Biosynthesis and Secretion of Human Tissue Kallikrein in Transgenic Chlamydomonas reinhardtii
Abstract
1. Introduction
2. Results
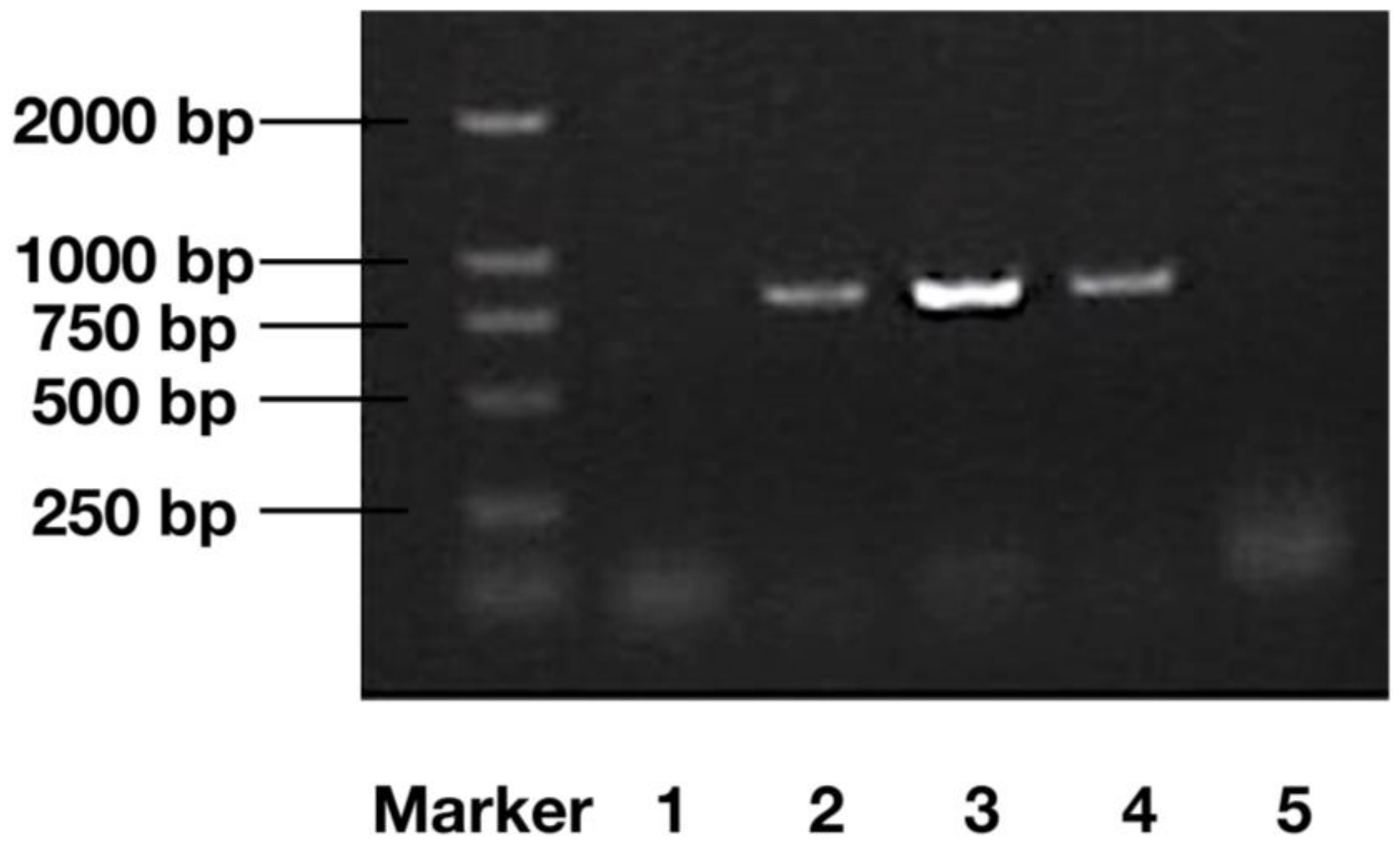
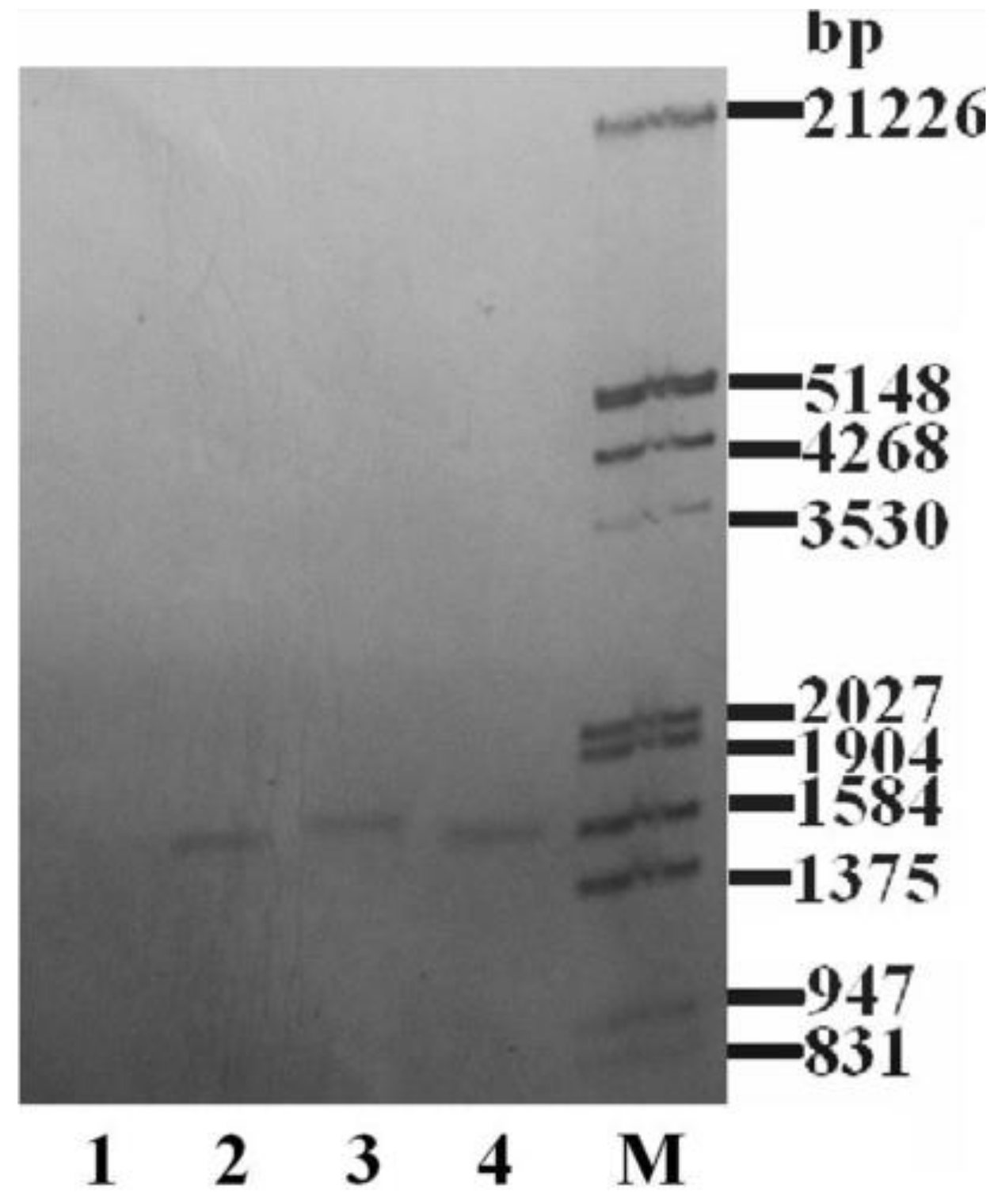
2.1. DNA Verification of Transgenic Algae
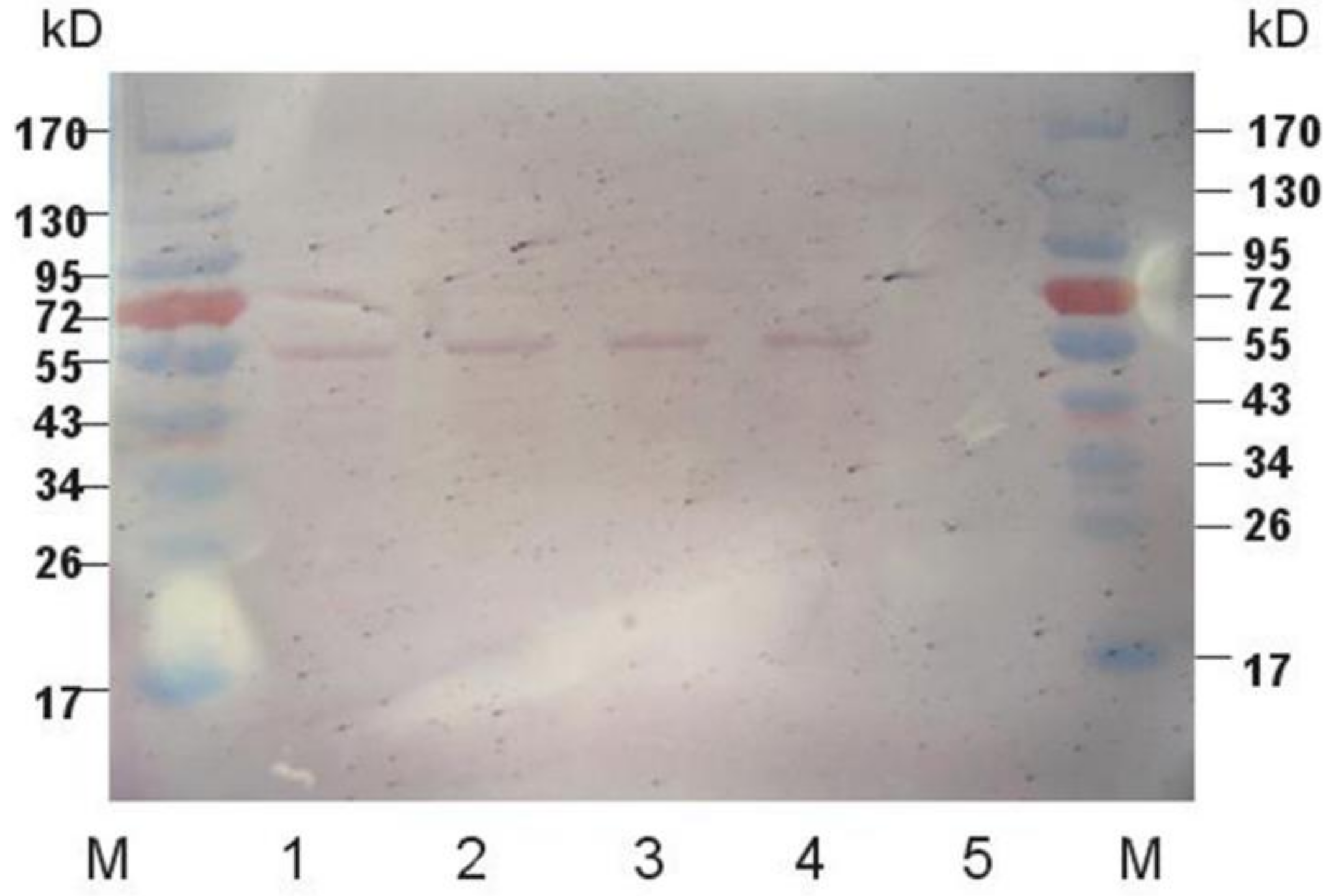
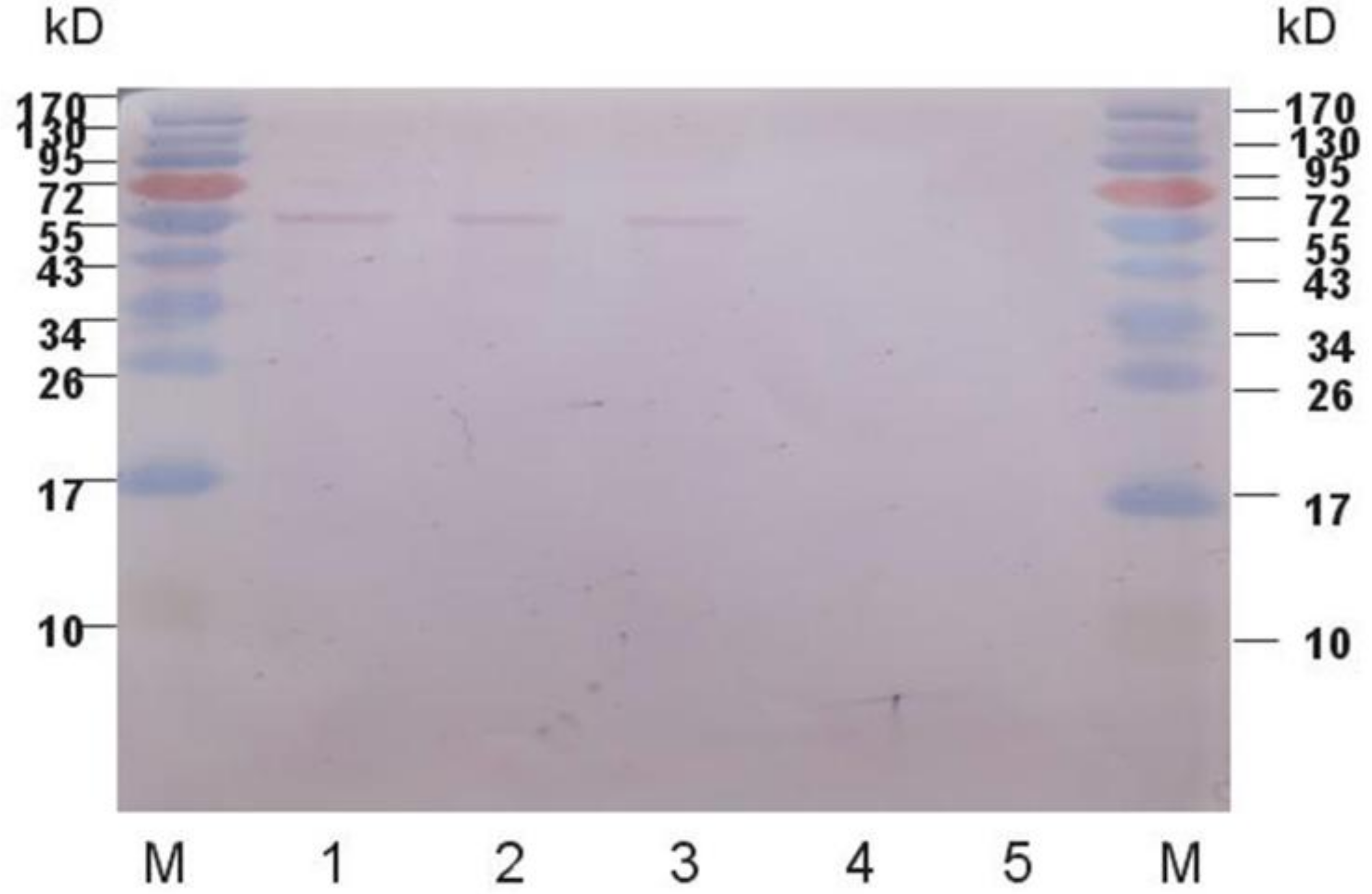
2.2. Western Blot Analysis of Expressed Protein
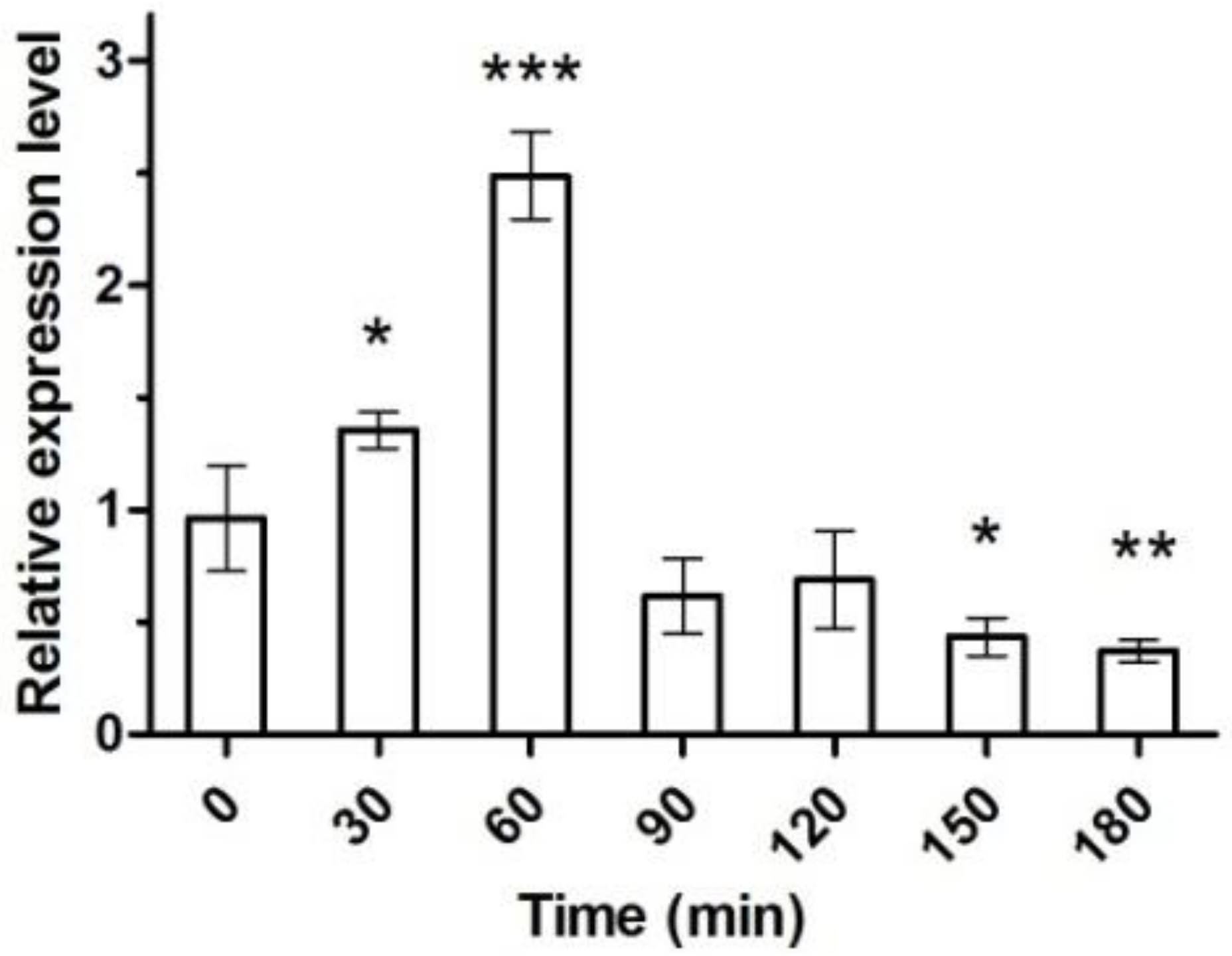
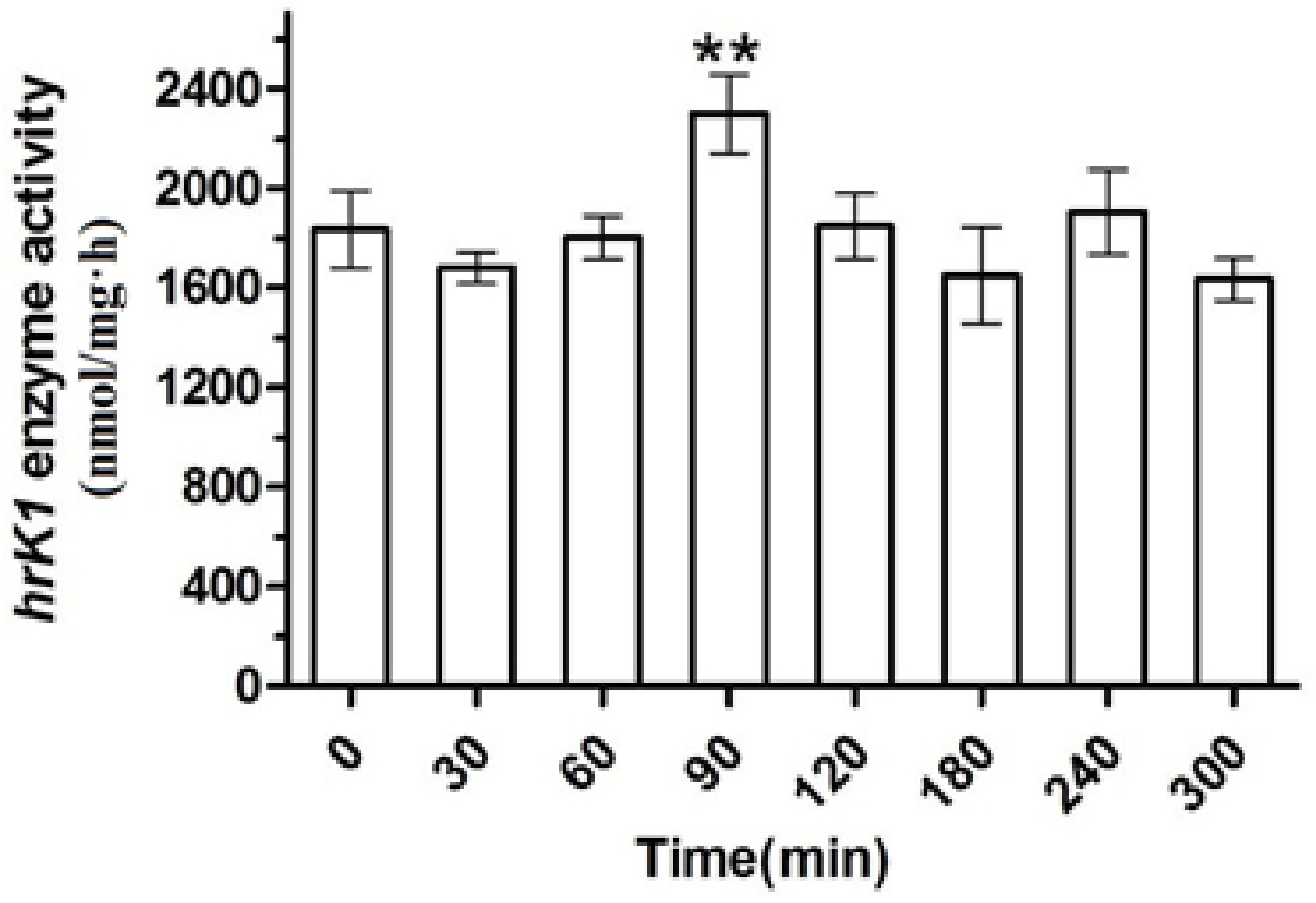
2.3. Gene Expression and Enzyme Activity under Heat Stress
3. Discussion
4. Materials and Methods
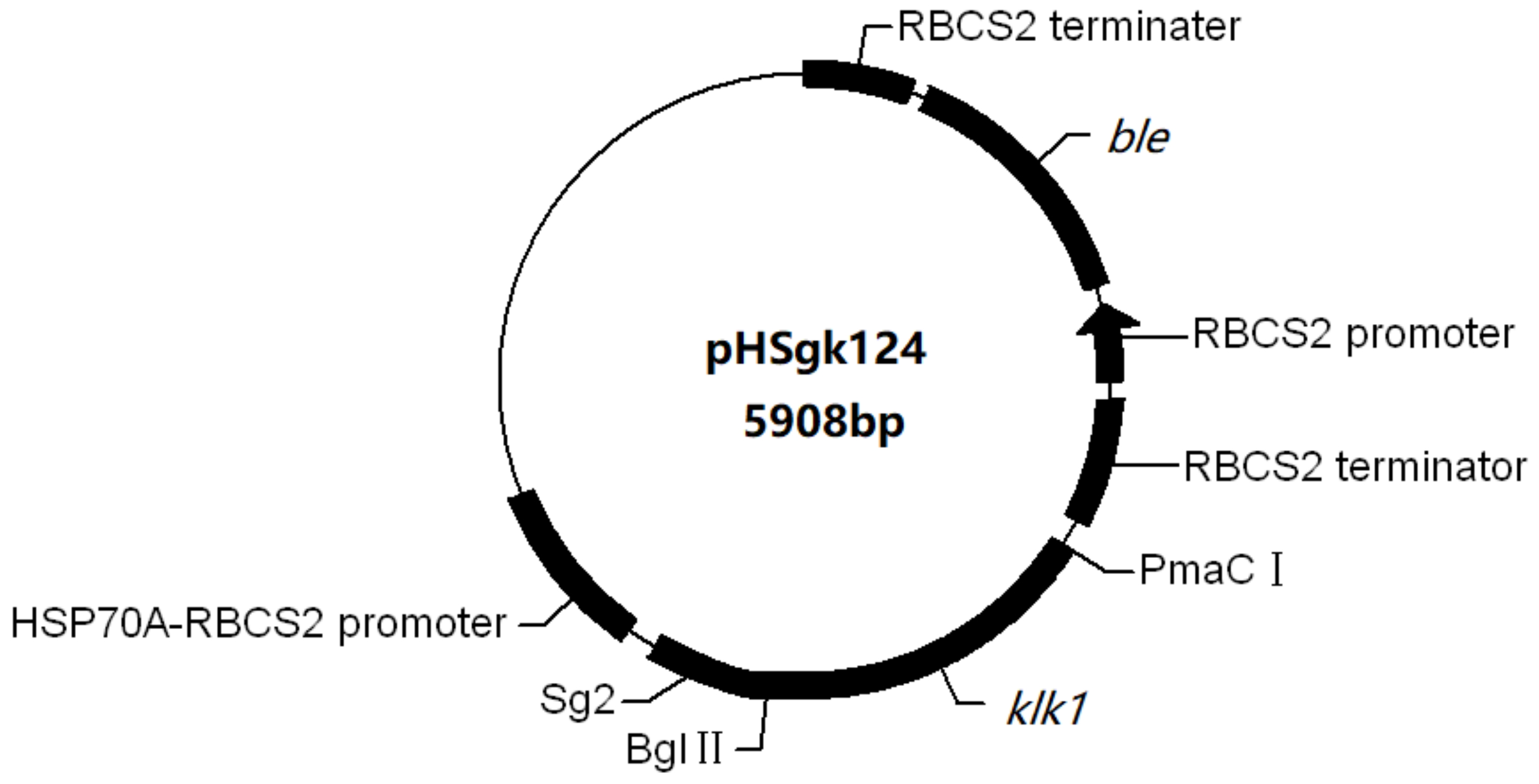
4.1. Construction of Plasmid
4.2. Materials and Culture Conditions
4.3. PCR Analysis
4.4. Total DNA Southern Blot
4.5. Fluorescence Quantitative PCR
4.6. Protein Western Blot Analysis and Enzyme Activity Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayfield, S.P.; Manuell, A.L.; Chen, S.; Wu, J.; Tran, M.; Siefker, D.; Muto, M.; Marin-Navarro, J. Chlamydomonas reinhardtii chloroplasts as protein factories. Curr. Opin. Biotechnol. 2007, 18, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Mayfield, S.P. The microalga Chlamydomonas reinhardtii as a platform for the production of human protein therapeutics. Bioeng. Bugs 2011, 2, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.Y.; Li, H.; Hu, Z.L. Expression of tandem repeat Cecropin B in Chlamydomonas reinhardtii and its antibacterial effect. Prog. Biochem. Biophys. 2012, 39, 344–351. [Google Scholar] [CrossRef]
- Wang, C.G.; Hu, Z.L.; Hu, W.; Lei, A.P. Expression and molecular analysis of phbB gene in Chlamydomonas reinhardtii. Chin. Sci. Bull. 2004, 49, 1713–1717. [Google Scholar]
- Zheng, K.J.; Wang, C.G.; Xiao, M.; Chen, J.; Li, J.C.; Hu, Z.L. Expression of bkt and bch genes from Haematococcus pluvialis in transgenic Chlamydomonas. Sci. China Life Sci. 2014, 57, 1028–1033. [Google Scholar] [CrossRef]
- Wang, C.G.; Hu, Z.L.; Lei, A.P. Biosynthesis of poly-3-hydroxybutyrate (phb) in the transgenic green alga Chlamydomonas reinhardtii. Phycol. Soc. Am. 2010, 46, 396–402. [Google Scholar]
- Wu, J.X.; Hu, Z.L.; Wang, C.G.; Li, S.F.; Lei, A.P. Efficient expression of green fluorescent protein (GFP) mediated by a chimeric promoter in Chlamydomonas reinhardtii. Chin. J. Oceanol. Limnol. 2008, 26, 242–247. [Google Scholar] [CrossRef]
- Surzycki, R.; Greenham, K.; Kitayama, K.; Dibal, F.; Wagner, R.; Rochaix, J.D.; Ajam, T.; Surzycki, S. Factors effecting expression of vaccines in microalgae. Biologicals 2009, 37, 133–138. [Google Scholar] [CrossRef]
- Hu, Z.L; Fan, Z.; Zhao, Z.L.; Chen, J.; Li, J.C. Stable expression of antibiotic-resistant gene ble from Streptoalloteichus hindustanus in the mitochondria of Chlamydomonas reinhardtii. PLoS ONE 2011, 7, e35542. [Google Scholar] [CrossRef]
- Hu, Z.L.; Zhao, Z.L.; Wu, Z.H.; Chen, J. Successful expression of heterologous egfp gene in the mitochondria of a photosynthetic eukaryote Chlamydomonas reinhardtii. Mitochondrion 2011, 5, 716–721. [Google Scholar] [CrossRef]
- Han, S.H.; Hu, Z.L.; Lei, A.P. Expression and function analysis of the metallothionein-like (MT-like) gene from Festuca rubra in Chlamydomonas reinhardtii chloroplast. Sci. China Ser. C 2008, 51, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Von Gunnar, H. Life and death of a signal peptide. Nature 1998, 396, 111–112. [Google Scholar]
- Uchia, H.; Naito, N.; Asadaa, N.; Wadaa, M.; Ikedaa, M.; Kobayashia, H.; Asanagia, M.; Moria, K.; Fujitaa, Y.; Kondaa, K.; et al. Secretion of authentic 20-kDa human growth hormone (20 kDa hGH) in Escherichia Coli and properties of the purified product. J. Biotechnol. 1997, 55, 101–112. [Google Scholar] [CrossRef]
- Kjeldsen, T.; Pettensson, A.F.; Hach, M. Secretory expression and characterization of insulin in Pichia pastoris. J. Biotechnol. Appl. Biochem. 1999, 29, 79–86. [Google Scholar]
- Rasala, B.A.; Lee, P.A.; Shen, Z.X.; Briggs, S.P.; Mendez, M.; Mayfield, S.P. Robust expression and secretion of Xylanase1 in Chlamydomonas reinhardtii by fusion to a selection gene and processing with the FMDV 2A peptide. PLoS ONE 2012, 7, e43349. [Google Scholar] [CrossRef] [PubMed]
- Yousef, G.M.; Diamandis, E.P. The new human tissue kallikrein gene family: structure, function and association to disease. Endocr. Rev. 2001, 22, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Borgono, C.A.; Michael, I.P.; Diamandis, E.P. Human tissue kallikreins: Physiologic roles and applications in cancer. Mol. Cancer Res. 2004, 2, 257–280. [Google Scholar] [PubMed]
- Bhoola, K.D.; Figueroa, C.D.; Worthy, K. Bioregulation of kinins: Kallikreins, kininogens, and kininases. Pharmacol. Rev. 1992, 44, 1–80. [Google Scholar]
- Clements, J. The molecular biology of the kallikreins and their roles in inflammation. In The Kinin System; Farmer, S., Ed.; Academic Press: New York, NY, USA, 1997; pp. 71–97. [Google Scholar]
- Scaife, M.A.; Nguyen, G.T.D.T.; Rico, J.; Lambert, D.; Helliwell, K.E.; Smith, A.G. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J. 2015, 82, 532–546. [Google Scholar] [CrossRef]
- Specht, E.; Miyake-Stoner, S.; Mayfield, S. Micro-algae come of age as a platform for recombinant protein production. Biotechnol. Lett. 2010, 32, 1373–1383. [Google Scholar] [CrossRef]
- Schroda, M.; Blcker, D.; Beck, C.F. The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J. 2000, 21, 121–131. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wu, J.; Wu, Q.; Hu, Z. Biosynthesis and Secretion of Human Tissue Kallikrein in Transgenic Chlamydomonas reinhardtii. Mar. Drugs 2018, 16, 493. https://doi.org/10.3390/md16120493
Chen J, Wu J, Wu Q, Hu Z. Biosynthesis and Secretion of Human Tissue Kallikrein in Transgenic Chlamydomonas reinhardtii. Marine Drugs. 2018; 16(12):493. https://doi.org/10.3390/md16120493
Chicago/Turabian StyleChen, Jun, Jinxia Wu, Qingyu Wu, and Zhangli Hu. 2018. "Biosynthesis and Secretion of Human Tissue Kallikrein in Transgenic Chlamydomonas reinhardtii" Marine Drugs 16, no. 12: 493. https://doi.org/10.3390/md16120493
APA StyleChen, J., Wu, J., Wu, Q., & Hu, Z. (2018). Biosynthesis and Secretion of Human Tissue Kallikrein in Transgenic Chlamydomonas reinhardtii. Marine Drugs, 16(12), 493. https://doi.org/10.3390/md16120493




