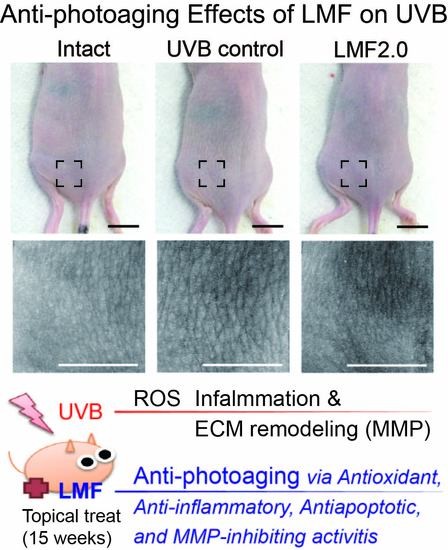
Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice
Abstract
1. Introduction
2. Results
2.1. Body Weight Changes
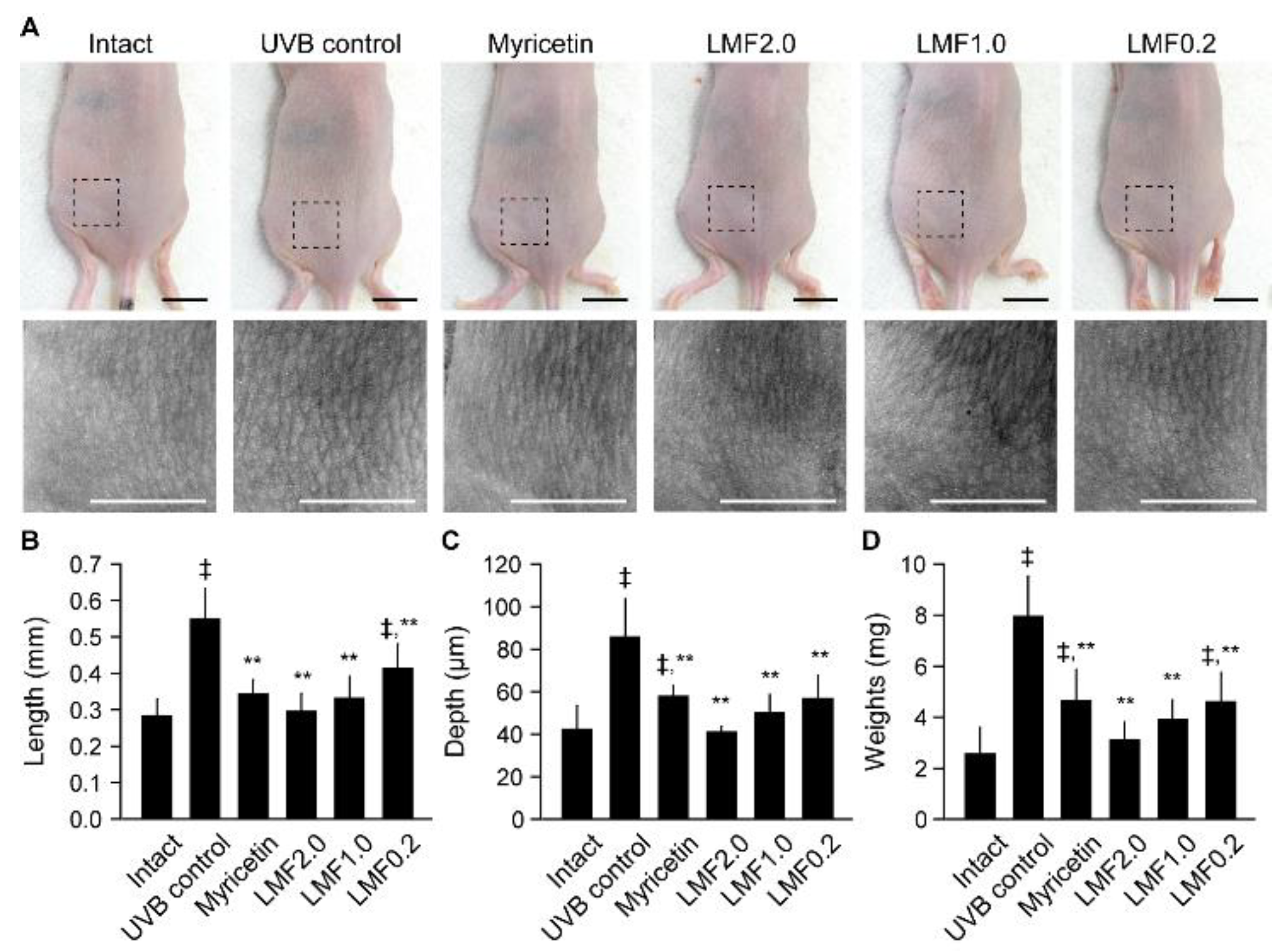
2.2. Wrinkle Formation and Edema in UVB-Irradiated Skin
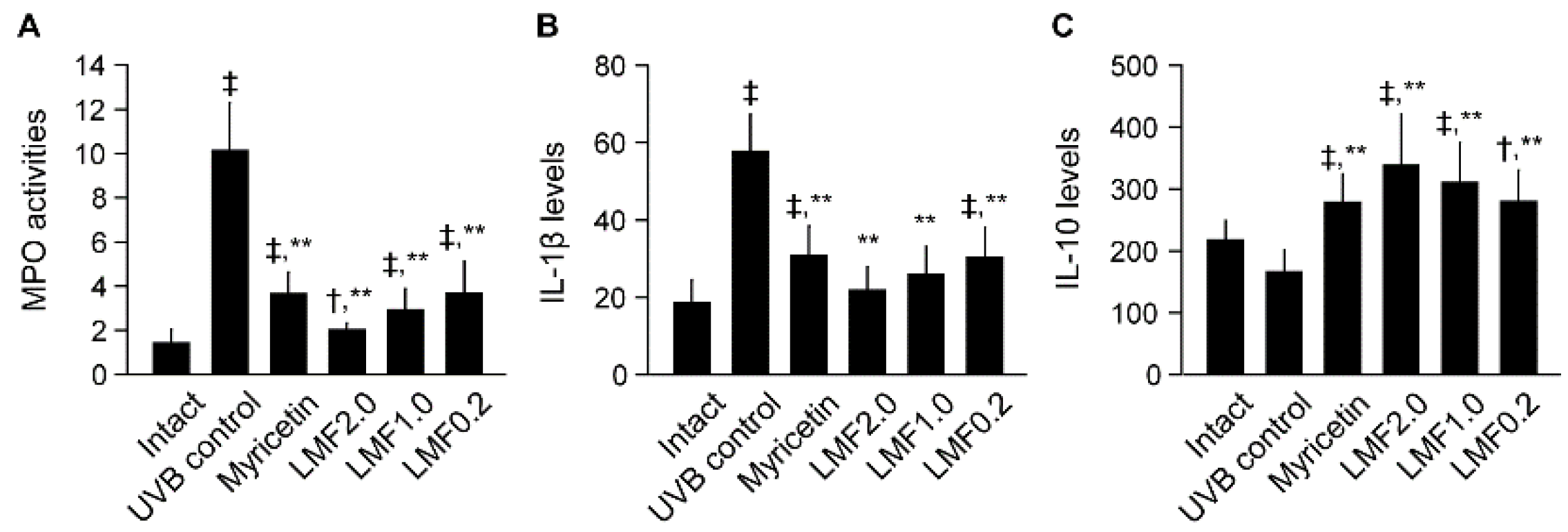
2.3. UVB-Irradiated Skin Inflammation
2.4. Antioxidant Activities in UVB-Irradiated Skin
2.5. mRNA Expression of MMPs Related to Skin Photoaging
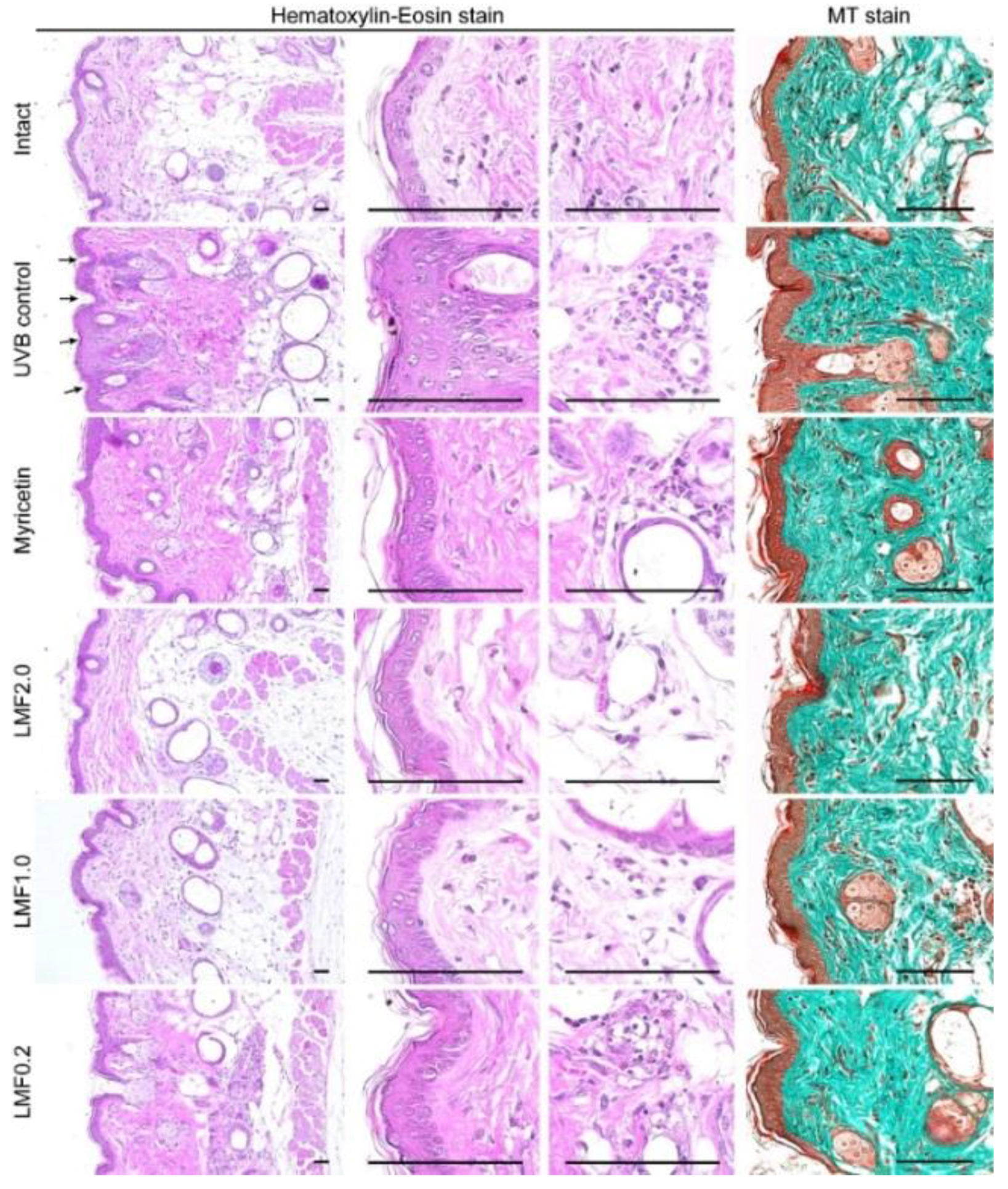
2.6. Histopathological Changes
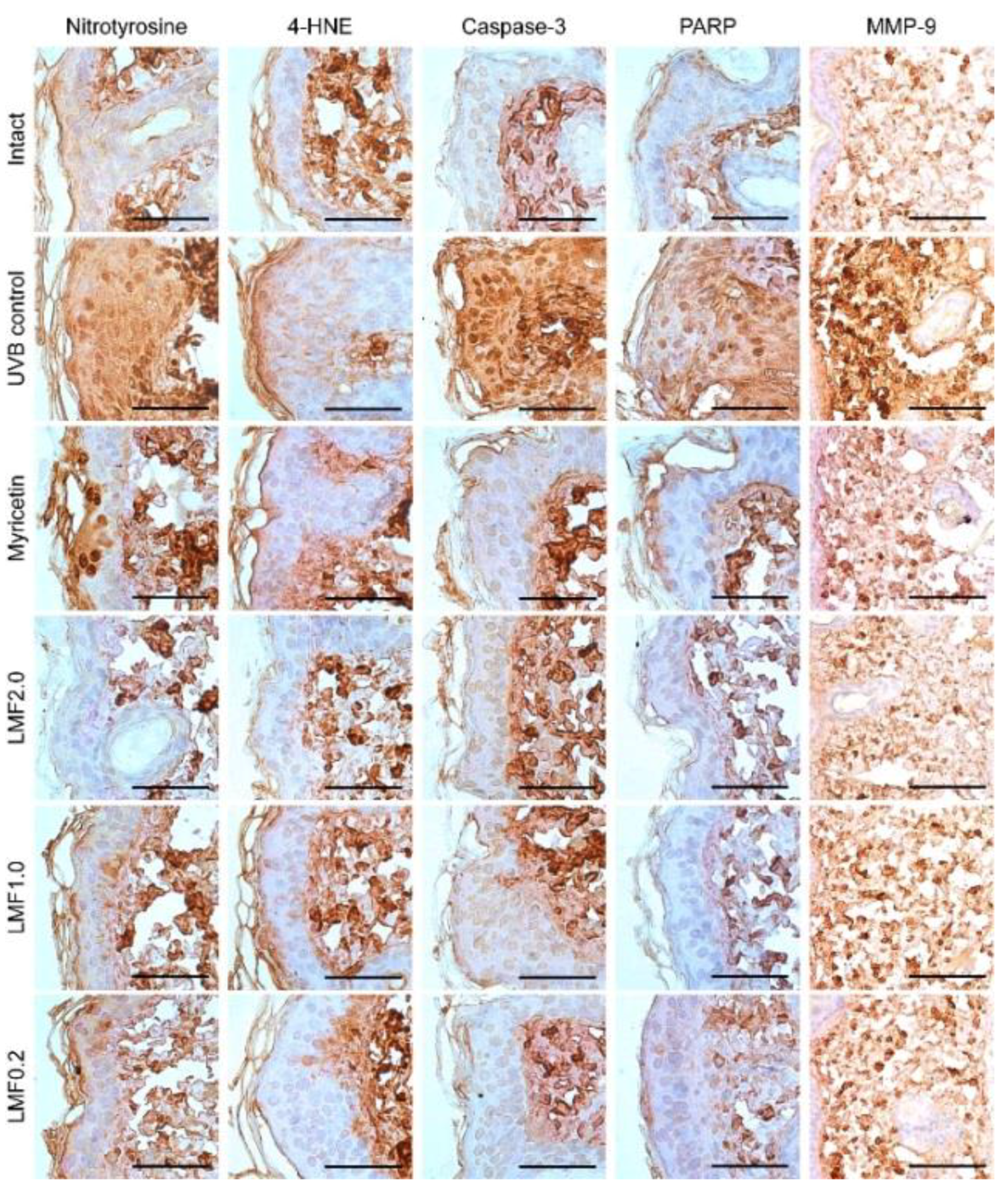
2.7. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Skin Photoaging Model and Treatment
4.4. Macroscopic Analysis of UVB-Irradiated Skin
4.5. Measurement of Leukocyte Migration to UVB-Irradiated Skin
4.6. Measurement of IL-1β and IL-10 in UVB-Irradiated Skin
4.7. Antioxidant Activities in UVB-Irradiated Skin
4.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
4.9. Histopathology
4.10. Immunohistochemistry
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2006, 150, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Krause, R. Vitamin D and UV exposure in chronic kidney disease. Dermatoendocrinol 2013, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Photoaging: Prevention and topical treatments. Am. J. Clin. Dermatol. 2010, 11, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.S.; Kang, N.J.; Bode, A.M.; Dong, Z.; et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Sim, G.S.; Lee, B.C.; Cho, H.S.; Lee, J.W.; Kim, J.H.; Lee, D.H.; Kim, J.H.; Pyo, H.B.; Moon, D.C.; Oh, K.W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm Res. 2007, 30, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-kappaB signaling pathways in SKH-1 mice skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, O.K.; Lee, J.; Lee, M.J.; Kang, N.; Hwang, J.K. Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutr. Res. Pract. 2014, 8, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, J.; Bhatt, P. Ultraviolet-B Protective Effect of Flavonoids from Eugenia caryophylata on Human Dermal Fibroblast Cells. Pharmacogn. Mag. 2015, 11 (Suppl. 3), S397–S406. [Google Scholar] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Durr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [PubMed]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Kawakami, F.; Yoshinaga, K.; Nakano, T. Suppressive Effect of Dietary Fucoidan on Proinflammatory Immune Response and MMP-1 Expression in UVB-Irradiated Mouse Skin. Planta Med. 2015, 81, 1370–1374. [Google Scholar] [PubMed]
- Park, J.H.; Choi, S.H.; Park, S.J.; Lee, Y.J.; Park, J.H.; Song, P.H.; Cho, C.M.; Ku, S.K.; Song, C.H. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs 2017, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Huang, Y.F.; Wang, L.; Xu, L.Q.; Yu, X.T.; Liu, Y.H.; Li, C.L.; Zhan, J.Y.; Su, Z.R.; Chen, J.N.; et al. Photo-protective activity of pogostone against UV-induced skin premature aging in mice. Exp. Gerontol. 2016, 77, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Campanini, M.Z.; Pinho-Ribeiro, F.A.; Ivan, A.L.; Ferreira, V.S.; Vilela, F.M.; Vicentini, F.T.; Martinez, R.M.; Zarpelon, A.C.; Fonseca, M.J.; Faria, T.J.; et al. Efficacy of topical formulations containing Pimenta pseudocaryophyllus extract against UVB-induced oxidative stress and inflammation in hairless mice. J. Photochem. Photobiol. B 2013, 127, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Tedder, T.F.; Steeber, D.A.; Chen, A.; Engel, P. The selectins: Vascular adhesion molecules. FASEB J. 1995, 9, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Mamelak, A.J.; La Morgia, S.; Wang, B.; Feliciani, C.; Tulli, A.; Sauder, D.N. The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J. Am. Acad. Dermatol. 2004, 50, 657–675, quiz 676–678. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, H.P. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med. 2007, 73, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Isnard, N.; Peterszegi, G.; Robert, A.M.; Robert, L. Regulation of elastase-type endopeptidase activity, MMP-2 and MMP-9 expression and activation in human dermal fibroblasts by fucose and a fucose-rich polysaccharide. Biomed. Pharmacother. 2002, 56, 258–264. [Google Scholar] [CrossRef]
- Robert, L.; Fodil-Bourahla, I.; Bizbiz, L.; Robert, A.M. Effect of L-fucose and fucose-rich polysaccharides on elastin biosynthesis, in vivo and in vitro. Biomed. Pharmacother. 2004, 58, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Fodil-Bourahla, I.; Bizbiz, L.; Schoevaert, D.; Robert, A.M.; Robert, L. Effect of L-fucose and fucose-rich oligo- and polysaccharides (FROP-s) on skin aging: Penetration, skin tissue production and fibrillogenesis. Biomed. Pharmacother. 2003, 57, 209–215. [Google Scholar] [CrossRef]
- Kulms, D.; Schwarz, T. Molecular mechanisms involved in UV-induced apoptotic cell death. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Lu, M.K.; Leng, P.J.; Tsao, S.M.; Wu, Y.C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017, 7, 44990. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Liu, P.Y.; Chen, Y.A.; Tseng, H.Y.; Shen, P.C.; Hwang, P.A.; Hsu, H.L. Oligo-Fucoidan prevents IL-6 and CCL2 production and cooperates with p53 to suppress ATM signaling and tumor progression. Sci. Rep. 2017, 7, 11864. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Q.; Cui, W.; Zeng, Z.; Yang, W.; Zhang, C.; Zhao, H.; Gao, W.; Wang, X.; Luo, D. Low molecular weight fucoidan alleviates cardiac dysfunction in diabetic Goto-Kakizaki rats by reducing oxidative stress and cardiomyocyte apoptosis. J. Diabetes Res. 2014, 2014, 420929. [Google Scholar] [CrossRef] [PubMed]
- Matou, S.; Helley, D.; Chabut, D.; Bros, A.; Fischer, A.M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002, 106, 213–221. [Google Scholar] [CrossRef]
- McCaffrey, T.A.; Falcone, D.J.; Vicente, D.; Du, B.; Consigli, S.; Borth, W. Protection of transforming growth factor-beta 1 activity by heparin and fucoidan. J. Cell. Physiol. 1994, 159, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Xu, W.; Liang, J.W.; Wang, C.S.; Kang, Y. Effect of Fucoidan on B16 Murine Melanoma Cell Melanin Formation and Apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Kusaykin, M.I.; Grachev, A.A.; Tsvetkova, E.A.; Zvyagintseva, T.N.; Nifantiev, N.E.; Usov, A.I. Effect of enzyme preparation from the marine mollusk Littorina kurila on fucoidan from the brown alga Fucus distichus. Biochemistry 2005, 70, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Choi, B.R.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Ku, S.K.; Lee, Y.J. Beneficial effects of dried pomegranate juice concentrated powder on ultraviolet B-induced skin photoaging in hairless mice. Exp. Ther. Med. 2017, 14, 1023–1036. [Google Scholar] [CrossRef] [PubMed]

| GSH (μM/mg) | MDA (nM/mg) | Superoxide Anion (NBT Reduction) | |
|---|---|---|---|
| Intact | 1.47 ± 0.62 | 0.34 ± 0.11 | 0.36 ± 0.08 |
| UVB control | 0.41 ± 0.09 ‡ | 1.68 ± 0.41 ‡ | 1.03 ± 0.18 ‡ |
| Myricetin | 0.95 ± 0.15 †,** | 0.69 ± 0.20 ‡,** | 0.56 ± 0.16 ‡,** |
| LMF2.0 | 1.44 ± 0.14 ** | 0.40 ± 0.10 ** | 0.36 ± 0.08 ** |
| LMF1.0 | 1.36 ± 0.20 ** | 0.51 ± 0.13 †,** | 0.49 ± 0.08 †,** |
| LMF0.2 | 0.94 ± 0.18 †,** | 0.67 ± 0.13 ‡,** | 0.56 ± 0.15 ‡,** |
| MMP-1 | MMP-9 | MMP-13 | GSH Reductase | Nox2 | |
|---|---|---|---|---|---|
| Intact | 1.04 ± 0.09 | 1.07 ± 0.10 | 1.06 ± 0.07 | 1.01 ± 0.09 | 1.01 ± 0.08 |
| UVB control | 2.02 ± 0.20 ‡ | 1.93 ± 0.23 ‡ | 2.29 ± 0.25 ‡ | 0.79 ± 0.15 ‡ | 1.71 ± 0.22 ‡ |
| Myricetin | 1.34 ± 0.18 ‡,** | 1.31 ± 0.14 ‡,** | 1.47 ± 0.21 ‡,** | 1.28 ± 0.16 ‡,** | 1.22 ± 0.14 ‡,** |
| LMF2.0 | 1.06 ± 0.09 ** | 1.12 ± 0.08 ** | 1.15 ± 0.07 †,** | 1.93 ± 0.19 ‡,** | 1.06 ± 0.08 ** |
| LMF1.0 | 1.20 ± 0.09 †,** | 1.16 ± 0.09 †,** | 1.26 ± 0.05 ‡,** | 1.58 ± 0.36 ‡,** | 1.12 ± 0.08 †,** |
| LMF0.2 | 1.33 ± 0.14 ‡,** | 1.28 ± 0.09 ‡,** | 1.47 ± 0.17 ‡,** | 1.30 ± 0.12 ‡,** | 1.22 ± 0.05 ‡,** |
| Microfolds (Folds/mm) | Epi. Thickness (μm) | IF Cells (Cells/mm2) | Collagen Fiber (%/mm2) | |
|---|---|---|---|---|
| Intact | 10.50 ± 3.63 | 20.54 ± 2.52 | 9.50 ± 2.98 | 45.31 ± 5.75 |
| UVB control | 74.75 ± 10.94 ‡ | 48.28 ± 5.04 ‡ | 269.50 ± 50.65 ‡ | 82.54 ± 8.20 ‡ |
| Myricetin | 39.50 ± 13.73 ‡,** | 30.26 ± 5.08 ‡,** | 204.50 ± 51.29 ‡,* | 59.32 ± 6.17 ‡,** |
| LMF2.0 | 19.63 ± 4.17 ‡,** | 26.75 ± 4.04 ‡,** | 31.00 ± 7.19 ‡,** | 48.44 ± 2.97 ** |
| LMF1.0 | 38.63 ± 5.10 ‡,** | 30.63 ± 2.04 ‡,** | 61.25 ± 14.57 ‡,** | 55.72 ± 7.89 ‡,** |
| LMF0.2 | 56.88 ± 5.99 ‡,** | 32.89 ± 3.83 ‡,** | 140.00 ± 38.37 ‡,** | 59.77 ± 8.94 ‡,** |
| Nitrotyrosine | 4-HNE | Caspase-3 | PARP | MMP-9 | |
|---|---|---|---|---|---|
| Intact | 12.00 ± 4.21 | 12.50 ± 3.07 | 17.38 ± 3.25 | 15.13 ± 3.04 | 31.25 ± 8.58 |
| UVB control | 86.88 ± 10.11 ‡ | 78.00 ± 10.72 ‡ | 83.50 ± 10.17 ‡ | 79.13 ± 11.00 ‡ | 81.75 ±11.62 ‡ |
| Myricetin | 50.00 ± 6.70 ‡,** | 43.13 ± 12.17 ‡,** | 32.00 ± 6.55 ‡,** | 38.88 ± 8.01 ‡,** | 51.88 ± 8.04 ‡,** |
| LMF2.0 | 21.38 ± 4.44 ‡,** | 19.50 ± 2.56 ‡,** | 21.13 ± 2.90 †,** | 21.13 ± 2.53 ‡,** | 43.00 ± 5.13 ‡,** |
| LMF1.0 | 36.25 ± 5.65 ‡,** | 33.00 ± 3.70 ‡,** | 26.13 ± 4.22 ‡,** | 23.88 ± 3.14 ‡,** | 48.63 ± 5.01 ‡,** |
| LMF0.2 | 50.25 ± 6.11 ‡,** | 39.75 ± 4.53 ‡,** | 30.25 ± 3.20 ‡,** | 36.75 ± 5.01 ‡,** | 53.00 ± 8.02 ‡,** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-I.; Oh, W.-S.; Song, P.H.; Yun, S.; Kwon, Y.-S.; Lee, Y.J.; Ku, S.-K.; Song, C.-H.; Oh, T.-H. Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice. Mar. Drugs 2018, 16, 286. https://doi.org/10.3390/md16080286
Kim Y-I, Oh W-S, Song PH, Yun S, Kwon Y-S, Lee YJ, Ku S-K, Song C-H, Oh T-H. Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice. Marine Drugs. 2018; 16(8):286. https://doi.org/10.3390/md16080286
Chicago/Turabian StyleKim, Young-In, Won-Seok Oh, Phil Hyun Song, Sungho Yun, Young-Sam Kwon, Young Joon Lee, Sae-Kwang Ku, Chang-Hyun Song, and Tae-Ho Oh. 2018. "Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice" Marine Drugs 16, no. 8: 286. https://doi.org/10.3390/md16080286
APA StyleKim, Y.-I., Oh, W.-S., Song, P. H., Yun, S., Kwon, Y.-S., Lee, Y. J., Ku, S.-K., Song, C.-H., & Oh, T.-H. (2018). Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice. Marine Drugs, 16(8), 286. https://doi.org/10.3390/md16080286