Anti-BACE1 and Antimicrobial Activities of Steroidal Compounds Isolated from Marine Urechis unicinctus
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Isolated Bioactivie Compounds
2.2. In Vitro BACE-1 Inhibitory Activity of Hecogenin and Cholest-4-en-3-one
2.3. Antimicrobial Activity of Cholesta-4,6-dien-3-ol and Hurgadacin
3. Materials and Methods
3.1. Experimental Materials and Chemicals
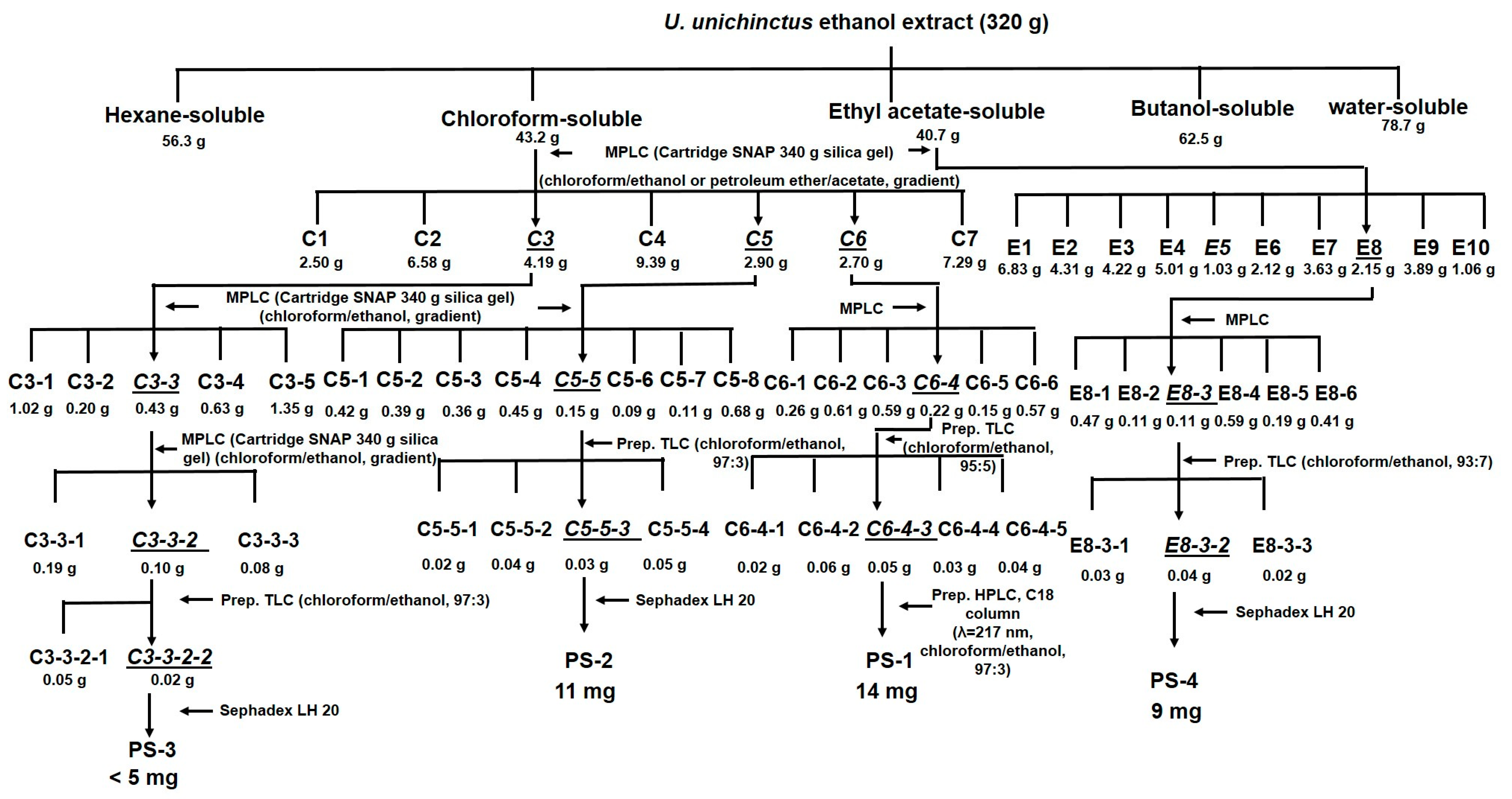
3.2. Bioassay-Directed Isolation of U. unicincuts Extracts
3.3. Identification of Isolated Bioactive Compounds
3.4. Fluorescence Resonance Energy Transfer (FRET)-Based Enzyme Assay
3.5. Antimicrobial Activity Assay
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BACE1 | β-site amyloid cleaving enzyme |
| FRET | fluorescence resonance energy transfer |
| HPLC | high-performance liquid chromatography |
| LB | lysogeny broth |
| MIC | minimal inhibitory concentration |
| MPLC | medium pressure liquid chromatography |
| MS | mass spectrometry |
| NMR | nuclear magnetic resonance spectroscopy |
| PDA | potato dextrose agar |
| PDB | potato dextrose broth |
References
- Kalaria, R.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.; Wu, Y.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Vassar, R.; Bennett, B.D.; Babu-Kahn, S. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Kovacs, D.M.; Yan, R.; Wong, P.C. The β-secretase enzyme BACE in health and Alzheimer’s disease: Regulation, cell biology, function, and therapeutic potential. J. Neurosci. 2009, 29, 12787–12794. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, J.L.; Lladó, A.; Rami, L. Memantine: Targeting glutamate excitotoxicity in Alzheimer’s disease and other dementias. Am. J. Alzheimers Dis. Other Dement. 2005, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Lanctôt, K.L.; Herrmann, N. The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer’s disease. Expert Opin. Saf. 2004, 3, 425–440. [Google Scholar] [CrossRef]
- Broadwell, R.D.; Sofroniew, M.V. Serum Proteins Bypass the Blood-Brain Fluid Barriers for Extracellular Entry to the Central Nervous System. Exp. Neurol. 1999, 120, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Bae, K.; Seong, Y.H.; Song, K.S. Green tea catechins as a BACE1 (β-secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kim, J.R.; Lee, S.B.; Kim, Y.J.; Jung, M.Y.; Kwon, H.W.; Ahn, Y.J. Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer’s disease Drosophila models. BMC Complement. Altern. Med. 2014, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yon, G.H.; Hong, K.S.; Yoo, D.S.; Choi, C.W.; Park, W.K.; Kong, J.Y.; Kim, Y.S.; Ryu, S.Y. In vitro BACE-1 inhibitory phenolic components from the seeds of Psoralea corylifolia. Planta Med. 2008, 74, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Jun, M. In vitro BACE1 inhibitory activity of geraniin and corilagin from Geranium thunbergii. Planta Med. 2013, 79, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, S.; Miyazawa, M. β-secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother. Res. 2010, 24, 510–513. [Google Scholar] [PubMed]
- Orhan, I.E. Current concepts on selected plant secondary metabolites with promising inhibitory effects against enzymes linked to Alzheimer’s disease. Curr. Med. Chem. 2012, 19, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Lee, E.J.; Kim, J.S.; Kang, S.S.; Lee, J.H.; Min, B.S.; Choi, J.S. Cholinesterase and BACE1 inhibitory diterpenoids from Aralia cordata. Arch. Pharm. Res. 2009, 32, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Schinke, C.; Martins, T.; Queiroz, S.C.N.; Melo, I.S.; Reyes, F.G.R. Antibacterial compounds from marine bacteria. J. Nat. Prod. 2017, 80, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, Y. A new marine microorganism strain L0804: Taxonomy and characterization of active compounds from its metabolite. World J. Microbiol. Biotechnol. 2010, 26, 1549–1556. [Google Scholar] [CrossRef]
- Haste, N.M.; Perera, V.R.; Maloney, K.N.; Tran, D.N.; Jensen, P.; Fenical, W.; Nizet, V.; Hensler, M.E. Activity of the streptogramin antibiotic etamycin against methicillin-resistant Staphylococcus aureus. J. Antibiot. 2010, 63, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.N.; Soliman, K.; Ayesh, A.; El-Wahed, A.A.; Hamza, Z.; Mansour, H.M.; Khalifa, S.A.M.; Ali, H.B.M.; El-Seedi, H.R. Antibacterial effect of the red sea soft coral Sarcophyton trocheliophorum. Nat. Prod. Res. 2016, 30, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jung, J.H.; Liu, Y. Antimicrobial compounds from marine invertebrates-derived microorganisms. Curr. Med. Chem. 2016, 23, 1–14. [Google Scholar] [CrossRef]
- Procopio, R.E.; De, L.; Da Silva, I.R.; Martins, M.K.; De Azevedo, J.C.; De Araujo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Passari, A.K.; Chandra, P.; Leo, V.V.; Mishra, B.K.; Singh, B.P. Production of potent antimicrobial compounds from Streptomyces cyaneofuscatus associated with fresh water sediment. Front. Microbiol. 2017, 8, 68. [Google Scholar]
- Li, F.L.; Wang, W.; Zhou, H. Studies on the echiurans (echiura) of the yellow sea (Huanghai) and Bohai sea. J. Ocean Univ. Qingdao 1994, 24, 203–210. (In Chinese) [Google Scholar]
- Yuan, C.; Liu, P.; Han, X.; Cui, Q. Hypoglycemic effects of glycosaminoglycan from Urechis unicinctus in diabetic mice. J. Med. Food 2015, 18, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Han, B.; Feng, Y.; Jiang, Z.; Yang, Y.; Liu, W. Antithrombotic effects of a newly purified fibrinolytic protease from Urechis unicinctus. Thromb. Res. 2013, 132, e135–e144. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.S.; Park, S.H.; Lee, D.G. Antimicrobial effect and membrane-active mechanism of urechistachykinins, neuropeptides derived from Urechis unicinctus. FEBS Lett. 2008, 582, 2463–2466. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Bae, W.J.; Kim, S.J.; Kang, K.-H.; Kim, S.-K.; Cho, H.J.; Hong, S.-H.; Lee, J.Y.; Kim, S.W. Improvement of erectile dysfunction by the active peptide from Urechis unicinctus by high temperature/pressure and ultra—Wave assisted lysis in Streptozotocin Induced Diabetic Rats. Int. Braz. J. Urol. 2016, 42, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K.; Jain, D.C.; Gupta, R.K.; Thakur, R.S. Carbon-13 NMR spectroscopy of steroidal sapogenins and steroidal saponins. Phytochemistry 1985, 24, 2479–2496. [Google Scholar] [CrossRef]
- Jin, J.M.; Liu, X.K.; Yang, C.R. Three new hecogenin glycosides from fermented leaves of Agave Americana. J. Asian Nat. Prod. Res. 2003, 5, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.G.; Branco, A. GC-MS characterisation of Sapogenins from sisal Waste and a method to isolate pure hecogenin. Bioresources 2014, 9, 1325–1333. [Google Scholar] [CrossRef]
- Chiang, Y.-B.; Ismail, W.; Muller, M.; Fuchs, G. Initial steps in the anoxic metabolism of cholesterol by the denitrifying Sterolibacterium denitrificans. J. Biol. Chem. 2007, 282, 13240–13249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Chen, G.Y.; Ge, F.L.; Li, W.; Zeng, L.H.; Cao, W.G. Efficient biotransformation of cholesterol to androsta-1,4-diene-3,17-dione by a newly isolated actinomycete Gordonia neofelifaecis. World Microbiol. Biotechnol. 2011, 27, 759–765. [Google Scholar] [CrossRef]
- Wu, K.; Li, W.; Song, J.; Li, T. Production, purification, and identification of cholest-4-en-3-one produced by cholesterol oxidase from Rhodococcus sp. in aqueous/organic biphasic system. Biochem. Insights 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahire, J.J.; Mokashe, N.U.; Chaudhari, B.L. Cholesterol biotransformation to cheolesta-4,6-dien-3-ol and effect of assimilation on adhesion properties of Lactobacillus helveticus CD6. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 398–401. [Google Scholar]
- Shaaban, M.; Shaaban, K.A.; Ghani, M.A. Hurgadacin: A new steroid from Sinularia polydactyla. Steroids 2013, 78, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Salmon, S.A.; Watts, J.L. Minimum inhibitory concentration determinations for various antimicrobial agents against 1570 bacterial isolates from Turkey Poults. Avian Dis. 2000, 44, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-Z.; Pu, X.; Luo, G.; Zhao, L.-X.; Xu, L.-H.; Li, W.-J.; Luo, Y. Isolation and characterization of new p-terphenyls with antifungal, antibacterial, and antioxidant activities from halophilic actinomycete Nocardiopsis gilva YIM 90087. J. Agric. Food Chem. 2013, 61, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yang, Q.Y.; Zhao, Y.; Yao, C.S.; Sun, Y.; Yang, E.J.; Song, K.S.; Mook-Jung, I.; Fang, W.S. BACE1 (β-secretase) inhibitory chromone glycosides from Aloe vera and Aloe nobilis. Planta Med. 2008, 74, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Perumalsamy, H.; Kwon, H.W.; Na, Y.-E.; Ahn, Y.J. Effects and possible mechanisms of action of acacetin on the behavior and eye morphology of Drosophila models of Alzheimer’s disease. Sci. Rep. 2015, 5, 16127. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
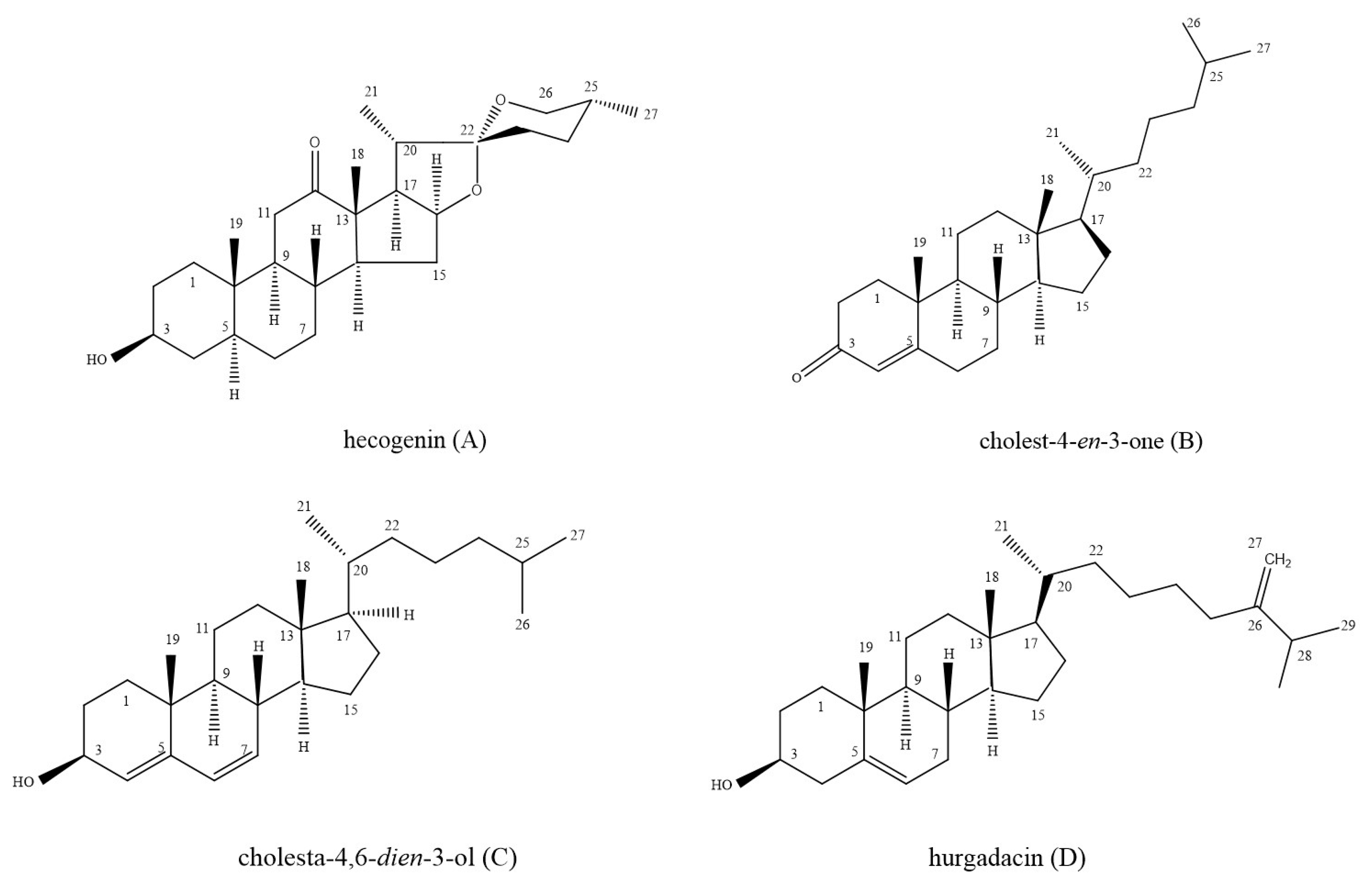
| Carbon Atom No. | PS-1 (Hecogenin) | Reference [34] | PS-2 (Cholest-4-en-3-one) | Reference [37] | PS-4 (Hurgadacin) | Reference [39] |
|---|---|---|---|---|---|---|
| 1 | 36.5 | 36.6 | 35.7 | 35.7 | 36.3 | 37.2 |
| 2 | 31.2 | 31.5 | 33.9 | 34.0 | 30.9 | 31.6 |
| 3 | 70.8 | 71.0 | 199.5 | 199.6 | 71.0 | 71.7 |
| 4 | 37.8 | 38.0 | 123.7 | 123.7 | 41.6 | 42.3 |
| 5 | 44.6 | 44.7 | 171.0 | 171.6 | 140.8 | 140.7 |
| 6 | 28.7 | 28.5 | 32.9 | 33.0 | 121.0 | 121.6 |
| 7 | 28.3 | 31.6 | 32.0 | 32.1 | 31.8 | 31.9 |
| 8 | 34.3 | 34.4 | 35.6 | 35.7 | 31.6 | 31.9 |
| 9 | 55.1 | 55.6 | 53.8 | 53.8 | 50.3 | 50.1 |
| 10 | 36.1 | 36.2 | 38.6 | 38.6 | 37.1 | 36.5 |
| 11 | 37.8 | 38.0 | 21.0 | 21.0 | 21.1 | 21.2 |
| 12 | 213.6 | 213.7 | 39.6 | 39.6 | 39.8 | 39.8 |
| 13 | 55.5 | 55.2 | 42.3 | 42.4 | 42.1 | 42.3 |
| 14 | 55.8 | 55.9 | 55.8 | 55.9 | 56.7 | 56.7 |
| 15 | 31.5 | 31.6 | 24.1 | 24.2 | 23.9 | 24.3 |
| 16 | 79.2 | 79.3 | 28.1 | 28.2 | 27.9 | 28.2 |
| 17 | 53.5 | 53.6 | 56.1 | 56.1 | 56.0 | 56.0 |
| 18 | 16.0 | 16.1 | 11.9 | 12.0 | 10.9 | 11.2 |
| 19 | 11.9 | 12.0 | 17.3 | 17.4 | 18.5 | 19.4 |
| 20 | 42.1 | 42.3 | 76.7 | 33.8 | 35.6 | 35.7 |
| 21 | 13.2 | 13.3 | 18.6 | 18.6 | 17.8 | 18.7 |
| 22 | 109.2 | 109.3 | 36.1 | 36.1 | 34.6 | 34.7 |
| 23 | 31.4 | 31.3 | 23.8 | 23.8 | 28.8 | 28.2 |
| 24 | 30.1 | 28.9 | 39.5 | 39.5 | 29.4 | 31.6 |
| 25 | 31.1 | 30.3 | 28.0 | 28.1 | 30.7 | 31.0 |
| 26 | 66.8 | 67.0 | 22.5 | 22.6 | 156.4 | 156.8 |
| 27 | 17.1 | 17.2 | 22.8 | 22.8 | 105.5 | 105.9 |
| 28 | - | - | - | - | 33.5 | 33.8 |
| 29 | - | - | - | - | 20.8 | 22.0 |
| 30 | - | - | - | - | 20.9 | 21.8 |
| Materials | E. coli (G−) | S. aureus (G+) | S. enterica (G−) | M. luteus (G+) | P. multocida (G−) | Cytospora sp. | P. piricola | F. oxysporum f. sp. cucumebrium |
|---|---|---|---|---|---|---|---|---|
| C3-3-2-2 a | 0.46 | - b | 0.46 | - | 0.46 | 0.46 | 0.94 | - |
| hurgadacin | 0.46 | 3.75 | 0.94 | >3.75 | 0.46 | >3.75 | 0.94 | >3.75 |
| Materials (Soluble Fraction) | % Inhibition | ||
|---|---|---|---|
| 0.5 (mg/mL) | 1.0 (mg/mL) | 2.0 (mg/mL) | |
| Curcumin | 88.03 ± 0.34 | 93.21 ± 0.52 | 98.51 ± 0.12 |
| Hexane | 24.3 ± 0.69 | 36.9 ± 0.13 | 39.1 ± 0.22 |
| Chloroform | 65.1 ± 0.21 | 75.2 ± 0.16 | 79.8 ± 0.06 |
| Ethyl acetate | 40.0 ± 0.10 | 48.3 ± 0.11 | 50.3 ± 0.12 |
| Butanol | 30.7 ± 0.07 | 37.4 ± 0.18 | 40.4 ± 0.25 |
| Water | 13.2 ± 0.28 | 14.9 ± 0.26 | 17.1 ± 0.11 |
| Materials (Soluble Fraction) | Concentration (mg/mL) | E. coli (G−) | S. aureus (G+) | S. enterica (G−) | M. luteus (G+) | P. multocida (G−) | Cytospora sp. | P. piricola | F. oxysporum f. sp. cucumebrium |
|---|---|---|---|---|---|---|---|---|---|
| Hexane | 7.5 | - | - | - | - | - | - | - | - |
| 15 | + | - | + | - | + | - | - | - | |
| Chloroform | 7.5 | + | + | + | - | + | + | + | + |
| 15 | + | + | + | - | + | + | + | + | |
| Ethyl acetate | 7.5 | + | - | + | - | + | + | + | + |
| 15 | + | + | + | - | + | + | + | + | |
| Butanol | 7.5 | - | - | - | - | - | - | - | - |
| 15 | - | - | - | - | - | - | - | - | |
| Water | 7.5 | - | - | - | - | - | - | - | - |
| 15 | - | - | - | - | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.-Z.; Liu, J.-W.; Wang, X.; Jeong, I.-H.; Ahn, Y.-J.; Zhang, C.-J. Anti-BACE1 and Antimicrobial Activities of Steroidal Compounds Isolated from Marine Urechis unicinctus. Mar. Drugs 2018, 16, 94. https://doi.org/10.3390/md16030094
Zhu Y-Z, Liu J-W, Wang X, Jeong I-H, Ahn Y-J, Zhang C-J. Anti-BACE1 and Antimicrobial Activities of Steroidal Compounds Isolated from Marine Urechis unicinctus. Marine Drugs. 2018; 16(3):94. https://doi.org/10.3390/md16030094
Chicago/Turabian StyleZhu, Yong-Zhe, Jing-Wen Liu, Xue Wang, In-Hong Jeong, Young-Joon Ahn, and Chuan-Jie Zhang. 2018. "Anti-BACE1 and Antimicrobial Activities of Steroidal Compounds Isolated from Marine Urechis unicinctus" Marine Drugs 16, no. 3: 94. https://doi.org/10.3390/md16030094
APA StyleZhu, Y.-Z., Liu, J.-W., Wang, X., Jeong, I.-H., Ahn, Y.-J., & Zhang, C.-J. (2018). Anti-BACE1 and Antimicrobial Activities of Steroidal Compounds Isolated from Marine Urechis unicinctus. Marine Drugs, 16(3), 94. https://doi.org/10.3390/md16030094






