Heteronemin Induces Anti-Proliferation in Cholangiocarcinoma Cells via Inhibiting TGF-β Pathway
Abstract
1. Introduction
2. Results
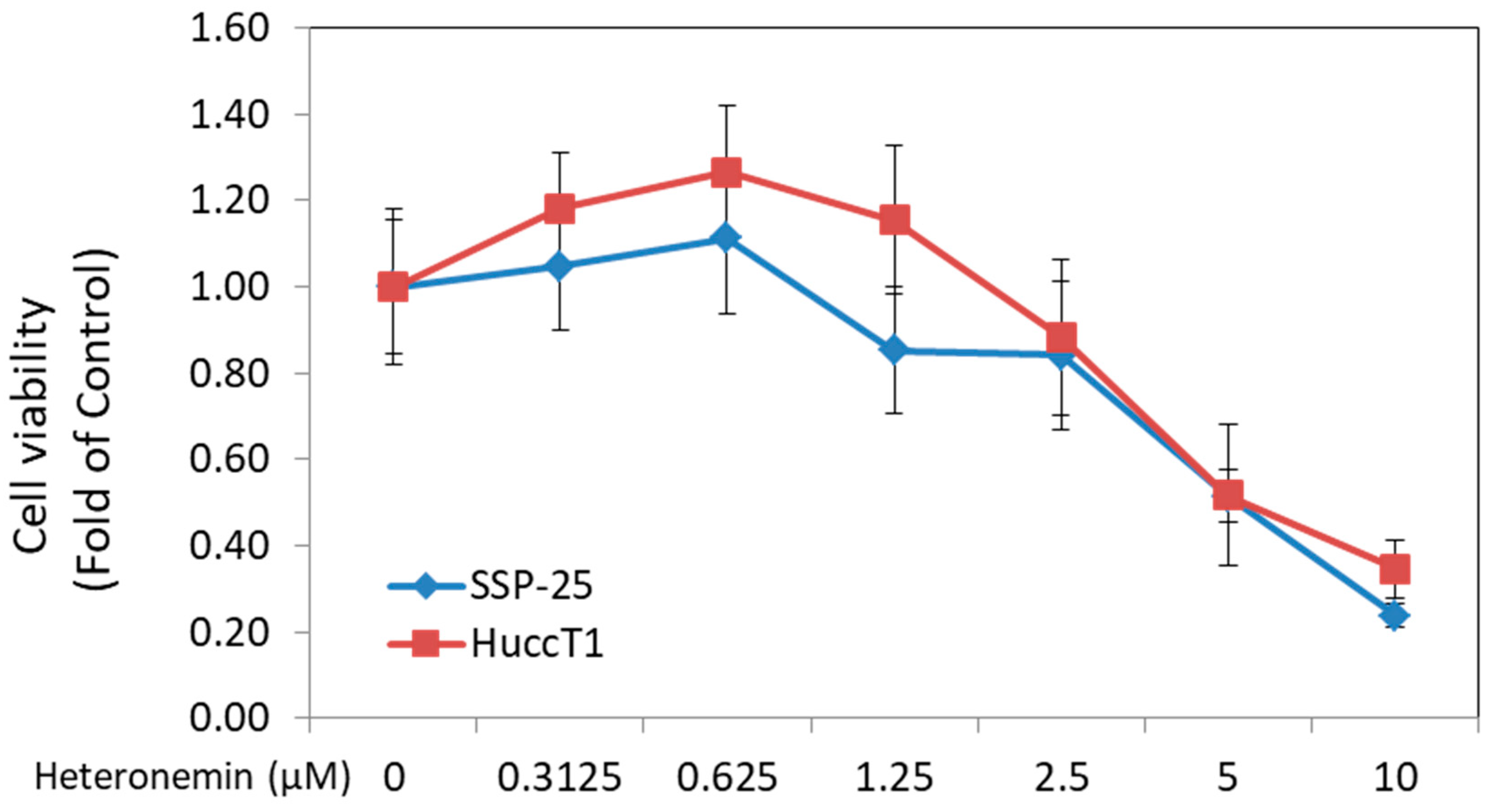
2.1. Heteronemin Inhibited Cell Proliferation of Cholangiocarcinoma Cells In Vitro
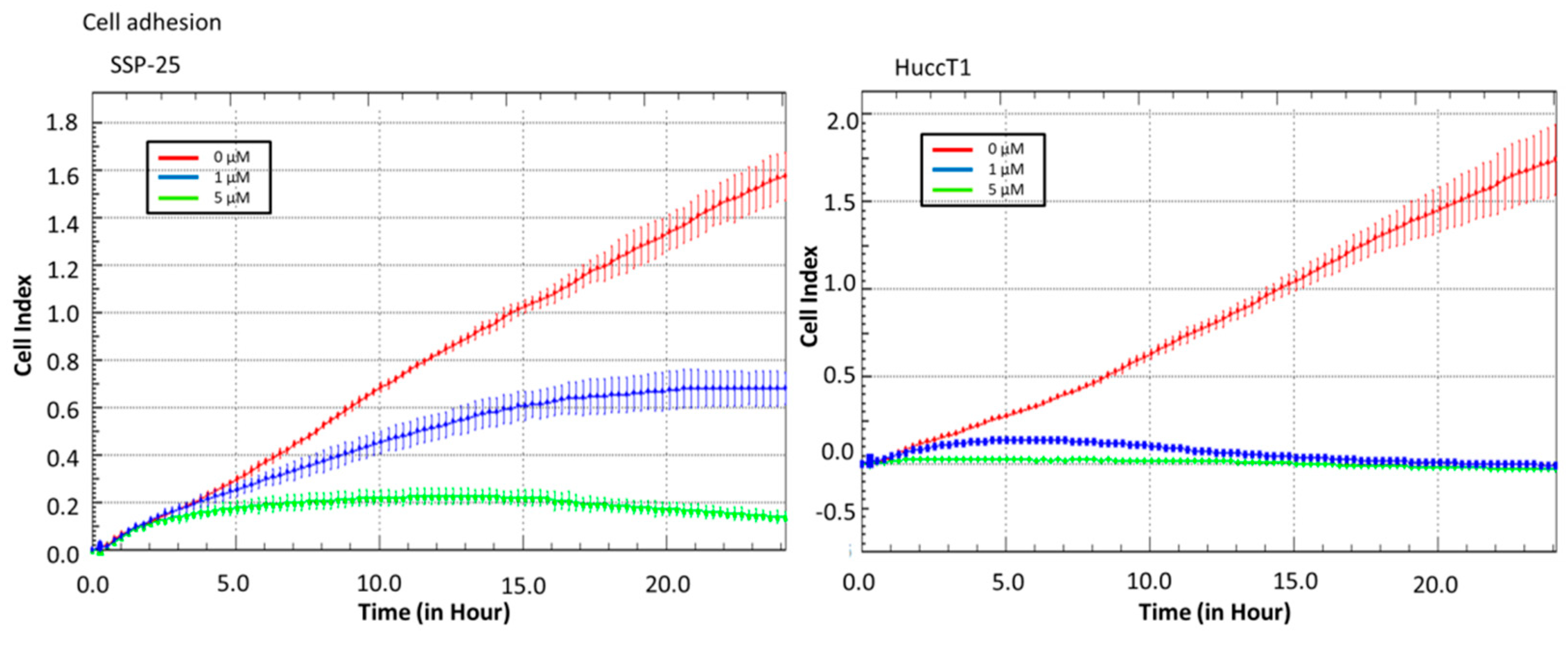
2.2. Heteronemin Affects Cell Migration and Cell Adhesion in Cholangiocarcinoma Cell Lines
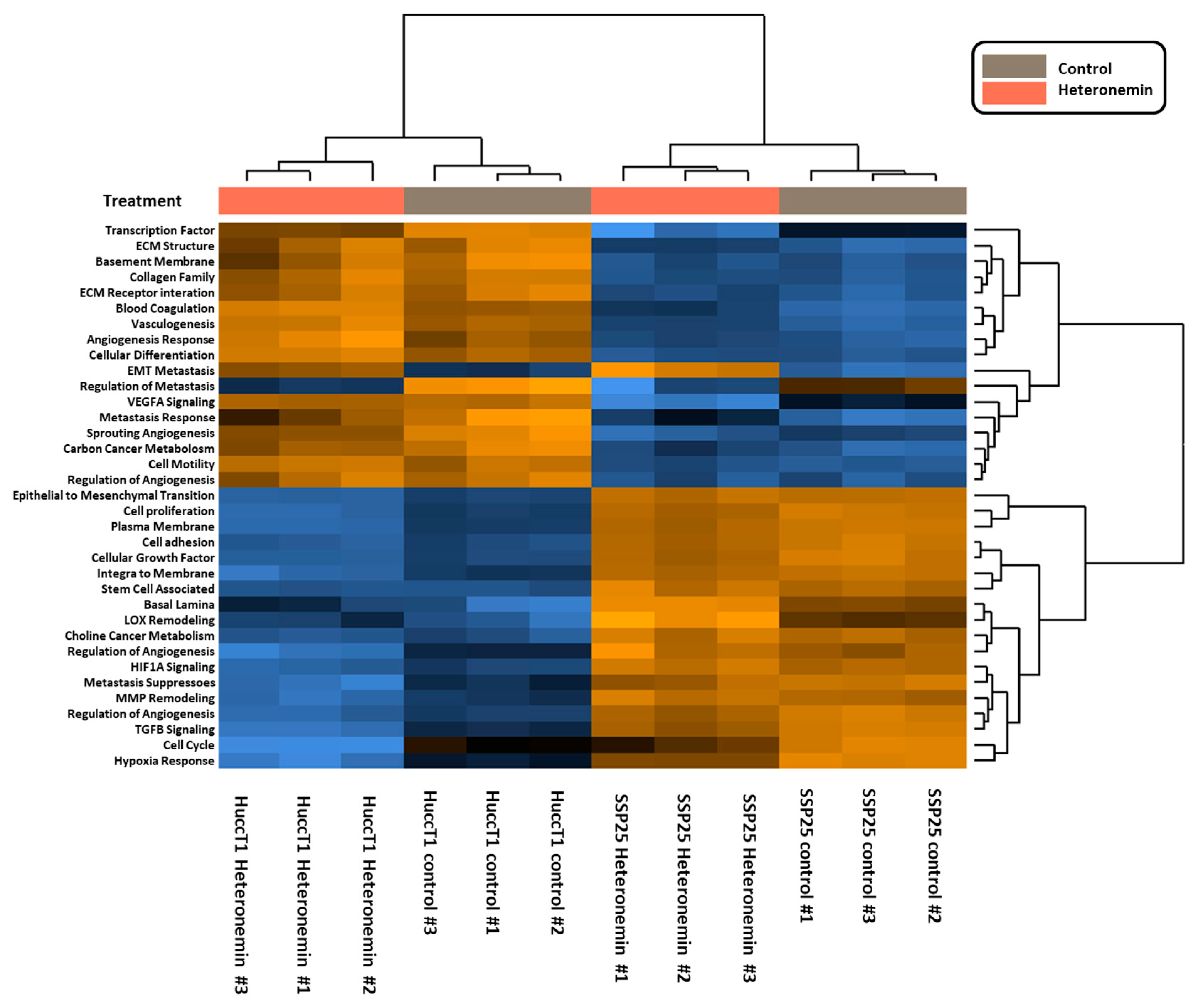
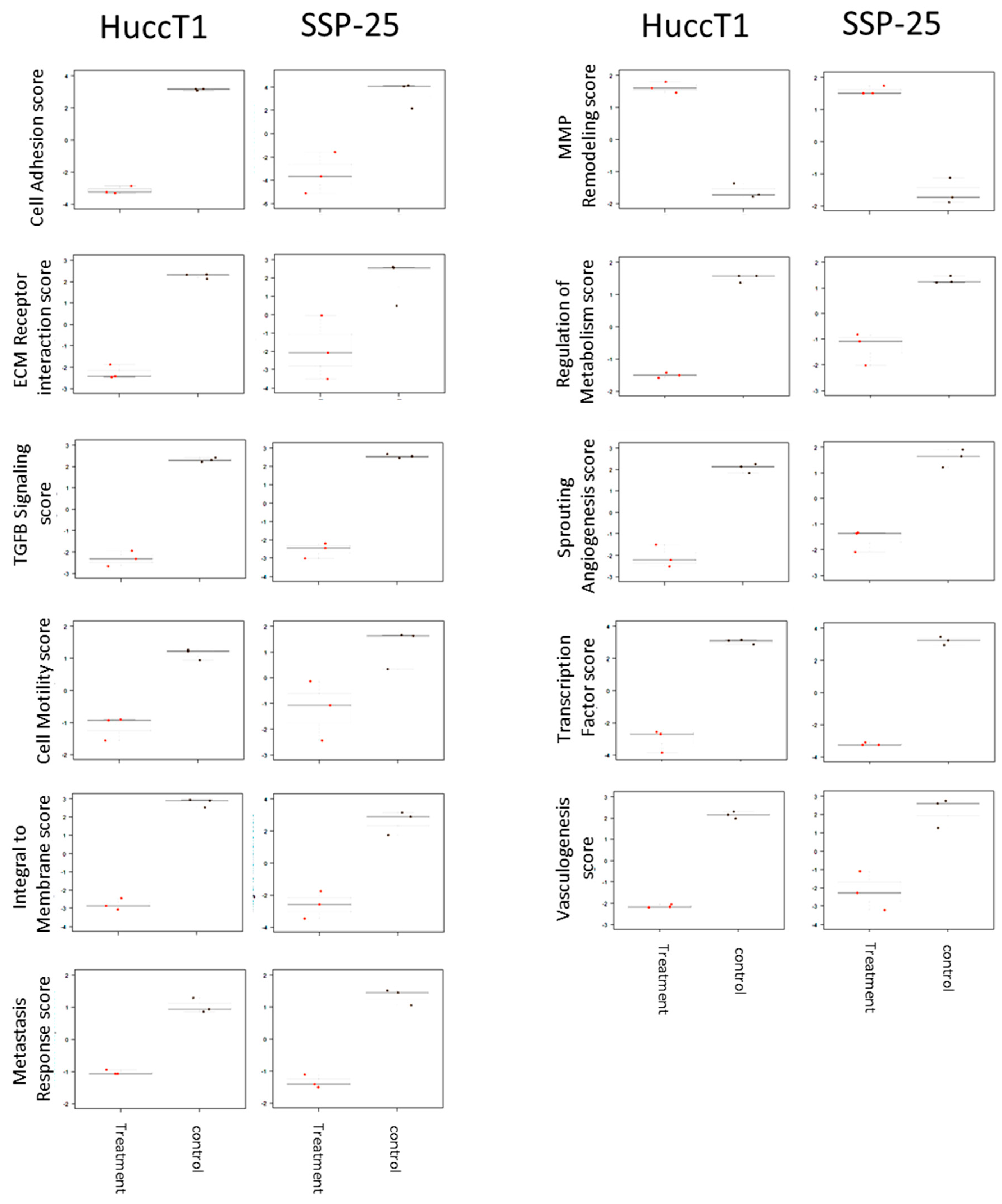
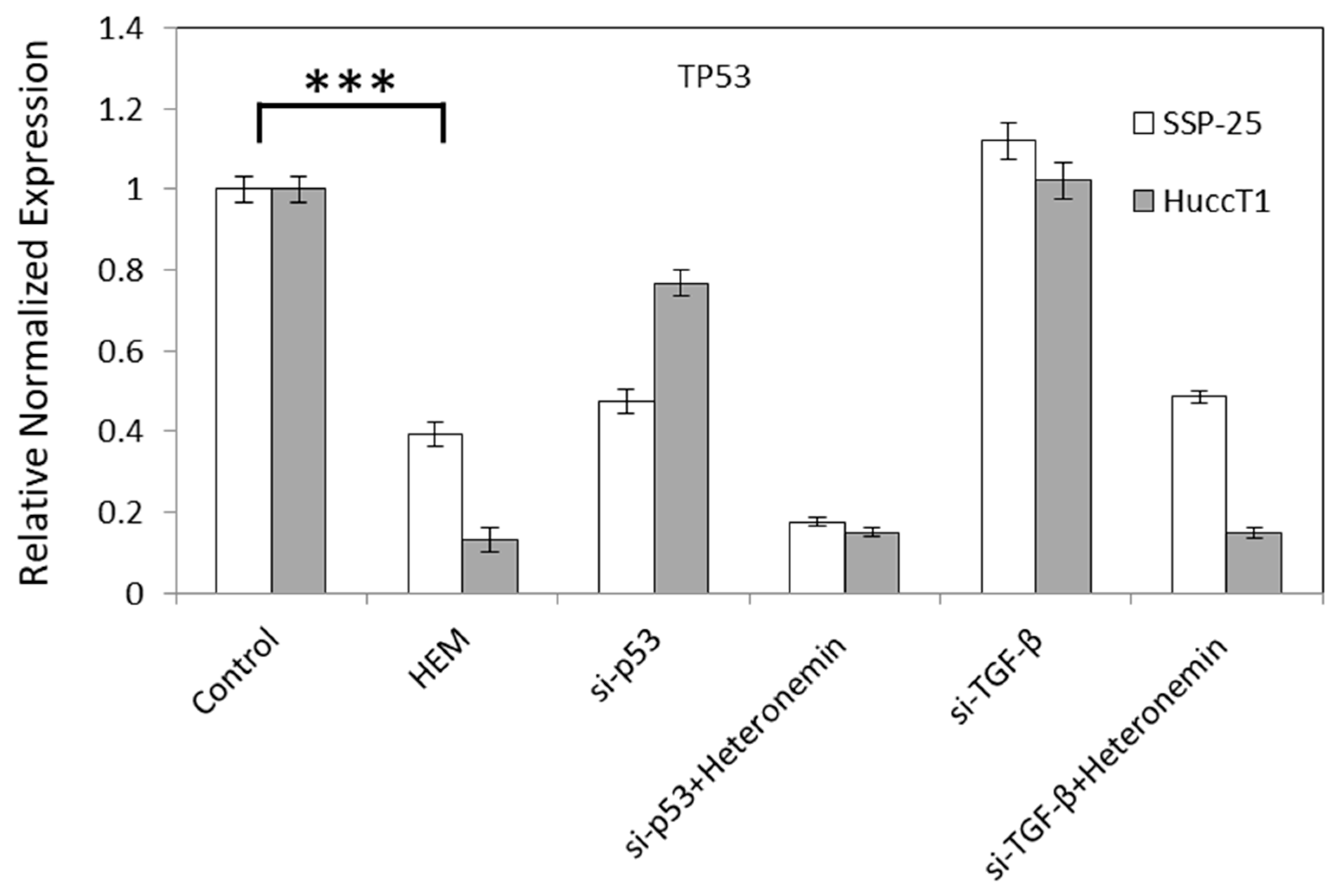
2.3. Heteronemin Regulates Expression of Genes in Cholangiocarcinoma Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. MTS Cell Proliferation Assay
4.3. Cell Migration and Cell Adhesion Assay
4.4. RNA Isolation and NanoString® Analysis
4.5. Quantitative Real-Time PCR
4.6. Quantification of Results and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leone, F.; Cavalloni, G.; Pignochino, Y.; Sarotto, I.; Ferraris, R.; Piacibello, W.; Venesio, T.; Capussotti, L.; Risio, M.; Aglietta, M. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin. Cancer Res. 2006, 12, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Sonongbua, J.; Thuwajit, P.; Chau-In, S.; Pairojkul, C.; Wongkham, S.; Thuwajit, C. Periostin activates integrin α5β1 through a PI3K/AKTdependent pathway in invasion of cholangiocarcinoma. Int. J. Oncol. 2012, 41, 1110–1118. [Google Scholar] [CrossRef]
- Wattanawongdon, W.; Hahnvajanawong, C.; Namwat, N.; Kanchanawat, S.; Boonmars, T.; Jearanaikoon, P.; Leelayuwat, C.; Techasen, A.; Seubwai, W. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int. J. Oncol. 2015, 47, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Lin, H.Y.; Changou, C.A.; Chen, C.H.; Liu, Y.R.; Wang, J.; Jiang, X.; Luh, F.; Yen, Y. Integrin β3 and LKB1 are independently involved in the inhibition of proliferation by lovastatin in human intrahepatic cholangiocarcinoma. Oncotarget 2016, 7, 362–373. [Google Scholar] [PubMed]
- Zhang, Y.E. Non-Smad pathways in β-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Huang, K.Y.; Lu, M.C.; Huang, H.L.; Chen, C.Y.; Cheng, Y.L.; Yu, H.C.; Liu, S.Q.; Lai, N.S.; Huang, H.B. TGF-β upregulates the translation of USP15 via the PI3K/AKT pathway to promote p53 stability. Oncogene 2017, 36, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGFβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Elston, R.; Inman, G.J. Crosstalk between p53 and TGF-β Signalling. J. Signal Transduct. 2012, 2012. [Google Scholar] [CrossRef]
- Senturk, S.; Mumcuoglu, M.; Gursoy-Yuzugullu, O.; Cingoz, B.; Akcali, K.C.; Ozturk, M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 2010, 52, 966–974. [Google Scholar] [CrossRef]
- Huang, T.; David, L.; Mendoza, V.; Yang, Y.; Villarreal, M.; De, K.; Sun, L.; Fang, X.; Lopez-Casillas, F.; Wrana, J.L.; et al. TGF-β signalling is mediated by two autonomously functioning TβRI: TβRII pairs. EMBO J. 2011, 30, 1263–1276. [Google Scholar] [CrossRef]
- Massague, J. A very private TGF-beta receptor embrace. Mol. Cell 2008, 29, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Groppe, J.; Hinck, C.S.; Samavarchi-Tehrani, P.; Zubieta, C.; Schuermann, J.P.; Taylor, A.B.; Schwarz, P.M.; Wrana, J.L.; Hinck, A.P. Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 2008, 29, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Moustakas, A. Role of Smads in TGFβ signaling. Cell Tissue Res. 2012, 347, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.H.; Derynck, R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lu, M.C.; El-Shazly, M.; Lai, K.H.; Wu, T.Y.; Hsu, Y.M.; Lee, Y.L.; Liu, Y.C. Breaking down Leukemia Walls: Heteronemin, a Sesterterpene Derivative, Induces Apoptosis in Leukemia Molt4 Cells through Oxidative Stress, Mitochondrial Dysfunction and Induction of Talin Expression. Mar. Drugs 2018, 16, 212. [Google Scholar] [CrossRef]
- Okamoto, A.; Tanaka, M.; Sumi, C.; Oku, K.; Kusunoki, M.; Nishi, K.; Matsuo, Y.; Takenaga, K.; Shingu, K.; Hirota, K. The antioxidant N-acetyl cysteine suppresses lidocaine-induced intracellular reactive oxygen species production and cell death in neuronal SH-SY5Y cells. BMC Anesthesiol. 2016, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.; Retnakumari, A.P.; Anwar, S.; Anto, N.P.; Mittal, R.; Shah, S.; Pillai, K.S.; Balachandran, V.S.; Peter, V.; Thomas, R.; et al. Heteronemin, a marine natural product, sensitizes acute myeloid leukemia cells towards cytarabine chemotherapy by regulating farnesylation of Ras. Oncotarget 2018, 9, 18115–18127. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Wang, C.T.; Hung, H.C.; Wu, W.J.; Wu, D.C.; Chang, M.C.; Sung, P.J.; Chou, Y.W.; Wen, Z.H.; Tai, M.H. Heteronemin Is a Novel c-Met/STAT3 Inhibitor Against Advanced Prostate Cancer Cells. Prostate 2016, 76, 1469–1483. [Google Scholar] [CrossRef]
- Inman, G.J. Switching TGFβ from a tumor suppressor to a tumor promoter. Curr. Opin. Genet. Dev. 2011, 21, 93–99. [Google Scholar] [CrossRef]
- Meulmeester, E.; Ten Dijke, P. The dynamic roles of TGF-beta in cancer. J. Pathol. 2011, 223, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, F.S.; Dawson, T.; Bond, J.A.; Goretzki, P.; Game, S.; Prime, S.; Wynford-Thomas, D. Correlated abnormalities of transforming growth factor-beta 1 response and p53 expression in thyroid epithelial cell transformation. Mol. Cell. Endocrinol. 1991, 76, 13–21. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Dupont, S.; Maretto, S.; Insinga, A.; Imbriano, C.; Piccolo, S. Links between tumor suppressors: P53 is required for TGF-beta gene responses by cooperating with Smads. Cell 2003, 113, 301–314. [Google Scholar] [CrossRef]
- Takebayashi-Suzuki, K.; Funami, J.; Tokumori, D.; Saito, A.; Watabe, T.; Miyazono, K.; Kanda, A.; Suzuki, A. Interplay between the tumor suppressor p53 and TGF beta signaling shapes embryonic body axes in Xenopus. Development 2003, 130, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Weisberg, E.; Fridmacher, V.; Watanabe, M.; Naco, G.; Whitman, M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature 1997, 389, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Cordenonsi, M.; Montagner, M.; Adorno, M.; Zacchigna, L.; Martello, G.; Mamidi, A.; Soligo, S.; Dupont, S.; Piccolo, S. Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science 2007, 315, 840–843. [Google Scholar] [CrossRef]
- Dupont, S.; Zacchigna, L.; Adorno, M.; Soligo, S.; Volpin, D.; Piccolo, S.; Cordenonsi, M. Convergence of p53 and TGF-beta signaling networks. Cancer Lett. 2004, 213, 129–138. [Google Scholar] [CrossRef]
- Mu, Y.; Gudey, S.K.; Landstrom, M. Non-Smad signaling pathways. Cell Tissue Res. 2012, 347, 11–20. [Google Scholar] [CrossRef]

| Gene Name | HuccT1: Treatment vs. Control | SSP-25: Treatment vs. Control | Gene Name | HuccT1: Treatment vs. Control | SSP-25: Treatment vs. Control | Gene Name | HuccT1: Treatment vs. Control | SSP-25: Treatment vs. Control |
|---|---|---|---|---|---|---|---|---|
| HSPB1 | 88.233 | 7.551 | CD24 | −1.568 | −3.733 | IL1B | −2.096 | −2.691 |
| HMOX1 | 71.408 | 3.531 | NRP1 | −1.572 | −2.019 | FSTL1 | −2.097 | −1.956 |
| SNAI1 | 34.199 | 4.227 | PTTG1 | −1.572 | −3.149 | MGAT5 | −2.121 | −1.854 |
| SERPINH1 | 25.425 | 2.579 | RAC2 | −1.587 | −2.025 | CHD4 | −2.128 | −2.105 |
| CREBBP | 13.219 | 2.045 | HPSE | −1.593 | −2.863 | SMC3 | −2.135 | −2.116 |
| COL7A1 | 9.126 | 2.896 | RORB | −1.597 | −3.446 | EPHA1 | −2.15 | −2.39 |
| JUN | 8.583 | 4.375 | ITGB6 | −1.602 | −3.286 | CEACAM1 | −2.152 | −1.723 |
| CLDN4 | 6.036 | 1.97 | EPHB4 | −1.626 | −1.674 | HOXB3 | −2.182 | −2.682 |
| NDRG1 | 4.916 | 2.429 | GPR124 | −1.633 | −1.831 | VAV3 | −2.187 | −2.392 |
| EIF2AK3 | 4.619 | 3.731 | LAMC1 | −1.637 | −1.767 | ILK | −2.22 | −2.484 |
| IL11 | 4.556 | 1.874 | MYLK | −1.641 | −4.584 | C3 | −2.278 | −1.906 |
| BTG1 | 4.158 | 2.085 | TMEM30B | −1.641 | −2.574 | SLC2A1 | −2.298 | −2.845 |
| NOTCH1 | 4.065 | 3.544 | CLIC4 | −1.667 | −2.164 | BMP5 | −2.302 | −7.016 |
| FGFR1 | 4.061 | 1.834 | P3H2 | −1.67 | −2.335 | DICER1 | −2.32 | −1.83 |
| VEGFA | 3.731 | 2.412 | SPOCK3 | −1.675 | −1.897 | EPHA2 | −2.444 | −2.341 |
| COL6A2 | 3.429 | 2.169 | EIF4E2 | −1.678 | −1.966 | CAV1 | −2.448 | −7.07 |
| CAMK2D | 3.256 | 3.202 | ENO1 | −1.685 | −2.548 | LDHA | −2.458 | −5.537 |
| HKDC1 | 3.224 | 1.896 | FREM2 | −1.685 | −2.227 | KDM1A | −2.498 | −2.083 |
| SIRT1 | 3.183 | 1.756 | CCDC80 | −1.7 | −4.522 | IGFBP4 | −2.533 | −2.556 |
| NFAT5 | 3.113 | 3.759 | CHI3L1 | −1.708 | −2.561 | GALNT7 | −2.553 | −2.494 |
| DST | 2.832 | 3.179 | PXDN | −1.708 | −3.924 | AHNAK | −2.639 | −2.638 |
| LAMA5 | 2.813 | 1.904 | CDH2 | −1.711 | −1.555 | LRG1 | −2.66 | −3.45 |
| CD44 | 2.7 | 1.933 | RBL1 | −1.713 | −3.098 | VWA2 | −2.677 | −3.884 |
| LTBP4 | 2.523 | 2.62 | ICAM1 | −1.717 | −2.351 | NFKB1 | −2.724 | −2.311 |
| HSP90B1 | 2.346 | 1.771 | ARHGDIB | −1.719 | −3.341 | GTF2I | −2.762 | −1.824 |
| SETD2 | 2.341 | 3.568 | RBM47 | −1.728 | −2.989 | TP53 | −2.782 | −5.449 |
| ZEB2 | 2.292 | 1.755 | CALD1 | −1.732 | −1.593 | DAG1 | −2.824 | −3.982 |
| ADAM17 | 2.271 | 2.214 | NME1 | −1.74 | −2.934 | CMA1 | −2.854 | −3.277 |
| CDKN1A | 2.215 | 2.449 | MMP17 | −1.746 | −2.125 | ITGA6 | −2.938 | −2.455 |
| ADM2 | 2.118 | 1.886 | RB1 | −1.757 | −1.919 | TFDP1 | −2.957 | −3.597 |
| PLEKHO1 | 2.087 | 3.115 | SDC4 | −1.763 | −1.732 | ITGB8 | −2.972 | −3.313 |
| MYC | 2.08 | 2.247 | RUNX1T1 | −1.783 | −5.442 | PDGFC | −3.001 | −3.104 |
| HIPK1 | 2.04 | 1.615 | CGN | −1.791 | −2.974 | KCNJ8 | −3.103 | −6.262 |
| PLXND1 | 1.813 | 1.683 | PKM | −1.805 | −2.413 | CDH11 | −3.256 | −3.584 |
| SERINC5 | 1.796 | 2.272 | TACSTD2 | −1.831 | −3.024 | EDN1 | −3.259 | −2.311 |
| ADAMTS1 | 1.79 | 6.932 | SACS | −1.832 | 2.214 | PLS1 | −3.263 | −2.762 |
| PLAUR | 1.778 | 2.422 | ITGA3 | −1.845 | −1.869 | F3 | −3.458 | −14.714 |
| SRPK2 | 1.656 | 2.519 | HK2 | −1.884 | −1.628 | SMAD3 | −3.521 | −4.024 |
| MMP1 | 1.612 | 5.34 | ALOX5 | −1.887 | −3.82 | THBS1 | −4.102 | −8.476 |
| ACHE | 1.59 | 2.054 | RBL2 | −1.915 | −2.354 | TGFBR2 | −4.281 | −1.759 |
| PFKFB4 | 1.588 | 1.725 | GLYR1 | −1.932 | −1.868 | PTX3 | −4.289 | −1.998 |
| ENPEP | −1.516 | −4.42 | STAT3 | −1.945 | −1.702 | BMP4 | −4.641 | −3.477 |
| IL1A | −1.516 | −1.985 | SRF | −2.057 | −2.931 | VCAN | −5.209 | −3.999 |
| ITGB2 | −1.521 | −3.986 | IL13RA2 | −2.058 | −2.512 | TGFB2 | −5.482 | −4.253 |
| SNRPF | −1.526 | −1.828 | DEN | −2.059 | −1.524 | LAMA3 | −5.659 | −2.226 |
| NRP2 | −1.527 | −1.952 | COL6A1 | −2.079 | −1.568 | |||
| CDC42 | −1.545 | −1.584 | CDS1 | −2.092 | −2.943 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Tey, S.-L.; Ho, Y.; Chin, Y.-T.; Wang, K.; Whang-Peng, J.; Shih, Y.-J.; Chen, Y.-R.; Yang, Y.-N.; Chen, Y.-C.; et al. Heteronemin Induces Anti-Proliferation in Cholangiocarcinoma Cells via Inhibiting TGF-β Pathway. Mar. Drugs 2018, 16, 489. https://doi.org/10.3390/md16120489
Lin H-Y, Tey S-L, Ho Y, Chin Y-T, Wang K, Whang-Peng J, Shih Y-J, Chen Y-R, Yang Y-N, Chen Y-C, et al. Heteronemin Induces Anti-Proliferation in Cholangiocarcinoma Cells via Inhibiting TGF-β Pathway. Marine Drugs. 2018; 16(12):489. https://doi.org/10.3390/md16120489
Chicago/Turabian StyleLin, Hung-Yun, Shu-Leei Tey, Yih Ho, Yung-Tang Chin, Kuan Wang, Jacqueline Whang-Peng, Ya-Jung Shih, Yi-Ru Chen, Yung-Ning Yang, Yu-Cheng Chen, and et al. 2018. "Heteronemin Induces Anti-Proliferation in Cholangiocarcinoma Cells via Inhibiting TGF-β Pathway" Marine Drugs 16, no. 12: 489. https://doi.org/10.3390/md16120489
APA StyleLin, H.-Y., Tey, S.-L., Ho, Y., Chin, Y.-T., Wang, K., Whang-Peng, J., Shih, Y.-J., Chen, Y.-R., Yang, Y.-N., Chen, Y.-C., Liu, Y.-C., Tang, H.-Y., & Yang, Y.-C. S. (2018). Heteronemin Induces Anti-Proliferation in Cholangiocarcinoma Cells via Inhibiting TGF-β Pathway. Marine Drugs, 16(12), 489. https://doi.org/10.3390/md16120489






