Abstract
Five new cembranoid-related diterpenoids, namely, flexibilisins D and E (1 and 2), secoflexibilisolides A and B (3 and 4), and flexibilisolide H (5), along with nine known compounds (6–14), were isolated from the soft coral Sinularia flexibilis. Their structures were established by extensive spectral analysis. Compound 3 possesses an unusual skeleton that could be biogenetically derived from cembranoids. The cytotoxicity and anti-inflammatory activities of the isolates were investigated, and the results showed that dehydrosinulariolide (7) and 11-epi-sinulariolide acetate (8) exhibited cytotoxicity toward a limited panel of cancer cell lines and 14-deoxycrassin (9) displayed anti-inflammatory activity by inhibition of superoxide anion generation and elastase release in N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLF/CB)-induced human neutrophils.
1. Introduction
Soft corals have been known to be the organisms possessing secondary metabolites with high diversity in chemical structures [1]. Since 1975, many cembranoid-type natural products with diverse and important biological activities have been isolated from Sinularia flexibilis [2,3,4,5,6,7,8,9,10,11,12,13]. In previous studies of the chemical constituents of Taiwanese soft corals, numerous marine metabolites with cytotoxic [14,15], neuroprotective [16], and anti-inflammatory activities [17,18,19] were also found from Sinularia flexibilis. Some cembranolides, possessing an α-methylene lactone ring, have been discovered as potent cytotoxic agents to a limited panel of cancer cell lines, for example, 14-deoxycrassin [20], and as significant anti-inflammatory agents, for example, sinulariolone acetate [21]. Additionally, cembranoids and related compounds from other coral species were also found to have notable antiviral [22], anti-inflammatory [23,24], and antiproliferative [25,26,27,28] activities. A wide variety of chemical diversity and biological activity of cembranoids encouraged us to search for more natural products from soft coral S. flexibilis, collected off the waters of Taiwan. Herein, we report the isolation of five new cembrane-related metabolites, namely, flexbilisins D and E (1 and 2), secoflexibilisolides A and B (3 and 4), and a cembranolide flexibilisolide H (5) (Figure 1), and nine known compounds, including 6R-hydroxysinulariolide (6) [3], 11-dehydrosinulariolide (7) [18], 11-epi-sinulariolide acetate (8) [13], 14-deoxycrassin (9) [20], 3,4:8,11-bisepoxy-7-acetoxycembra-15(17)-en-1,12-olide (10) [6], sinulariolide (11) [2], sinulaflexiolide E (12) [10], querciformolide A (13) [21], and flexibilisquinone (14) [19] (Figure 2). Their structures were established by spectroscopic analysis including infrared (IR), mass spectrometry (MS), and nuclear magnetic resonance (NMR) data (Figures S1–S14), as well as chemical transformation. The biogenetic origins of 3 and 4 were postulated to demonstrate the relationship between stereochemistry and biogenetic implications. The cytotoxicity of the isolates toward a limited panel of cancer cell lines and their inhibition of superoxide anion generation and elastase release in N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLF/CB)-induced human neutrophils were also investigated.
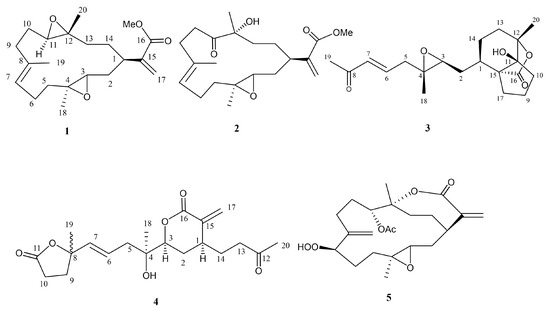
Figure 1.
Structures of 1–5.
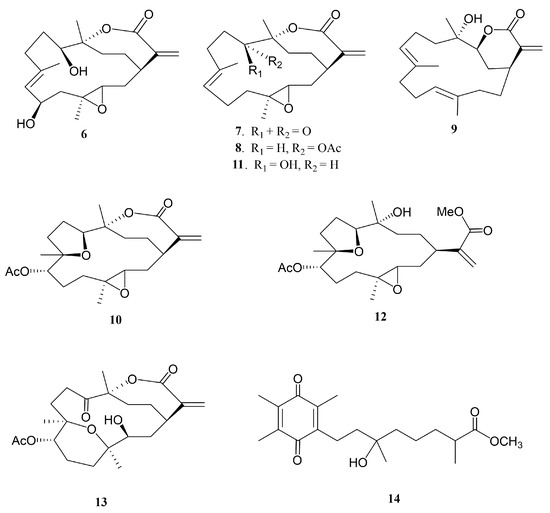
Figure 2.
Structures of metabolites 6−14.
2. Results and Discussion
Flexbilisin D (1) was obtained as a colorless oil and its high-resolution electrospray ionization mass spectrometry (HRESIMS) (m/z 371.2196 [M + Na]+) data established the molecular formula of C21H32O4, indicating six degrees of unsaturation. The IR spectrum showed the absorption bands of carbonyl group (1716 cm−1) and double bond (1647 cm−1). The NMR data (Table 1), including 13C NMR and distortion less enhancement by polarization transfer (DEPT) spectra, displayed 21 carbin signals which can be classified as three methyls (δc 15.3, 17.0, 17.8), one methoxy (δc 52.0), seven sp3 methylene (δc 22.2, 24.6, 27.4, 31.5, 33.0, 36.0, 36.9), one sp2 methylene (δc 124.4), three sp3 methine (δc 35.2, 60.1, 61.9), one sp2 methine (δc 126.5), two sp3 quaternary (δc 60.2, 60.8), and three sp2 quaternary (134.2, 142.7, 167.3) carbons. The 13C NMR signals at δc 142.7 (C), δc 124.4 (CH2) and the 1H NMR signals at δH 6.26 (1H, s) and δH 5.48 (1H, s) revealed the presence of a 1,1-disubstituted double bond, while those at δc 134.2 (CH), δc 126.5 (C), and δH 5.18 (1H, t, J = 5.6 Hz) are indicative of a trisubstituted double bond. Two trisubstituted epoxides were identified from the NMR signals at δc 61.9 CH, 60.8 C and 60.2 C, 60.1 CH; δH 2.65 dd, J = 10.0 and 3.2 Hz; 2.81 dd, J = 10.0 and 4.0 Hz. The molecular skeleton of 1 was established by the above results, as well as the correlations spectroscopy (COSY) and heteronuclear multiple bond correlation (HMBC) correlations as shown in Figure 3. By analysis of the COSY correlations, it was possible to identify three partial structures (a–c). The fact that the methyl ester group [CO (δc 167.3)/OMe (δc 52.0; δH 3.76)] was on C-15 (δc 142.7) was confirmed by the HMBC correlation from H2-17 (δH 6.26, 5.48) to C-16 (δc 167.3). The two epoxides were assigned at 3,4- and 11,12-positions with methyl substituents at C-4 (δc 60.2) and C-12 (δc 60.8) according to HMBC correlations from H3-18 (δH 1.29) to C-3 (δc 60.1), C-4, and C-5 (δc 36.9), as well as H3-20 (δH 1.23) to C-11(δc 61.9), C-12, and C-13 (δc 33.0), respectively. Together with other key HMBC correlations from H3-19 (δH 1.65) to C-7 (δc 126.5), C-8 (δc 134.2), and C-9 (δc 36.0), as well as H2-17 to C-1 (δc 35.2), C-15, and C-16 permitted the establishment of the carbon skeleton.

Table 1.
1H and 13C NMR spectroscopic data of 1 and 2.
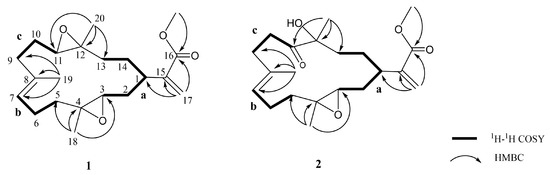
Figure 3.
Key COSY and HMBC correlations for 1 and 2.
The relative configuration of 1 was determined by the nuclear Overhauser effect spectroscopy (NOESY) experiment as shown in Figure 4. Assuming that H-1 is α-oriented, which showed NOESY correlations to one of H2-2 (δH 1.36) and one of H2-13 (δH 1.70); thus, another of H2-2 (δH 2.05) and H2-13 (δH 1.20) were β-oriented. Two trans epoxides were then assigned according to the NOESY correlations from H-11 to H-13β and from H-13α to H3-20, as well as those from H3-18 to H-2α. The proposed structure of 1 is in agreement with the most stable conformation (Figure 4) generated by an energy-minimized (MM2) force field calculation [29]. Consequently, the relative configurations of C-1, C-3, C-11, and C-12 were determined as 1R*, 3S*, 4S*, 11R*, 12R* (Figure 4).
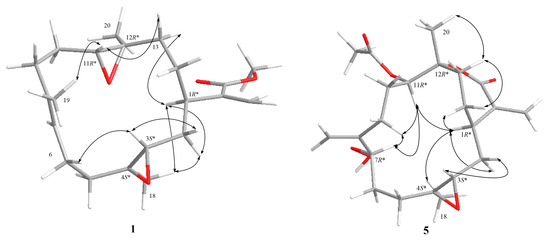
Figure 4.
Selective NOESY correlations for 1 and 5.
Flexibilisin E (2) had a molecular formula C21H32O5 as deduced from HRESIMS data. The 1H and 13C NMR spectra showed that the structure of 2 closely resembled that of 1. By comparing their NMR data (Table 1), significant differences in chemical shifts were observed at C-11 (δc 61.9 for 1; 213.6 for 2), C-12 (δc 60.8 for 1; 78.8 for 2), and C-20 (δc 17.0 for 1; 25.7 for 2), suggesting that 2 is a 12-hydroxy-11-oxo derivative of 1. This was supported by the COSY and HMBC correlations (Figure 3). The structure and absolute configurations of the stereogenic centers of 2 were confirmed by an alkaline hydrolysis of 11-dehydrosinulariolide (7), whose absolute configurations at C-1, C-3, C-4, and C-12 were determined as 1R, 3S, 4S, and 12R, respectively, based on a single-crystal X-ray diffraction analysis [18]. Accordingly, the structure of 2 was determined as shown in Figure 1.
Secoflexibilisolide A (3) was isolated as a colorless oil and its molecular formula was established as C20H28O5 by the observation of a sodiated molecular ion peak at m/z 371.1831 (calcd. for 371.1829 [M + Na]+) in the HRESIMS, indicating seven degrees of unsaturation. Its IR absorption bands suggested the presence of hydroxy (3450 cm−1) and carbonyl (1765 and 1671 cm−1) groups. Three spin systems (a–c), inferred by analysis of the COSY correlations, were constructed as shown in Figure 5. A bicyclo[4.3.0]nonane ring system was established by the HMBC correlations from H3-20 (δH 1.39) to C-11 (δc 85.3), C-12 (δc 84.7), and C-13 (δc 30.4); from H2-10 (δH 2.05, 1.68) to C-11 and C-12; and from H2-17 (δH 2.26, 1.66) to C-1 (δc 34.5), C-15 (δc 60.9), and C-16 (δc 177.6). In addition, a γ–lactone ring between C-16 and C-11 was evidenced by the IR absorption band at 1765 cm−1, which is consistent with perhydroindan analogues [30]. A methyl-substituted epoxy group was evidenced by the HMBC correlations from H3-18 (δH 1.28) to C-3 (δc 61.9), C-4 (δc 58.8), and C-5 (δc 41.2), while an acetyl group was found to be located at C-7 by the HMBC correlations from H3-19 (δH 2.26) to C-7 (δc 134.0) and C-8 (δc 198.3). Accordingly, the planar structure of 3 was deduced as shown in Figure 5.
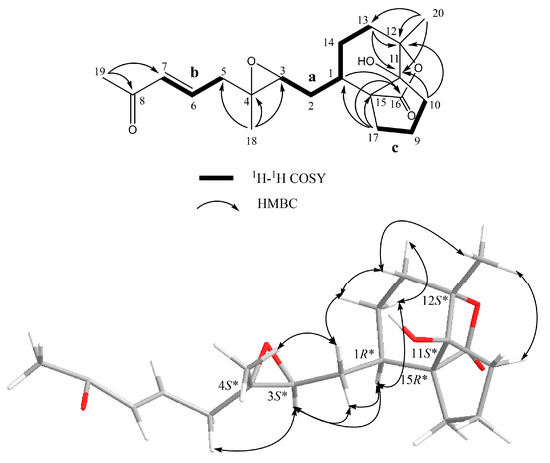
Figure 5.
Key COSY and HMBC correlations and selective NOESY correlations for 3.
Compound 3 can be hypothesized to derive from a precursor with the cembranoid-type skeleton. As shown in Scheme 1, flexibilisolide D, which was also isolated from this coral [17], was suggested as a precursor. Flexibilisolide D was converted to intermediate I by oxidative cleavage and Michael addition. Reduction of the carboxylic acid of the cyclobutane intermediate (II), derived from I by the aldol condensation, would produce an alcohol functionality in III. Rearrangement of the hydroxymethyl cyclobutane through a reductive ring opening in III resulted in a formation of the cyclopentane ring in 3. This suggested that the configurations of C-1, C-3, C-4, and C-12 should be the same as those of flexibilisolide D and related analogues possessing a 3,4-epoxide group, isolated previously from this soft coral [17]. The relative configurations of C-11 and C-15 were determined by a combination of NOESY correlations (Figure 5) and pyridine-induced solvent shift experiment [31]. The NOESY correlations from H3-20 to H2-10 allowed the assignment of the lactone ring as α-oriented. However, the present NOESY data were unable to fully confirm the orientation of OH-11. As a result, the pyridine-induced solvent shift experiment was applied on 3. H-2β (δH 1.66 in pyridine), which is 1,3-diaxial to OH-11, was found to be downfield shifted by 0.28 ppm with respect to H-2β, measured in CDCl3 (Table 2), suggesting the β-orientation of OH-11. Consequently, the relative configurations 1R*, 3S*, 4S*, 11S*, 12S*, 15R* were suggested for 3.
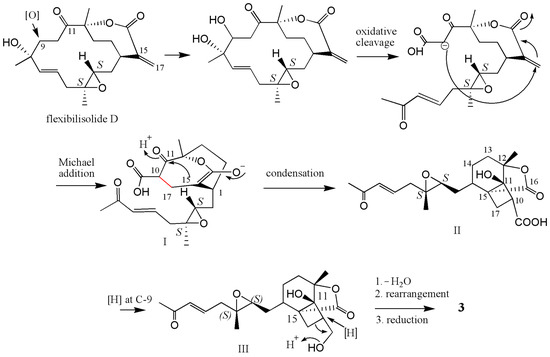
Scheme 1.
Proposed biosynthetic pathway for 3.

Table 2.
1H, 13C NMR data, COSY, and HMBC correlations of 3.
The molecular formula of 4 was found to be C20H28O6 as deduced from the HRESIMS and 13C NMR data, suggesting seven degrees of unsaturation. The 13C NMR data of 4 (Table 3) showed signals attributable to two double bonds (δc 139.5, 126.0, 136.9, 124.1), one ketone carbonyl (δc 205.6), and two ester carbonyls (δc 175.4, 165.3), which accounted for five degrees of unsaturation. Accordingly, the structure of 4 may contain two rings. From the COSY spectrum of 4, it was possible to suggest the presence of three proton sequences for H2-13/H2-14/H-1/H-2/H-3, H2-5/H-6/H-7, and H2-9/H2-10 (a–c, respectively; Figure 6). Key HMBC correlations from H3-20 (δH 1.64) to C-12 (δc 205.6) and C-13 (δc 39.4); from H2-17 (δH 6.40, 5.12) to C-15 (δc 139.5), C-16 (δc 165.3), and C-1 (δc 35.9); from H3-18 (δH 1.00) to C-3 (δc 83.0), C-4 (δc 72.5), and C-5 (δc 41.3); and from H3-19 (δH 1.04) to C-7 (δc 136.9), C-8 (δc 83.9), and C-9 (δc 33.9); as well as H2-10 (δH 2.05, 1.94) to C-11 (δc 175.4) were observed, allowing to establish the planar structure of 4 as shown in Figure 6.

Table 3.
1H and 13C NMR data of compounds 4 and 5.
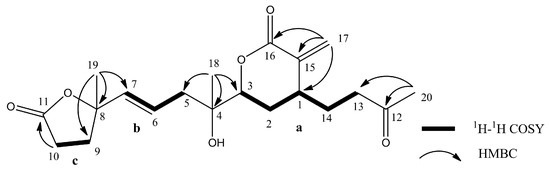
Figure 6.
Key COSY and HMBC correlations for 4.
In the NOESY spectrum of 4, correlations from H-2α to both H-1 and H-3, and from H3-18 to H-3 revealed that H-1 and H-3 are on the same face of the lactone ring. Biogenetically, 4 could be derived from sinuflexolide, which has been reported from the same coral [12], via an oxidative cleavage of the diol groups, epimerization of the vinyl alcohol, and subsequent esterification (Scheme 2). This suggested that the configuration of C-4 should be the same as that of sinuflexolide. Consequently, the relative configurations 1S*, 3S*, 4R* were suggested for 4. The configuration of C-8 remains undetermined.
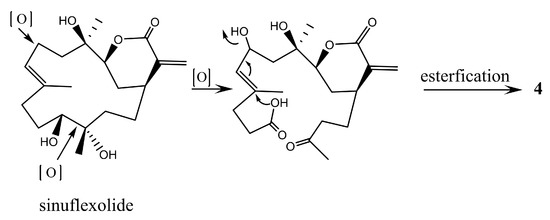
Scheme 2.
Proposed biosynthetic pathway for 4.
The 13C NMR data (Table 3) and the HRESIMS of 5 showed that it has the same molecular formula as flexibilisolide G [17], that is, C22H32O7. They also have similar functional groups, including an α-exomethylene-ε-lactone ring, an acetoxyl group, and a methyl-substituted epoxy group. However, the obvious difference between the two compounds is that the 6,7-double bond in flexibilisolide G is isomerized to an exocyclic double bond at C-8 in 5. In addition, the hydroperoxy group at C-7 in flexibilisolide G was found to migrate to C-8 in 5. The above findings were further confirmed by COSY and HMBC correlations. The 1R*, 12R* configuration of the α-exomethylene-ε-lactone ring was deduced based on the NOESY correlations from H-1 to H-14α, from H3-20 to H-13β, and from H-14β to H-13β. In addition, NOESY correlations from H-11 to both H-7 and H-1 as well as H-1 to H-4 suggested the relative configurations of C-3, C-4, C-7, and C-11 as shown in Figure 4.
The cytotoxicitiy of 1, 2, and 4–14 against P-388 (murine leukemia), K-562 (human erythromyeloblastoid leukemia), and HT-29 (human colon carcinoma cells) cell lines was investigated. The results showed that 7–9 and 11 exhibited cytotoxic activity toward P-388 and K-562 cancer cell lines with half maximal inhibitory concentration (IC50) values ranging from 6.9 μM to 26.7 μM (Table 4). Among them, 7 showed selective cytotoxicity toward P-388, while 8 was found to show potent activity and selectivity toward P-388 and HT-29 cancer cell lines. Compounds 1, 2, 4–6, 10, and 12–14 were nontoxic toward these cancer cell lines (IC50 values > 40 μM). The anti-inflammatory effect of 1, 2, and 4–14 was also studied by measuring their ability to suppress N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLF-CB)-induced superoxide anion (O2−•) generation and elastase release in human neutrophils. The results revealed that, at a concentration of 10 μM, 9 exhibited significant inhibition toward superoxide anion generation and elastase release with IC50 values of 10.8 ± 0.38 and 11.0 ± 1.52 μM, respectively.

Table 4.
The cytotoxic activity of 7–9 and 11 a.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a JASCO P-1020 polarimeter (JASCO Corpotation, Tokyo, Japan). IR spectra were recorded on a JASCO FT/IR-4100 infrared spectrophotometer (JASCO Corporation) (Varian Inc., Palo Alto, CA, USA). NMR spectra were recorded on 400 MHz (or 500 MHz) for 1H and 100 MHz (or 125 MHz) for 13C in CDCl3 or C6D6. LRMS or HRMS were obtained by electrospray ionization (ESI) on a Bucker APEX II mass spectrometer (Bruker, Bremen, Germany). Silica gel (230–400 mesh, Merck, Darmstadt, Germany) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254, 0.2 mm) were used for analytical thin-layer chromatography (TLC). High-performance liquid chromatography was performed on a Hitachi L-7100 (HPLC) (Hitachi Ltd., Tokyo, Japan) apparatus with a Merck Hibar Si-60 column (250 mm × 21.2 mm, 8 μm) and on a Hitachi L-2455 (HPLC) (Hitachi Ltd., Tokyo, Japan) apparatus with a Sciences Inc. (GL Science, Tokyo, Japan) ODS-3 C18 column (250 mm × 20 mm, 5 μm).
3.2. Animal Material
The soft coral Sinularia flexibilis was collected by scuba diving off the coast of Liuqiu, Taiwan, in October 2011, at a depth of 10–15 m, and stored in the freezer until extraction. A voucher specimen was deposited in the Department of Marine Biotechnology and Resource, National Sun Yet-sen University.
3.3. Extraction and Separation
Sliced bodies of S. flexibilis were exhaustively extracted with EtOAc (2 L × 5). The EtOAc extract (40.0 g) was chromatographed over silica gel by column chromatography using hexane, EtOAc–hexane (1:100 and gradually increasing the proportion of EtOAc to 10:1), EtOAc, and then Me2CO–EtOAc (1:100 and gradually increasing the proportion of Me2CO to 10:1), and subsequently Me2CO as eluents to yield 26 fractions. Fraction 16, eluting with hexane–EtOAc (3:1), was further applied on a silica gel column (240 g) using hexane–EtOAc (8:1) to yield five subfractions (A–E). Subfraction 16-C was purified by normal-phase HPLC using hexane–Me2CO (8:1) to afford 1 (3.4 mg). Fraction 18, eluting with hexane-EtOAc (1:1), was further purified by RP-18 silica gel and using a mixture of MeOH-H2O (1.5:1) to yield seven subfractions (A–G). Subfraction 18-B was purified by reversed-phase HPLC using MeCN-H2O (1:2) to afford 3 (1.0 mg). Subfraction 18-D was purified over silica gel column (50 g) using hexane–EtOAc (2:1) to obtain 4 (3.2 mg). Fraction 19, eluting with hexane–EtOAc (1:2), was chromatographed on silica gel (240 g) using hexane–EtOAc (5:1) to yield six subfractions (A–F). Subfraction 19-B was purified on RP-18 silica gel using MeOH–H2O (1:1) and subsequently by RP-HPLC with MeOH–H2O (1.5:1) to afford 7 (280.4 mg) and 14 (2.9 mg). Subfraction 19-E was purified by RP-HPLC with MeCN–H2O (2:1) to yield 2 (94.6 mg), 8 (84.5 mg), and 9 (11.2 mg). Fraction 20, eluting with hexane–EtOAc (2:1), was further purified over silica gel column (200 g) and eluted with hexane–EtOAc (2:1) to yield five subfractions (A–E). Compounds 5 (2.1 mg) and 10 (123.7 mg) were obtained from subfraction 20-C using NP-HPLC (hexane–Me2CO, 4:1). Subfraction 20-E was isolated repeatedly over silica gel column (50 g) using hexane–Me2CO (3:1) as eluent, followed by RP-HPLC (MeCN–H2O, 1:1.5) to afford 6 (4.8 mg), 11 (9.5 mg), 12 (5.1 mg), and 13 (1.0 mg).
Flexibilisin D (1): colorless oil; −245 (c 0.85, CHCl3); IR (KBr) vmax 2933, 1716, 1647, 1453, 1244, 1149, and 1074 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 371 [M + Na]+; HRESIMS m/z 371.2196 [M + Na]+ (calcd. for C21H32O4Na, 371.2198).
Flexibilisin E (2): colorless oil; +26 (c 0.57, CHCl3); IR (KBr) vmax 3481, 2927, 1713, 1627, 1460, 1438, 1387, 1252, 1159, 946, 816, and 755 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 387 [M + Na]+; HRESIMS m/z 387.2145 [M + Na]+ (calcd. for C21H32O5Na, 387.2147).
Secoflexibilisolide A (3): colorless oil; −580 (c 0.28, CHCl3); IR (KBr) vmax 3450, 2924, 1766, 1672, 1627, 1380, 1232, 1175, 1101, 1078, 1030, and 1026 cm−1; 1H and 13C NMR data, see Table 2; ESIMS m/z 371 [M + Na]+; HRESIMS m/z 371.1831 [M + Na]+ (calcd. for C20H28O5Na, 371.1829).
Secoflexibilisolide B (4): colorless oil; −56 (c 0.91, CHCl3); IR (KBr) vmax 3501, 2924, 1705, 1647, 1515, 1267, and 1153 cm−1; 1H and 13C NMR data, see Table 3; ESIMS m/z 387 [M + Na]+; HRESIMS m/z 387.1779 [M + Na]+ (calcd. for C20H28O6Na, 387.1778).
Flexibilisolide H (5): white powder; −12 (c 0.60, CHCl3); IR (KBr) vmax 3391, 2934, 1739, 1711, 1621, 1239, 1236, 1141, 1065, and 1048 cm−1; 1H and 13C NMR data, see Table 3; ESIMS m/z 431 [M + Na]+; HRESIMS m/z 431.2047 [M + Na]+ (calcd. for C22H32O7Na, 431.2046).
3.4. Alkaline Hydrolysis of 7
Compound 7 (3.5 mg) was dissolved in 1 N methanolic NaOH solution (1 mL), and the mixture was stirred at 0 °C for 12 h. The reaction mixture was neutralized with 0.1 N HCl (aq). After evaporation of the solvent, the residue was extracted with CHCl3, and subsequently purified by HPLC using MeOH–H2O (3:1) as eluent to yield a methyl ester (1.1 mg, 31.4%; +48 (c 0.28, CHCl3; 1H and 13C NMR spectra, Supplementary materials, Figures S4 and S5), which was identified as 2.
3.5. Cytotoxicity Testing
Cell lines were purchased from the American Type Cultural Collection (ATCC). Cytotoxicity assay of 1, 2, and 4–14 were performed using Alamar Blue Assay [32,33].
3.6. In Vitro Anti-Inflammatory Assay
Human neutrophils were obtained from whole blood using dextran sedimentation and Ficoll centrifugation. Measurements of superoxide anion generation and elastase release were performed according to previously described procedures [34,35]. Idelalisib was used as a positive control, of which the IC50 values for inhibition of superoxide anion generation and elastase release were 0.07 ± 0.01 and 0.28 ± 0.09 μM, respectively.
4. Conclusions
Five new diterpenoids and nine known compounds were isolated and characterized from the marine soft coral Sinularia flexibilis. The previously unreported 3, containing a bicyclo[4.3.0]nonane ring system, was proposed be derived from flexibilisolide D. Compounds 7 and 8 showed selective cytotoxicity toward P388 cancer cell line, while 8 also exhibited significant cytotoxicity toward HT-29 cancer cells. Compound 9 displayed weaker cytotoxicity than 7 and 8, but displayed potent inhibitory activities for superoxide anion generation and elastase release in (fMLF-CB)-induced human neutrophils.
Supplementary Materials
1H and 13C spectra of compounds 1–14 and the hydrolyzed product of 7 are available online at http://www.mdpi.com/1660-3397/16/8/278/s1. Figure S1: 1H NMR spectrum of compound 1 in CDCl3, Figure S2: 13C NMR spectrum of compound 1 in CDCl3, Figure S3: 1H NMR spectrum of compound 2 in CDCl3, Figure S4: 1H NMR spectra of compound 2 and hydrolyzed product of 7 in CDCl3, Figure S5: 13C NMR spectra of compound 2 and hydrolyzed product of 7 in CDCl3, Figure S6: 13C NMR spectrum of compound 2 in CDCl3, Figure S7: 1H NMR spectrum of compound 3 in CDCl3, Figure S8: 1H NMR spectrum of compound 3 in pyridine-d5, Figure S9: 13C NMR spectrum of compound 3 in CDCl3, Figure S10: 1H–1H COSY spectrum of 3 in pyridine-d5, Figure S11: 1H NMR spectrum of compound 4 in C6D6, Figure S12: 13C NMR spectrum of compound 4 in C6D6, Figure S13: 1H NMR spectrum of compound 5 in CDCl3, Figure S14: 13C NMR spectrum of compound 5 in CDCl3, Figure S15: 1H NMR spectrum of compound 6 in CDCl3, Figure S16: 13C NMR spectrum of compound 6 in CDCl3, Figure S17: 1H NMR spectrum of compound 7 in CDCl3, Figure S18: 13C NMR spectrum of compound 7 in CDCl3, Figure S19: 1H NMR spectrum of compound 8 in CDCl3, Figure S20: 13C NMR spectrum of compound 8 in CDCl3, Figure S21: 1H NMR spectrum of compound 9 in CDCl3, Figure S22: 13C NMR spectrum of compound 9 in CDCl3, Figure S23: 1H NMR spectrum of compound 10 in CDCl3, Figure S24: 13C NMR spectrum of compound 10 in CDCl3, Figure S25: 1H NMR spectrum of compound 11 in CDCl3, Figure S26: 13C NMR spectrum of compound 11 in CDCl3, Figure S27: 1H NMR spectrum of compound 12 in CDCl3, Figure S28: 13C NMR spectrum of compound 12 in CDCl3, Figure S29: 1H NMR spectrum of compound 13 in CDCl3, Figure S30: 13C NMR spectrum of compound 13 in CDCl3, Figure S31: 1H NMR spectrum of compound 14 in CDCl3, Figure S32: 13C NMR spectrum of compound 14 in CDCl3.
Author Contributions
J.-H.S. designed and guided the whole experiment and contributed to manuscript preparation. T.-Z.H. and C.-H.C. make the structure elucidation and manuscript preparation. C.-H.W. and C.-Y.H. perform the experiment. C.-Y.H. and T.-L.H. performed bioassays. C.-F.D. identified the soft coral.
Funding
This work was supported by grants from Ministry of Science and Technology of Taiwan (MOST104-2113-M-110-006 and 105-2113-M-110-002), and the National Sun Yat-sen University-Kaohsiung Medical University (NSYSU-KMU) Joint Research Projects (NSYSUKMU 105-I008 and 106-I007) awarded to J.-H.S.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Herin, M.; Karlsson, R.; Losman, D. Chemical studies of the marine invertebrates XI. Sinulariolide, a new cembranolide diterpene from the soft coral Sinularia flexibilis. Tetrahedron 1975, 31, 129–133. [Google Scholar] [CrossRef]
- Herin, M.; Colin, M.; Tursch, B. Chemical studies of marine invertebrates. XXV. Flexibilene, an unprecedented fifteen-membered ring diterpene hydrocarbon from the soft coral Sinularia flexibilis (Coelenterata, Octocorallia, Alcyonacea). Bull. Soc. Chim. Belg. 1976, 85, 801–803. [Google Scholar] [CrossRef]
- Weinheimer, A.J.; Matson, J.A.; Hossain, M.B.; van der Helm, D. Marine anticancer agents: Sinularin and dihydrosinularin, new cembranolides from the soft coral Sinularia flexibilis. Tetrahedron Lett. 1977, 34, 2923–2926. [Google Scholar] [CrossRef]
- Kazlauskao, R.; Murphy, P.I.; Wells, R.J.; Schonholzer, P.; Coll, J.C. Cembranoid constituents from an Australian collection of the soft coral Sinularia flexibilis. Aust. J. Chem. 1978, 31, 1817–1824. [Google Scholar] [CrossRef]
- Mori, K.; Suzuki, S.; Iguchi, K.; Yamada, Y. 8,11-Epoxy bridged cembranolide diterpene from the soft coral Sinularia flexibilis. Chem. Lett. 1983, 10, 1515–1516. [Google Scholar] [CrossRef]
- Guerrero, P.P.; Read, R.W.; Batley, M.; Janairo, G.C. The structure of a novel cembranoid diterpene from a Philipine collection of the soft coral Sinularia flexibilis. J. Nat. Prod. 1995, 58, 1185–1191. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Sagar, K.S. Flexibiolide and dihydroflexibiolide, the first trihydroxycembranolide lactones from the soft coral Sinularia flexibilis of the Indian Ocean. Nat. Prod. Lett. 1996, 9, 127–135. [Google Scholar] [CrossRef]
- Su, C.C.; Wong, B.S.; Chin, C.; Wu, Y.J.; Su, J.H. Oxygenated cembranoids from the soft coral Sinularia flexibilis. Int. J. Mol. Sci. 2013, 14, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Ding, Y.; Deng, Z.W.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Sinulaflexiolides A–K, cembrane-type diterpenoids from the Chinese soft coral Sinularia flexibilis. J. Nat. Prod. 2008, 71, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.Y.; Wang, S.K.; Tseng, H.K.; Sheu, J.H. A novel cytotoxic biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 1998, 39, 7121–7122. [Google Scholar] [CrossRef]
- Duh, C.Y.; Wang, S.K.; Tseng, H.K.; Sheu, J.H.; Chiang, M.Y. Novel cytotoxic cembranoids from the Formosan soft coral Sinularia flexibilis. J. Nat. Prod. 1998, 61, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.W.; Chang, F.R.; Mcphail, A.T.; Lee, K.H.; Wu, Y.C. New cembranolide analogues from the Formosan soft coral Sinularia flexibilis and their cytotoxicity. Nat. Prod. Res. 2003, 17, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.L.; Khalil, A.T.; Chen, M.H.; Shen, Y.C. Sinuladiterpenes A–F, new cembrane diterpenes from Sinularia flexibilis. Chem. Biodivers. 2009, 6, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Chen, C.H.; Liaw, C.C.; Chen, Y.C.; Kuo, Y.H.; Shen, Y.C. Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis. Tetrahedron 2009, 65, 9157–9164. [Google Scholar] [CrossRef]
- Chen, B.W.; Chao, C.H.; Su, J.H.; Huang, C.Y.; Dai, C.F.; Wen, Z.H.; Sheu, J.H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010, 51, 5764–5766. [Google Scholar] [CrossRef]
- Shih, H.J.; Tseng, Y.J.; Huang, C.Y.; Wen, Z.H.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis. Tetrahedron 2012, 68, 244–249. [Google Scholar] [CrossRef]
- Hu, L.C.; Yen, W.H.; Su, J.H.; Michael Chiang, Y.N.; Wen, Z.H.; Chen, W.F.; Lu, T.J.; Chang, Y.W.; Chen, Y.H.; Wang, W.H.; et al. Cembrane derivatives from the soft corals, Sinularia gaweli and Sinularia flexibilis. Mar. Drugs 2013, 11, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Kuo, C.Y.; Wen, Z.H.; Lin, Y.Y.; Wang, W.H.; Su, J.H.; Sheu, J.H.; Sung, P.J. Flexibilisquinone, a new anti-inflammatory quinone from the cultured soft coral Sinularia flexibilis. Molecules 2013, 18, 8160–8167. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Martinez, N. Marine antitumor agents: 14-deoxycrassin and pseudoplexaurol, new cembranoid diterpenes from the Caribbean gorgonian Pseudoplexaura porosa. Experientia 1993, 49, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, C.Y.; Lin, Y.F.; Wen, Z.H.; Su, J.H.; Chiang, M.Y.; Sheu, J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.K.; Tseng, C.K.; Liaw, C.C.; Huang, C.Y.; Wei, C.K.; Sheu, J.H.; Lee, J.C. Lobohedleolide suppresses hepatitis C virus replication via JNK/c-Jun-C/EBP-mediated downregulation of cyclooxygenase-2 expression. Sci. Rep. 2018, 8, 8676. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Tsai, C.R.; Huang, C.Y.; Wang, S.Y.; Sheu, J.H. Klyflaccicembranols A–I, new cembranoids from the soft coral Klyxum flaccidum. Mar. Drugs 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Teng, Y.J.; Chokkalingam, U.; Hwang, T.L.; Hu, C.H.; Dai, C.F.; Sung, P.J.; Sheu, J.H. Bioactive isoprenoid-derived natural products from a Dongsha Atoll soft coral Sinularia erecta. J. Nat. Prod. 2016, 79, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.R.; Lu, M.C.; El-Shazly, M.; Huang, C.Y.; Wu, S.L.; Lai, K.H.; Su, J.H. Aquaculture soft coral Lobophytum crassum as a producer of anti-proliferative cembranoids. Mar. Drugs 2018, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; Din, Z.H.; Su, J.H.; Wu, Y.J.; Liu, C.I. Sinulariolide suppresses cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 and urokinase through the PI3K/AKT/mTOR signaling pathway in human bladder cancer cells. Mar. Drugs 2017, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Lai, K.H.; Su, J.H.; Wu, Y.J.; Sheu, J.H. 7-Acetylsinumaximol B induces apoptosis and autophagy in human gastric carcinoma cells through mitochondria dysfunction and activation of the PERK/eIF2α/ATF4/CHOP signaling pathway. Mar. Drugs 2018, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.X.; Lin, S.H.; Li, Y.R.; Chao, Y.H.; Lin, C.H.; Su, J.H.; Lin, C.C. Lobocrassin B induces apoptosis of human lung cancer and inhibits tumor xenograft growth. Mar. Drugs 2017, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Li, W.L.; Huang, C.Y.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Lu, M.C.; Liaw, C.C.; Sheu, J.H. Isoprenoids from the soft coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.P.; Gallucci, J.C.; Hart, D.J. Preparation of functionalized trans-perhydroindans from substituted benzoic acids: reductive alkylation-halolactonization-free radical cyclization. J. Org. Chem. 1988, 53, 3210–3218. [Google Scholar] [CrossRef]
- Demarco, P.V.; Farkas, E.; Doddrell, D.; Mylari, B.L.; Wenkert, E. Pyridine-induced solvent shifts in the nuclear magnetic resonance spectra of hydroxylic compounds. J. Am. Chem. Soc. 1968, 90, 5480–5486. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Hwang, T.L.; Wang, C.C.; Kuo, Y.H.; Huang, H.C.; Wu, Y.C.; Kuo, L.M.; Wu, Y.H. The hederagenin saponin SMG-1 is a natural FMLF receptor inhibitor that suppresses humanneutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Leu, Y.L.; Kao, S.H.; Tang, M.C.; Chang, H.L. Viscolin, a new chalcone fromViscum coloratum, inhibits human neutrophil superoxide anion and elastase release via acAMP-dependent pathway. Free Radic. Biol. Med. 2006, 41, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).