Abstract
Isolation of marine compounds from living invertebrates represents a major challenge for sustainable and environmentally friendly exploitation of marine bio-resources. To develop innovative technology to trap invertebrate compounds in the open sea, the proof of concept of a system combining external continuous circulation of water with XAD-amberlite solid-phase extraction was validated in an aquarium. In this work, we reported the elicitation of guanidine alkaloid production of Crambe crambe in the presence of Anemonia sulcata, both collected from the Mediterranean Sea. Besides the previously reported crambescidin 359 (1), and crambescidin acid (2), three new compounds were isolated; one carboxylated analog of 1 named crambescidin 401 (3), and two analogs of crambescin B, crambescin B 281 (4) and crambescin B 253 (5). Based on these results, a technology named Somartex® for “Self Operating MARine Trapping Extractor” was patented and built to transfer the concept from closed aquarium systems to open marine ecosystems.
1. Introduction
The marine environment represents a diverse and abundant reservoir of valuable molecules. Oceans cover more than 70% of the planet, extending to depths of more than 3 km and host an exceptional biodiversity consisting of more than 90% of animal phyla as well as a rich biodiversity of plants, algae and microorganisms. Technical diving, remote control vehicles and deep-sea exploration vehicles remaining of limited use due to their cost, autonomy and complexity. As a result, major discoveries have been concentrated around the shallow waters, while the greater depths of the ocean remain far less explored and understood.
During the recent decades, not only terrestrial biodiversity but also marine species, especially marine invertebrates and microorganisms, have been investigated for their potential pharmaceutical applications. However, compared to their terrestrial counterparts, few marine-derived drugs have progressed through to the later stages of clinical trials [1].
The major limiting factor in marine invertebrate chemistry is the difficulty to access the bioresource. In addition to the difficulties involved in harvesting the invertebrates, these sessile animals face various environmental and anthropogenic threats. Many are endangered species and protected through national, regional and international conventions. Attempts to cultivate marine invertebrates or their cells have not, to date, provided a satisfactory solution to the problem of sourcing [2,3,4]. This is due not only to the low yield and slow growth rate but also a result of taking the invertebrates out of their natural environment, which implies removing natural physico-chemical constraints and the presence of symbionts, resulting in the cultivated invertebrates not being able to produce the desired target compounds.
The future challenge is to ensure the protection of marine invertebrate biodiversity while meeting the need to provide the drug pipeline with marine natural scaffolds and bioactive compounds.
The trapping of invertebrate molecules in their natural habitat, without harvesting the organism, represents a novel approach and a potential solution to the problem at hand to which no efforts have yet been dedicated.
In this paper, we report a proof of concept for an innovative strategy enabling the trapping of molecules from invertebrates maintained alive in an aquarium. This approach allows the isolation and the structural elucidation of the produced compounds without applying any stress or injury either to the animal or to its environment. To validate the approach in open marine ecosystems, a specific technology, named Somartex® (Self Operating MARine Trapping Extractor, Maximator SAS, Rantigny, France) was patented and built for testing in different marine locations and depths.
2. Results
2.1. From the Observation to the Proof of Concept
Our strategy is based on the observation that when the red encrusting sponge Crambe crambe from the Mediterranean Sea was introduced into an aquarium containing the cnidarian Anemonia sulcata, the anemonia died within 48 h, whereas the Crambe crambe sponge seemed not to be affected. One possible explanation is that Crambe crambe produces compounds in these conditions that are toxic to anemonia.
To verify this hypothesis, the trapping technology was applied, in similar conditions, to four aquariums. The first aquarium (Aq-1) did not contain any invertebrate and served as a control. The second aquarium (Aq-2) contained 20 Anemonia sulcata, the third aquarium (Aq-3) contained stones holding Crambe crambe sponge samples, the fourth (Aq-4) contained 20 Anemonia sulcata to which stones holding Crambe crambe sponge were added.
In each aquarium, water was circulated continuously in a filter containing Amberlite XAD-16 resin polymeric absorbent (Figure 1).
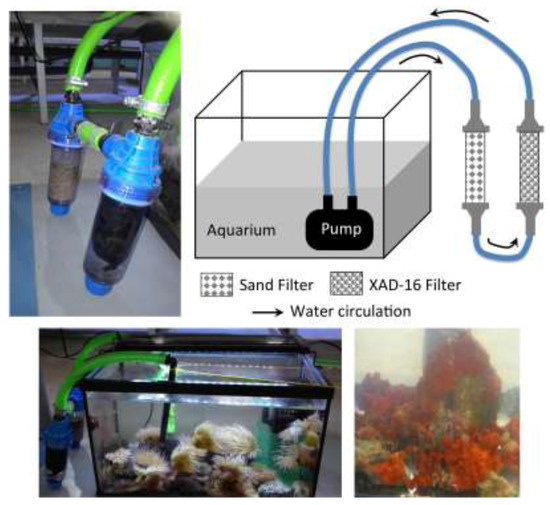
Figure 1.
Design and pictures of the experimental assembly.
After 48 h, the Amberlite XAD-16 (≈50 g) is recovered from the filters and extensively washed with distilled water. According to the recovered quantities and analytical profiles, only the MeOH extract of Aq-4 offered analytical profiles and yielded quantities sufficient for advanced isolation and structural elucidation tasks (160 mg). In general, we applied the in situ XAD-16 solid-phase extraction to microbial secondary metabolites. Among the many advantages of this method, it allows the isolation of the metabolic intermediates and the proposal of biosynthetic pathways [5,6].
As shown in Figure 2, the HPLC-ELSD chromatogram of the MeOH extract shows various peaks, which were isolated by a combination of flash chromatography and semi-preparative HPLC (see materials and methods section).
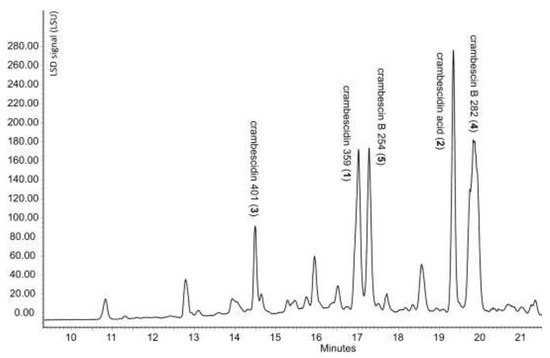
Figure 2.
HPLC analysis of the MeOH extract of aquarium 4 (Aq-4) under LSD detection.
2.2. Isolation and Structural Elucidation
Chromatographic separation of major compounds present in the extract (Figure 2) led to the isolation of five compounds in mg scale. Their structure elucidation was performed based on 1D, 2D NMR and high-resolution mass spectrometry experiments (Figure 3).
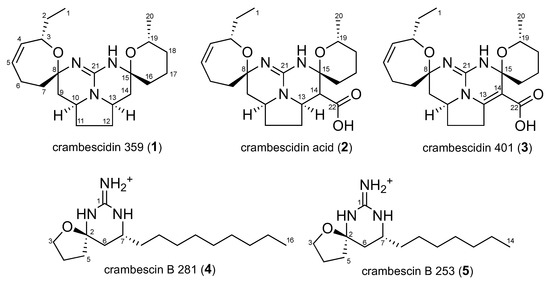
Figure 3.
Structures of the isolated compounds 1 to 5.
Molecular formula C21H33N3O2 of 1 was established based on HR-ESI-MS (m/z 360.2651 [M+H]+). This compound was identified as crambescidin 359 based on the identical NMR data (Supplementary Material, Table S30) to those previously reported by Breakman et al. [7].
Compound 2 had a HR-ESI-MS of m/z 404.2549 [M+H]+ corresponding to the molecular formula C22H33N3O4. The 1H and 13C NMR spectra (Table 1) are identical to those reported for crambescidin acid, previously reported by McMahon et al. [8]. Crambescidin acid (2) is herein reported for the first time to be isolated from Crambe crambe but has also been reported by Overman et al. as the key intermediate in the total synthesis of crambescidins 359 and 431 [9]. The authors demonstrated that the zwitterionic guanidinium carboxylate at 2 readily decarboxylates to form crambescidin 359 (1), more easily than under basic conditions.

Table 1.
1H NMR (600 MHz, cryoprobe) and 13C NMR (150 MHz) data for crambescidin acid (2) and crambescidin 401 (3) in CD3OD.
The new crambescidin derivative 3, named crambescidin 401 had a molecular formula C22H31N3O4 as deduced from HR-ESI-MS (m/z 402.2393 [M+H]+). Its 1H and 13C NMR spectra were very similar to those of 2. The main difference was the absence of 1H NMR signals corresponding to H-13 and H-14 in 3. This observation, together with the comparison of molecular formulae, suggested the presence of a double bond in C-13/C-14 position. This was supported by the chemical shifts of C-13 and C-14 δC 160.7 and δC 123.6 respectively. as well as by HMBC correlations (Figure 4) from H-12 (δH 3.38) to C-13 and from H-16 (δH 3.01-3.08) to C-14 (Table 1).
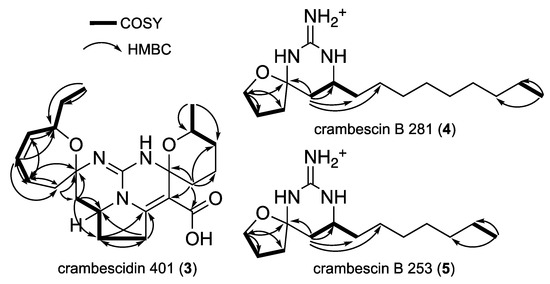
Figure 4.
COSY and key HMBC correlations for compounds 3 to 5.
Compound 3 is, to our knowledge, the first representative of the pentacyclic guanidinium alkaloids with a double bond between C-13 and C-14. Only the tricyclic batzelladine C, isolated from Poecilosclerida sponges, bears the double bond at the same position (Figure 5) [10].
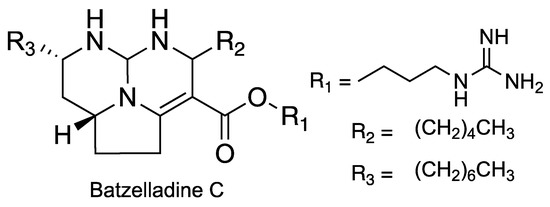
Figure 5.
Structures of batzelladine C.
The molecular formula C16H31N3O of 4 was determined by HR-ESI-MS (m/z 282.2545 [M+H]+). The NMR data are listed in Table 2. The molecular formula accounts for three degrees of unsaturation, corresponding to a spiroaminal moiety (C-2, δC 88.0 ppm) linked to a cyclic guanidine moiety (C-1, δC 154.4 ppm). The 13C NMR revealed the presence of 16 carbons which, in combination with HSQC spectrum, can be categorized as one methyl carbon (δC 14.2), eleven sp3 methylene groups (including one oxygen-bearing methylene (δC 67.3), one nitrogen-bearing methine (δC 47.1), one spiro (δC 88.0) and one guanidine (δC 154.4). HMBC correlations are listed in Table 2.

Table 2.
1H NMR (500 MHz) and 13C NMR (125 MHz) Data for crambescin B 281 (4) and crambescin B 253 (5) in CD3OD.
The COSY correlations from H-3 to H-5 allowed building the tetrahydrofuran moiety (Figure 4). HMBC correlations from H-3 and H-6 to C-2 showed the connection of the spiroaminal moiety with the cyclic guanidine through the spiro carbon C-2 (δC 88.0). The aliphatic from C-8 to C-16 was then connected to the cyclic guanidine, based on COSY correlations from H-6 to H-8 and by HMBC correlations from H-6 to C-6, C-7 and C-9.
Molecular formula C14H27N3O of 5, a new analog of 4, was determined by HR-ESI-MS (m/z 254.2232 [M+H]+). The main difference between the two compounds is the length of the carbon chain holding 7 carbons in 5 and 9 in 4. The 1H, 13C NMR data and the HBMC correlations of 5 are shown in Table 2.
Compounds 4 and 5 have never been previously described and are related to crambescin B which was isolated in 1990 by Breakman et al. from the sponge Crambe crambe [11]. In crambescin B, a guanidino-alkyl ester attached to C-6 indicating that 4 and 5 should be considered as originated from a common biosynthetic precursor (Scheme 1).
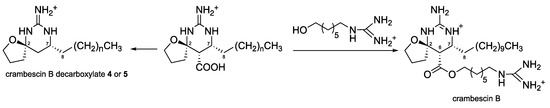
Scheme 1.
Structure of crambescin B and hypothetic biosynthesis of 4 or 5.
Crambescin B derivatives were synthetized by Nishikawa et al. in 2016 [12]. Among them, the crambescin decarboxylate is similar to 4 and 5 but the alkyl chain at C-7 consisted of 11 carbons. The authors highlighted that the relative stereochemistry of crambescin B decarboxylate could not be determined by NOESY analysis, which was confirmed by our data. However, they assumed that the stereochemistry at the C-2 spiro center would be R based on the reaction mechanism of the key synthetic steps. Nishikawa et al. [12] synthetized the (+) and (-)-crambescin B decarboxylate. The α25D + 103.8 (c 0.1, CHCl3) for 4 and + 124.2 (c 0.05, CHCl3) for 5, combined with the supposed R configuration of the spiro center C-2 indicate that the absolute configuration of C-2 and C-7 in 4 and 5 is probably 2R-7R. Attempts to record ECD spectra for 4 and 5 failed to offer exploitable results confirming the difficulty to establish the absolute configuration of crambescin B derivatives since their discovery in 1990.
2.3. Building Innovative Equipement for Open Sea Investigation
Beyond the isolated compounds and the three new natural products identified, the design of this experiment offers a proof of concept that molecules could be isolated from marine invertebrates without harming the bio-resources. The invertebrate brought from the sea and maintained in an aquarium for the duration of the experiment could be returned to its original habitat. However, the challenge, that represents the greatest interest in terms of environmental sustainability, is to transpose such a process to the open sea. The invertebrates will be kept in their natural habitat, together with their natural symbionts, and the extraction device will be brought to them with a minimal injury to the animal and minimal harm to the environment. This is the idea behind the development, patenting and construction of Somartex® [13], which will soon be operational and will be tested in diverse locations (Figure 6).
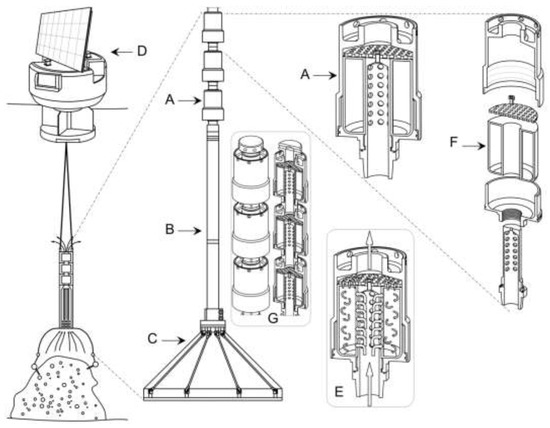
Figure 6.
Drawing of Somartex®. (A) trapping module, (B) submersible pump, (C) cover dome, (D) Somartex platform, (E) water circulation inside the module. The water is forced to pass through the trapping material (ex. XAD resin) present in the (F) compartment, through the holed tube. The water can then reach the second module through the holed grid, (G) superposition of different modules that may contain different means of molecular trapping.
3. Discussion
This study shows that the production of secondary metabolites by Crambe crambe is probably elicited by the presence of Anemonia sulcata, leading to intoxication by crambescidin and crambescin derivatives followed by a rapid death of all anemonia present. None of the compounds reported herein were detected in aquariums containing separately the two invertebrates, and no injury was observed. These experiments prove that secondary metabolites are secreted by the invertebrate to the surrounding water and could be captured by trapping materials, such as XAD resins. This represents the fundamental principle and the proof of concept for the development of Somartex® technology.
The key step in the design of our aquarium experiment is the external continuous circulation of water. This strategy, often used for microbial cultivation, involves a gradual depletion of the compounds from the medium, leading to the trapping of early metabolic intermediates, which unlock the metabolic repression and increased the recovery yield of the resin. In their efforts to investigate sponge-bacteria interactions (Aplysilla rosea challenged by its associated bacterium Streptomyces ACT-52A), Zhang et al. has used XAD7 packed in cheesecloth bags and introduced in an aquarium that contained the sponge and to which a microorganism was added [14]. Unfortunately, in this batch experiment, no compounds were isolated in sufficient quantity to be fully identified.
The continuous circulation, also applied in Somartex®, has another advantage. The dome covering the sampling area can be extended from 40 cm diameter and 40 cm height to 124 cm diameter and 100 cm height. This allows the selection of a unique specimen or a colony of invertebrates. The molecules collected can either come from a single invertebrate or from several species, each containing its own symbionts. This will provide information about the intra- and intermolecular communication network. This will hopefully mark the start of a new era in the growing field of chemical ecology, as well as promoting the sustainable valorization of marine compounds.
4. Materials and Methods
4.1. General Experimental Procedures
Optical rotations [α]D were measured using an Anton Paar MCP-300 polarimeter at 589 nm (Anton Paar, Les Ulis, France). The IR spectra were obtained using a Perkin-Elmer Spectrum 100 model instrument (Perkin-Elmer, Villebon-sur-Yvette, France). NMR experiments were performed using a Bruker Avance III 600 MHz spectrometer equipped with a TCi cryo-probe head for compounds 2 and 3, and a Bruker Avance 500 MHz spectrometer for compounds 1, 4 and 5 (Bruker, Vienna, Austria). All the spectra were acquired in CD3OD (δH 3.31 ppm and δC 49.15 ppm) at 300 K. High-resolution mass spectra were obtained on a Waters LCT Premier XE spectrometer equipped with an ESI-TOF (electrospray-time of flight) by direct infusion of the purified compounds (Waters SAS, Saint-Quentin-en-Yvelines, France). Pre-packed silica gel Redisep columns were used for flash chromatography using a Combiflash-Companion chromatogram (Serlabo, Entraigues-sur-la-Sorgue, France). All other chemicals and solvents were purchased from SDS (SdS, Peypen, France).
Analytical HPLC system consisted of an Alliance Waters 2695 controller coupled with a PhotoDiode Array Waters 2996, an evaporative light-scattering detector ELSD Waters 2424 detector and a mass detector Waters QDa. Sunfire C18 column (4.6 × 150 mm, 3.5 μm) was used with a flow rate of 0.7 mL/min. The elution gradient consisted of a linear gradient from 100 % solvent A to 100% solvent B in 40 min, then 10 min at 100% B (Solvent A: H2O + 0,1 HCOOH, Solvent B: ACN + 0,1% HCOOH). Preparative HPLC was performed on a semi-preparative Sunfire C18 column (10 × 250 mm, 5 μm) using a Waters autosampler 717, a pump 600, a photodiode array detector 2996 and an ELSD detector 2420 (Waters SAS, Saint-Quentin-en-Yvelines, France). XAD resin (AMBERLITE™ XAD™16HP N) was purchased from DOW (DOW France SAS, Saint-Denis, France).
4.2. Invertebrate Collection, Transport and Experimental Design
Anemonia sulcata individuals (two years old, adult size of 30 g wet weight per unit) were selected from the cultivation facilities of iMare Natural (www.imarenatural.com). Crambe crambe individuals (10 to 15 cm length) were carefully collected with their supporting stones from Salobreña, Granada, Spain, at a depth of 8 m, in June 2017 (diving site La Caleta de Salobreña, N 36. 74419, W −3.602065). Sponges were harvested just before starting the experiments. Crambe crambe individuals were introduced into plastic bags and kept immersed in seawater. Once on board, plastic bags were introduced in isothermal box, with no aeration and no sunlight exposure.
Transportation from the collection site to iMare facilities took about 2 h. The temperature of the seawater was 24 °C and the salinity was 37‰.
The aquarium experiments were implemented in 35 L aquariums equipped with an 800 L/h submersible pump and a 3 W led light lamp. The pump ensured the continuous circulation of the aquarium water into the trapping filters during 48 h. Each aquarium was equipped with two filters, the first one containing 50 g of silica sand and the second 50 g of Amberlite XAD-16. During the experiment, the invertebrates were not fed and there were no other additives. The experimental system was designed to simulate the environmental conditions of the Summer season, including a high temperature between 25–28 °C and a Summer photoperiod of 12L:12D.
Four aquariums were set up:
- Aquarium 1: was a control without invertebrates,
- Aquarium 2: contained 20 Anemonia sulcata
- Aquarium 3: contained Crambe crambe individuals covering in total an average surface of 250 to 300 cm2.
- Aquarium 4: contained 20 Anemonia sulcata and Crambe crambe individuals covering in total 250 to 300 cm2.
After 48 h, the filters containing the Amberlite XAD-16 resin were recovered and frozen without any treatment until extraction and chemical analysis.
4.3. Isolation of Compounds and Struture Elucidation
The Amberlite XAD-16 resin (≈50 g) was poured in a Buchner funnel equipped with a paper filter and washed with 3 × 100 mL of distilled water. The resins were dried under vacuum and transferred to an Erlenmeyer. The compounds trapped by the resin were eluted by a gentle shaking with 3 × 100 mL of ethyl acetate (EtOAc) followed by 3 × 100 mL of methanol (MeOH). The EtOAc and the MeOH fractions were evaporated in vacuo weighed and analyzed by TLC and HPLC. Aquariums containing a single invertebrate species as well as the control aquarium without invertebrates gave extracts weights ranging from 15 to 20 mg. TLC and HPLC analysis gave flat chromatograms without any exploitable signals.
The XAD-16 resin recovered from the aquarium in which Crambe crambe individuals were added to Anemonia sulcata (Aq 4) yielded 22 mg of crude EtOAc extract and 160 mg of MeOH extract. The EtOAc extract did not show any exploitable signals after TLC and HPLC analysis. The MeOH extract (160 mg) was submitted to flash chromatography on a Combiflash Companion using a Redisep 12 g silica column eluted with a gradient of methylene dichloromethane (DCM) and MeOH. Based on the TLC and HPLC profiles, 17 fractions were pooled and submitted to single peak preparative HPLC purification. The pure compounds were further analyzed by LC-MS and the peaks with similar mass and chromatographic data were pooled to obtain the maximum quantities for structural elucidation. Thereby we isolated 1.5 mg of crambescidin 359 (1), 1.1 mg of crambescidin acid (2), 1.1 mg of crambescidin 401 (3), 1.6 mg of crambescin B 281 (4) and 1.8 mg of crambescin B 253 (5).
Crambescidin 359 (1), α25D − 135.0 (c 0.2, MeOH). IR (neat) νmax 2967, 2933, 1620, 1449, 1344, 1010, 847, 755. NMR data Table S30 (supporting information); HR-ESI-MS for C21H33N3O2 (m/z 360.2651 [M+H]+) (calcd for C21H34N3O2, 360.2573).
Crambescidin acid (2), α25D − 45.0 (c 0.06, CHCl3). IR (neat) νmax 3230, 2961, 2931, 1622, 1446, 1398, 1342, 1047, 1011, 755, 716. NMR data, Spectra S6 and S7 and Table S30 (supporting information); HR-ESI-MS for C22H33N3O4 (m/z 404.2549 [M+H]+) (calcd for C22H34N3O4, 404.2471).
Crambescidin 401 (3), α25D − 48.6 (c 0.07, CHCl3). IR (neat) νmax 3358, 2963, 2929, 1620, 1580, 1360, 1053, 1026, 754 NMR data, Spectra S10 to S14 and Table S30 (supporting information); HR-ESI-MS for C22H31N3O4 (m/z 402.2393 [M+H]+) (calcd for C22H32N3O4, 402.2315).
Crambescin B 281 (4), α25D + 103.8 (c 0.1, CHCl3). IR (neat) νmax 3236, 3067, 2924, 2855, 2776, 1650, 1610, 1582, 1464, 1346, 1027, 759.NMR data, Spectra S17 to S21 and Table S31 (supporting information); HR-ESI-MS for C16H32N3O (m/z 282.2544 [M+H]+) (calcd for C16H32N3O, 282.2545).
Crambescin B 253 (5), α25D + 124.2 (c 0.05, CHCl3). IR (neat) νmax 3224, 3062, 2956, 2856, 1650, 1609, 1581, 1462, 1346, 1028, 761. NMR data, Spectra S24 to S28 and Table S31 (supporting information); HR-ESI-MS for C14H28N3O (m/z 254.2227 [M+H]+) (calcd for C14H28N3O1, 254.2232).
5. Patents
Somartex® (Self Operating MARine Trapping Extractor) is a unique technology inspired by the results of this paper. The technology was built to allow the trapping of the compounds released by an invertebrate or a community of invertebrates directly into their marine natural habitat. It is formed by (1) a cover cone with various diameters and heights which delimits the area of interest, (2) a submerged pump that drives the water around the invertebrate into the cone. The water is expected to contain valuable compounds secreted by the invertebrate (3) a trapping module in which the water is brought into contact with one or more trapping matrix which also increases the concentration of the compounds. The trapping material is then recovered after a defined period of contact and the target compounds are desorbed. The power for the pump can be supplied by renewable solar energy, associated with an accumulator to allow continuous and autonomous operations. Solar panels will be installed on a 2.4 m diameter floating solid platform. The operator can reach the platform (using a dinghy) to carry out any maintenance, recover/replace the trapping material or move the equipment to another area.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/5/152/s1.
Author Contributions
P.V. and G.L.G. contributed equally to this work and oversaw the isolation and structural elucidation of the compounds. C.A. and P.A.A. were involved in the marine invertebrate experiments and the specific aquarium design. J.-F.G. recorded the 600 MHz cryoprobe NMR spectra of the compounds. N.F. contributed to the analysis of the publication results and the discussion of the article. J.O. coordinated the work reported in this article.
Acknowledgments
This research was supported and conducted in the frame of the H2020 TASCMAR project, which is funded by the European Union under grant agreement number 634674 (www.tascmar.eu).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Drugs and drug candidates from Marine sources: An assessment of the current “State of Play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Schippers, K.J.; Sipkema, D.; Osinga, R.; Smidt, H.; Pomponi, S.A.; Martens, D.E.; Wijffels, R.H. Chapter six—Cultivation of sponges, sponge cells and symbionts: Achievements and future prospects. Adv. Mar. Biol. 2012, 62, 273–337. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A. Farming sponges to supply bioactive metabolites and bath sponges. Mar. Biotechnol. 2009, 11, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.R.; Battershill, C.N. Developing farming structures for production of biologically active sponge metabolites. Aquaculture 2003, 217, 139–156. [Google Scholar] [CrossRef]
- Adelin, E.; Servy, C.; Martin, M.-T.; Arcile, G.; Bogdan, I.I.; Retailleau, P.; Bonfill, M.; Ouazzani, J. Bicyclic and tetracyclic diterpenes from a Trichoderma symbiont of Taxus baccata. Phytochemistry 2014, 97, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, G.; Martin, M.-T.; Bogdan, I.I.; Adelin, E.; Servy, C.; Cortial, S.; Ouazzani, J. Isolation and characterization of unusual hydrazides from Streptomyces sp. Impact of the cultivation support and extraction procedure. J. Nat. Prod. 2013, 76, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Braekman, J.C.; Daloze, D.; Tavares, R.; Hajdu, E.; Van Soest, R.W.M. Novel polycyclic guanidine alkaloids from two Marine sponges of the genus. Monanchora. J. Nat. Prod. 2000, 63, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Meragelman, K.M.; McKee, T.C.; McMahon, J.B. Monanchorin, a bicyclic alkaloid from the sponge Monanchora ungiculata. J. Nat. Prod. 2004, 67, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Aron, Z.D.; Overman, L.E. Total synthesis and properties of the crambescidin core zwitterionic acid and crambescidin 359. J. Am. Chem. Soc. 2005, 127, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Kumar, N.V.; Kokke, W.C.; Bean, M.F.; Freyer, A.J.; De Brosse, C.; Mai, S.; Truneh, A.; Faulkner, D.J.; Carte, B.; et al. Novel alkaloids from the sponge Batzella sp.: Inhibitors of HIV gp120-human CD4 binding. J. Org. Chem. 1995, 60, 1182–1188. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Braekman, J.C.; Daloze, D.; Hallenga, K.; Ottinger, R.; Bruno, I.; Riccio, R. Two new guanidine alkaloids from the mediterranean sponge crambe crambe. Tetrahedron Lett. 1990, 31, 6531–6534. [Google Scholar] [CrossRef]
- Atsuo Nakazaki, A.; Nakane, Y.; Ishikawa, Y.; Yotsu-Yamashita, M.; Nishikawa, T. Asymmetric synthesis of crambescin A–C carboxylic acids and their inhibitory activity on voltage-gated sodium channels. Org. Biomol. Chem. 2016, 14, 5304–5309. [Google Scholar] [CrossRef] [PubMed]
- Ouazzani, J.; Le Goff, G. Somartex. Self Operating MARine trapping EXtractor. INPI Patent 1850669, 2018. [Google Scholar]
- Mohammad, F.; Mehbub, M.F.; Jason, E.; Tanner, J.E.; Stephen, J.; Barnett, S.J.; Franco, C.M.M.; Zhang, W. The role of sponge-bacteria interactions: the sponge Aplysilla rosea challenged by its associated bacterium Streptomyces ACT-52A in a controlled aquarium system. Appl. Microbiol. Biotechnol. 2016, 100, 10609–10626. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).