d (11.1) | 3.82 d (11.1) |
| 6 | 2.42 m | 2.14, m | 2.46 m | 2.34 m |
| 7 | 2.72 dd (6.4, 10.6) 2.29 dd (11.0, 15.4) | 2.52, dd (18.8, 11.6) 2.40, dd (18.8, 6.3) | 2.74 dd (6.3, 19.3) 2.32 dd (10.7, 19.2) | 2.69 m |
| 11 | 1.17 d (5.9) | 1.18, d (6.3) | 1.18 d (6.4) | 1.08 d (6.3) |
| 13 | 3.72 s | 3.69 s | 3.70 s | 3.66 |
| 1-OH | -- | 11.49 s | 11.41 s | |
| 8-OH | 15.78 s | 16.0, s | 13.75 brs | 13.68 brs |
| 2′ | -- | -- | 6.51 s | -- |
| 3′ | 7.22 d (8.9) | 7.22, d (8.7) | -- | -- |
| 4′ | 6.57 d (8.9) | 6.57, d (8.7) | -- | 6.56 s |
| 5′ | 3.94 d (11.3) | 3.94, d (11.1) | 4.14 dd (5.0, 12.6) | 4.33 dd (5.0, 12.4) |
| 6′ | 2.43 m | 2.40, m | 2.12 m; 2.00, m | 2.23 m; 2.10, m |
| 7′ | 2.76 dd (6.1, 10.4); 2.32 dd (4.1, 8.2) | 2.75, dd (18.7, 5.8) 2.32, dd (18.7, 10.9) | 2.62 m | 2.66 m |
| 11′ | 1.18 d (5.7) | 1.17, d (6.8) | 2.12 s | 2.23 s |
| 13′ | 3.70 s | 3.72, s | 3.74 s | 3.73 s |
| 1′-OH | 11.35 s | 11.4, s | 11.25 brs | 11.44 brs |
| 8′-OH | 13.75 s | 13.7, s | 13.77 brs | 13.86 brs |
| No. | 1 | Asperdi- chrome | 2 | 3 |
|---|---|---|---|---|
| 1 | 158.7 | 160.0 | 159.3 | 161.9 |
| 2 | 109.5 | 110.9 | 117.1 | 110.5 |
| 3 | 135.6 | 136.6 | 141.0 | 140.2 |
| 4 | 111.9 | 113.0 | 107.8 | 114.7 |
| 4a | 160.2 | 160.8 | 158.5 | 155.9 |
| 5 | 77.9 | 72.4 | 76.9 | 76.6 |
| 6 | 29.2 | 30.1 | 29.3 | 29.2 |
| 7 | 37.8 | 35.1 | 36.2 | 36.1 |
| 8 | 182.5 | 186.4 | 177.7 | 177.3 |
| 8a | 102.7 | 103.2 | 101.6 | 101.4 |
| 9 | 180.2 | 181.7 | 187.1 | 187.2 |
| 9a | 110.8 | 112.0 | 106.9 | 107.0 |
| 10a | 84.9 | 86.2 | 85.0 | 84.7 |
| 11 | 18.0 | 17.9 | 18.0 | 17.9 |
| 12 | 170.1 | 173.5 | 170.5 | 169.9 |
| 13 | 53.0 | 53.7 | 53.2 | 53.0 |
| 1′ | 158.7 | 154.1 | 159.3 | 161.9 |
| 2′ | 136.8 | 137.8 | 111.8 | 118.4 |
| 3′ | 130.3 | 131.5 | 150.2 | 149.8 |
| 4′ | 107.6 | 109.1 | 115.7 | 109.1 |
| 4a′ | 155.5 | 157.9 | 155.7 | 157.8 |
| 5′ | 77.9 | 77.6 | 71.5 | 71.9 |
| 6′ | 29.3 | 31.1 | 23.5 | 23.9 |
| 7′ | 36.3 | 37.1 | 27.5 | 27.6 |
| 8′ | 178.3 | 179.6 | 177.3 | 177.8 |
| 8a′ | 101.6 | 103.3 | 101.1 | 101.3 |
| 9′ | 186.9 | 188.7 | 186.8 | 186.7 |
| 9a′ | 107.9 | 109.1 | 105.1 | 104.5 |
| 10a′ | 84.9 | 86.8 | 84.7 | 84.5 |
| 11′ | 18.0 | 18.3 | 21.1 | 21.5 |
| 12′ | 170.2 | 172.1 | 169.8 | 170.4 |
| 13′ | 53.2 | 53.4 | 53.1 | 53.2 |
| Comp. | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| MGC803 | HL-60 | HO-8910 | H1975 | K562 | A549 | |
| 1 | >30 | >30 | >30 | >30 | >30 | >30 |
| 2 | 1.7 | 2.7 | 8.5 | 8.8 | 9.1 | >30 |
| 3 | 1.8 | 8.1 | 6.7 | 8.5 | 16.1 | >30 |
| Dox a | 0.2 | 0.02 | 0.5 | 0.8 | 0.3 | 0.2 |
| Comp. | MIC (μM) | |||||
|---|---|---|---|---|---|---|
| Vibrio parahemolyticus | Bacillus subtilis | Mycobacterium phlei | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | |
| 1 | 100 | 200 | 200 | >200 | 100 | >200 |
| Positive Drug | 0.781 a | 0.391 a | 0.781 a | 0.391 a | 1.56 a | 3.13 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Wu, G.; Sun, Z.; Zhang, X.; Che, Q.; Gu, Q.; Zhu, T.; Li, D.; Zhang, G. Cytotoxic Tetrahydroxanthone Dimers from the Mangrove-Associated Fungus Aspergillus versicolor HDN1009. Mar. Drugs 2018, 16, 335. https://doi.org/10.3390/md16090335
Yu G, Wu G, Sun Z, Zhang X, Che Q, Gu Q, Zhu T, Li D, Zhang G. Cytotoxic Tetrahydroxanthone Dimers from the Mangrove-Associated Fungus Aspergillus versicolor HDN1009. Marine Drugs. 2018; 16(9):335. https://doi.org/10.3390/md16090335
Chicago/Turabian StyleYu, Guihong, Guangwei Wu, Zichao Sun, Xiaomin Zhang, Qian Che, Qianqun Gu, Tianjiao Zhu, Dehai Li, and Guojian Zhang. 2018. "Cytotoxic Tetrahydroxanthone Dimers from the Mangrove-Associated Fungus Aspergillus versicolor HDN1009" Marine Drugs 16, no. 9: 335. https://doi.org/10.3390/md16090335
APA StyleYu, G., Wu, G., Sun, Z., Zhang, X., Che, Q., Gu, Q., Zhu, T., Li, D., & Zhang, G. (2018). Cytotoxic Tetrahydroxanthone Dimers from the Mangrove-Associated Fungus Aspergillus versicolor HDN1009. Marine Drugs, 16(9), 335. https://doi.org/10.3390/md16090335