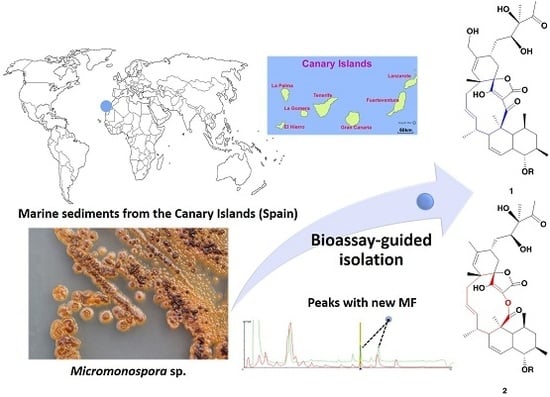
Phocoenamicins B and C, New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp.
Abstract
1. Introduction
2. Results and Discussion
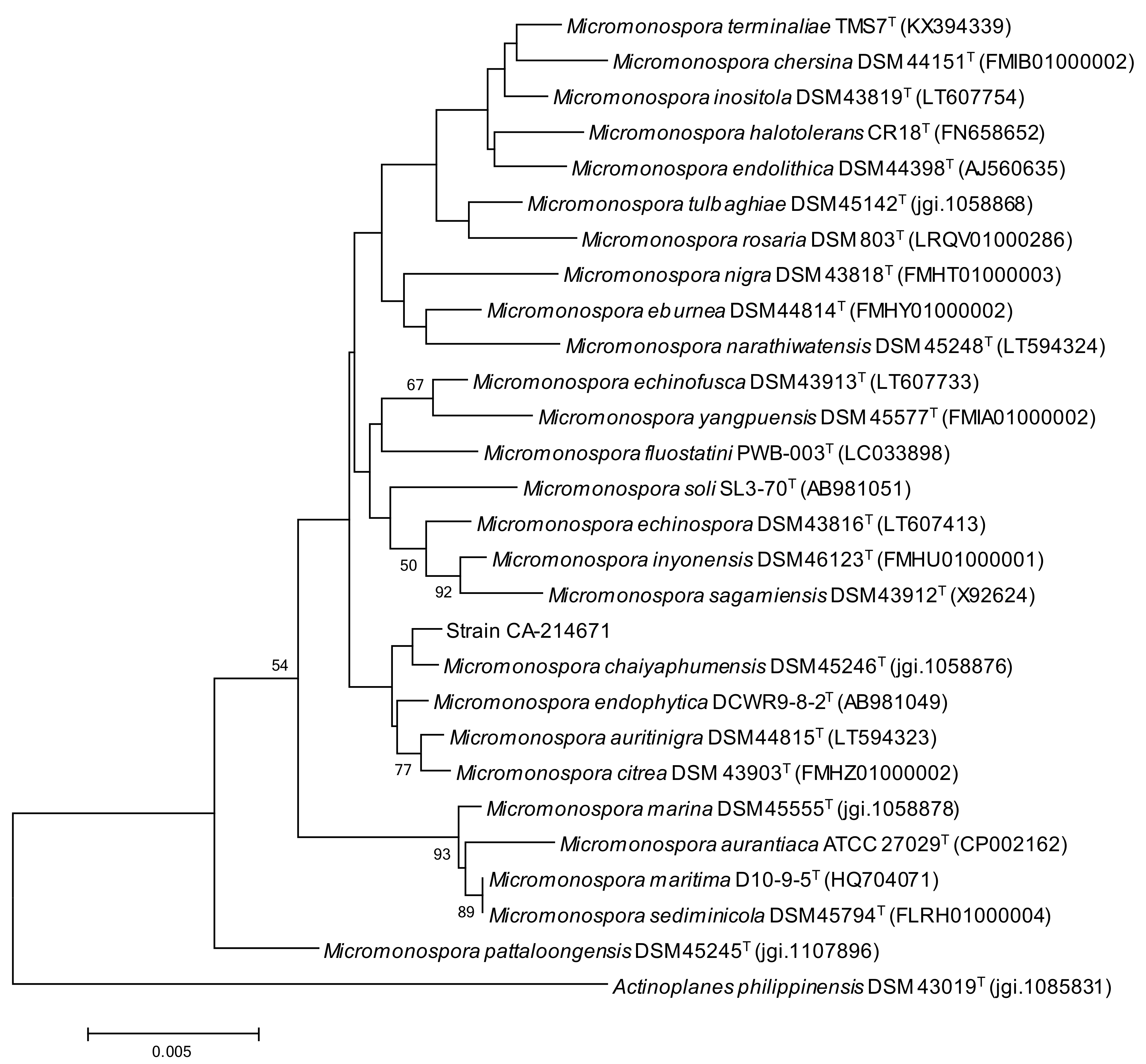
2.1. Isolation and Taxonomy of the Producing Microorganism
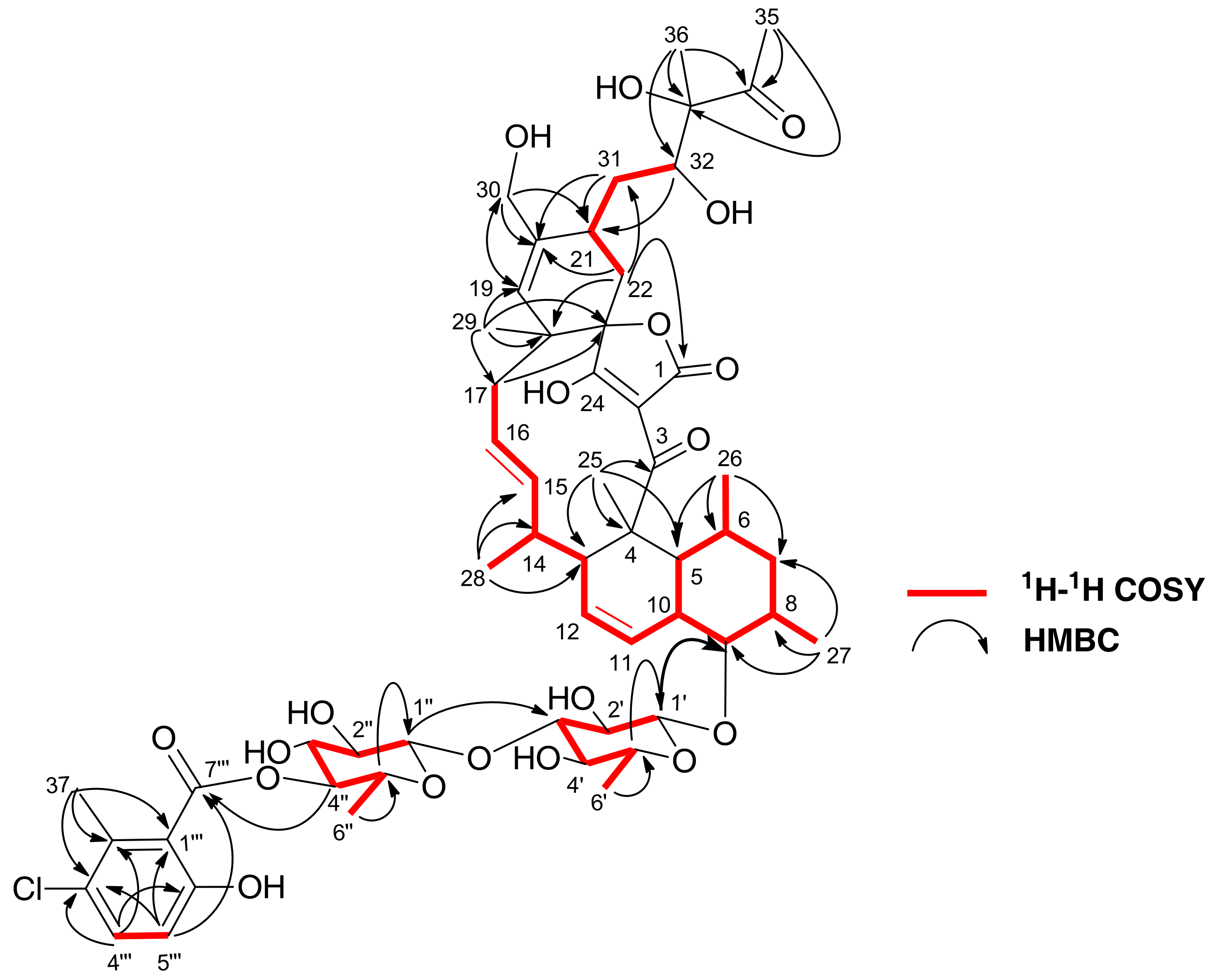
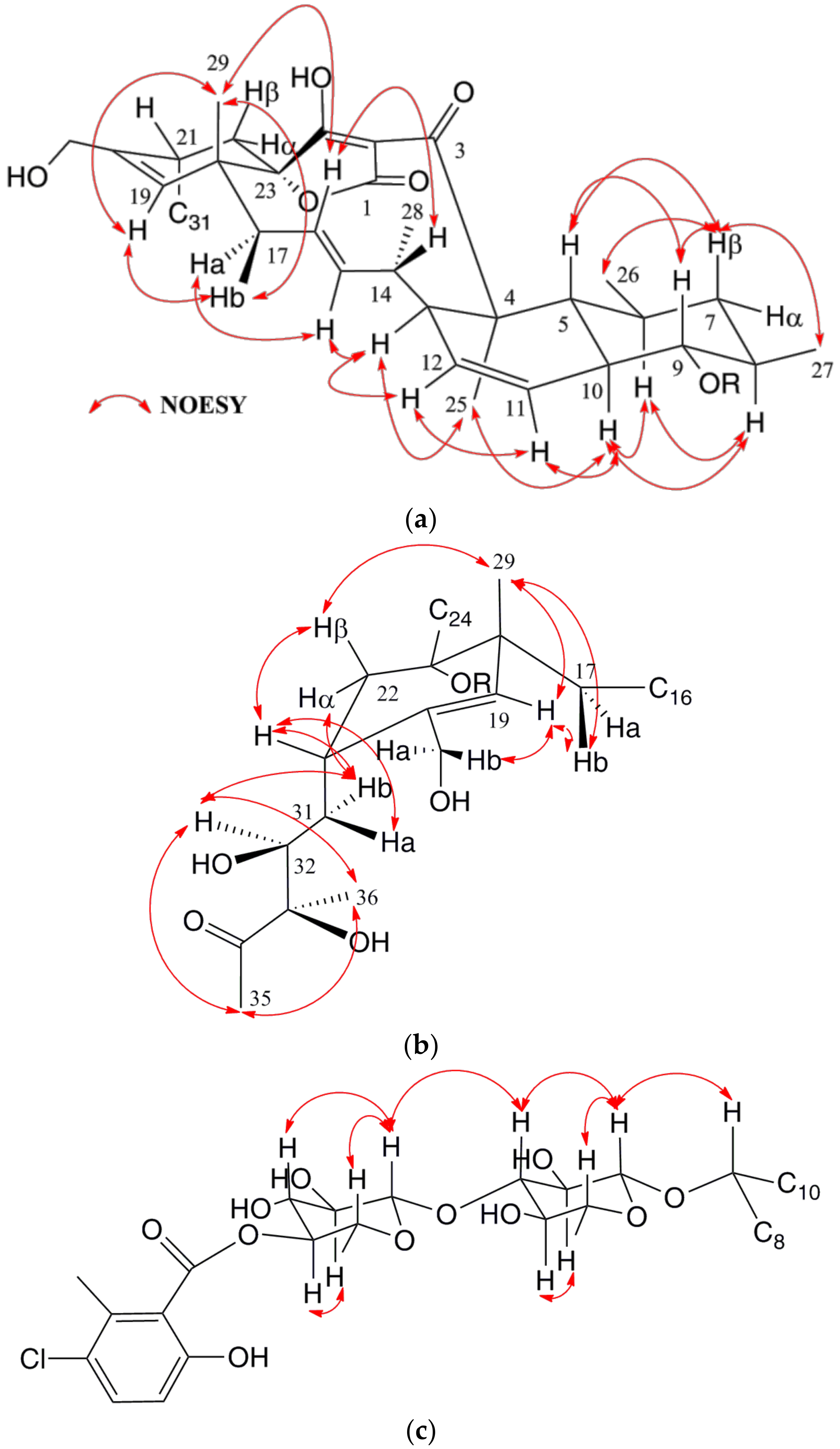
2.2. Extraction, Bioassay-Guided Isolation and Structural Elucidation
2.3. Antimicrobial Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Taxonomical Identification of the Producing Microorganism
3.3. Fermentation of the Producing Microorganism
3.4. Extraction and Bioassay-Guided Isolation
3.5. Antibacterial Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Manivasagan, P.; Kanga, K.-H.; Sivakumarb, K.; Li-Chanc, E.C.Y.; Oha, H.-M.; Kim, S.-K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Fact Sheet 194, Updated November 2017. Available online: http://who.int/mediacentre/factsheets/fs194/en/ (accessed on 17 January 2018).
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Austin, B.; Mitchell, W.J.; Morris, P.C.; Jamieson, D.J.; Adams, D.R.; Spragg, A.M.; Schweizer, M. Novel Anti-Infective Compounds from Marine Bacteria. Mar. Drugs 2010, 8, 498–518. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Saha, M.; Sana, B. Antimicrobial Agents from Marine Cyanobacteria and Actinomycetes. Mar. Microbiol. 2013, 191–205. [Google Scholar] [CrossRef]
- Crowley, S.P.; O’Gara, F.; O’Sullivan, O.; Cotter, P.D.; Dobson, A.D.W. Marine Pseudovibrio sp. as a Novel Source of Antimicrobials. Mar. Drugs 2014, 12, 5916–5929. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Kumari, P.; Reddy, C.R.K. Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Schinke, C.; Martins, T.; Queiroz, S.C.N.; Melo, I.S.; Reyes, F.G.R. Antibacterial Compounds from Marine Bacteria, 2010–2015. J. Nat. Prod. 2017, 80, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H.; Rosselet, J.P.; Marquez, J.A.; Coniglio, C.T.; Charney, W.; Herzog, H.L.; Black, J. Gentamicin, a new antibiotic complex from Micromonospora. J. Med. Chem. 1963, 6, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Hong, K.; Genilloud, O. The Family Micromonosporaceae. In The Prokaryotes, 4th ed.; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: New York, NY, USA, 2014; pp. 499–569. ISBN 978-3-642-30137-7. [Google Scholar]
- Vieweg, L.; Reichau, S.; Schobert, R.; Leadlayc, P.F.; Sussmuth, R.D. Recent advances in the field of bioactive tetronates. Nat. Prod. Rep. 2014, 31, 1554–1584. [Google Scholar] [CrossRef] [PubMed]
- Lacoske, M.H.; Theodorakis, E.A. Spirotetronate Polyketides as Leads in Drug Discovery. J. Nat. Prod. 2015, 78, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Ogura, H.; Furihata, K.; Oku, N.; Indananda, C.; Thamchaipenet, A. Maklamicin, an Antibacterial Polyketide from an Endophytic Micromonospora sp. J. Nat. Prod. 2011, 74, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Iida, T.; Odu, N.; Watanabe, H.; Furihata, K.; Miyanouchi, K. Nomimicin, a new spirotetronate-class polyketide from an actinomycete of the genus Actinomadura. J. Antibiot. 2012, 65, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kawamura, Y.; Yoshimura, Y.; Terui, Y.; Nakai, H.; Yoshida, T.; Shoji, J. Isolation, characterization and structures of PA-46101 A and B. J. Antibiot. 1990, 43, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.L.; Sanchez, L.M.; Koo, B.-M.; Doherty, J.S.; Rajendram, M.; Huang, K.C.; Gross, C.A.; Linington, R.G. Marine Mammal Microbiota Yields Novel Antibiotic with Potent Activity Against Clostridium difficile. ACS Infect. Dis. 2018, 4, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C. Evolution of proteins molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Yamamoto, I.; Nakagawa, M.; Hayakawa, Y.; Adachi, K.; Kobayashi, E. Hydroxychlorothricin, a New Antitumor Antibiotic. J. Antibiot. 1987, 40, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Nur-e-alam, M.; Lipata, F.; Oliveira, M.A.; Rohr, J. Characterization of Kinetics and Products of the Baeyer-Villiger Oxygenase MtmOIV, The Key Enzyme of the Biosynthetic Pathway toward the Natural Product Anticancer Drug Mithramycin from Streptomyces argillaceus. J. Am. Chem. Soc. 2005, 127, 17594–17595. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Crespo, G.; González-Menéndez, V.; Pérez-Moreno, G.; Sánchez-Carrasco, P.; Pérez-Victoria, I.; Ruiz-Pérez, L.M.; González-Pacanowska, D.; Vicente, F.; Genilloud, O.; et al. MDN-0104, an Antiplasmodial Betaine Lipid from Heterospora chenopodii. J. Nat. Prod. 2014, 77, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Lacret, R.; Perez-Victoria, I.; Oves-Costales, D.; de la Cruz, M.; Domingo, E.; Martin, J.; Diaz, C.; Vicente, F.; Genilloud, O.; Reyes, F. MDN-0170, a New Napyradiomycin from Streptomyces sp. Strain CA-271078. Mar. Drugs 2016, 14, 188–199. [Google Scholar] [CrossRef]
- Martín, J.; Sousa, T.S.; Crespo, G.; Palomo, S.; González, I.; Tormo, J.R.; de la Cruz, M.; Anderson, M.; Hill, R.T.; Vicente, F.; et al. Kokurin, the True Structure of PM181104, an Anti-Methicillin-Resistant Staphylococcus aureus (MRSA) Thiazolyl Peptide from the Marine-Derived Bacterium Kokuria pallustris. Mar. Drugs 2013, 11, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Suay, I.; Arenal, F.; Asensio, F.J.; Basilio, A.; Angeles Cabello, M.; Teresa Díez, M.; García, J.B.; González del Val, A.; Gorrochategui, J.; Hernández, P.; et al. Screening of basidiomycetes for antimicrobial activities. Antonie Leeuwenhoek 2000, 78, 129–140. [Google Scholar] [CrossRef] [PubMed]

| Position | Phocoenamicin B (1) | Phocoenamicin C (2) | ||
|---|---|---|---|---|
| δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | |
| 1 | 178.1, C a | 172.7, C a | ||
| 2 | 107.4, C | n.d * | ||
| 3 | 201.3, C a | 176.2, C | ||
| 4 | 51.2, C | 47.1, C | ||
| 5 | 43.9, CH | 1.85, m | 44.1, CH | 1.60, m |
| 6 | 39.6, CH | 1.42, m | 38.6, CH | 1.52, m |
| 7α | 46.0, CH2 | 1.70, m | 46.0, CH2 | 1.75, m |
| 7β | 1.20, m | 1.11, m | ||
| 8 | 40.9, CH | 1.60, m | 40.8, CH | 1.63, m |
| 9 | 88.9, CH | 3.03, t (9.2) | 88.0, CH | 3.03, t (9.8) |
| 10 | 48.4, CH | 1.93, m | 48.3, CH | 1.92, m |
| 11 | 126.4, CH | 6.22, d (10.4) | 126.4, CH | 6.27, dd (10.3, 2.5) |
| 12 | 127.9, CH | 5.55, dd (9.8, 5.9) | 128.2, CH | 5.63, ddd (10.3, 6.2, 2.4) |
| 13 | 42.1, CH | 2.72, m | 50.6, CH | 2.00, m |
| 14 | 39.8, CH | 2.10, m | 40.7, CH | 2.21, m |
| 15 | 141.5, CH | 5.27, m | 136.3, CH | 4.90, m |
| 16 | 123.7, CH | 5.18, dd (15.2, 11.8) | 129.3, CH | 5.27, ddd (15.7, 11.6, 3.1) |
| 17a | 44.1, CH2 | 2.34, m | 43.1, CH2 | 2.32, m |
| 17b | 1.85, m | 1.70, m | ||
| 18 | 41.1, C | 44.3, C | ||
| 19 | 133.6, CH | 5.26, s | 133.5, CH | 4.99, brs |
| 20 | 138.0, C | 134.8, C | ||
| 21 | 30.1, CH | 2.58, m | 34.6, CH | 2.33, m |
| 22α | 30.5, CH2 | 1.68, m | 29.8, CH2 | 1.73, m |
| 22β | 2.26, m | 2.38, m | ||
| 23 | 87.1, C | 87.0, C | ||
| 24 | 204.4, C a | n.d * | ||
| 25 | 16.8, CH3 | 1.53, s | 17.3, CH3 | 1.31, s |
| 26 | 24.0, CH3 | 0.80, brs | 22.1, CH3 | 0.93, d (6.8) |
| 27 | 20.2, CH3 | 1.02, d (6.2) | 20.0, CH3 | 1.02, d (6.4) |
| 28 | 21.5, CH3 | 0.80, brs | 22.5, CH3 | 0.89, d (7.2) |
| 29 | 24.6, CH3 | 1.23, brs | 23.7, CH3 | 1.26, s |
| 30a | 65.4, CH2 | 4.14, d (13.2) | 22.4, CH3 | 1.73, s |
| 30b | 3.98, d (13.2) | |||
| 31a | 33.5, CH2 | 2.07, m | 33.9, CH2 | 1.94, m |
| 31b | 1.69, m | 1.73, m | ||
| 32 | 74.1, CH | 3.86, dd (11.2, 1.4) | 74.1, CH | 3.84, dd (10.9, 1.9) |
| 33 | 83.5, C | 83.5, C | ||
| 34 | 215.3, C | 215.2, C | ||
| 35 | 25.7, CH3 | 2.24, s | 25.6, CH3 | 2.23, s |
| 36 | 22.2, CH3 | 1.24, s | 22.1, CH3 | 1.20, s |
| 1′ | 104.0, CH | 4.36, d (7.3) | 104.0, CH | 4.35, d (7.3) |
| 2′ | 75.4, CH | 3.45, m | 75.4, CH | 3.45, m |
| 3′ | 88.6, CH | 3.46, m | 88.6, CH | 3.48, m |
| 4′ | 75.7, CH | 3.11, t (8.8) | 75.6, CH | 3.11, t (8.8) |
| 5′ | 72.8, CH | 3.22, m | 72.9, CH | 3.24, m |
| 6′ | 18.3, CH3 | 1.27, d (6.0) | 18.3, CH3 | 1.28, d (6.0) |
| 1″ | 105.4, CH | 4.61, d (7.8) | 105.4, CH | 4.61, d (7.9) |
| 2″ | 76.1, CH | 3.42, t (8.5) | 76.1, CH | 3.42, t (8.6) |
| 3″ | 75.3, CH | 3.64, t (9.3) | 75.4, CH | 3.64, t (9.4) |
| 4″ | 77.9, CH | 4.89, ** | 77.9, CH | 4.87, ** |
| 5″ | 71.7, CH | 3.69, dq (9.7, 6.1) | 71.7, CH | 3.69, dq (9.7, 6.2) |
| 6″ | 18.0, CH3 | 1.35, d (6.1) | 18.0, CH3 | 1.35, d (6.2) |
| 1′′′ | 124.3, C | 124.3, C | ||
| 2′′′ | 135.6, C | 135.6, C | ||
| 3′′′ | 126.0, C | 126.0, C | ||
| 4′′′ | 132.4, CH | 7.25, d (8.7) | 132.4, CH | 7.25, d (8.7) |
| 5′′′ | 115.9, CH | 6.70, d (8.7) | 115.8, CH | 6.70, d (8.7) |
| 6′′′ | 155.3, C | 155.3, C | ||
| 7′′′ | 169.3, C | 169.3, C | ||
| 37 | 17.3, CH3 | 2.36, s | 17.9, CH3 | 2.36, s |
| Compounds | MIC (μg/mL) | ZOI * mm(μg) | |||
|---|---|---|---|---|---|
| S. aureus MB5393 | M. tuberculosis ATCC 25177 | M. bovis ATCC 35734 | E. faecium MB5571 | B. subtilis MB964 | |
| phocoenamicin B (1) | 8–16 | >128 | >128 | >128 | 7 (2) |
| phocoenamicin C (2) | 32–64 | 32 | >128 | >128 | 7 (4) |
| phocoenamicin (3) | 4–8 | 16–32 | >128 | 32–64 | 7 (4) |
| vancomycin | 2–4 | >128 | |||
| streptomycin | 1.6–3.2 | 0.4-0.8 | |||
| gentamicin | 8 (0.25) | ||||
| penicillin G | 19 (0.06) | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Bonilla, M.; Oves-Costales, D.; De la Cruz, M.; Kokkini, M.; Martín, J.; Vicente, F.; Genilloud, O.; Reyes, F. Phocoenamicins B and C, New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp. Mar. Drugs 2018, 16, 95. https://doi.org/10.3390/md16030095
Pérez-Bonilla M, Oves-Costales D, De la Cruz M, Kokkini M, Martín J, Vicente F, Genilloud O, Reyes F. Phocoenamicins B and C, New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp. Marine Drugs. 2018; 16(3):95. https://doi.org/10.3390/md16030095
Chicago/Turabian StylePérez-Bonilla, Mercedes, Daniel Oves-Costales, Mercedes De la Cruz, Maria Kokkini, Jesús Martín, Francisca Vicente, Olga Genilloud, and Fernando Reyes. 2018. "Phocoenamicins B and C, New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp." Marine Drugs 16, no. 3: 95. https://doi.org/10.3390/md16030095
APA StylePérez-Bonilla, M., Oves-Costales, D., De la Cruz, M., Kokkini, M., Martín, J., Vicente, F., Genilloud, O., & Reyes, F. (2018). Phocoenamicins B and C, New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp. Marine Drugs, 16(3), 95. https://doi.org/10.3390/md16030095