UV-Protective Compounds in Marine Organisms from the Southern Ocean
Abstract
1. Introduction
1.1. Antarctic Marine Environment
1.2. UV Radiation, Penetration, Photoperiod
1.3. Sea Ice Dynamics
1.4. The Ozone Hole and the Impacts of a Changing Environment
2. Effects of Light in Marine Organisms
2.1. Beneficial Effects of Light
2.2. Negative Effects of Light
3. UV Photoprotection in Marine Organisms: Antarctic and Non-Antarctic Strategies
3.1. Physical Structures for Light Avoidance
3.2. UV-Absorbing Substances (Sunscreen)
3.2.1. Mycosporine-Like Amino Acids (MAAs)
Structure, Biosynthetic Pathways of MAAs and Their Regulation
Biological Functions of MAAs
Environmental Distribution of MAAs and Their Occurrence in Organisms
MAAs in Antarctic Marine Organisms
3.2.2. Scytonemin
3.2.3. Erebusinone
3.2.4. Pigments
3.2.5. Other Secondary Metabolites
Phlorotannins
Flavonoids
Tridentatols
3.2.6. Fluorescent Proteins
3.3. Quenching Mechanisms
3.3.1. Non-Enzymatic Antioxidants
Carotenoids
α-Tocopherol
Ascorbic Acid
Glutathione (GSH)
3.3.2. Enzymatic Antioxidants
3.4. Mechanisms of Repair from Photodamage
3.4.1. DNA Repair
Photoreactivation
Dark Repairs
3.4.2. Other Mechanisms of Repair and Defence
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Antarctic Circumpolar Current |
| ACCE | Antarctic Climate Change and the Environment |
| APF | Antarctic Polar Front |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| CDOM | Coloured dissolved organic matter |
| CFCs | Chlorofluorocarbons |
| Chl | Chlorophyll |
| CPDs | Cyclobutane pyrimidine dimers |
| DOM | Dissolved organic matter |
| EPS | Extracellular polysaccharides |
| GHG | Greenhouse gases |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| HPLC | High pressure liquid chromatography |
| MAAs | Mycosporine-like amino acids |
| MDT | Marine-derived tocopherol |
| my | Million of years |
| mya | Million of years ago |
| NASA | National Aeronautics and Space Administration |
| NER | Nucleotide excision repair |
| NOAA | National Oceanic and Atmospheric Administration |
| ODSs | Ozone depleting substances |
| PAR | Photosynthetically active radiation |
| 6-4PPs | Pyrimidine 6-4 pyrimidone photoproducts |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| UNEP | United Nations Environment Programme |
| UV, UV-R | Ultraviolet, UV radiation |
| WMO | World Meteorological Organization |
Appendix A
| Taxonomy | Compound | Reference |
|---|---|---|
| Phylum: Heterokontophyta | ||
| Class: Bacillariophyceae | ||
| Chaetoceros sp. 1 | Porphyra-334, Shinorine | [152] |
| Chaetoceros sp. 2 | Porphyra-334, Shinorine | [152] |
| Coretron cryophilum | Porphyra-334, Shinorine | [213] |
| Coscinodiscus centralis | Porphyra-334, Shinorine | [152] |
| Fragilariopsis cylindrus | Porphyra-334, Shinorine | [152,213] |
| Fragilariopsis linearis | Porphyra-334, Shinorine | [152] |
| Porosira glacialis | Porphyra-334, Shinorine | [152] |
| Porosira pseudodenticulata | Porphyra-334, Shinorine | [152] |
| Proboscia inermis | Porphyra-334, Shinorine | [152] |
| Pseudonitzschia sp. | Porphyra-334, Shinorine | [213] |
| Stellarima microtrias | Porphyra-334, Shinorine | [152] |
| Thalassiosira antarctica | Porphyra-334, Shinorine | [152] |
| Thalassiosira tumida | Porphyra-334, Shinorine | [152] |
| Thalassiosira sp. | Porphyra-334, Shinorine | [213] |
| Diatom mat (mixture of Achnantes sp., Licmophora sp., Navicula sp.) | Porphyra-334, Shinorine, Palythine | [84] |
| Phylum: Chlorophyta | ||
| Class: Chlorophyceae | ||
| Enteromorpha bulbosa | Porphyra-334 | [84] |
| Monostroma hariotii | Shinorine | [220] |
| Green algal mat (mixture of Ulothrix cf. australis, Urospora cf. penicilliformis) | Porphyra-334, Shinorine, Palythine, Asterina-330 | [84] |
| Phylum: Ochrophyta | ||
| Class: Phaeophyceae | ||
| Desmarestia menziesii | Porphyra-334 | [84] |
| Himantothallus grandifolius | Porphyra-334 | [220] |
| Phylum: Rhodophyta | ||
| Class: Rhodophyceae | ||
| Bangia atropurpurea | Porphyra-334 | [220] |
| Curdiea racovitzae | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Palythene, Palythinol | [84,220] |
| Georgiella confluens | Porphyra-334, Shinorine, Palythine | [220] |
| Gigartina skottsbergii | Porphyra-334, Shinorine, Palythine, Asterina-330 | [220] |
| Gymnogongrus antarctica | Shinorine, Palythine, Asterina-330 | [220,221] |
| Gymnogongrus turquetii | Porphyra-334, Shinorine, Mycosporine-glycine | [221,222] |
| Iridaea cordata | Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Palythene, Palythinol | [84,219,220] |
| Kallymenia antarctica | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Palythinol | [221,222] |
| Lithothamnion cf. antarcticum | Porphyra-334, Shinorine | [84] |
| Myriogramme manginii | Porphyra-334, Shinorine, Palythine, Asterina-330 | [220] |
| Neuroglossum ligulatum | Porphyra-334, Shinorine, Palythine | [220,221,222] |
| Notophycus fimbriatus | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine | [220] |
| Pachymenia orbicularis | Porphyra-334, Palythine, Mycosporine-glycine | [220] |
| Palmaria decipiens | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Palythene, Palythinol, Usujirene | [222] |
| Phyllophora antarctica | Shinorine, Palythine | [219] |
| Phyllophora appendiculata | Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Palythene | [84] |
| Plocamium cartilagineum | Porphyra-334, Shinorine, Palythine, Asterina-330, Palythinol | [220,221] |
| Porphyra endiviifolium | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Palythinol | [222] |
| Porphyra plocamiestris | Porphyra-334, Shinorine | [221] |
| Rhodymenia subantarctica | Porphyra-334 | [220] |
| Sarcothalia papillosa | Shinorine, Palythine | [220] |
| Phylum: Porifera | ||
| Class: Demospongia | ||
| Latrunculia (Latrunculia) apicalis | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine-valine | [219] |
| Cinachyra antarctica | Porphyra-334, Shinorine, Palythine | [219] |
| Tetilla leptoderma | Porphyra-334, Shinorine, Palythine | [219] |
| Haliclona (Gellius) benedeni | Shinorine, Palythine, Mycosporine-glycine-valine | [219] |
| Haliclona sp. | Palythine, Mycosporine-glycine | [219] |
| Homaxinella balfourensis | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine-valine | [219] |
| Inflatella belli | Palythine | [219] |
| Isodictya erinacea | Palythine, Palythinol | [219] |
| Kirkpatrickia variolosa | Shinorine, Palythine, Mycosporine-glycine-valine | [219] |
| Mycale (Oxymycale) acerata | Palythine | [219] |
| Sphaerotylus antarcticus | Porphyra-334 | [219] |
| Dendrilla membranosa | Porphyra-334, Shinorine, Palythine | [219] |
| Polymastia invaginata | Porphyra-334, Shinorine, Palythine | [219] |
| Unidentified Sponge #1 | Porphyra-334, Shinorine, Palythine, Palythene, Palythinol, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Unidentified Sponge #3 | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine | [84] |
| Unidentified Sponge #5 | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Unidentified Sponge #6 | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Class: Hexactinellida | ||
| Rossella nuda | Palythine | [219] |
| Rossella racovitzae | Palythine, Mycosporine-glycine | [219] |
| Phylum: Cnidaria | ||
| Class: Anthozoa | ||
| Isotealia antarctica | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Mycosporine-glycine-valine | [219] |
| Unidentified Cnidarian #1 | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine-valine | [219] |
| Phylum: Platyhelminthes | ||
| Class: Rhabditophora | ||
| Obrimoposthia wandeli | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine-valine | [84] |
| Plagiostomum n. sp. | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine-valine | [84] |
| Phylum: Nemertea | ||
| Class: Anopla | ||
| Parborlasia corrugatus | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine-valine | [84] |
| Parborlasia fueguina | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine-valine | [84] |
| Amphiporus michaelseni | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine-valine | [84] |
| Phylum: Mollusca | ||
| Class: Polyplacophora | ||
| Tonicina zschaui | Porphyra-334, Shinorine, Palythine | [84] |
| Class: Gastropoda | ||
| Margarella antarctica | Porphyra-334, Shinorine, Palythine, Asterina-330 | [84] |
| Nacella concinna | Porphyra-334, Shinorine | [407] |
| Marseniopsis mollis | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine | [219] |
| Paludestrina antarctica | Porphyra-334, Shinorine, Palythine, Asterina-330, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Trophon cf. geversianus | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Palythene, Palythinol | [84] |
| Limacina helicina | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Palythenic acid | [135] |
| Limacina helicina ssp. antarctica | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Clione antarctica | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Palythenic acid | [135] |
| Telarma antarctica | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Notaeolidia gigas | Mycosporine-glycine | [219] |
| Tritoniella belli | Shinorine | [219] |
| Mycosporine-glycine | [219] | |
| Class: Bivalvia | ||
| Limatula hodgsoni | Shinorine, Palythine | [219] |
| Limatula cf. ovalis | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Cyamium cf. commune | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Phylum: Anellida | ||
| Class: Polychaeta | ||
| Aglaophamus ornatus | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine | [84] |
| Neanthes kerguelensis | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Tomopteris carpenteri | Porphyra-334, Palythine, Mycosporine-glycine | [84] |
| Terebella ehlersi | Porphyra-334, Shinorine, Palythine, Palythene, Asterina-330, Palythinol, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Unidentified Polychaete #2 | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Class: Clitellata | ||
| Trachelobdella autralis | Porphyra-334, Shinorine, Palythine, Asterina-330, Palythene, Palythinol, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Phylum: Artropoda | ||
| Class: Crustacea | ||
| Calanus propinquus | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine | [84] |
| Euphasia superba | Porphyra-334, Shinorine, Palythine, Asterina-330, Palythene, Palythinol, Mycosporine-glycine, Mycosporine-glycine:valine, Palythenic acid | [84] |
| Cymodocella tubicauda | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Notasellus sarsii | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Glyptonotus antarcticus | Palythine | [219] |
| Bovallia gigantea | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Halirages sp. | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Jassa sp. | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Orchomene sp. | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Paraceradocus sp. | Porphyra-334, Shinorine, Palythine, Asterina-330, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Pariphimedia integricauda | Porphyra-334, Shinorine, Palythine, Asterina-330, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine, Palythinol | [84] |
| Pontogeneia sp. | Porphyra-334, Shinorine, Palythine, Asterina-330, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine, Palythinol | [84] |
| Class: Pycnogonida | ||
| Achelia spicata | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Unidentified Pycnogonid | Shinorine, Palythine | [219] |
| Phylum: Bryozoa | ||
| Class: Gymnolaemata | ||
| Beania livingstonei | Porphyra-334, Shinorine | [84] |
| Inversiula nutrix | Porphyra-334, Shinorine, Palythine, Palythene, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Phylum: Echinodermata | ||
| Class: Crinoidea | ||
| Promachocrinus kerguelensis | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine | [219] |
| Class: Asteroidea | ||
| Granaster nutrix | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Odontaster validus | Palythine | [219] |
| Mycosporine-glycine | [219] | |
| Class: Echinoidea | ||
| Sterechinus neumayeri | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine | [191,219] |
| Class: Holothuroidea | ||
| Cucumaria ferrari | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [219] |
| Ekmocucumis steineni | Porphyra-334, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine | [84] |
| Amphioplus affinis | Palythine | [84] |
| Phylum: Chordata | ||
| Class: Ascidiacea | ||
| Cnemidocarpa verrucosa | Porphyra-334, Shinorine, Palythine | [219] |
| Molgula enodis | Porphyra-334, Shinorine, Palythine, Asterina-330, Mycosporine-glycine, Mycosporine-glycine:valine, Palythene | [84] |
| Unidentified ascidia | Shinorine, Mycosporine-glycine | [219] |
| Class: Actinopterygii | ||
| Unidentified ice-fish larvae | Porphyra-334, Shinorine, Palythine, Mycosporine-glycine, Mycosporine-glycine:valine, Palythene | [84] |
| Trematomus bernacchii | Shinorine, palythine | [219] |
| Taxonomy | Compound | Reference |
|---|---|---|
| Phylum: Porifera | ||
| Class: Demospongia | ||
| Isodictya erinacea | Erebusinone | [228] |
| Phylum: Proteobacteria | ||
| Class: Gammaproteobacteria | ||
| Alteromonas stellipolaris sp. nov. | Melanin | [241] |
| Phylum: Rhodophyta | ||
| Class: Florideophyceae | ||
| Palmaria decipiens | UV-adsorbing pigment | [245] |
| Phylum: Chlorophyta | ||
| Class: Ulvophyceae | ||
| Enteromorpha bulbosa | UV-adsorbing pigment | [245] |
| Phylum: Ochrophyta | ||
| Class: Phaeophyceae | ||
| Adenocystis utricularis | Phlorotannins | [266] |
| Ascoseira mirabilis | Phlorotannins | [266] |
| Chordaria linearis | Phlorotannins | [266] |
| Cystosphaera jacquinotii | Phlorotannins | [266] |
| Desmarestia anceps | Phlorotannins | [266,408,409] |
| Desmarestia antarctica | Phlorotannins | [266] |
| Desmarestia menziesii | Phlorotannins | [266,408,409] |
| Himantothallus grandifolius | Phlorotannins | [266] |
| Phaeurus antarcticus | Phlorotannins | [266] |
| Taxonomy | Compound | Reference |
|---|---|---|
| Phylum: Bacteroidetes | ||
| Class: Flavobacteria | ||
| Antarcticimonas flava | Carotenoids | [298] |
| Muricauda antarctica | Carotenoids | [299] |
| Phylum: Proteobacteria | ||
| Class: Gammaproteobacteria | ||
| Pseudoalteromonas haloplanktis TAC125 | Enzymatic antioxidant defence | [338] |
| Phylum: Cyanobacteria | ||
| Class: Hormogoneae | ||
| Nostoc sp. | Carotenoids | [294] |
| Nostoc sp. | Carotenoids | [295] |
| Nostoc commune | Carotenoids | [296] |
| Nostoc sp. | Carotenoids | [301] |
| Class: Cyanophyceae | ||
| Anabaena sp. | Carotenoids | [301] |
| Phormidium sp. | Carotenoids | [301] |
| Oscillatoria sp. | Carotenoids | [295] |
| Phylum: Bacillariophyta | ||
| Class: Mediophyceae | ||
| Thalassiosira antarctica | Diadinoxantin, diatoxanthin | [293] |
| Thalassiosira weissflogii | Diadinoxantin, diatoxanthin | [293] |
| Thalassiosira sp. | α-tocopherol | [312] |
| Chaetoceros brevis | SOD | [331] |
| Phylum: Euglenozoa | ||
| Class: Euglenophyceae | ||
| Eutreptiella sp. | Xanthophylls | [292] |
| Phylum: Miozoa | ||
| Class: Dinophyceae | ||
| Polarella glacialis | Xanthophylls | [291] |
| Phylum: Chlorophyta | ||
| Class: Ulvophyceae | ||
| Enteromorpha bulbosa | UV-absorbing pigments | [245] |
| Class: Trebouxiophyceae | ||
| Chlorella sp. | Ascorbic acid | [322] |
| Class: Chlorophyceae | ||
| Chlamydomonas sp. ICE-L | SOD, CAT, peroxidase | [105] |
| Phylum: Rhodophyta | ||
| Class: Florideophyceae | ||
| Palmaria decipiens | UV-absorbing pigments | [245] |
| Leptosomia simplex | Carotenoids | [410] |
| Delesseria lancifolia | Violaxanthin, antheraxanthin, zeaxanthin | [302] |
| Phylum: Cnidaria | ||
| Class: Hydrozoa | ||
| Errina antarctica | Astaxanthin | [307] |
| Phylum: Mollusca | ||
| Class: Bivalvia | ||
| Laternula elliptica | α-tocopherol | [313] |
| Enzymatic antioxidant defence | [332] | |
| Phylum: Arthropoda | ||
| Class: Malacostraca | ||
| Euphausia superba | Astaxantin | [217] |
| Astaxanthin | [305] | |
| Vitamin A | [305] | |
| Vitamin E | [305] | |
| α-tocomonoenol | [317] | |
| Phylum: Chordata | ||
| Class: Chondrichthyes | ||
| Raja georgiana | Carotenoids | [306] |
| Class: Actinopterygii | ||
| Muraenolepis microps | Carotenoids | [306] |
| Dissostichus eleginoides | Carotenoids | [306] |
| Notothenia gibberifrons | Carotenoids | [306] |
| Notothenia rossi-marmorata | Carotenoids | [306] |
| Trematomus hansoni | Carotenoids | [306] |
| Chaenocephalus aceratus | Carotenoids | [306] |
| Champsocephalus gunnari | Carotenoids | [306] |
| Pseudochaenichthys georgianus | Carotenoids | [306] |
| Pagothenia borchgrevinki | Vitamin E | [316] |
| Trematomus bernacchii | Vitamin E | [316] |
| Champsocephalus gunnari | α-tocomonoenol | [317] |
| Gobionotothen gibberifrons | α-tocomonoenol | [317] |
| Chaenocephalus aceratus | α-tocomonoenol | [317] |
| Antarctic notothenioid fishes | Enzymatic antioxidant defence | [333,334,335,336,337] |
| Taxonomy | Compound | Activity | Reference |
|---|---|---|---|
| Phylum: Proteobacteria, Bacteroidetes | |||
| Class: Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Flavobacteriia | |||
| Pseudomonas, Janthinobacterium, Flavobacterium, Hymenobacter, Sphingomonas | Photolyase | DNA repair | [3,355] |
| Phylum: Ciliophora | |||
| Class: Spirotrichea | |||
| Euplotes focardii | Heat shock protein, Hsp70 | Chaperone in the UV stress | [382] |
| Phylum: Chlorophyta | |||
| Class: Chlorophyceae | |||
| Chlamydomonas sp. ICE-L | Photolyase | DNA repair | [356] |
| Heat shock protein, Hsp70 | Chaperone in the UV stress | [381] | |
| Phylum: Ochrophyta | |||
| Class: Bacillariophyceae | |||
| Chaetoceros neglectus, Corethron cryophilum, Coscinodiscus ocutus-iridis, Odontella weissflogii, Porosira pseudodenticulata, Thalassiosira subtilis, Eucampia antarctica, Chaetoceros convolutus, Chaetocerois socialis, Thalassiosira australis, Licmophora decora, Nitzchia kerguelensis | Photolyase/NER | DNA repair | [96] |
| Phylum: Echinodermata | |||
| Class: Echinoidea | |||
| Sterechinus neumayeri | Photolyase | DNA repair | [359] |
| Phylum: Arthropoda | |||
| Class: Crustacea | |||
| Euphausia superba | Photolyase | DNA repair | [103] |
| Phylum: Chordata | |||
| Class: Actinopterygii | |||
| Chaenocephalus aceratus, Notothenia coriiceps | Photolyase | DNA repair | [103] |
| Organismal assemblages | |||
| Antarctic marine bacterioplankton communities | RecA protein | DNA repair | [367] |
| Antarctic phytoplankton communities | Photosystem II (PSII) repair cycle | de novo synthesis | [368] |
References
- Häder, D.P.; Helbling, E.W.; Williamson, C.E.; Worrest, R.C. Effects of UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2011, 10, 242–260. [Google Scholar] [CrossRef] [PubMed]
- Karentz, D.; Bosch, I. Influence of Ozone-Related Increases in Ultraviolet Radiation on Antarctic Marine Organisms. Am. Zool. 2001, 41, 3–16. [Google Scholar] [CrossRef]
- Karentz, D. Ultraviolet tolerance mechanisms in Antarctic marine organisms. Ultrav. Radiat. Antarct. Meas. Boil. Eff. 1994, 62, 93–110. [Google Scholar]
- Eastman, J.T. Antarctic notothenioid fishes as subjects for research in evolutionary biology. Antarct. Sci. 2000, 12, 276–287. [Google Scholar] [CrossRef]
- Rogers, A.D. Evolution and biodiversity of Antarctic organisms: A molecular perspective. Philos. Trans. R. Soc. B 2007, 362, 2191–2214. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.T. The nature of the diversity of Antarctic fishes. Pol. Biol. 2005, 28, 94–107. [Google Scholar] [CrossRef]
- Barbante, C.; Fischer, H.; Masson-Delmotte, V.; Waelbroeck, C.; Wolff, E.W. Climate of the last million years: New insights from EPICA and other records. Quat. Sci. Rev. 2010, 29, 1–7. [Google Scholar] [CrossRef]
- Anderson, J.B.; Shipp, S.S.; Lowe, A.L.; Smith Wellner, J.; Mosola, A.B. The Antarctic ice sheet during the Last Glacial Maximum and its subsequent retreat history: A review. Quat. Sci. Rev. 2002, 21, 49–70. [Google Scholar] [CrossRef]
- Sura, P.; Gille, S.T. Stochastic dynamics of sea surface height variability. J. Phys. Oceanogr. 2010, 40, 1582–1596. [Google Scholar] [CrossRef]
- Convey, P.; Bindschadler, R.; di Prisco, G.; Fahrbach, E.; Gutt, J.; Hodgson, D.A.; Mayewski, P.A.; Summerhayes, C.P.; Turner, J.; the ACCE Consortium. Antarctic climate change and the environment. Antarct. Sci. 2009, 21, 541–563. [Google Scholar] [CrossRef]
- Littlepage, J.L. Oceanographic Observations in McMurdo Sound, Antarctica. In Biology of the Antarctic Seas; Llano, G.A., Ed.; American Geophysical Union: Washington, DC, USA, 1965; pp. 1–37. [Google Scholar]
- DeWitt, H.H. The character of the midwater fish fauna of the Ross Sea, Antarctica. In Antarctic Ecology; Holdgate, M.W., Ed.; Academic Press: London, UK, 1970; pp. 305–314. [Google Scholar]
- Hunt, B.M.; Hoefling, K.; Cheng, C.H.C. Annual warming episodes in seawater temperatures in McMurdo Sound in relationship to endogenous ice in notothenioid fish. Antarct. Sci. 2003, 15, 333–338. [Google Scholar] [CrossRef]
- Gordon, A.L. Oceanography of Antarctic waters. Antarct. Res. Ser. 1971, 15, 169–203. [Google Scholar]
- Eastman, J.T. Antarctic Fish Biology: Evolution in a Unique Environment; Academic Press: San Diego, CA, USA, 1993; p. 322. [Google Scholar]
- Thatje, S.; Hillenbrand, C.D.; Mackensen, A.; Larter, R. Life hung by a thread: Endurance of Antarctic fauna in glacial periods. Ecology 2008, 89, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Census of Marine Life (CAML). Available online: http://www.coml.org/census-antarctic-marine-life-caml/ (accessed on 15 May 2018).
- Beers, J.M.; Jayasundara, N. Antarctic notothenioid fish: What are the future consequences of losses and gains acquired during long-term evolution at cold and stable temperatures? J. Exp. Biol. 2015, 218, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Clarke, A. Antarctic marine biology. Curr. Biol. 2011, 21, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Arntz, W.; Thatje, S.; Gerdes, D.; Gili, J.M.; Gutt, J.; Jacob, U.; Montiel, A.; Orejas, C.; Teixido, N. The Antarctic-Magellan connection: Macrobenthos ecology on the shelf and upper slope, a progress report. Sci. Mar. 2005, 69, 237–269. [Google Scholar] [CrossRef]
- Aronson, R.B.; Thatje, S.; Clarke, A.; Peck, L.S.; Blake, D.B.; Wilga, C.D.; Seibel, B.A. Climate change and invasibility of the antarctic benthos. Ann. Rev. Ecol. Evol. Syst. 2007, 38. [Google Scholar] [CrossRef]
- Peck, L.S.; Convey, P.; Barnes, D.K.A. Environmental constraints on life histories in Antarctic ecosystems: Tempos, timings and predictability. Biol. Rev. 2006, 81, 75–109. [Google Scholar] [CrossRef] [PubMed]
- C.I.E. Standardization of the Terms UV-A1, UV-A2 and UV-B. Commission Internationale de l’Eclairage (International Commission on Illumination) Collection in Photobiology and Photochemistry 134-1999: TC 6-26. 1999. Available online: http://www.cie.co.at/publications/cie-collection-photobiology-photochemistry-1999/ (accessed on 23 August 2018).
- Cordero, R.R.; Damiani, A.; Ferrer, J.; Jorquera, J.; Tobar, M.; Labbe, F.; Carrasco, J.; Laroze, D. UV irradiance and albedo at Union Glacier Camp (Antarctica): A case study. PLoS ONE 2014, 9, e90705. [Google Scholar] [CrossRef] [PubMed]
- Tedetti, M.; Sempéré, R. Penetration of Ultraviolet Radiation in the marine environment. A Review. Photochem. Photobiol. 2006, 82, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, A.T.; Lesser, M.P. Effects of solar ultraviolet radiation on coral reef organisms. Photochem. Photobiol. Sci. 2009, 8, 1276–1294. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, P.; Ramírez, J.; Gómez, I.; Ban, S. Underwater Optics in Sub-Antarctic and Antarctic Coastal Ecosystems. PLoS ONE 2016, 11, e0154887. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Prézelin, B.B.; Baker, K.S.; Bidigare, R.R.; Boucher, N.P.; Coley, T.L.; Karentz, D.; Macintyre, S.; Matlick, H.A.; Menzies, D.; et al. Ozone depletion: Ultraviolet radiation and phytoplankton biology in Antarctic waters. Science 1992, 255, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P.; Lamare, M.D.; Barker, M.F. Transmission of ultraviolet radiation through the Antarctic annual sea ice and its biological effects on sea urchin embryos. Limnol. Oceanogr. 2004, 49, 1957–1963. [Google Scholar] [CrossRef]
- Clark, G.F.; Stark, J.S.; Johnston, E.L.; Runcie, J.W.; Goldsworthy, P.M.; Raymond, B.; Riddle, M.J. Light-driven tipping points in polar ecosystems. Glob. Chang. Biol. 2013, 19, 3749–3761. [Google Scholar] [CrossRef] [PubMed]
- McMinn, A.; Martin, A. Dark Survival in a Warming World. Proc. R. Soc. B Biol. Sci. 2013, 280, 1755. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Tarling, G.A. Polar oceans in a changing climate. Curr. Biol. 2017, 27, R431–R510. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.L. Oceanography-the brawniest retroflection. Nature 2003, 421, 904–905. [Google Scholar] [CrossRef] [PubMed]
- Smale, D.A. Ice disturbance intensity structures benthic communities in nearshore Antarctic waters. Mar. Ecol. Prog. Ser. 2007, 349, 89–102. [Google Scholar] [CrossRef]
- Constable, A.J.; Melbourne-Thomas, J.; Corney, S.P.; Arrigo, K.R.; Barbraud, C.; Barnes, D.K.A.; Bindoff, N.L.; Boyd, P.W.; Brandt, A.; Costa, D.P.; et al. Climate change and Southern Ocean ecosystems I: How changes in physical habitats directly affect marine biota. Glob. Chang. Biol. 2014, 20, 3004–3025. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.F.; Stark, J.S.; Palmer, A.S.; Riddle, M.J.; Johnston, E.L. The Roles of Sea-Ice, Light and Sedimentation in Structuring Shallow Antarctic Benthic Communities. PLoS ONE 2017, 12, e0168391. [Google Scholar] [CrossRef] [PubMed]
- Smetacek, V.; Nicol, S. Polar ocean ecosystems in a changing world. Nature 2005, 437, 362–368. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.W.; Ambrose, W.G.J.; Johnson, B.J.; Sun, M.; Lopez, G.R.; Clough, L.M.; Carrol, M.L. Benthic community response to ice algae and phytoplankton in Ny Alesund, Svalbard. Mar. Ecol. Prog. Ser. 2006, 310, 1–14. [Google Scholar] [CrossRef]
- Gutt, J.; Isla, E.; Bertler, A.N.; Bodeker, G.E.; Bracegirdle, T.J.; Cavanagh, R.D.; Comiso, J.C.; Convey, P.; Cummings, V.; De Conto, R.; et al. Cross-disciplinarity in the advance of Antarctic ecosystem research. Mar. Gen. 2018. [Google Scholar] [CrossRef] [PubMed]
- Massom, R.A.; Eicken, H.; Hass, C.; Jeffries, M.O.; Drinkwater, M.R.; Sturm, M.; Worby, A.P.; Wu, X.; Lytle, V.I.; Ushio, S.; et al. Snow on Antarctic Sea ice. Rev. Geophys. 2001, 39, 413–445. [Google Scholar] [CrossRef]
- Buckley, R.; Trodahl, H. Scattering and absorption of visible light by sea ice. Nature 1987, 326, 867–869. [Google Scholar] [CrossRef]
- Arndt, S.; Meiners, K.M.; Ricker, R.; Krumpen, T.; Katlein, C.; Nicolaus, M. Influence of snow depth and surface flooding on light transmission through Antarctic pack ice. J. Geophys. Res. Oceans 2017, 122, 2108–2119. [Google Scholar] [CrossRef]
- Brierley, A.S.; Thomas, D.N. Ecology of southern ice pack. Adv. Mar. Biol. 2002, 43, 171–276. [Google Scholar] [PubMed]
- Smith, W., Jr.; Comiso, J.C. The influence of sea ice on primary production in the Southern Ocean: A satellite perspective. J. Geophys. Res. 2008, 113, C05S93. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Bindschadler, R.; Convey, P.; di Prisco, G.; Fahrbach, E.; Gutt, J.; Hodgson, D.; Mayewski, P.; Summerhayes, C. Antarctic Climate Change and the Environment; SCAR & Scott Polar Research Institute: Cambridge, UK, 2009; pp. 1–526. [Google Scholar]
- Holland, M.M.; Landrum, L.; Raphael, M.; Stammerjohn, S. Springtime winds drive Ross Sea ice variability and change in the following autumn. Nat. Commun. 2017, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Crampton, J.S.; Cody, R.D.; Levy, R.; Harwood, D.; McKay, R.; Naish, T.R. Southern Ocean phytoplankton turnover in response to stepwise Antarctic cooling over the past 15 million years. Proc. Natl. Acad. Sci. USA 2016, 113, 6868–6873. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Lu, H.; White, I.; King, J.C.; Phillips, T.; Hosking, J.S.; Bracegirdle, T.J.; Marshall, G.J.; Mulvaney, R.; Deb, P. Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 2016, 535, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Murphy, E.J.; Meredith, M.P.; King, J.C.; Peck, L.S.; Barnes, D.K.A.; Smith, R.C. Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.J.; Miller, S.L. Oceanic protection of prebiotic organic compounds from UV radiation. Proc. Natl. Acad. Sci. USA 1998, 95, 7260–7263. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.D. Out of Thin Air: Dinosaurs, Birds, and Earth’s Ancient Atmosphere; Joseph Henry Press: Washington, DC, USA, 2006; pp. 1–296. [Google Scholar]
- Scientific Assessment of Ozone Depletion: 2010. WMO Global Ozone Research and Monitoring Project—Report No. 52. World Meteorological Organization: Geneva, Switzerland. Available online: https://www.wmo.int/pages/prog/arep/gaw/ozone_2010/documents/Ozone-Assessment-2010-complete.pdf (accessed on 5 June 2018).
- Molina, M.J.; Rowland, F.S. Stratospheric sink for chlorofluoromethanes: Chlorine atom-catalysed destruction of ozone. Nature 1974, 249, 810–812. [Google Scholar] [CrossRef]
- Manney, G.L.; Santee, M.L.; Rex, M.; Livesey, N.J.; Pitts, M.C.; Veefkind, P.; Nash, E.R.; Wohltmann, I.; Lehmann, R.; Froidevaux, L.; et al. Unprecedented Arctic ozone loss in 2011. Nature 2011, 478, 467–475. [Google Scholar] [CrossRef] [PubMed]
- National Oceanic and Atmospheric Administration NOAA, USA. Available online: http://www.ozonelayer.noaa.gov/data/antarctic.htm (accessed on 18 May 2018).
- Farman, J.; Gardiner, B.; Shanklin, J. Large losses of total ozone in Antarctica reveal seasonal CIO/NO Interaction. Nature 1985, 315, 207–210. [Google Scholar] [CrossRef]
- Robinson, S.A.; Erickson III, D.J. Not just about sunburn—The ozone hole’s profound effect on climate has significant implications for Southern Hemisphere ecosystems. Glob. Chang. Biol. 2015, 21, 515–527. [Google Scholar] [CrossRef] [PubMed]
- National Aeronautics and Space Administration (NASA). Available online: https://www.nasa.gov/content/goddard/2014-antarctic-ozone-hole-holds-steady/ (accessed on 23 April 2018).
- United Nations Environment Programme (UNEP). Available online: http://ozone.unep.org/ (accessed on 23 April 2018). update 2014.
- Velders, G.J.M.; Andersen, S.O.; Daniel, J.S.; Fahey, D.W.; McFarland, M. The importance of the Montreal Protocol in protecting climate. Proc. Natl. Acad. Sci. USA 2007, 104, 4814–4819. [Google Scholar] [CrossRef] [PubMed]
- Estrada, F.; Perron, P.; Martínez-López, B. Statistically derived contributions of diverse human influences to twentieth-century temperature changes. Nat. Geosci. 2013, 6, 1–6. [Google Scholar] [CrossRef]
- Kuttippurath, J.; Nair, P.J. The signs of Antarctic ozone hole recovery. Sci. Rep. 2017, 7, 585. [Google Scholar] [CrossRef] [PubMed]
- Portmann, R.W.; Daniel, J.S.; Ravishankara, A.R. Stratospheric ozone depletion due to nitrous oxide: Influences of other gases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.P.; Williamson, C.E.; Wängberg, S.A.; Rautio, M.; Rose, K.C.; Gao, K.; Helbling, E.W.; Sinhah, R.P.; Worresti, R. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem. Photobiol. Sci. 2015, 14, 108–126. [Google Scholar]
- United Nations Environment Programme (UNEP). Environmental effects of ozone depletion and its interactions with climate change: Progress report 2016. Environmental Effects Assessment Panel. Photochem. Photobiol. Sci. 2017, 16, 107–145. [Google Scholar] [CrossRef] [PubMed]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, Z.; Stambler, N. Coral Reefs: An. Ecosystem in Transition; Springer: Dordrecht, The Netherlands, 2011; p. 552. [Google Scholar]
- Marbà, N.; Jordà, G.; Agustí, S.; Girard, C.; Duarte, C.M. Footprints of climate change on Mediterranean Sea biota. Front. Mar. Sci. 2015, 2, 56. [Google Scholar] [CrossRef]
- Meredith, M.; King, J. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Peck, L.S. Prospects for surviving climate change in Antarctic aquatic species. Front. Zool. 2005, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Peck, L.S.; Conway, L.Z. The myth of metabolic cold adaptation: Oxygen consumption in stenothermal Antarctic bivalves. In The Evolutionary Biology of the Bivalvia; Harper, E.M., Taylor, J.D., Crame, J.A., Eds.; Special Publication; Geological Society: London, UK, 2000; pp. 441–445. [Google Scholar]
- Peck, L.S.; Morley, S.A.; Richard, J.; Clark, M.S. Acclimation and thermal tolerance in Antarctic marine ectotherms. J. Exp. Biol. 2014, 217, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Barrand, N.E.; Bracegirdle, T.J.; Convey, P.; Hodgson, D.A.; Jarvis, M.; Jenkins, A.; Marshall, G.; Meredith, M.P.; Roscoe, H.; et al. Antarctic climate change and the environment: An update. Polar Rec. 2014, 50, 237–259. [Google Scholar] [CrossRef]
- Oliva, M.; Navarro, F.; Hrbáček, F.; Hernández, A.; Nývlt, D.; Pereira, P.; Ruiz-Fernández, J.; Trigo, R. Recent regional climate cooling on the Antarctic Peninsula and associated impacts on the cryosphere. Sci. Total Environ. 2017, 580, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Bednaršek, N.; Tarling, G.A.; Bakker, D.C.E.; Fielding, Z.S.; Jones, E.M.; Venables, H.J.; Ward, P.; Kuzirian, A.; Lézé, B.; Feely, R.A.; et al. Extensive dissolution of live pteropods in the Southern Ocean. Nat. Geosci. 2012, 5, 881–885. [Google Scholar] [CrossRef]
- Margalef, R. Ecología; Ediciones Omega: Barcelona, Spain, 1998; pp. 1–968. [Google Scholar]
- Diffey, B.L. Solar ultraviolet radiation effects on biological systems. Phys. Med. B 1991, 36, 299–328. [Google Scholar] [CrossRef]
- Leech, D.M.; Johnsen, S. Behavioral responses: UV avoidance and vision. In UV Effects in Aquatic Organisms and Ecosystems; Helbling, H., Zagarese, H., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 455–481. [Google Scholar]
- Valiela, I. Marine Ecological Processes; Springer Advanced Texts in Life Sciences; Springer: New York, NY, USA, 1984; pp. 1–702. [Google Scholar]
- Franklin, L.A.; Osmond, C.B.; Larkum, A.W.D. Photoinhibition, UV-B and algal photosynthesis. In Photosynthesis in algae; Larkum, A.W., Douglas, S.E., Raven, J.A., Eds.; Springer: Kluwer, The Netherlands, 2003; pp. 351–384. [Google Scholar]
- Levinton, J.S. Marine Biology: Function, Biodiversity, Ecology; Oxford University Press: Oxford, UK, 2009; pp. 1–588. [Google Scholar]
- Kingsley, R.J.; Corcoran, M.L.; Krider, K.L.; Kriechbaum, K.L. Thyroxine and vitamin D in the gorgonian Leptogorgia virgulata. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 129, 897–907. [Google Scholar] [CrossRef]
- Karentz, D.; McEue, F.S.; Land, M.C.; Dunlap, W.C. Survey of mycosporine like amino acid compounds in Antarctic marine organisms: Potential protection from ultraviolet exposure. Mar. Biol. 1991, 108, 157–166. [Google Scholar] [CrossRef]
- Leech, D.M.; Johnsen, S. Light, Biological Receptors. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Elsevier: Oxford, UK, 2009; pp. 671–681. [Google Scholar]
- Biscontin, A.; Frigato, E.; Sales, G.; Mazzotta, G.M.; Teschke, M.; De Pittà, C.; Jarman, S.; Meyer, B.; Costa, R.; Bertolucci, C. The opsin repertoire of the Antarctic krill Euphausia superba. Mar. Gen. 2016, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Lemonovich, T.L.; Salata, R.A. An update on the association of vitamin D deficiency with common infectious diseases. Can. J. Physiol. Pharmacol. 2015, 93, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Hume, E.M.; Lucas, N.S.; Smith, H.H. On the Absorption of Vitamin D from the Skin. Biochem. J. 1927, 21, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.F.; Neale, P.J. Mechanisms of UV damage to aquatic organisms. In The Effects of UV Radiation in the Marine Environment; de Mora, S., Demers, S., Vernet, M., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 149–176. [Google Scholar]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, R.M. UV-A (320–380 nm) as an oxidative stress. In Oxidative Stress: Oxidants and Antioxidants; Sies, H., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 57–83. [Google Scholar]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Bischof, K.; Krábs, G.; Wiencke, C.; Hanelt, D. Solar ultraviolet radiation affects the activity of ribulose-1,5-bisphosphate carboxylase-oxygenase and the composition of photosynthetic and xanthophyll cycle pigments in the intertidal green alga Ulva lactuca L. Planta 2002, 215, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Orostegui, M.; Huovinen, P. Morpho-functional patterns of photosynthesis in the South Pacific kelp Lessonia nigrescens: Effects of UV radiation on 14C fixation and primary photochemical reactions. J. Phycol. 2007, 43, 55–64. [Google Scholar] [CrossRef]
- Dring, M.J.; Wagner, A.; Boeskov, J.; Lüning, K. Sensitivity of intertidal and subtidal red algae to UV-A and UV-B radiation, as monitored by chlorophyll fluorescence measurements: Influence of collection depth, and season, and length of irradiance. Eur. J. Phycol. 1996, 31, 293–302. [Google Scholar] [CrossRef]
- Karentz, D.; Cleaver, J.E.; Mitchell, D.L. Cell survival characteristics and molecular responses of Antarctic phytoplankton to Ultraviolet-B radiation. J. Phycol. 1991, 27, 326–341. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Survival and sex ratios of the intertidal copepod, Tigriopus californicus, following ultraviolet-B (290–320 nm) radiation exposure. Mar. Biol. 1995, 123, 799–804. [Google Scholar] [CrossRef]
- Alonso Rodríguez, C.; Browman, H.I.; Runge, J.A.; St-Pierre, J.F. Impact of solar ultraviolet radiation on hatching of a marine copepod, Calanus finmarchicus. Mar. Ecol. Progr. Ser. 2000, 193, 85–93. [Google Scholar] [CrossRef]
- Hader, D.P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Aquatic ecosystems: Effects of solar ultraviolet radiation and interactions with other climatic change factors. Photochem. Photobiol. Sci. 2003, 2, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Nahon, S.; Pruski, A.M.; Duchene, J.C.; Mejanelle, L.; Vetion, G.; Desmalades, M.; Charles, F. Can UV radiation affect benthic deposit-feeders through biochemical alteration of food resources? An experimental study with juveniles of the benthic polychaete Eupolymnia nebulosa. Mar. Environ. Res. 2011, 71, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P.; Kruse, V.A.; Barry, T.M. Exposure to ultraviolet radiation causes apoptosis in developing sea urchin embryos. J. Exp. Biol. 2003, 206, 4097–4103. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, M.F. Harmful effects of UV radiation in Algae and aquatic macrophytes—A review. Emir. J. Food Agric. 2012, 24, 510–526. [Google Scholar] [CrossRef]
- Malloy, K.D.; Holman, M.A.; Mitchell, D.; Detrich, H.W., III. Solar UV-B-induced DNA damage and photoenzymatic DNA repair in antarctic zooplankton. Proc. Natl. Acad. Sci. USA 1997, 94, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Helbling, E.W.; Marguet, E.R.; Villafañe, V.E.; Holm-Hansen, O. Bacterioplankton viability in Antarctic waters as affected by solar ultraviolet radiation. Mar. Ecol. Prog. Ser. 1995, 126, 293–298. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, Y.; Miao, J.; Li, G. Effect of UV-B radiation on the growth and antioxidant enzymes of Antarctic sea ice microalgae Chlamydomonas sp. ICE-L. Acta Physiol. Plant. 2009, 31, 1097–1102. [Google Scholar] [CrossRef]
- Zacher, K. The susceptibility of spores and propagules of Antarctic seaweeds to UV and photosynthetically active radiation—Field versus laboratory experiments. J. Exp. Mar. Biol. Ecol. 2014, 458, 57–63. [Google Scholar] [CrossRef]
- Zacher, K.; Roleda, M.Y.; Hanelt, D.; Wiencke, C. UV effects on photosynthesis and DNA in propagules of three Antarctic seaweeds (Adenocystis utricularis, Monostroma hariotii and Porphyra endiviifolium). Planta 2007, 225, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.F.; Quesada, A. Ultraviolet Radiation Effects on Cyanobacteria: Implications for Antarctic Microbial Ecosystems. In Ultraviolet Radiation in Antarctica: Measurements and Biological Effects; Weiler, C.S., Penhale, P.A., Eds.; Washington Geophysical Union: Washington, DC, USA, 1994; pp. 111–124. [Google Scholar]
- Nadeau, T.L.; Howard-Williams, C.; Castenholz, R.W. Effects of solar UV and visible irradiance on photosynthesis and vertical migration of Oscillatoria sp. (Cyanobacteria) in an Antarctic microbial mat. Aquat. Microb. Ecol. 1999, 20, 231–239. [Google Scholar] [CrossRef]
- Van Den Broecke, L.; Martens, K.; Pieri, V.; Schön, I. Ostracod valves as efficient UV protection. J. Limnol. 2012, 71. [Google Scholar] [CrossRef]
- Rawlings, T.A. Adaptations to Physical Stresses in the Intertidal Zone: The Egg Capsules of Neogastropod Molluscs. Am. Zool. 1999, 39, 230–243. [Google Scholar] [CrossRef]
- Wraith, J.; Przeslawski, R.; Davis, A.R. UV-Induced Mortality in Encapsulated Intertidal Embryos: Are Mycosporine-Like Amino Acids an Effective Sunscreen? J. Chem. Ecol. 2006, 32, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Molina, F.J.; Cubillos, V.M.; Montory, J.A.; Andrade-Villagrán, P.A. Are embryonic developing modes determinant in the acquisition and levels of photoprotective compounds in slipper limpets of the Crepipatella genus? J. Photochem. Photobiol. B Biol. 2016, 162, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Hirabayashi, S.; Hori, K.; Kasai, F.; Watanabe, M.M. UV protection in the photosymbiotic ascidian Didemnum molle inhabiting different depths. Zool. Sci. 2006, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hirose, E.; Ohtsuka, K.; Ishikura, M.; Maruyama, T. Ultraviolet absorption in ascidian tunic and ascidian-Prochloron simbiosis. J. Mar. Biol. Assoc. UK 2004, 84, 789–794. [Google Scholar] [CrossRef]
- Marchant, H.J.; Davidson, A.T.; Kelly, G.J. UV-B protecting compounds in the marine alga Phaeocystis pouchetii from Antarctica. Mar. Biol. 1991, 109, 391–395. [Google Scholar] [CrossRef]
- Drollet, J.H.; Glaziou, P.; Martin, P.M.V. A study of mucus from the solitary coral Fungia fungites (Scleractinia: Fungiidae) in relation to photobiological UV adaptation. Mar. Biol. 1993, 115, 263–266. [Google Scholar] [CrossRef]
- Banaszak, A.T. Photoprotective physiological and biochemical responses of aquatic organisms. In UV Effects in Aquatic Organisms and Ecosystems; Helbling, E.W., Zagarese, H., Eds.; Royal Society of Chemistry: Cambridge, UK, 2003; pp. 329–356. [Google Scholar]
- Mandal, S.; Rath, J. Extremophilic cyanobacteria for novel drug development. In Springer Brief in Pharmaceutical Science & Drug Development; Springer: Basel, Switzerland, 2014; pp. 1–89. ISBN 978-3-319-12009-6. [Google Scholar]
- de los Ríos, A.; Ascaso, C.; Wierzchos, J.; Fernández-Valiente, E.; Quesada, A. Microstructural Characterization of Cyanobacterial Mats from the McMurdo Ice Shelf, Antarctica. Appl. Environ. Microbiol. 2004, 70, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Mancuso Nichols, C.A.; Garon, S.; Bowman, J.P.; Raguénès, G.; Guézennec, J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 2004, 96, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Extracellular polymeric substances with metal adsorption capacity produced by Pseudoalteromonas sp. MER144 from Antarctic seawater. Environ. Sci. Pollut. Res. Int. 2018, 25, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Komenda, J.; Kopecky, J.; Nedbal, L. Strategies of ultraviolet-B protection in microscopic algae. Physiol. Plant. 1997, 100, 378–388. [Google Scholar] [CrossRef]
- Rozema, J.; Noordjik, A.J.; Broekman, R.A.; van Beem, A.; Meijkamp, B.M.; de Bakker, N.V.J.; van de Staaij, J.W.M.; Stroetenga, M.; Bohncke, S.J.P.; Konert, M.; et al. (Poly)phenolic compounds in pollen and spores of Antarctic plants as indicators of UV-B: A new proxy for the reconstruction of past solar UV-B? Plant. Ecol. 2001, 154, 11–26. [Google Scholar]
- Banaszak, A.T.; Trench, R.K. Effects of ultraviolet (UV) radiation on marine microalgal-invertebrate symbioses. I.Response, of the algal symbionts in culture and in hospite. J. Exp. Mar. Biol. Ecol. 1995, 194, 213–232. [Google Scholar] [CrossRef]
- Taira, H.; Goes, J.I.; Gomes, H.R.; Yabe, K.; Taguchi, S. Photoinduction of mycosporine-like amino acids and cell volume increases by ultraviolet radiation in the marine dinoflagellate Scrippsiella sweeneyae. Plankton Biol. Ecol. 2004, 51, 82–94. [Google Scholar]
- Dunlap, W.C. Sunscreens, oxidative stress and antioxidant functions in marine organisms of the Great Barrier Reef. Redox Rep. 1999, 4, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.P. Ultraviolet absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J. Photochem. Photobiol. B 1998, 47, 83–94. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C.; Buettner, G.R. Ultraviolet (UV) protection in marine organisms II. Biosynthesis, accumulation, and sunscreening function of mycosporine-like amino acids. In Free Radicals in Chemistry, Biology and Medicine; OICA International: London, UK, 2000; pp. 215–228. [Google Scholar]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed]
- Trione, E.J.; Leach, C.M.; Mutch, J.T. Sporogenic substances isolated from fungi. Nature 1966, 212, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Karentz, D.; Hedges, J.I. Mycosporine-like amino acids (MAAs) in phytoplankton, a herbivorous pteropod (Limacina helicina), and its pteropodpredator (Clione antarctica) in McMurdo Bay, Antarctica. Mar. Biol. 2001, 139, 1013–1019. [Google Scholar]
- Teai, T.; Drollet, J.H.; Bianchini, J.P.; Cambon, A.; Martin, M.V.P. Widespread occurrence of mycosporine-like amino acid compounds in scleractinians from French Polynesia. Coral Reefs 1997, 16, 169–176. [Google Scholar] [CrossRef]
- Bentley, R. The shikimate pathway—A metabolic tree with many branches. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 307–384. [Google Scholar] [CrossRef] [PubMed]
- Portwich, A.; Garcia-Pichel, F. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium Chlorogloeopsis sp. strain PCC 6912. Phycologia 2003, 42, 384–392. [Google Scholar] [CrossRef]
- Starcevic, A.; Dunlap, W.C.; Shick, J.M.; Hranueli, D.; Cullum, J.; Long, P.F. Enzymes of the shikimic acid pathway encoded in the genome of a basal metazoan, Nematostella vectensis, have microbial origins. Proc. Natl. Acad. Sci. USA 2008, 105, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, A.T.; Barba Santos, M.G.; LaJeunesse, T.C.; Lesser, M.P. The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean. J. Exp. Mar. Biol. Ecol. 2006, 337, 131–146. [Google Scholar] [CrossRef]
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. eLife 2015, 4, e05919. [Google Scholar] [CrossRef] [PubMed]
- García-Pichel, F.; Wingard, C.E.; Castenholz, R.W. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the Cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium. Nostoc Commun. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef]
- Portwich, A.; Garcia-Pichel, F. Ultraviolet and osmotic stresses induce and regulate the synthesis of mycosporines in the cyanobacterium Chlorogloeopsis PCC 6912. Arch. Microbiol. 1999, 172, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Portwich, A.; Garcia-Pichel, F. A novel prokaryotic UV-B photoreceptor in the cyanobacterium Chlorogloeopsis PCC 6912. Photochem. Photobiol. 2000, 71, 493–498. [Google Scholar] [CrossRef]
- Kräbs, G.; Watanabe, M.; Wiencke, C. A Monochromatic Action Spectrum for the Photoinduction of the UV-Absorbing Mycosporine-like Amino Acid Shinorine in the Red Alga Chondrus crispus. Photochem. Photobiol. 2004, 79, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Carreto, J.I.; De Marco, S.G.; Lutz, V.A. UV-absorbing pigments in the dinoflagellates Alexandrium excavatum and Prorocentrum micans. Effects of light intensity. In Red Tides: Biology, Environmental Science, and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier Science: New York, NY, USA, 1989; pp. 333–336. [Google Scholar]
- Banaszak, A.T.; Trench, R.K. Effects of ultraviolet (UV) radiation on marine microalgal-invertebrate symbioses. II. The, synthesis of mycosporine-like amino acids in response to exposure to UV in Anthopleura elegantissima and Cassiopeia xamachana. J. Exp. Mar. Biol. Ecol. 1995, 194, 233–250. [Google Scholar] [CrossRef]
- Gröniger, A.; Hallier, C.; Häder, D.P. Influence of UV radiation and visible light on Porphyra umbilicalis: Photoinhibition and MAA concentration. J. Appl. Phycol. 1999, 11, 437–445. [Google Scholar] [CrossRef]
- Zudaire, L.; Roy, S. Photoprotection and long-term acclimation to UV radiation in the marine diatom Thalassiosira weissflogii. J. Photochem. Photobiol. B Biol. 2001, 62, 26–34. [Google Scholar] [CrossRef]
- Riegger, L.; Robinson, D. Photoinduction of UV-absorbing compounds in Antarctic diatoms and Phaeocystis antarctica. Mar. Ecol. Prog. Ser. 1997, 160, 13–25. [Google Scholar] [CrossRef]
- Hannach, G.; Sigleo, A.C. Photoinduction of UV-absorbing compounds in six species of marine phytoplankton. Mar. Ecol. Prog. Ser. 1998, 174, 207–222. [Google Scholar] [CrossRef]
- Hernando, M.; Carreto, J.I.; Carignan, M.O.; Ferreyra, G.A.; Gross, C. Effects of solar radiation on growth and mycosporine-like amino acids content in Thalassiosira sp., an Antarctic diatom. Polar Biol. 2002, 25, 12–20. [Google Scholar]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pages, C.; Gattuso, J.P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef]
- Lesser, M.P. Depth-dependent photoacclimatization to solar ultraviolet radiation in the Caribbean coral Montastrea faveolata. Mar. Ecol. Prog. Ser. 2000, 192, 137–151. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C.; Chalker, B.E.; Banaszak, A.T.; Rosenzweig, T.K. Survey of ultraviolet radiation-absorbing mycosporinelike amino acids in organs of coral reef holothuroids. Mar. Ecol. Prog. Ser. 1992, 90, 139–148. [Google Scholar] [CrossRef]
- Carroll, A.K.; Shick, J.M. Dietary accumulation of UV-absorbing mycosporine-like amino acids (MAAs) by the green sea urchin (Strongylocentrotus droebachiensis). Mar. Biol. 1996, 124, 561–569. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Shick, J.M. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: A biochemical and environmental perspective. J. Phycol. 1998, 34, 418–430. [Google Scholar] [CrossRef]
- Mason, D.S.; Schafer, F.; Shick, J.M.; Dunlap, W.C. Ultraviolet radiation-absorbing mycosporine-like amino acids (MAAs) are acquired from their diet by medaka fish (Oryzias latipes) but not by SKH-1 hairless mice. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 587–598. [Google Scholar] [CrossRef]
- Shick, J.M. The continuity and intensity of ultraviolet irradiation affect the kinetics of biosynthesis, accumulation, and conversión of mycosporine-like amino acids (MAAs) in the coral Stylophora pistillata. Limnol. Oceanogr. 2004, 49, 442–458. [Google Scholar] [CrossRef]
- Yakovleva, I.; Hidaka, M. Diel fluctuations of mycosporine-likeamino acids in shallow-water scleractinian corals. Mar. Biol. 2004, 145, 863–873. [Google Scholar] [CrossRef]
- Rezanka, T.; Temina, M.; Tolstikov, A.G.; Dembitsky, V.M. Natural Microbial UV Radiation Filters—Mycosporine-like Amino Acids. Folia Microbiol. 2004, 49, 339–352. [Google Scholar] [CrossRef]
- Misonou, T.; Saitoh, J.; Oshiba, S.; Tokitomo, Y.; Maegawa, M.; Inoue, Y.; Hori, H.; Sakurai, T. UV-absorbing substance in the red alga Porphyra yezoensis (Bangiales, Rhodophyta) block thymine photodimer production. Mar. Biotechnol. 2003, 5, 194–200. [Google Scholar]
- Dunlap, W.C.; Chalker, B.E. Identification and quantitation of near-UV absorbing compounds (S-320) in a hermatypic scleractinian. Coral Reefs 1986, 5, 155–159. [Google Scholar] [CrossRef]
- Shick, J.M.; Lesser, M.P.; Stochaj, W.R. Ultraviolet radiation and photooxidative stress in zooxanthellate Anthozoa: The sea anemone Phyllodiscus semoni and the octocoral Clavularia sp. Symbiosis 1991, 10, 145–173. [Google Scholar]
- Shick, J.M.; Lesser, M.P.; Dunlap, W.C.; Stochaj, W.R.; Chalker, B.E.; Wu Won, J. Depth-dependent responses to solar ultraviolet radiation and oxidative stress in the zooxanthellate coral Acropora microphthalma. Mar. Biol. 1995, 122, 41–51. [Google Scholar] [CrossRef]
- Gleason, D.F. Differential effects of ultraviolet radiation on Green and brown morphs of the Caribbean coral Porites astreoides. Limnol. Oceanogr. 1993, 38, 1452–1463. [Google Scholar] [CrossRef]
- Teai, T.; Raharivelomanana, P.; Bianchini, J.P.; Faura, R.; Martín, P.M.V.; Cambon, A. Structure de deux nouvelles iminomycosporines isolées de Pocillopora eydouxy. Tetrahedron Lett. 1997, 38, 5799–5800. [Google Scholar] [CrossRef]
- Banaszak, A.T.; Lesser, M.P.; Kuffner, I.B.; Ondrusek, M. Relationship between ultraviolet (UV) radiation and mycosporinelike amino acids (MAAs) in marine organisms. Bull. Mar. Sci. 1998, 63, 617–628. [Google Scholar]
- Yakovleva, I.; Bhagooli, R.; Takemura, A.; Hidaka, M. Differential susceptibility to oxidative stress of two scleractinian corals: Antioxidant functioning of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Suh, H.J.; Lee, H.W.; Jung, J. Mycosporine glycine protects biological systems against photodynamic damage by quenching singlet oxygen with a high efficiency. Photochem. Photobiol. 2003, 78, 109–113. [Google Scholar] [CrossRef]
- Oren, A. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol. J. 1997, 14, 231–240. [Google Scholar] [CrossRef]
- Neale, P.J.; Banaszak, A.T.; Jarriel, C.R. Ultraviolet sunscreens in Gymnodinium sanguineum (Dynophyceae): Mycosporine-like amino acids protect against inhibition of photosynthesis. J. Phycol. 1998, 938, 928–938. [Google Scholar] [CrossRef]
- Peinado, N.K.; Abdala Díaz, R.T.; Figueroa, F.L.; Helbling, E.W. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J. Phycol. 2004, 40, 248–259. [Google Scholar] [CrossRef]
- Korbee, N.; Figueroa, F.L.; Aguilera, J. Accumulation of mycosporine-like amino acids (MAAs): Biosynthesis, photocontrol and ecophysiological functions. Rev. Chil. Hist. Nat. 2006, 79, 119–132. [Google Scholar]
- Shick, J.M.; Ferrier-Pagés, C.; Grover, R.; Allemand, D. Effects of starvation, ammonium concentration, and photosynthesis on the UV dependent accumulation of mycosporine-like amino acids (MAAs) in the coral Stylophora pistillata. Mar. Ecol. Prog. Ser. 2005, 295, 135–156. [Google Scholar] [CrossRef]
- Cook, C.B.; Davy, S.K. Are free amino acids responsible for the ‘host factor’ effects on symbiotic zooxanthellae in extracts of host tissue? Hydrobiologia 2001, 461, 71–78. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 66, 47–53. [Google Scholar] [CrossRef]
- Shibata, K. Pigments and a UV-absorbing substance in corals and a blue–green alga living on the Great Barrier Reef. Plant. Cell. Physiol. 1969, 10, 325–335. [Google Scholar]
- Llewellyn, C.A.; Airs, R.L. Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Bernillon, J.; Bouillant, M.L.; Pittet, J.L.; Favre-Bonvin, J.; Arpin, N. Mycosporine glutamine and related mycosporines in the fungus Pyronema omphalodes. Phytochemistry 1984, 23, 1083–1087. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Hanelt, D.; Bischof, K.; Figueroa, F.L.; Flores-Moya, A.; Wiencke, C. An inventory of UV-absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot. Mar. 1998, 41, 443–453. [Google Scholar] [CrossRef]
- Karentz, D. Chemical defences of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scytonemin. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 481–520. [Google Scholar]
- Dunlap, W.C.; Williams, D.M.; Chalker, B.E.; Banaszak, A.T. Biochemical photoadaptations in vision: UV-absorbing pigments in fish eye tissues. Comp. Biochem. Physiol. 1989, 93, 601–607. [Google Scholar] [CrossRef]
- Arai, T.; Nishijima, M.; Adachi, K.; Sano, H. Isolation and structure of a UV absorbing substance from the marine bacterium Micrococcus sp. AK-334. MBI Rep. 1992, 88–94. [Google Scholar]
- Miyamoto, K.T.; Komatsu, M.; Ikeda, H. Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. J. Appl. Environ. Microbiol. 2014, 80, 5028–5036. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Michalek-Wagner, K. Seasonal and sex-specific variations in levels of photo-protecting mycosporine-like amino acids (MAAs) in soft corals. Mar. Biol. 2001, 139, 651–660. [Google Scholar]
- Karentz, D.; Dunlap, W.C.; Bosch, I. Temporal and spatial occurrence of UV-absorbing mycosporine-like amino acids in tissues of the Antarctic sea urchin Sterechinus neumayeri during springtime ozone-depletion. Mar. Biol. 1997, 129, 343–353. [Google Scholar] [CrossRef]
- Corredor, J.E.; Bruckner, A.W.; Muszynski, F.Z.; Armstrong, R.A.; Garcia, R.; Morell, J.M. UV-absorbing compounds in three species of Caribbean zooxanthellate corals: Depth distribution and spectral response. Bull. Mar. Sci. 2000, 67, 821–830. [Google Scholar]
- Muszynski, F.Z.; Bruckner, A.; Armstrong, R.A.; Morell, J.M.; Corredor, J.E. Within-colony variations of UV absorption in a reef building coral. Bull. Mar. Sci. 1998, 63, 589–594. [Google Scholar]
- Vernet, M.; Whitehead, K. Release of ultraviolet-absorbing compounds by the red-tide dinoflagellate Lingulodinium polyedra. Mar. Biol. 1996, 127, 35–44. [Google Scholar] [CrossRef]
- Cubillos, V.M.; Burritt, D.J.; Lamare, M.D.; Peake, B.M. The relationship between UV-irradiance, photoprotective compounds and DNA damage in two intertidal invertebrates with contrasting mobility characteristics. J. Photochem. Photobiol. B Biol. 2015, 149, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.; Taboada, S.; Núñez-Pons, L. Antarctic marine chemical ecology: What is next? Mar. Ecol. 2008, 29, 1–71. [Google Scholar] [CrossRef]
- Teai, T.; Drollet, J.H.; Bianchini, J.P.; Cambon, A.; Martin, P.M.V. Occurrence of ultraviolet radiation-absorbing mycosporinelike amino acids in coralmucus and whole corals of French Polynesia. Mar. Freshw. Res. 1998, 49, 127–132. [Google Scholar] [CrossRef]
- Yakovleva, I.M.; Baird, A.H. Ontogenetic change in the abundance of mycosporine-like amino acids in non-zooxanthellate coral larvae. Coral Reefs 2005, 24, 443–452. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Ishikura, M.; Maruyama, T.; Miyachi, S. UV absorbing substances in the tunic of a colonial ascidian protect its symbiont, Prochloron sp. from damage by UV-B radiation. Mar. Biol. 1997, 128, 455–461. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar] [CrossRef]
- Adams, N.L.; Shick, J.M. Mycosporine-like amino acids prevent UVB-induced abnormalities during early development of the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2001, 138, 267–280. [Google Scholar] [CrossRef]
- Franklin, L.A.; Yakovleva, I.; Karsten, U.; Lüning, K. Synthesis of mycosporine-like amino acids in Chondrus crispus (Florideophyceae) and the consequences for sensitivity to ultraviolet B radiation. J. Phycol. 1999, 35, 682–693. [Google Scholar] [CrossRef]
- Michalek-Wagner, K.; Willis, B.L. Impacts of bleaching on the soft coral Lobophytum compactum. II. Biochemical, changes in adults and their eggs. Coral Reefs 2001, 19, 240–246. [Google Scholar] [CrossRef]
- Adams, N.L.; Shick, J.M. Mycosporine-like amino acids provide protection against ultraviolet radiation in eggs of the sea urchin Strongylocentrotus droebachiensis. Photochem. Photobiol. 1996, 64, 149–158. [Google Scholar] [CrossRef]
- Ishikura, M.; Kato, C.; Maruyama, T. UV-absorbing substances in zooxanthellate and azooxanthellate clams. Mar. Biol. 1997, 128, 649–655. [Google Scholar] [CrossRef]
- Maruyama, T.; Hirose, E.; Ishikura, M. Ultraviolet-light-absorbing tunic cells in didemnid ascidians hosting a symbiotic photo-oxygenic prokaryote. Prochloron. Biol. Bull. 2003, 204, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Drollet, J.H.; Teai, T.; Faucon, M.; Martin, P.M.V. Field study of compensatory changes in UV-absorbing compounds in the mucus of the solitary coral Fungia repanda (Scleractinia: Fungiidae) in relation to solar UV radiation, sea-water temperature, and other coincident physico-chemical parameters. Mar. Freshw. Res. 1997, 48, 329–333. [Google Scholar] [CrossRef]
- Bidigare, R. Potential effects of UV-B Radiation on marine organisms of the Southern Ocean: Distributions of phytoplankton and krill during spring. Photochem. Photobiol. 1989, 50, 469–477. [Google Scholar] [CrossRef]
- Ferreyra, G.A.; Schloss, I.; Demers, S.; Neale, P.J. Phytoplankton responses to natural ultraviolet irradiance during early spring in the Weddell-Scotia Confluence: An experimental approach. Antarct. J. U. S. 1994, 29, 268–270. [Google Scholar]
- Davidson, A.T.; Marchanht, H.J. The impact of ultraviolet radiation on Phaeocystis and selected species of Antarctic marine diatoms. Antarc. Res. Ser. 1994, 62, 187–205. [Google Scholar]
- Vernet, M.; Brody, E.A.; Holm-Hansen, O.; Mitchell, B.G. The response of Antarctic phytoplankton to ultraviolet radiation: Absorption, photosynthesis, and taxonomic composition. Antarct. Res. Ser. 1994, 62, 143–158. [Google Scholar]
- Helbling, E.W.; Villafan, V.; Holm-Hansen, O. Effects of ultraviolet radiation on Antarctic marine phytoplankton photosynthesis with particular attention to the influences of mixing. Antarc. Res. Ser. 1994, 62, 207–227. [Google Scholar]
- Helbling, E.W.; Chalker, B.E.; Dunlap, W.C.; Holm-Hansen, O.; Villafañe, V.E. Photoacclimation of antarctic marine diatoms to solar ultraviolet radiation. J. Exp. Mar. Biol. Ecol. 1996, 204, 85–101. [Google Scholar] [CrossRef]
- Bidigare, R.R.; Iriarte, J.L.; Kangs, S.H.; Ondrusemk, M.E.; Karentdz, D.; Fryxell, G.A. Phytoplankton: Quantitative and qualitative assessments. Antarct. Res. Ser. 1996, 70, 173–198. [Google Scholar]
- Frame, E.R. Mycosporine-Like Amino Acids (MAAs) in Bloom Forming Phytoplankton: The Influence of Nitrogen, Ultraviolet Radiation and Species Composition. Ph.D. Thesis, University of California, San Diego, CA, USA, 2004. [Google Scholar]
- Ryan, K.G.; McMinn, A.; Mitchell, K.A.; Trenerry, L. Mycosporine-like amino acids inAntarctic Sea ice algae, and their response to UV-B radiation. Z. Fuer Naturforschung C J. Biosci. 2002, 57, 471–477. [Google Scholar] [CrossRef]
- Newman, S.J.; Dunlap, W.C.; Nicol, S.; Ritz, D. Antarctic krill (Euphausia superba) acquire a UV-absorbing mycosporine-like amino acid from dietary algae. J. Exp. Mar. Biol. Ecol. 2000, 255, 93–110. [Google Scholar] [CrossRef]
- Nakamura, H.; Kobayashi, J.; Hirata, Y. Separation of mycosporine-like amino acids in marine organisms using reversed-phase high-performance liquid chromatography. J. Chromatogr. 1982, 250, 113–118. [Google Scholar] [CrossRef]
- McClintock, J.; Karentz, D. Mycosporine-like amino acids in 38 species of subtidal marine organisms from McMurdo Sound, Antarctica. Antarc. Sci. 1997, 4, 392–398. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Wiencke, C. Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar. Biol. 2002, 141, 619–627. [Google Scholar]
- Hoyer, K.; Karsten, U.; Wiencke, C. Inventory of UV-absorving mycosporine-like amino acids in polar macroalgae and factors controlling their content. In Antarctic Biology in a Global Context; Huiskes, A.H.L., Gieskes, W.W.C., Rozema, J., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 56–62. [Google Scholar]
- Helbling, E.W.; Barbieri, E.S.; Sinha, R.J.; Villafañe, V.E.; Häder, D.P. Dynamics of potentially protective compounds in Rhodophyta species from Patagonia (Argentina) exposed to solar radiation. J. Photochem. Photobiol. B 2004, 75, 63–71. [Google Scholar] [CrossRef] [PubMed]
- García-Pichel, F.; Castenholz, R.W. Characterization and biological implications of scytonemin, and cyanobacterial sheath pigment. J. Phycol. 1991, 27, 395–409. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289. [Google Scholar] [CrossRef]
- Dillon, J.G.; Tatsumi, C.M.; Tandingan, P.G.; Castenholz, R.W. Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.). Arch. Microbiol. 2002, 177, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.Y.; Cowie, R.O.; Simpson, A.M.; O’Toole, R.; Ryan, K.G. The origin of cyanobacteria in Antarctic sea ice: Marine or freshwater? Environ. Microbiol. Rep. 2012, 4, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.; Park, Y.C.; McClintock, J.B.; Bakera, B.J. Structure and bioactivity of erebusinone, a pigment from the antarctic sponge Isodictya erinacea. Tetrahedron 2000, 56, 9057–9062. [Google Scholar] [CrossRef]
- Truscott, R.J.W.; Carver, J.A.; Thorpe, A.; Douglas, R.H. Identification of 3-Hydroxykynurenine as the lens pigment in the Gourami Trichogaster trichopterus. Exp. Eye Res. 1992, 54, 1015–1017. [Google Scholar] [CrossRef]
- Thorpe, A.; Douglas, R.H.; Truscott, R.J.W. Spectral transmission and shortwave absorbing pigments in the fish lens—I. Phylogenetic distribution and identity. Vis. Res. 1993, 33, 289–300. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. The nature and role of pigments of marine invertebrates. Nat. Prod. Rep. 2006, 23, 223–255. [Google Scholar] [CrossRef] [PubMed]
- Meredith, P.; Riesz, J. Radiative relaxation quantum yields for synthetic eumelanin. Photochem. Photobiol. 2004, 79, 211–216. [Google Scholar] [CrossRef]
- Tartarotti, B.; Laurion, I.; Sommaruga, R. Large variability in the concentration of mycosporine-like amino acids among zooplankton from lakes located across an altitude gradient. Limnol. Oceanogr. 2001, 46, 1546–1552. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Setlow, R.B. Ultraviolet radiation-induced DNAdamage and its photorepair in the skin of the platyfish Xiphophorus. Cancer Res. 1993, 53, 2249–2255. [Google Scholar] [PubMed]
- Hamilton, A.J.; Gomez, B.L. Melanins in fungal pathogens. J. Med. Microbiol. 2002, 51, 189–191. [Google Scholar] [PubMed]
- Castelvecchi, D. Dark Power: Pigment seems to put radiation to good use. Sci. News 2007, 171, 325. [Google Scholar] [CrossRef]
- Dadachova, E.; Bryan, R.A.; Huang, X.; Moadel, T.; Andrew, D.; Schweitzer, A.D.; Aisen, P.; Nosanchuk, J.; Casadevall, A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2007, 2, e457. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.C. Biology of the Arthropod Cuticle; Springer: Berlin, Germany, 1975; pp. 1–450. [Google Scholar]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, R.T.; Messenger, J.B. Cephalopod Behaviour; Cambridge University Press: Cambridge, UK, 1999; pp. 1–365. [Google Scholar]
- Van Trappen, S.; Tan, T.L.; Yang, J.; Mergaert, J.; Swings, J. Alteromonas stellipolaris sp. nov., a novel, budding, prosthecate bacterium from Antarctic seas, and emended description of the genus Alteromonas. Int. J. Syst. Evol. Microbiol. 2004, 54, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Fukuda, W.; Sanada, T.; Imanaka, T. Characterization of water-soluble dark-brown pigment from Antarctic bacterium, Lysobacter oligotrophicus. J. Biosci. Bioeng. 2015, 120, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Tashirev, A.B.; Romanovskaia, V.A.; Rokitko, P.V.; Matveeva, N.A.; Shilin, S.O.; Tashireva, A.A. Synthesis of melanin pigments by Antarctic black yeast. Mikrobiol. Z. 2012, 74, 2–8. [Google Scholar] [PubMed]
- Tashirev, A.B.; Romanovskaia, V.A.; Shilin, S.O.; Chernaia, N.A. Screening of yeast-producers of melanin in the Antarctic terrestrial biotopes. Mikrobiol. Z. 2010, 72, 3–8. [Google Scholar] [PubMed]
- Post, A.; Larkum, A.W. UV-absorbing pigments, photosynthesis and UV exposure in Antarctica: Comparison of terrestrial and marine algae. Aquat. Bot. 1993, 45, 231–243. [Google Scholar] [CrossRef]
- Ragan, A.M.; Glombitza, K.W. Phlorotannins, brown algal polyphenols. Prog. Phycol. Res. 1986, 4, 129–241. [Google Scholar]
- Targett, N.M.; Arnold, T.M. Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 1998, 834, 195–205. [Google Scholar] [CrossRef]
- Pavia, H.; Cervin, G.; Lindgren, A.; Aberg, P. Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 1997, 157, 139–146. [Google Scholar] [CrossRef]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291. [Google Scholar] [CrossRef]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2006, 43, 1–91. [Google Scholar]
- Rice–Evans, R.E.; Miller, C.A.; Paganga, N.J.G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E.A.; Clayton, M.N. Secretion of phenolic substances into the zygote wall and cell plate in embryos of Hormosira and Acrocarpis (Fucales, Phaeophyceae). J. Phycol. 1998, 34, 969–980. [Google Scholar] [CrossRef]
- Lüder, U.H.; Clayton, M.N. Induction of phlorotannins in the brown macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to simulated herbivory—The first microscopic study. Planta 2004, 218, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Shibata, T.; Fujimoto, K.; Honj, T.; Nakamura, T. Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 2003, 218, 601–611. [Google Scholar] [CrossRef]
- Ragan, M.A.; Smidsrød, O.; Bjørn, L. Chelation of divalent metal ions by brown algal polyphenols. Mar. Chem. 1979, 7, 265. [Google Scholar] [CrossRef]
- Cruces, E.; Rautenberger, R.; Rojas-Lillo, Y.; Cubillos, V.M.; Arancibia-Miranda, N.; Ramírez-Kushel, E.; Gómez, I. Physiological acclimation of Lessonia spicata to diurnal changing PAR and UV radiation: Differential regulation among down- regulation of photochemistry, ROS scavenging activity and phlorotannins as major photoprotective mechanisms. Photosynth. Res. 2017, 131, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Amsler, C.D.; Rowley, R.J.; Laur, D.R.; Quetin, L.B.; Ross, R.M. Vertical distribution of Antarctic peninsular macroalgae: Cover, biomass and species composition. Phycologia 1995, 34, 424–430. [Google Scholar] [CrossRef]
- Klöser, H.; Quartino, M.L.; Wiencke, C. Distribution of macroalgae and macroalgal communities in gradients of physical conditions in Potter Cove, King George Island, Antarctica. Hydrobiologia 1996, 333, 1–17. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Connan, S.; Deslandes, E.; Gall, E. Influence of day-night and tidal cycles on phenol content and antioxidant capacity in three temperate intertidal brown seaweeds. J. Exp. Mar. Biol. Ecol. 2007, 349, 359–369. [Google Scholar] [CrossRef]
- Rautenberger, R.; Huovinen, P.; Gómez, I. Effects of increased seawater temperature on UV tolerance of Antarctic marine macroalgae. Mar. Biol. 2015, 162, 1087–1097. [Google Scholar] [CrossRef]
- Gómez, I.; Huovinen, P. Lack of physiological depth patterns in conspecifics of endemic antarctic brown algae: A trade-off between UV stress tolerance and shade adaptation? PLoS ONE 2015, 10, e0134440. [Google Scholar] [CrossRef] [PubMed]
- Flores-Molina, M.R.; Rautenberger, R.; Muñoz, P.; Huovinen, P.; Gómez, I. Stress tolerance of the endemic antarctic brown alga Desmarestia anceps to UV radiation and temperature is mediated by high concentrations of phlorotannins. Photochem. Photobiol. 2016, 92, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Iken, K.; Amsler, C.D.; Amsler, M.O.; McClintock, J.B.; Baker, B.J. Field studies on deterrent properties of phlorotannins in Antarctic brown algae. Bot. Mar. 2009, 52, 547–557. [Google Scholar] [CrossRef]
- Iken, K.; Amsler, C.D.; Hubbard, J.M.; McClintock, J.B.; Baker, B.J. Allocation patterns of phlorotannins in Antarctic brown algae. Phycologia 2007, 46, 386–395. [Google Scholar] [CrossRef]
- Cruces, E.; Huovinen, P.; Gómez, I. Phlorotannin and antioxidant responses upon short-term exposure to UV radiation and elevated temperature in three south Pacific kelps. Photochem. Photobiol. 2012, 88, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, P.; Gómez, I. UV sensitivity of vegetative and reproductive tissues of two Antarctic Brown Algae is related to differential allocation of phenolic substances. Photochem. Photobiol. 2015, 91, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Español, S.; Véliz, K.; Huovinen, P. Spatial distribution of phlorotannins and its relationship with photosynthetic UV tolerance and allocation of storage carbohydrates in blades of the kelp Lessonia spicata. Mar. Biol. 2016, 163, 1–14. [Google Scholar] [CrossRef]
- Tala, F.; Penna-Díaz, M.A.; Luna-Jorquera, G.; Rothäusler, E.; Thiel, M. Daily and seasonal changes of photobiological responses in floating bull kelp Durvillaea antarctica (Chamisso) Hariot (Fucales: Phaeophyceae). Phycologia 2017, 56, 271–283. [Google Scholar] [CrossRef]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Winter, F.C.; Estes, J.A. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 28, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, J.J.; Lindquist, N. Chemical defence among hydroids on pelagic Sargassum: Predator deterrence and absorption of solar UV radiation by secondary metabolites. Mar. Ecol. Prog. Ser. 1997, 155, 115–126. [Google Scholar] [CrossRef]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent proteins from nonbiolumninescent Anthozoa species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan. Aequorea J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Tsein, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Bou-Abdallah, F.; Chasteen, N.D.; Lesser, M.P. Quenching of superoxide radicals by green fluorescent protein. Biochim. Biophys. Acta 2006, 1760, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford Science Publications: Oxford, UK, 1999; pp. 1–936. [Google Scholar]
- McCord, J.M. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Intern. Hormesis Soc. 2008, 6, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Staniek, K.; Nohl, H. H2O2 detection from intact mitochondria as a measure for one-electron reduction of dioxygen requires a non-invasive assay system. Biochim. Biophys. Acta 1999, 1413, 70–80. [Google Scholar] [CrossRef]
- Barata, C.; Lekumberri, I.; Vila-Escale, M.; Prat, N.; Porte, C. Trace metal concentration, antioxidant enzyme activities and susceptibility to oxidative stress in the trichoptera larvae Hydropsyche exocellata from the Llobregat river basin (NE Spain). Aquat. Toxicol. 2005, 74, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Storelli, C.; Acierno, R.; Maffia, M. Membrane lipid and protein adaptations in Antarctic fish. In Cold Ocean Physiology; Pörtner, H., Playle, R., Eds.; Cambridge University Press: Cambridge, UK, 1998; pp. 166–189. [Google Scholar]
- Avila, C.; Fontana, A.; Esposito, M.; Ciavatta, M.L.; Cimino, G. Fatty acids of Antarctic gastropods: Distribution and comparison with Mediterranean species. Iberus 2004, 22, 33–44. [Google Scholar]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Marine natural pigments: Chemistry, distribution and analysis. Dyes Pigments 2014, 111, 124–134. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Magdaong, N.C.M.; Blankenship, R.E. Photoprotective, excited-state quenching mechanisms in diverse photosynthetic organisms. J. Biol. Chem. 2018, 293, 5018–5025. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.M. Mechanistic aspects of xanthopyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol. Plant. 1997, 99, 197–209. [Google Scholar] [CrossRef]
- Verhoeven, A.S.; Adams, W.W.; Demmig-Adams, B.; Croce, R.; Bassi, R. Xanthophyll cycle pigment localization and dynamics during exposure to low temperatures and light stress in Vinca major. Plant Physiol. 1999, 120, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Buma, A.G.J.; Wright, S.W.; van den Enden, R.; van de Poll, W.H.; Davidson, A.T. PAR acclimation and UVBR-induced DNA damage in Antarctic marine microalgae. Mar. Ecol. Prog. Ser. 2006, 315, 33–42. [Google Scholar] [CrossRef]
- Hernando, M.; Schloss, I.; Roy, S.; Ferreyra, G. Photoacclimation to Long-Term Ultraviolet Radiation Exposure of Natural Sub-Antarctic Phytoplankton Communities: Fixed Surface Incubations versus Mixed Mesocosms. Photochem. Photobiol. 2006, 82, 923–935. [Google Scholar] [CrossRef] [PubMed]
- van de Poll, W.H.; Alderkamp, A.C.; Janknegt, P.J.; Roggeveld, J.; Buma, A.G.J. Photoacclimation modulates excessive photosynthetically active and ultraviolet radiation effects in a temperate and an Antarctic marine diatom. Limnol. Oceanogr. 2006, 51, 1239–1248. [Google Scholar] [CrossRef]
- Wynn-Williams, D.D.; Edwards, H.G.M. Environmental UV Radiation: Biological Strategies for Protection and Avoidance. In Astrobiology; Horneck, G., Baumstark-Khan, C., Eds.; Springer: Berlin, Heidelberg, Germany, 2002; pp. 245–260. [Google Scholar]
- Palmisano, A.C.; Wharton, R.A.; Cronin, S.E.; des Marais, D.J. Lipophilic pigments from the benthos of a perennially ice-covered Antarctic lake. Hydrobiologia 1989, 178, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Potts, M.; Olie, J.J.; Nickels, J.S.; Parsons, J.; White, D.C. Variation in phospholipid ester-linked fatty acids and carotenoids of dessicated Nostoc commune (Cyanobacteria) from different geographic locations. Appl. Environ. Microbiol. 1987, 53, 4–9. [Google Scholar] [PubMed]
- Órdenes-Aenishanslins, N.; Anziani-Ostuni, G.; Vargas-Reyes, M.; Alarcón, J.; Tello, A.; Pérez-Donoso, J.M. Pigments from UV-resistant Antarctic bacteria as photosensitizers in Dye Sensitized Solar Cells. J. Photochem. Photobiol. B Biol. 2016, 162, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Oh, H.M.; Chung, S.; Cho, J.C. Antarcticimonas flava gen. nov., sp. nov., isolated from Antarctic coastal seawater. J. Microbiol. 2009, 47, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Yu, P.S.; Zhou, Y.D.; Xu, L.; Wang, C.S.; Wu, M.; Oren, A.; Xu, X.W. Muricauda antarctica sp. nov., a marine member of the Flavobacteriaceae isolated from Antarctic seawater. Int. J. Syst. Evol. Microbiol. 2013, 63, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid Pigmentation in Antarctic Heterotrophic Bacteria as a Strategy to Withstand Environmental Stresses. Arct. Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Shukla, S.P.; Kashyap, A.K. An assessment of biopotential of three cyanobacterial isolates from Antarctic for carotenoid production. Indian J. Biochem. Biophys. 2003, 40, 362–366. [Google Scholar] [PubMed]
- Marquardt, J.; Hanelt, D. Carotenoid composition of Delesseria lancifolia and other marine red algae from polar and temperate habitats. Eur. J. Phycol. 2004, 39, 285–292. [Google Scholar] [CrossRef]
- Matsuno, T. Aquatic animal carotenoids. Fish. Aquat. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Rangarao, A.; Moi, P.S.; Sarada, R.; Ravishankar, G.A. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar]
- Ulven, S.M. Metabolic Effects of Krill Oil. In Fish and Fish Oil in Health and Disease Prevention; Raatz, S.K., Bibus, D.M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 333–339. [Google Scholar]
- Czeczuga, B.; Klyszejko, B. Carotenoids in fish XIV. The carotenoid content in the flesh of certain species from the Antarctic. Hydrobiologia 1978, 60, 173–175. [Google Scholar] [CrossRef]
- Czeczuga, B. Carotenoid-calcium carbonate complex from the hydrocoral Errina antarctica. Biochem. Syst. Ecol. 1985, 13, 455–457. [Google Scholar] [CrossRef]
- Mène-Saffrané, L. Vitamin E Biosynthesis and Its Regulation in Plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.J. Evidence for the photoprotective effects of vitamin E. Photochem. Photobiol. 1993, 58, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.; Witt, E.; Goldman, R.; Scita, G.; Packer, L. Ultraviolet light-induced generation of vitamin E radicals and their recycling. A possible photosensitizing effect of vitamin E in skin. Free Radic. Res. Commun. 1992, 16, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Podhaisky, H.P.; Wohlrab, W. Is the photoprotective effect of vitamin E based on its antioxidative capacity? J. Dermatol. Sci. 2002, 28, 84–86. [Google Scholar] [CrossRef]
- Hernando, M.; Malanga, G.; Puntarulo, S.; Ferreyra, G. Non-enzymatic antioxidant photoprotection against potential UVBR-induced damage in an Antarctic diatom (Thalassiosira sp.). Lat. Am. J. Aquat. Res. 2011, 39, 397–408. [Google Scholar] [CrossRef]
- Estevez, M.S.; Abele, D.; Puntarulo, S. Lipid radical generation in polar (Laternula elliptica) and temperate (Mya arenaria) bivalves. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 729–737. [Google Scholar] [CrossRef]
- Woodward, B. Dietary vitamin requirements of cultured young fish, with emphasis on quantitative estimates for salmonids. Aquaculture 1994, 124, 133–168. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujisawa, A.; Hara, A.; Dunlap, W.C. An unusual vitamin E constituent provides antioxidant protection in marine organisms adapted to coldwater environments. Proc. Natl. Acad. Sci. USA 2001, 98, 13144–13148. [Google Scholar] [CrossRef] [PubMed]
- Gieseg, S.P.; Cuddihy, S.; Hill, J.V.; Davison, W. A comparison of plasma vitamin C and E levels in two Antarctic and two temperate water fish species. Comp. Biochem. Physiol. B 2000, 125, 371–378. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Fujisawa, A.; Yamamoto, Y.; Moylan, T.J.; Sidell, B.D. Notothenioid fish, krill and phytoplankton from Antarctica contain a vitamin E constituent (α-tocomonoenol) functionally associated with cold-water adaptation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 133, 299–305. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Maita, N.; Fujisawa, A.; Takashima, J.; Ishii, Y.; Dunlap, W.C. A new vitamin E (α-tocomonoenol) from eggs of the Pacific salmon Oncorhynchus keta. J. Nat. Prod. 1999, 62, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochem. Biophys. Acta Bioenerg. 2009, 1787, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.S.; Malanga, G.; Puntarulo, S. UV-B effects on Antarctic Chlorella sp cells. J. Photochem. Photobiol. B Biol. 2001, 62, 19–25. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 191–212. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetics and molecular approaches. Annu. Rev. Plant Physiol. 1999, 50, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Laureau, C.; Bligny, R.; Streb, P. The significance of glutathione for photoprotection at contrasting temperatures in the alpine plant species Soldanella alpine and Ranunculus glacialis. Physiol. Plant. 2011, 143, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Häder, D.P.; Sinha, R.P. Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res. Rev. 2010, 9, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Tel-Or, E.; Huflejt, M.E.; Packer, L. Hydroperoxide metabolism in cyanobacteria. Arch. Biochem. Biophys. 1986, 246, 396–402. [Google Scholar] [CrossRef]
- Alscher, R.G.; Donahue, J.H.; Cramer, C.L. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol. Plant. 1997, 100, 224–233. [Google Scholar] [CrossRef]
- Janknegt, P.J.; van de Poll, W.H.; Visser, R.J. Oxidative stress responses in the marine Antarctic diatom Chaetoceros brevis (bacillariophyceae) during photoacclimation. J. Phycol. 2008, 44, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Philipp, E.; Brey, T.; Pörtner, H.O.; Abele, D. Chronological and physiological ageing in a polar and a temperate mud clam. Mech. Ageing Dev. 2005, 126, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Favero, M.; Albergoni, V. Comparative studies of antioxidant enzymes in red-blooded and white-blooded Antarctic teleost fish Pagothenia bernacchii and Chionodraco hamatus. Comp. Biochem. Physiol. 1993, 106, 333–336. [Google Scholar] [CrossRef]
- Ansaldo, M.; Luquet, C.M.; Evelson, P.A.; Polo, J.M.; Llesuy, S. Antioxidant levels from different Antarctic fish caught around South Georgia Island and Shag Rocks. Polar Biol. 2000, 23, 160–165. [Google Scholar] [CrossRef]
- Abele, D.; Puntarulo, S. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp. Biochem. Physiol. Part A 2004, 138, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.; Zaleski, T.; Rodrigues, E.; Carvalho, C.; Cadena, S.M.; Gozzi, G.; Krebsbach, P.; Rios, F.S.; Donatti, L. Effect of temperature acclimation on the liver antioxidant defence system of the Antarctic nototheniids Notothenia coriiceps and Notothenia rossii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 172–173, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Coppola, D.; Russo, R.; Tinajero-Trejo, M.; di Prisco, G.; Lauro, F.; Ascenzi, P.; Verde, C. The globins of cold-adapted Pseudoalteromonas haloplanktis TAC125: From the structure to the physiological functions. Adv. Microb. Physiol. 2013, 63, 329–389. [Google Scholar] [PubMed]
- Giordano, D.; Russo, R.; Coppola, D.; Altomonte, G.; di Prisco, G.; Bruno, S.; Verde, C. “Cool” adaptations to cold environments: Globins in Notothenioidei. Hydrobiologia 2015, 761, 293–312. [Google Scholar] [CrossRef]
- Cortat, B.; Garcia, C.C.M.; Quinet, A.; Schuch, A.P.; de Lima-Bessa, K.M.; Menck, C.F.M. The relative roles of DNA damage induced by UVA irradiation in human cells. Photochem. Photobiol. Sci. 2013, 12, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.R.P.; Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef] [PubMed]
- Richa; Sinha, R.P.; Häder, D.P. Physiological aspects of UV-excitation of DNA. In Photoinduced Phenomena in Nucleic Acids; Barbatti, B., Borin, A.C., Ullrich, A.C., Eds.; Springer: Berlin, Germany, 2014; pp. 1–46. [Google Scholar]
- Karentz, D. Beyond Xeroderma pigmentosum: DNA Damage and repair in an ecological context. A tribute to James, E. Cleaver. Photochem. Photobiol. 2015, 91, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Buma, A.G.J.; Karin de Boer, M.; Boelen, P. Depth distributions of DNA damage in antarctic marine phyto and bacterioplankton exposed to summertime UV radiation. J. Phycol. 2001, 37, 200–208. [Google Scholar] [CrossRef]
- Eisen, J.A.; Hanawalt, P.C. A phylogenomic study of DNA repair genes, proteins, and processes. Mutat. Res. 1999, 435, 171–213. [Google Scholar] [CrossRef]
- O’Brien, P.J. Catalytic promiscuity and the divergent evolution of DNA repair enzymes. Chem. Rev. 2006, 106, 720–752. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.P.; Patil, A.; Joseph, F.; Endres, L.; Begley, U.; Zappala, M.R.; Cunningham, R.P.; Begley, T.J. Cross-species functionome analysis identifies proteins associated with DNA repair, translation and aerobic respiration as conserved modulators of UV-toxicity. Genomics 2011, 97, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Essen, L.O.; Klar, T. Light-driven DNA repair by photolyases. Cell. Mol. Life Sci. 2006, 63, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Todo, T.; Ayaki, H.; Ishizaki, K.; Morita, T.; Mitra, S.; Ikenaga, M. Cloning of a marsupial DNA photolyase gene and the lack of related nucleotide sequences in placental mammals. Nucleic Acids Res. 1994, 22, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, J.E. Photoreactivation: A radiation repair mechanism absent from mammalian cells. Biochem. Biophys. Res. Commun. 1966, 24, 569–576. [Google Scholar] [CrossRef]
- Johnson, J.L.; Hamm-Alvarez, S.; Payne, G.; Sancar, G.B.; Rajagopalan, K.V.; Sancar, A. Identification of the second chromophore of Escherichia coli and yeast DNA photolyases as 5,10-methenyltetrahydrofolate. Proc. Natl. Acad. Sci. USA 1988, 85, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Eker, A.P.M.; Kooiman, P.; Hessels, J.K.C.; Yasui, A. DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J. Biol. Chem. 1990, 265, 8009–8015. [Google Scholar] [PubMed]
- Wang, J.; Du, X.; Pan, W.; Wang, X.; Wu, W. Photoactivation of the cryptochrome/photolyase superfamily. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 84–102. [Google Scholar] [CrossRef]
- Sancar, A.; Sancar, G.B. DNA repair enzymes. Annu. Rev. Biochem. 1988, 57, 29–67. [Google Scholar] [CrossRef] [PubMed]
- Marizcurrena, J.J.; Morel, M.A.; Braña, V.; Morales, D.; Martinez-López, W.; Castro-Sowinski, S. Searching for novel photolyases in UVC-resistant Antarctic bacteria. Extremophiles 2017, 21, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, L.; Mou, S.; Wang, Y.; Zheng, Z.; Liu, F.; Qi, X.; An, M.; Chen, H.; Miao, J. Cyclobutane pyrimidine dimers photolyase from extremophilic microalga: Remarkable UV-B resistance and efficient DNA damage repair. Mutat. Res. 2015, 773, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lud, D.; Moerdijk, T.C.W.; van de Poll, W.H.; Buma, A.G.J.; Huiskes, A.H.L. DNA damage and photosynthesis in Antarctic and Arctic Sanionia uncinata (Hedw.) Loeske under ambient and enhanced levels of UV-B radiation. Plant Cell Environ. 2002, 25, 1579–1589. [Google Scholar] [CrossRef]
- Lamare, M.D.; Barker, M.F.; Lesser, M.P.; Marshall, C. DNA photorepair in echinoid embryos: Effects of temperature on repair rate in Antarctic and non-Antarctic species. J. Exp. Biol. 2006, 209, 5017–5028. [Google Scholar] [CrossRef] [PubMed]
- Isely, N.; Lamare, M.; Marshall, C.; Barker, M. Expression of the DNA repair enzyme, photolyase, in developmental tissues and larvae, and in response to ambient UV-R in the Antarctic sea urchin Sterechinus neumayeri. Photochem. Photobiol. 2009, 85, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Dantuma, N.P.; Heinen, C.; Hoogstraten, D. The ubiquitin receptor Rad23: At the crossroads of nucleotide excision repair and proteasomal degradation. DNA Repair 2009, 8, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Bergink, S.; Toussaint, W.; Luijsterburg, M.S.; Dinant, C.; Alekseev, S.; Hoeijmakers, J.H.J.; Dantuma, N.P.; Houtsmuller, A.B.; Vermeulen, W. Recognition of DNA damage by XPC coincides with disruption of the XPC–RAD23 complex. J. Cell Biol. 2012, 196, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.N. An Investigation of Sunlight Stress Response Genes in the Intertidal Sea Anemone, Actinia tenebrosa. Master’s Thesis, Queensland University of Technology, Queensland, New Zealand, 2016. [Google Scholar]
- Janion, C. Inducible SOS Response System of DNA Repair and Mutagenesis in Escherichia coli. Int. J. Biol. Sci. 2008, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Courcelle, J.; Khodursky, A.; Peter, B.; Brown, P.O.; Hanawalt, P.C. Comparative gene expression profiles following UV exposure in wild-type and SOS deficient Escherichia coli. Genetics 2001, 58, 41–64. [Google Scholar]
- Walker, G.C. Mutagenesis and induced responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol. Rev. 1984, 48, 60–93. [Google Scholar] [PubMed]
- Miller, R.V. recA: The gene and its protein product. In Encyclopedia of Microbiology, 2nd ed.; Luria, S., Ed.; Academic Press: San Diego, CA, USA, 2000; Volume 4, pp. 43–54. [Google Scholar]
- Booth, M.G.; Hutchinson, L.; Brumsted, M.; Aas, P.; Coffin, R.B.; Downer, R.C.; Kelley, C.A.; Lyons, M.M.; Pakulski, J.D.; Sandvik, S.L.H.; et al. Quantification of recA gene expression as an indicator of repair potential in marine bacterioplankton communities of Antarctica. Aquat. Microb. Ecol. 2001, 24, 51–59. [Google Scholar] [CrossRef]
- Bouchard, J.N.; Roy, S.; Ferreyra, G.; Campbell, D.A.; Curtosi, A. Ultraviolet-B effects on photosystem II efficiency of natural phytoplankton communities from Antarctica. Polar Biol. 2005, 28, 607–618. [Google Scholar] [CrossRef]
- Apostolova, E.L.; Pouneva, I.; Rashkov, G.; Dankov, K.; Grigorova, I.; Misra, A.N. Effect of UV-B radiation on photosystem II functions in Antarctic and mesophilic strains of a green alga Chlorella vulgaris and a cyanobacterium Synechocystis salina. Ind. J. Plant Physiol. 2014, 19, 111–118. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Hoffman-Falk, H.; Marder, J.B.; Edelman, M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of chloroplast membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Prásil, O.; Adir, N.; Ohad, I. Dynamics of photosystem II: Mechanism of photoinhibition and recovery processes. In The photosystems: Structure, Function and Molecular Biology. Topics in Photosynthesis; Barber, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 11, pp. 295–348. [Google Scholar]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Sass, L.; Spetea, C.; Maté, Z.; Nagy, F.; Vass, I. Repair of UV-B induced damage of photosystem II via de novo synthesis of D1 and D2 reaction centre subunits in Synechocystis sp. PCC 6803. Photosynth. Res. 1997, 54, 55–62. [Google Scholar] [CrossRef]
- Verschooten, L.; Claerhout, S.; Laethemii, A.V.; Agostinis, P.; Garmyn, M. New strategies of photoprotection. Photochem. Photobiol. 2006, 82, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Tsokos, G.C. Heat shock protein 70 kDa: Molecular biology, biochemistry, and physiology. Pharmacol. Ther. 1998, 80, 183–201. [Google Scholar] [CrossRef]
- Bierkens, J.G. Applications and pitfalls of stress-proteins in biomonitoring. Toxicology 2000, 153, 61–72. [Google Scholar] [CrossRef]
- La Terza, A.; Barchetta, S.; Buonanno, F.; Ballarini, P.; Miceli, C. The protozoan ciliate Tetrahymena thermophila as biosensor of sublethal levels of toxicants in the soil. Fresenius Environ. Bull. 2008, 17, 1144–1150. [Google Scholar]
- Sarkar, S.; Singh, D.M.; Yadav, R.; Arunkumar, K.P.; Pittman, G.W. Heat shock proteins: Molecules with assorted functions. Front. Biol. 2011, 6, 312–327. [Google Scholar]
- Torok, Z.; Goloubinoff, P.; Horvath, I.; Tsvetkova, N.M.; Glatz, A.; Balogh, G.; Varvasovszki, V.; Los, D.A.; Vierling, E.; Crowe, J.H.; et al. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. USA 2001, 98, 3098–3103. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Glatz, A.; Varvasovszki, V.; Török, Z.; Páli, T.; Balogh, G.; Kovács, E.; Nádasdi, L.; Benkö, S.; Joó, F.; et al. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: Identification of hsp17 as a “fluidity gene”. Proc. Natl. Acad. Sci. USA 1998, 95, 3513–3518. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, P.; Cong, B.; Liu, C.; Lin, X.; Shen, J.; Huang, X. Molecular cloning and expression analysis of a cytosolic Hsp70 gene from Antarctic ice algae Chlamydomonas sp. ICE-L. Extremophiles 2010, 14, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Fulgentini, L.; Passini, V.; Colombetti, G.; Miceli, C.; La Terza, A.; Marangoni, R. UV Radiation and Visible Light Induce hsp70 Gene Expression in the Antarctic Psychrophilic Ciliate Euplotes focardii. Microb. Ecol. 2015, 70, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Dahms, H.U.; Lee, J.S. UV radiation in marine ectotherms: Molecular effects and responses. Aquat. Toxicol. 2010, 97, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.P.; et al. Ultraviolet radiation and cyanobacteria. J. Photochem. Photobiol. B 2014, 141, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, R.; Poma, V.; Costa, C.; Matranga, V. UV-B radiation prevents skeleton growth and stimulates the expression of stress markers in sea urchin embryos. Biochem. Biophys. Res. Commun. 2005, 328, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D.J.; Brussaard, C.P.D.; Berges, J.A. What is the role and nature of programmed cell death in phytoplankton ecology? Eur. J. Phycol. 2006, 41, 1–14. [Google Scholar] [CrossRef]
- Vardi, A.; Berman-Frank, I.; Rozenberg, T.; Hadas, O.; Kaplan, A.; Levine, A. Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxide stress. Curr. Biol. 1999, 9, 1061–1064. [Google Scholar] [CrossRef]
- Häder, D.P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Effects on aquatic ecosystems. J. Photochem. Photobiol. B 1998, 46, 53–68. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Mango, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Domit, C.; Azevedo, A.; Lailson-Brito, J.; et al. First determination of UV filters in marine mammals. Octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 2013, 47, 5619–5625. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, P.R.; Vinebrooke, R.D.; Donald, D.B.; Smol, J.P.; Schindler, D.W. Past ultraviolet radiation environments in lakes derived from fossil pigments. Nature 1997, 388, 457–459. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.; Fitzmaurice, N.; Dunlap, W.C.; Chalker, B.E.; Bandaranayake, W.M. Sunscreen Compositions and Compounds for Use Therein. Australian Patent 595,075, 26 September 1986. [Google Scholar]
- Chalmers, P.J.; Fitzmaurice, N.; Rigg, D.J.; Thang, S.H.; Bird, G. UV Absorbing Compounds and Compositions. Australian Patent 653,495, 7 September 1990. [Google Scholar]
- Schmid, D.; Schürch, C.; Zülli, F.; Nissen, H.P.; Prieur, H. Mycosporine-like amino acids: Natural UV-screening compounds from red algae to protect the skin against photoaging. SÖFW-J. 2003, 129, 1–5. [Google Scholar]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a potential natural source for UVA protective sunscreens. Photochem. Photobiol. Sci. 2006, 5, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Oyamada, C.; Kaneniwa, M.; Ebitani, K.; Murata, M.; Ishihara, K. Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar. Biotechnol. 2008, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Eur. Cosmet. 2006, 9, 1–4. [Google Scholar]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Andre, G.; Pellegrini, M.; Pellegrini, L. Algal Extracts Containing Amino Acid Analogs of Mycosporin Are Useful as Dermatological Protecting Agents against Ultraviolet Radiation. Patent No. FR2803201, 6 July 2001. [Google Scholar]
- Sathasivam, R.; Ki, J.S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- The Global Market for Carotenoids. Available online: https://www.bccresearch.com/market-research/food-and-beverage/carotenoids-global-market-report-fod025e.html (accessed on 15 April 2018).
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- CODIF. Available online: http://www.codif-tn.com/en?s=dermochlorella (accessed on 18 April 2018).
- Frutarom. Available online: http://www.frutarom.com/FrutaromNew/Templates/showpage.asp?DBID=1&LNGID=1&TMID=84&FID=534 (accessed on 18 April 2018).
- Lipotec. Available online: https://www.lipotec.com/en/products/seacode-trade-marine-ingredient/ (accessed on 18 April 2018).
- Lipotec. Available online: https://www.lipotec.com/en/products/antarcticine-reg-marine-ingredient/ (accessed on 18 April 2018).
- Karentz, D.; Bosch, I.; Dunlap, W.C. Distribution of UV-absorbing compounds in the Antarctic limpet, Nacella concinna. Antarct. J. U. S. 1992, 27, 121–122. [Google Scholar]
- Fairhead, V.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Within-thallus variation in chemical and physical defences in two species of ecologically dominant brown macroalgae from the Antarctic Peninsula. J. Exp. Mar. Biol. Ecol. 2005, 322, 1–12. [Google Scholar] [CrossRef]
- Fairhead, V.A.; Amsler, C.D.; McCLintock, J.B.; Baker, B.J. Lack of defence or phlorotannin induction by UV radiation or mesograzers in Desmarestia anceps and D. menziesii (phaeophyceae). J. Phycol. 2006, 42, 1174–1183. [Google Scholar] [CrossRef]
- Döhler, G. Effect of ultraviolet radiation on pigmentation and nitrogen metabolism of antarctic phytoplankton and ice algae. J. Plant Physiol. 1998, 153, 603–609. [Google Scholar] [CrossRef]
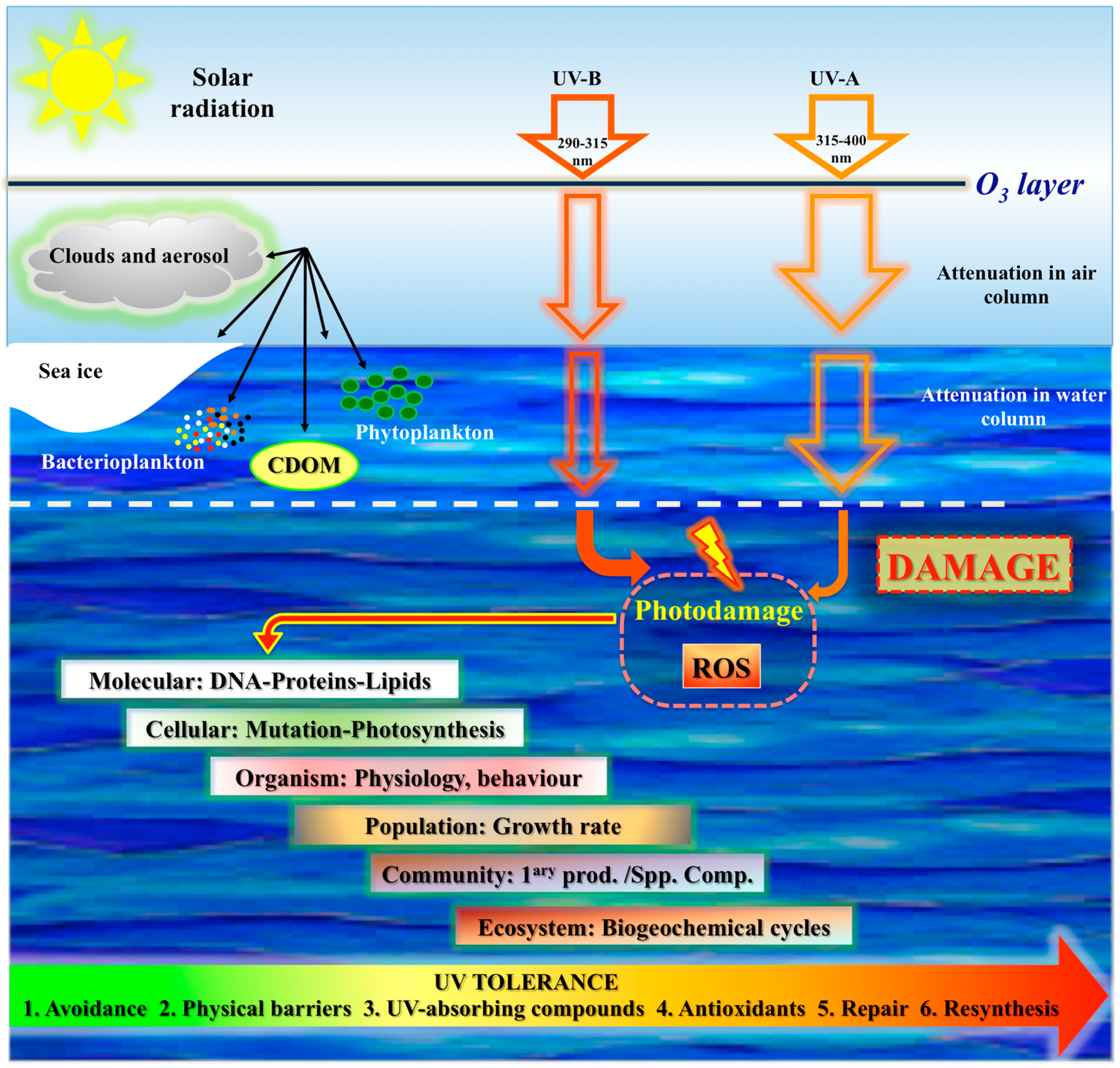
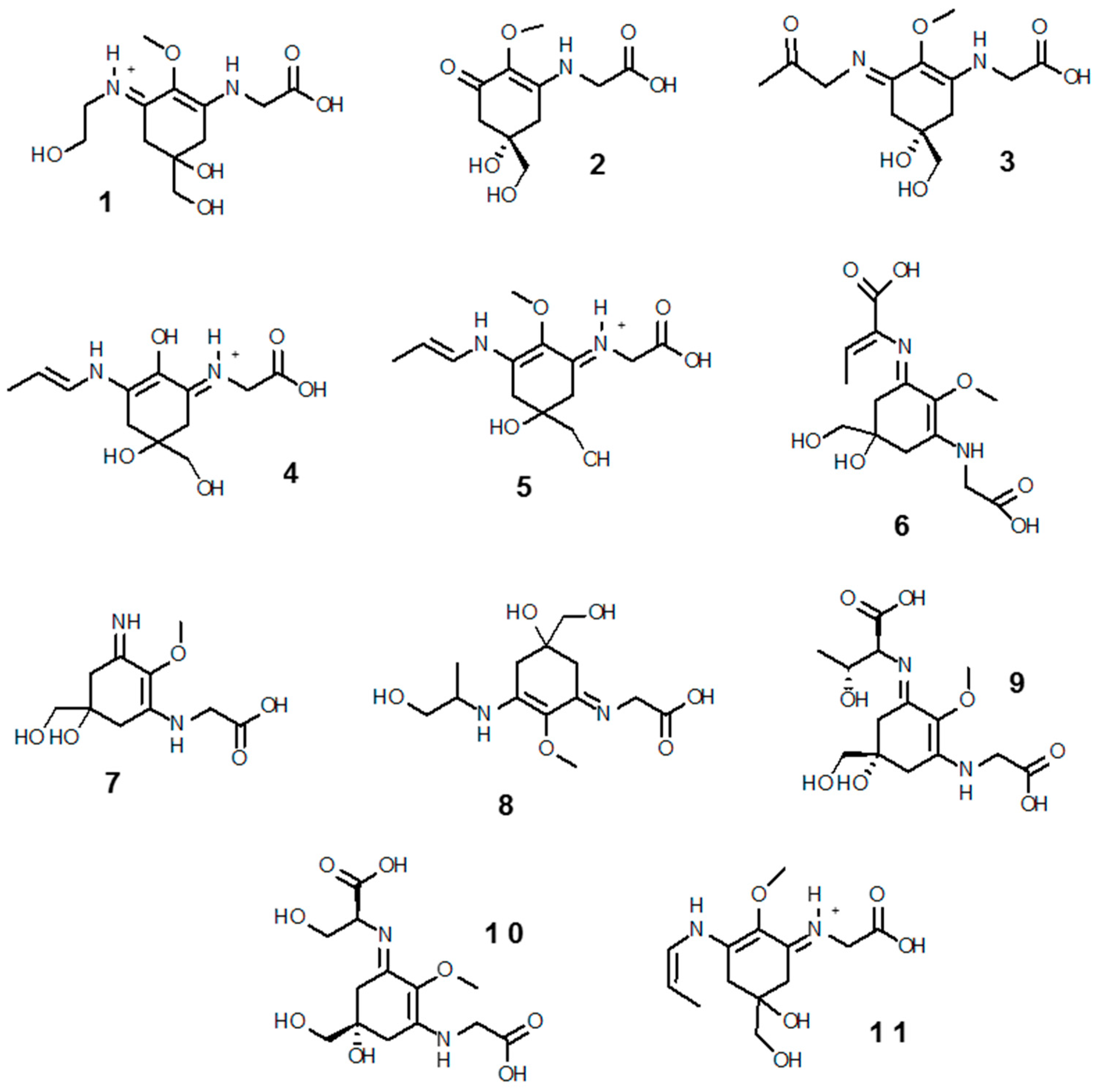
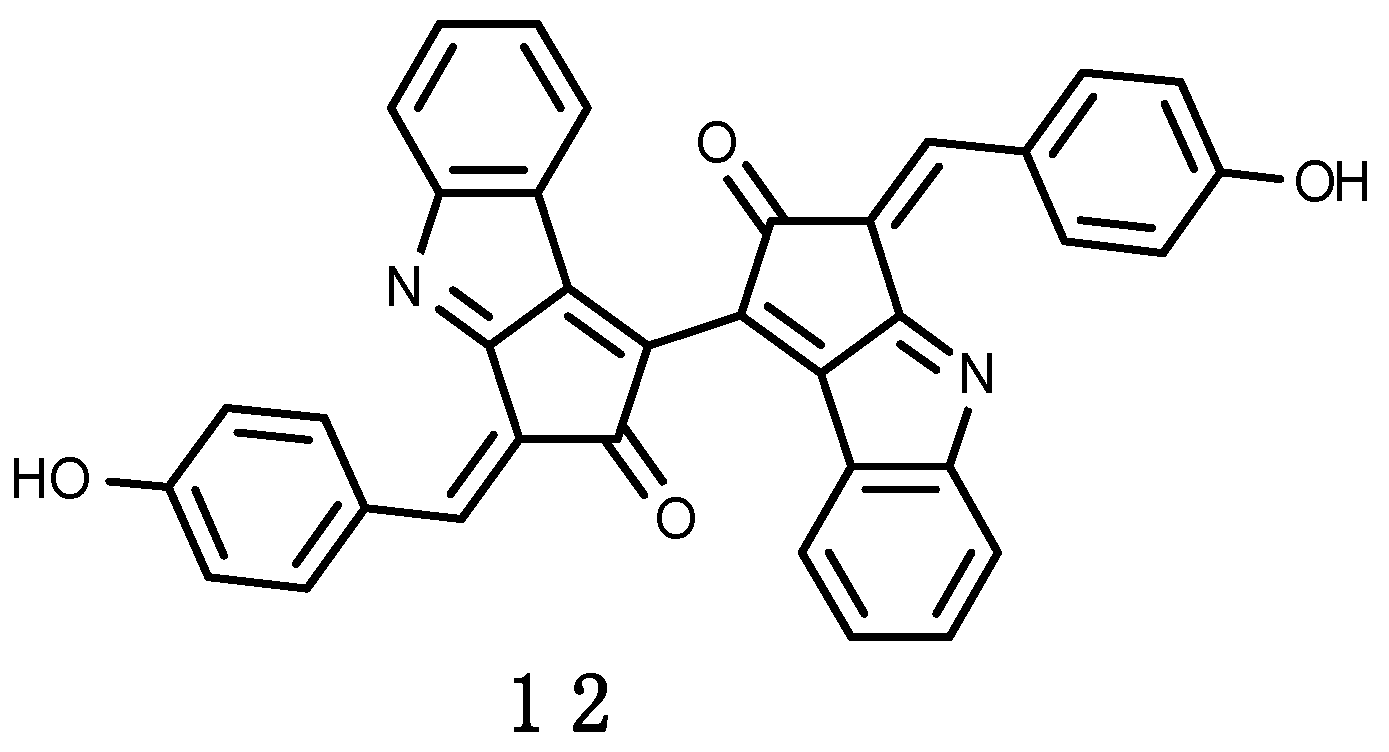
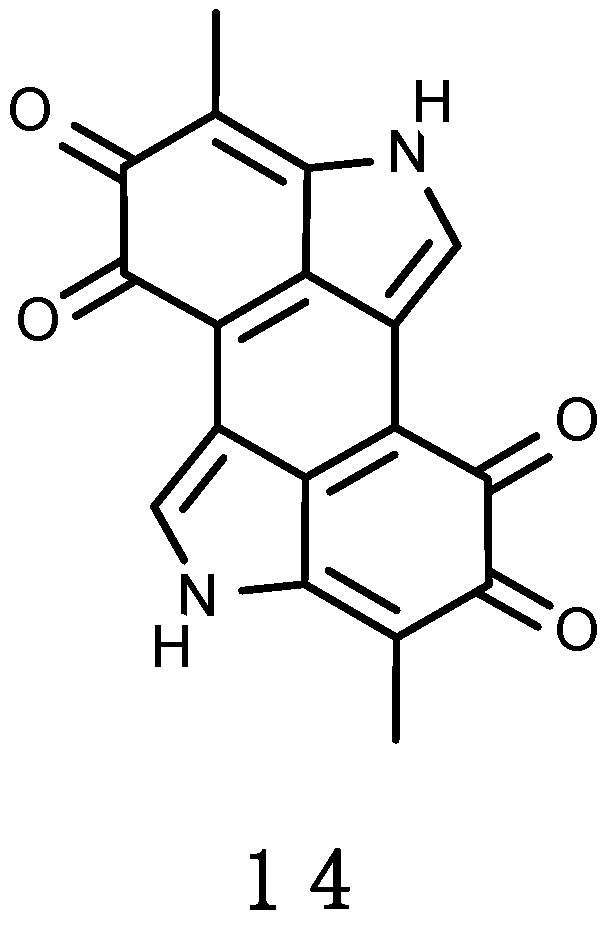
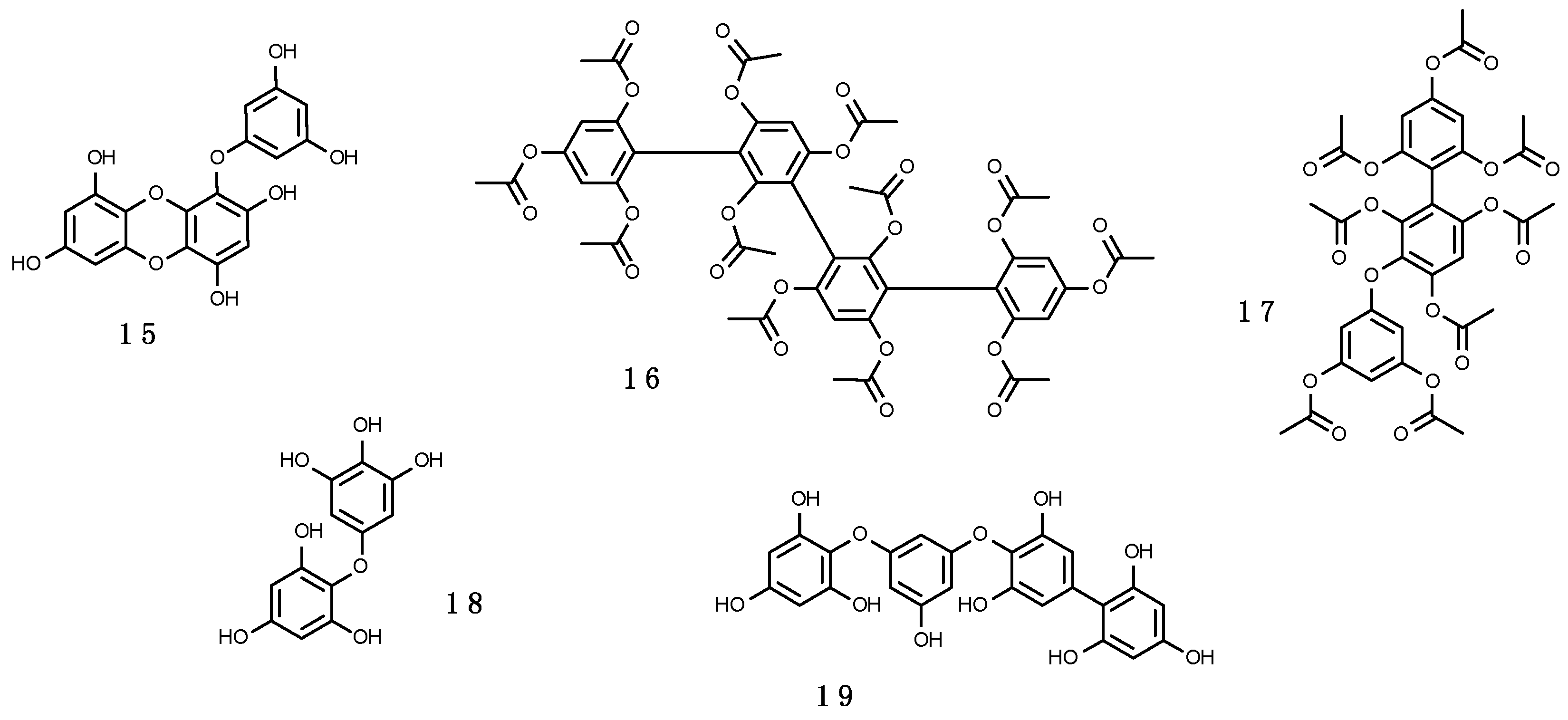
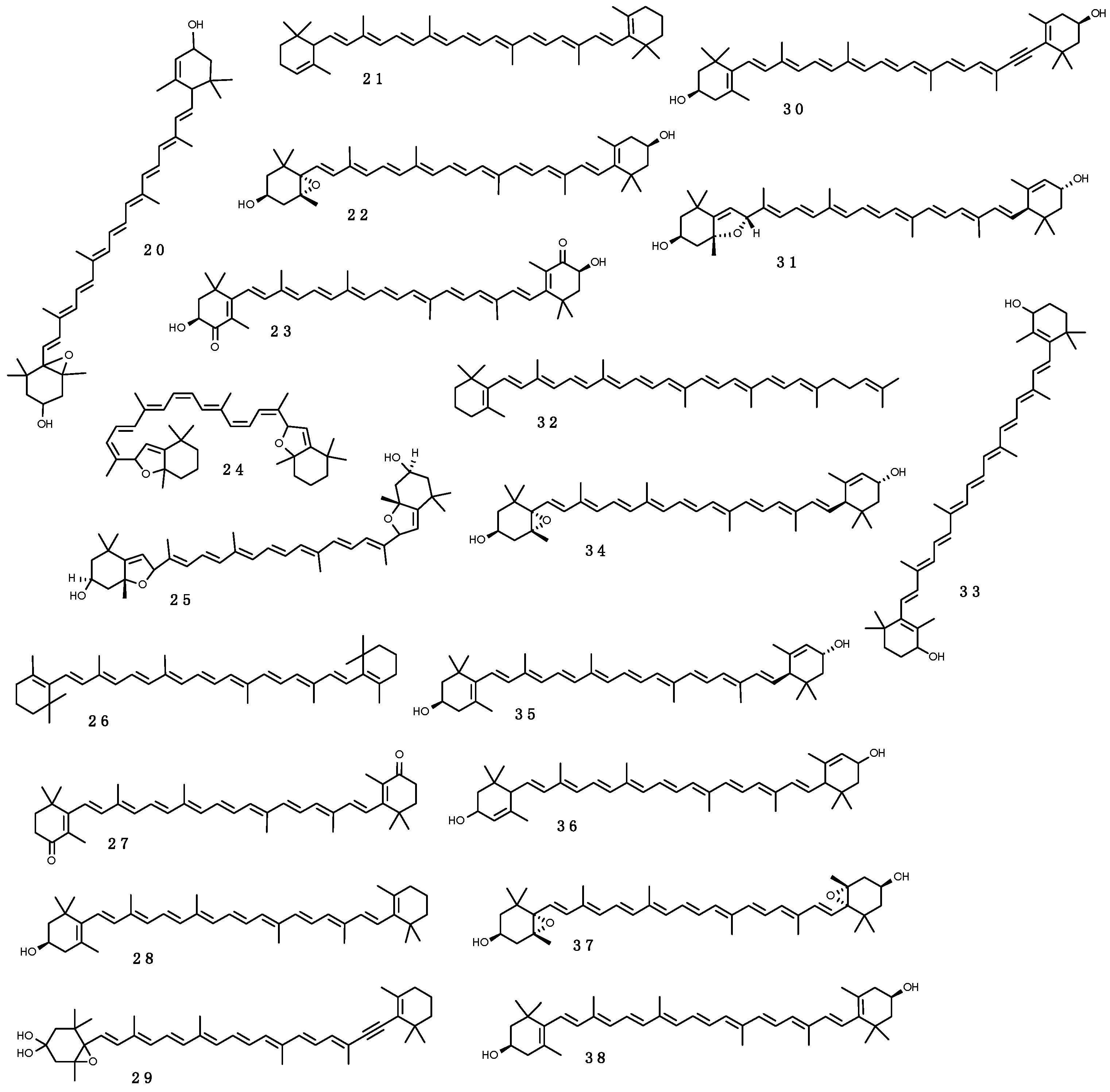
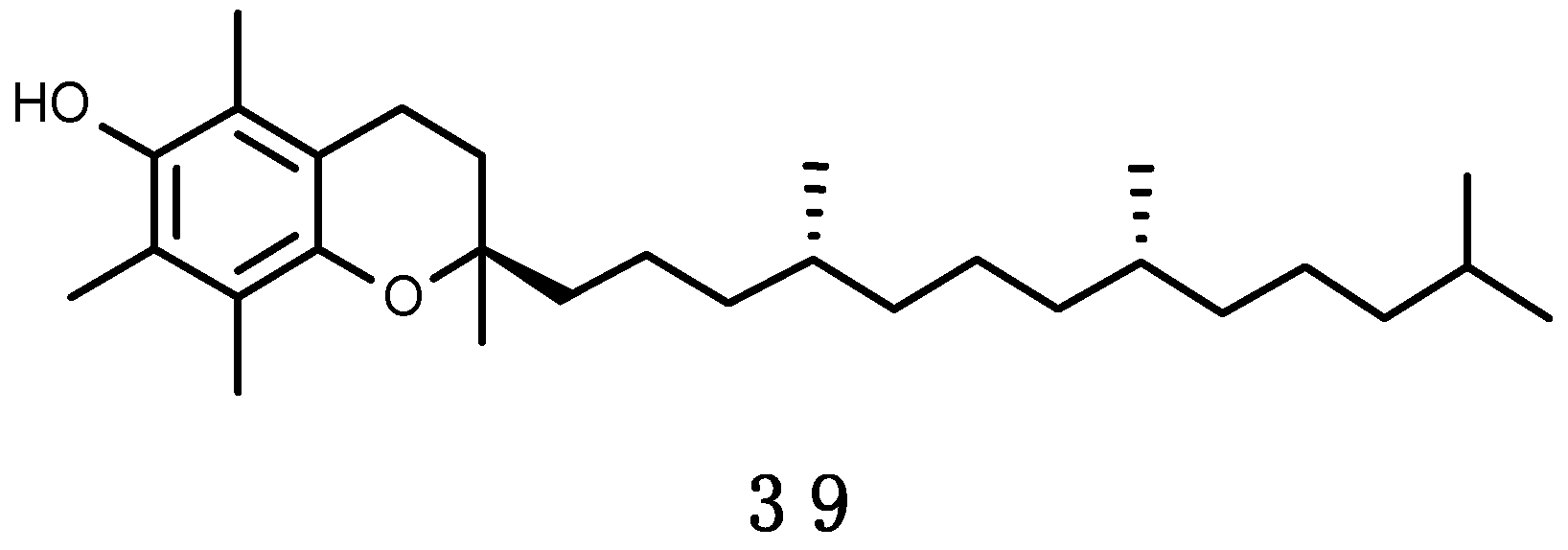
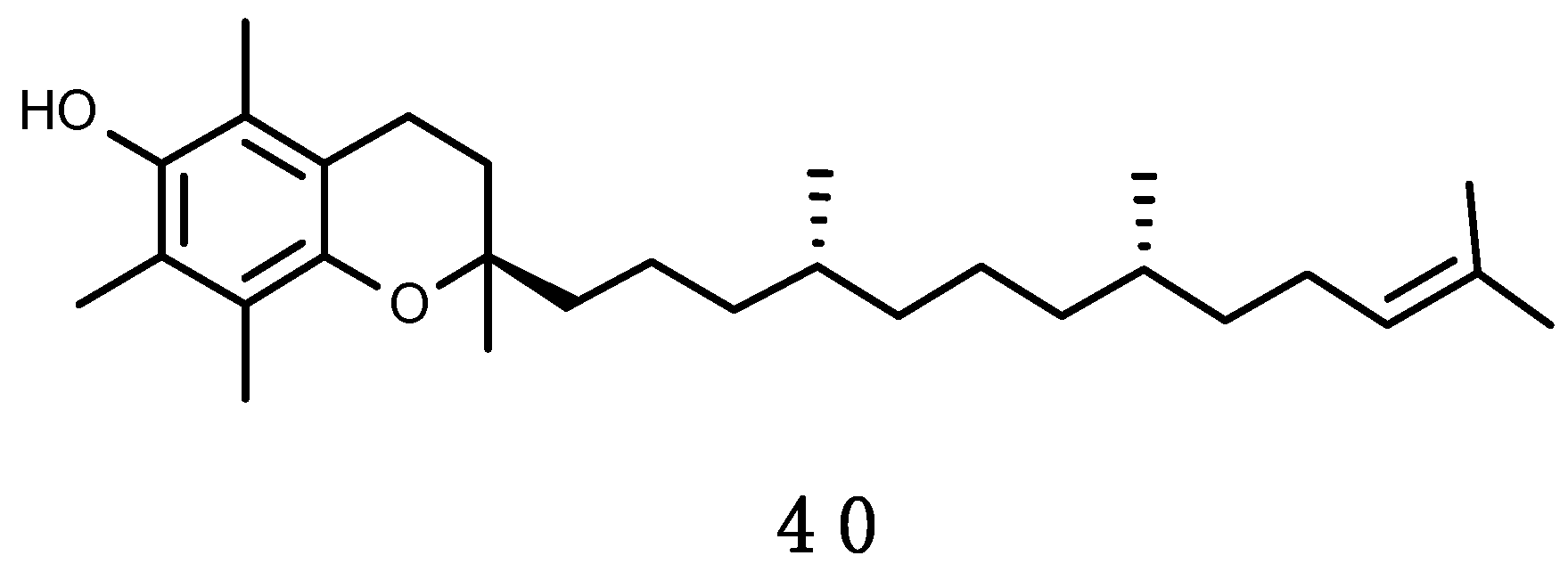
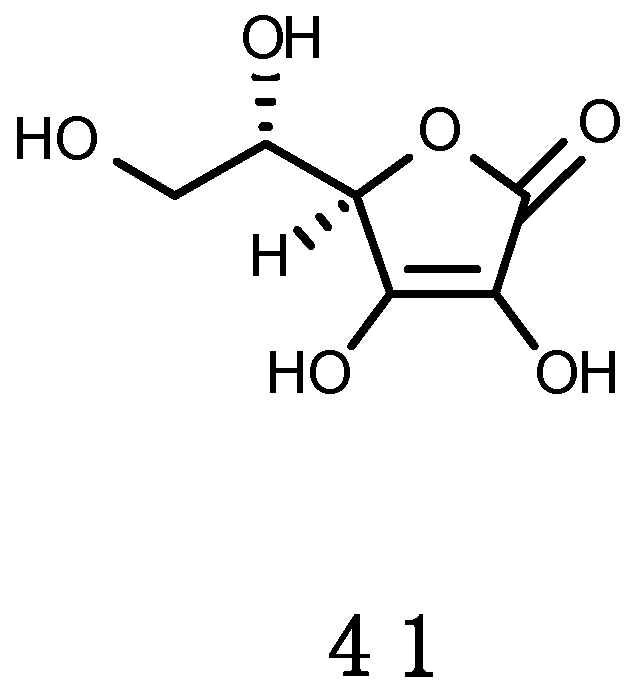
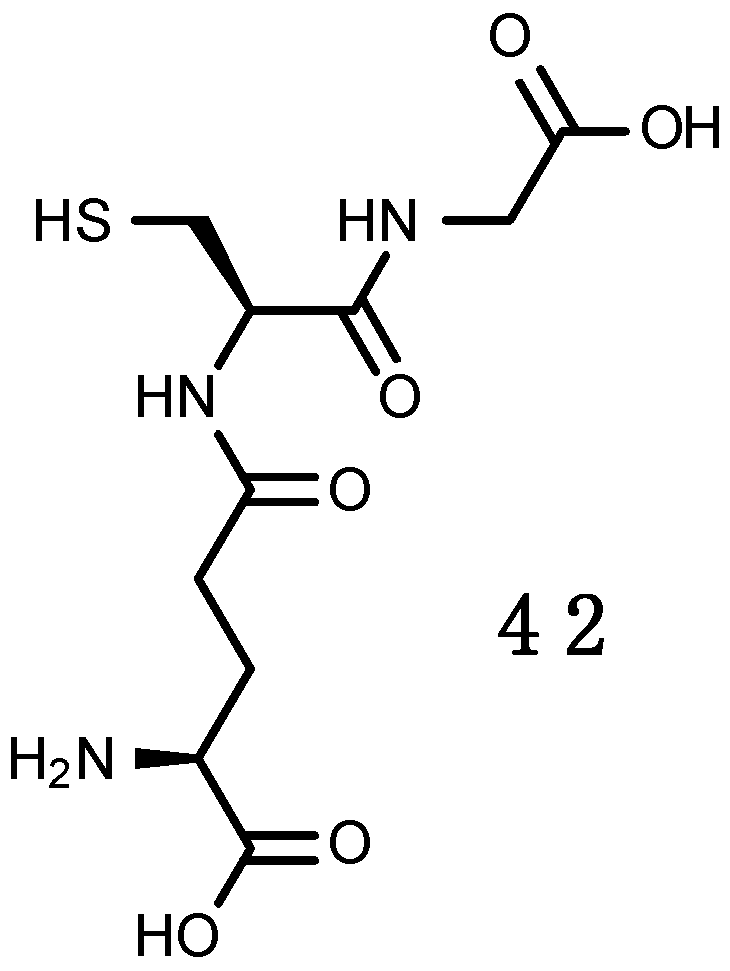
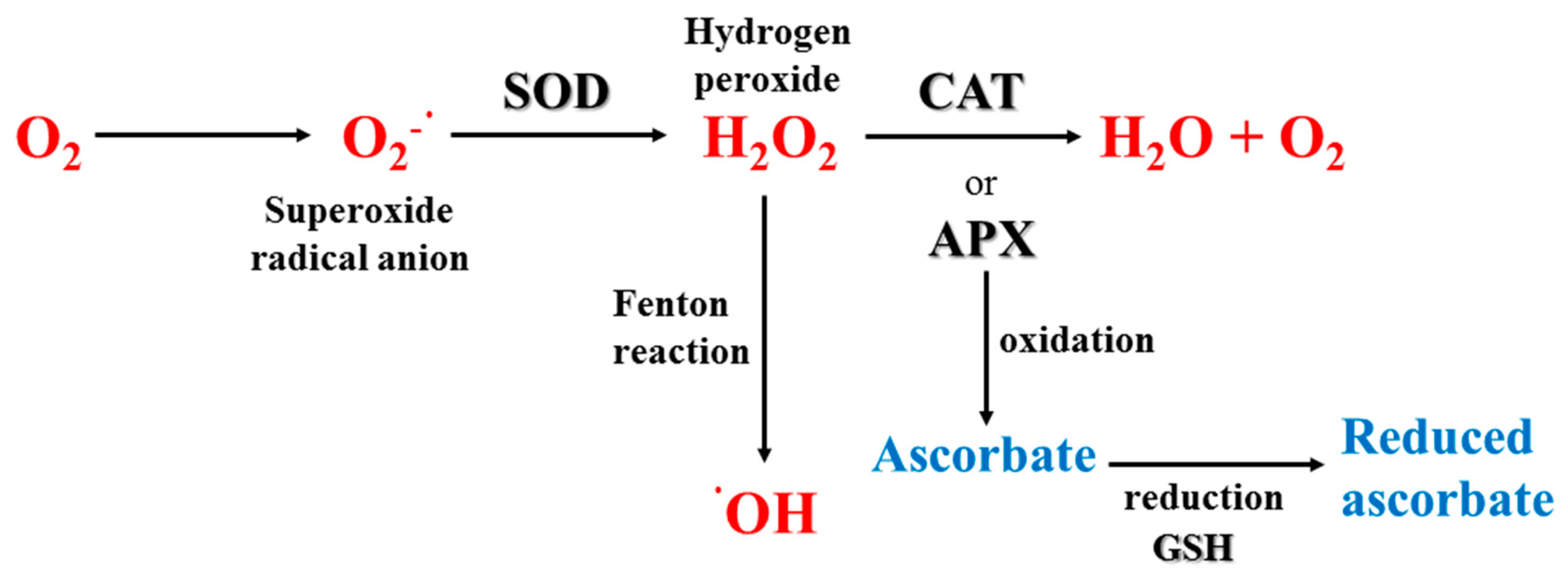
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Mar. Drugs 2018, 16, 336. https://doi.org/10.3390/md16090336
Núñez-Pons L, Avila C, Romano G, Verde C, Giordano D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Marine Drugs. 2018; 16(9):336. https://doi.org/10.3390/md16090336
Chicago/Turabian StyleNúñez-Pons, Laura, Conxita Avila, Giovanna Romano, Cinzia Verde, and Daniela Giordano. 2018. "UV-Protective Compounds in Marine Organisms from the Southern Ocean" Marine Drugs 16, no. 9: 336. https://doi.org/10.3390/md16090336
APA StyleNúñez-Pons, L., Avila, C., Romano, G., Verde, C., & Giordano, D. (2018). UV-Protective Compounds in Marine Organisms from the Southern Ocean. Marine Drugs, 16(9), 336. https://doi.org/10.3390/md16090336









