Astaxanthin Prevents Human Papillomavirus L1 Protein Binding in Human Sperm Membranes
Abstract
1. Introduction
2. Results and Discussion
2.1. L1 Treatment: Effect on Sperm L1 Location
2.2. L1 Treatment: Effect on Sperm Motility
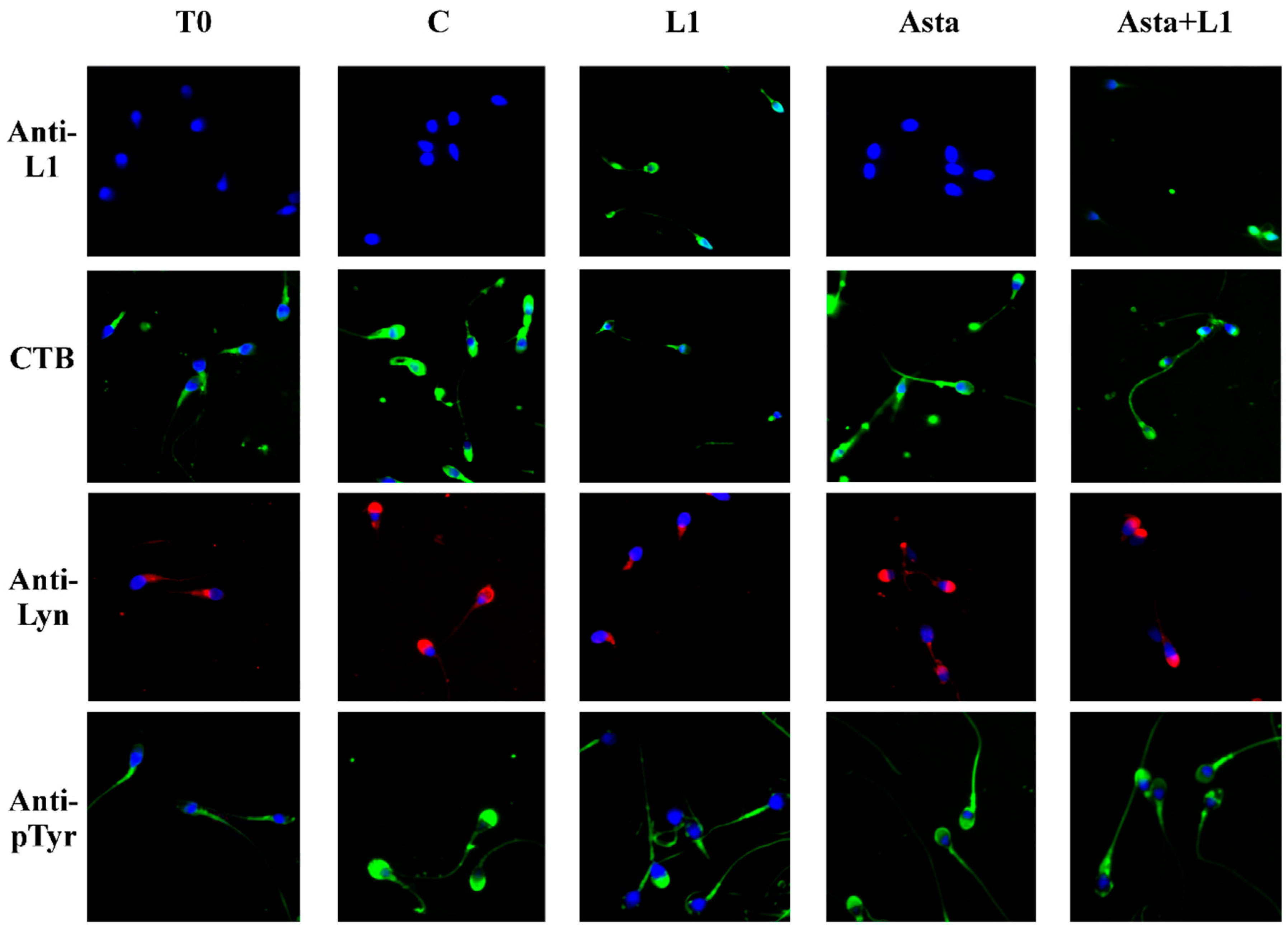
2.3. Effect of L1 and Asta Alone or in Association on Sperm Capacitation
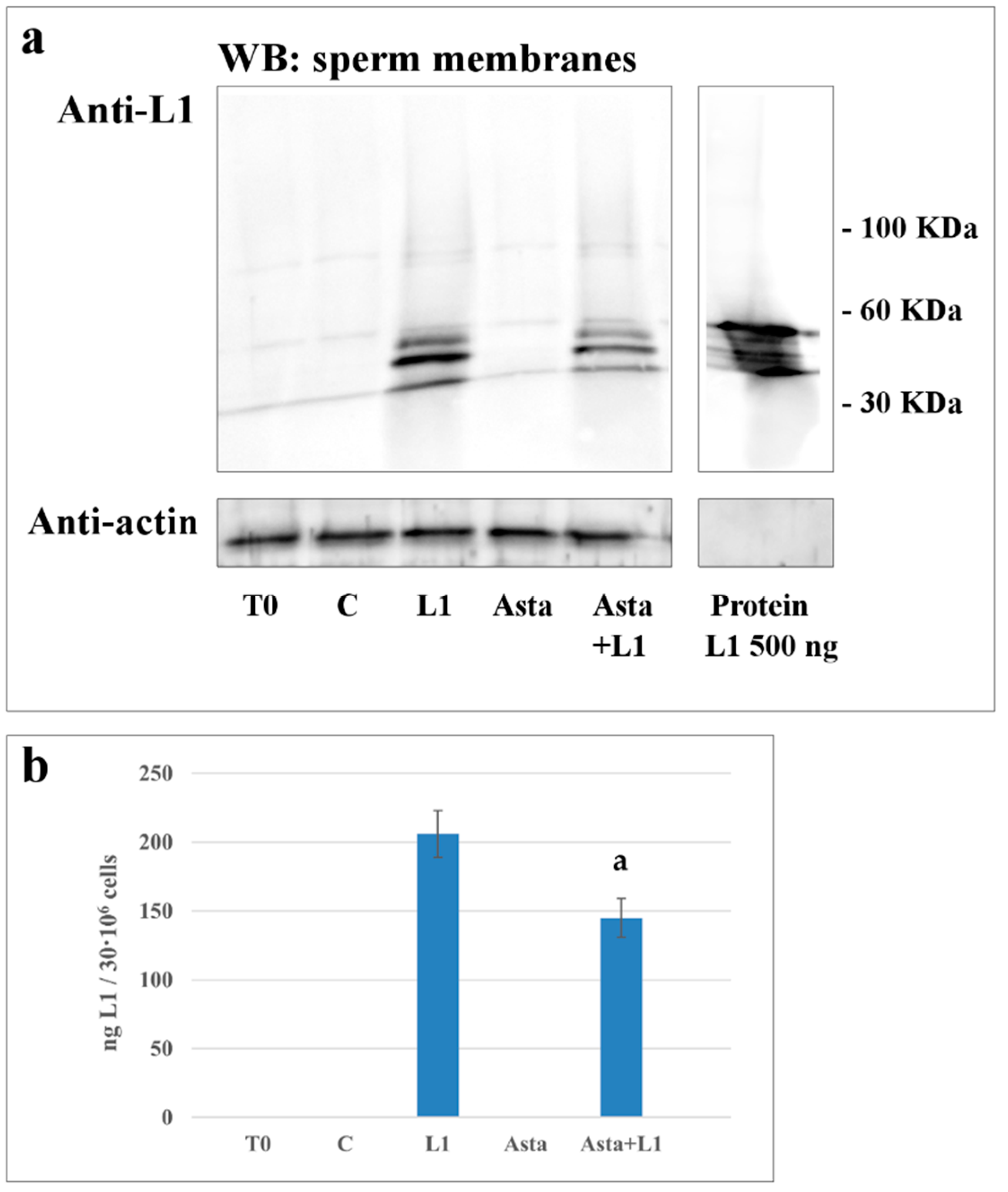
2.4. Effect of Asta on L1 Binding to Sperm Membranes
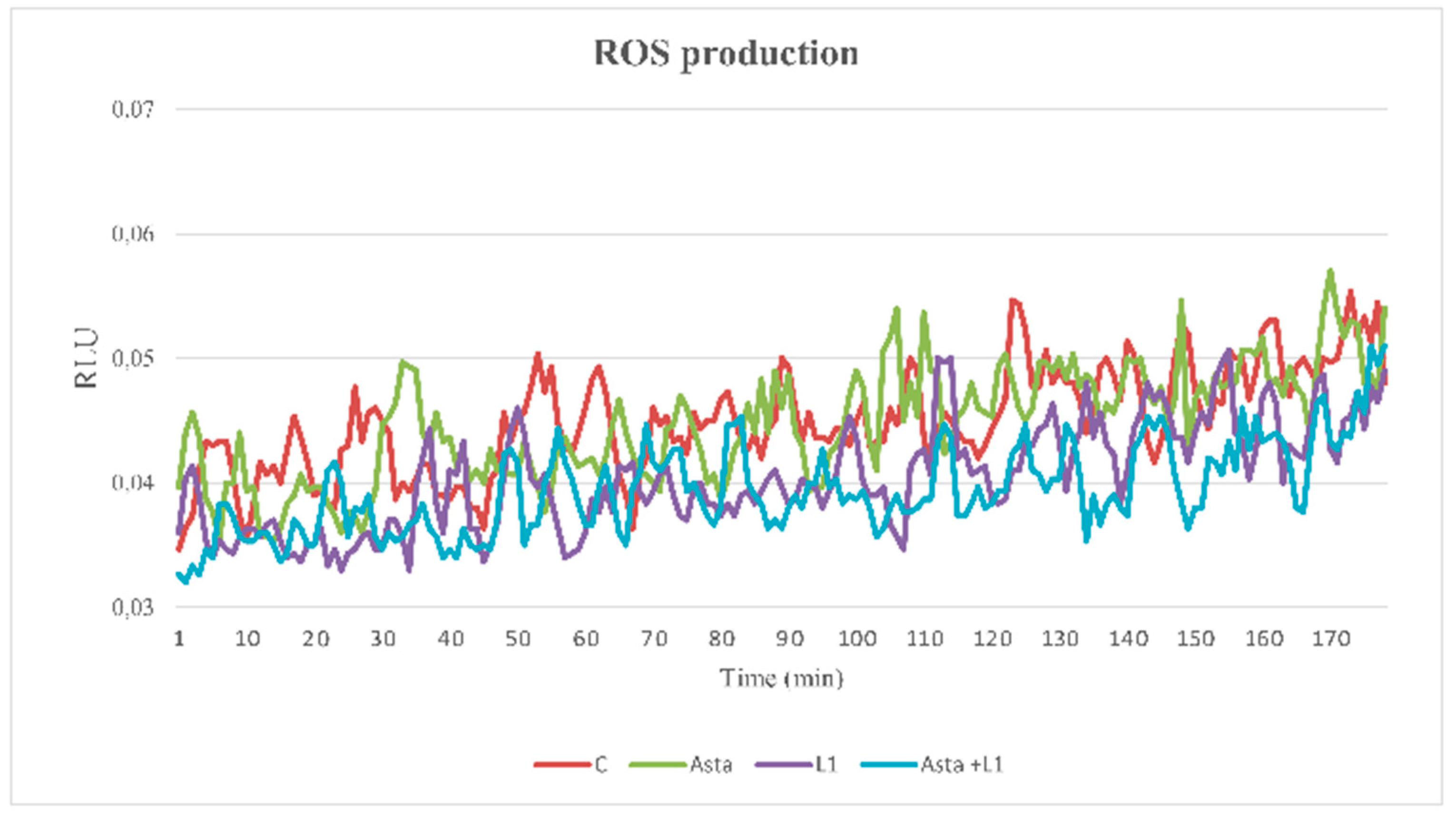
2.5. Effect of Asta and L1 on ROS Production
3. Experimental Section
3.1. Chemicals
3.2. Semen Collection and Analysis
3.3. Sample Preparation
3.4. Computer Assisted Sperm Analysis (CASA)
3.5. Anti-L1, Anti-P-Tyr and Anti-Lyn Evaluations with Confocal Microscopy
3.6. Evaluation of Membrane Rafts
3.7. Evaluation of Acrosome Reaction
3.8. Protein L1 Distribution
3.9. Western Blotting
3.10. ROS Enhanced Chemiluminescence (ECL)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Biswas, M.; Jose, T. HPV vaccine: Current status and future directions. Med. J. Armed. Forces India 2015, 71, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Meijer, C.J. Do HPV-negative cervical carcinomas exist. J. Pathol. 1997, 181, 253–254. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, H.; Clifford, G.M.; Nascimento, M.C.; Madeleine, M.M.; Franceschi, S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: A meta-analysis. Int. J. Cancer 2009, 124, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Chan, P.J.; Kalugdan, T.H.; King, A. Transfection of the inner cell mass and lack of a unique DNA sequence affecting the uptake of exogenous DNA by sperm as shown by dideoxy sequencing analogues. J. Assist. Reprod. Genet. 1997, 14, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.J.; Seraj, I.M.; Kalugdan, T.H.; King, A. Blastocysts exhibit preferential uptake of DNA fragments from the E6-E7 conserved region of the human papillomavirus. Gynecol. Oncol. 1995, 58, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, C.E.; Beert, J.; Bosmans, E.; Salembier, G. Human Papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. Facts Views Vis. ObGyn 2016, 8, 211–222. [Google Scholar] [PubMed]
- Foresta, C.; Noventa, M.; De Toni, L.; Gizzo, S.; Garolla, A. HPV-DNA sperm infection and infertility: From a systematic literature review to a possible clinical management proposal. Andrology 2015, 3, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Kucharczyk, K.M.; Estes, J.L.; Gerber, R.S.; Lekovich, J.P.; Elias, R.T.; Spandorfer, S.D. Human Papillomavirus Infection, Infertility, and Assisted Reproductive Outcomes. J. Pathog. 2015, 2015, 578423. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, P.; Gottschalk, E.Y.; Meneses, P.I. HPV entry into cells. Mutat. Res. Rev. Mutat. Res. 2017, 772, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Raff, A.B.; Woodham, A.W.; Raff, L.M.; Skeate, J.G.; Yan, L.; Da Silva, D.M.; Schelhaas, M.; Kast, W.M. The evolving field of human papillomavirus receptor research: A review of binding and entry. J. Virol. 2013, 87, 6062–6072. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Bing, W.; Yuan, J.P.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing IκB kinase-dependentNF-κB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Yasui, Y. Chemopreventive effects of astaxanthin on inflammatory bowel disease and inflammation-related colon carcinogenesis. In Carotenoids and Vitamin A in Translational Medicine; Sommerburg, O., Siems, W., Kraemer, K., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 289–304. [Google Scholar]
- Kochi, T.; Shimizu, M.; Sumi, T.; Kubota, M.; Shirakami, Y.; Tanaka, T.; Moriwaki, H. Inhibitory effects of astaxanthin on azoxymethaneinduced colonic preneoplastic lesions in C57/BL/KsJdb/db mice. BMC Gastroenterol. 2014, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthinrich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, M.; Gao, Z.; Cai, X.; Dixon, W.; Chen, X.; Cao, Y.; Xiao, H. Stereoisomers of Astaxanthin Inhibit Human Colon Cancer Cell Growth by Inducing G2/M Cell Cycle Arrest and Apoptosis. J. Agric. Food Chem. 2016, 64, 7750–7759. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Kožuh, I.; Brunati, A.M.; Andrisani, A.; Ambrosini, G.; Bonanni, G.; Ragazzi, E.; Armanini, D.; Clari, G.; Bordin, L. Effect of astaxanthin on human sperm capacitation. Mar. Drugs 2013, 11, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, A.; Donà, G.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Alvisi, G.; Gizzo, S.; Ambrosini, G.; Ragazzi, E.; et al. Astaxanthin improves human sperm capacitation by inducing Lyn displacement and activation. Mar. Drugs 2015, 13, 5533–5551. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, A.; Donà, G.; Ambrosini, G.; Bonanni, G.; Bragadin, M.; Cosmi, E.; Clari, G.; Armanini, D.; Bordin, L. Effect of various commercial buffers on sperm viability and capacitation. Syst. Biol. Reprod. Med. 2014, 60, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Fiore, C.; Tibaldi, E.; Frezzato, F.; Andrisani, A.; Ambrosini, G.; Fiorentin, D.; Armanini, D.; Bordin, L.; Clari, G. Endogenous reactive oxygen species content and modulation of tyrosine phosphorylation during sperm capacitation. Int. J. Androl. 2011, 34, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Brunati, A.; Ragazzi, E.; Armanini, D.; Bordin, L.; Clari, G. Evaluation of correct endogenous reactive oxygen species content for human sperm capacitation and involvement of the NADPH oxidase system. Hum. Reprod. 2011, 26, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Surviladze, Z.; Dziduszko, A.; Ozbun, M.A. Essential roles for soluble virion-associated heparan sulfonated proteoglycans and growth factors in human papillomavirus infections. PLoS Pathog. 2012, 8, e1002519. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Popa, A.; Pimienta, G.; Wyler, M.; Bhan, A.; Kuruvilla, L.; Guie, M.A.; Poffenberger, A.C.; Nelson, C.D.; Atwood, W.J.; et al. Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus. Proc. Natl. Acad. Sci. USA 2013, 110, 7452–7457. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Ferlin, A.; Bertoldo, A.; Patassini, C.; Zuccarello, D.; Garolla, A. Human papilloma virus in the sperm cryobank: An emerging problem? Int. J. Androl. 2011, 34, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Patassini, C.; Bertoldo, A.; Menegazzo, M.; Francavilla, F.; Barzon, L.; Ferlin, A. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS ONE 2011, 6, e15036. [Google Scholar] [CrossRef] [PubMed]
- Carey, D.J. Syndecans: Multifunctional cell-surface co-receptors. Biochem. J. 1997, 327, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Williams, K.J. Molecular mediators for raft-dependent endocytosis of syndecan-1, a highly conserved, multifunctional receptor. J. Biol. Chem. 2013, 288, 13988–13999. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kwon, W.S.; Rahman, M.S.; Yoon, S.J.; Park, Y.J.; Pang, M.G. Actin-related protein 2/3 complex-based actin polymerization is critical for male fertility. Andrology 2015, 3, 937–946. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Mortimer, S.T.; Swan, M.A.; Mortimer, D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum. Reprod. 1998, 13, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Mitchell, L.A.; Anderson, A.L.; McLaughlin, E.A.; O’bryan, M.K.; Aitken, R.J. Proteomic and functional analysis of human sperm detergent resistant membranes. J. Cell. Physiol. 2011, 226, 2651–2665. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Clarke, G.N.; Baker, H.W. Exposure of actin on the surface of the human sperm head during in vitro culture relates to sperm morphology, capacitation and zona binding. Hum. Reprod. 2005, 20, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.M.; Lee, J.F.; Huang, H.Y.; Soong, Y.K.; Yang, F.P.; Pao, C.C. The effect of human papillomavirus infection on sperm cell motility. Fertil. Steril. 1997, 67, 1152–1155. [Google Scholar] [CrossRef]
- Rintala, M.A.; Grenman, S.E.; Pollanen, P.P.; Suominen, J.J.; Syrjänen, S.M. Detection of high risk HPV DNA in semen and its association with the quality of semen. Int. J. STD AIDS 2004, 15, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jia, C.W.; Ma, Y.M.; Zhou, L.Y.; Wang, S.Y. Correlation between HPV sperm infection and male infertility. Asian J. Androl. 2013, 15, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Henneberg, A.A.; Patton, W.C.; Jacobson, J.D.; Chan, P.J. Human papilloma virus DNA exposure and embryo survival is stage-specific. J. Assist. Reprod. Genet. 2006, 23, 255–259. [Google Scholar] [CrossRef] [PubMed]
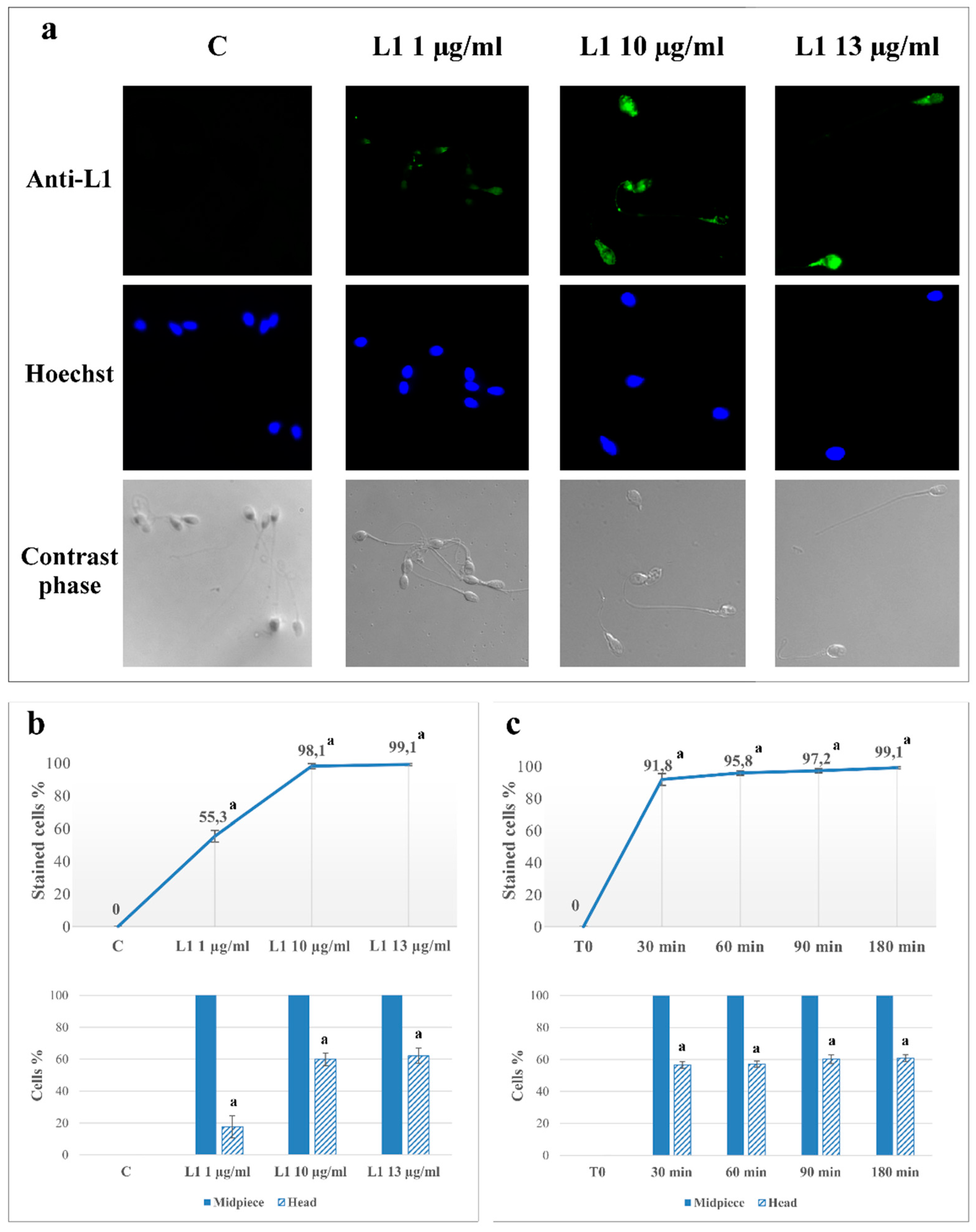
| Motility (%) | VSL (µm/s) | VAP (µm/s) | ALH (µm) | |
|---|---|---|---|---|
| T0 | 68 ± 9 | 58.4 ± 8.9 | 54.0 ± 6.7 | 3.1 ± 0.5 |
| C | 75 ± 11 | 77.8 ± 13.9 a | 67.6 ± 11.0 a | 4.9 ± 0.9 a |
| L1 1 µg/mL | 74 ± 10 | 76.9 ± 9.4 a | 67.3 ± 9.0 a | 4.7 ± 0.6 a |
| L1 10 µg/mL | 73 ± 8 | 76.3 ± 8.8 a | 67.5 ± 7.2 a | 4.8 ± 0.7 a |
| L1 13 µg/mL | 73 ± 8 | 76.4 ± 8.9 a | 67.4 ± 8.1 a | 4.8 ± 0.6 a |
| Asta | 75 ± 9 | 78.2 ± 13.6 a | 67.9 ± 9.3 a | 5.0 ± 0.6 a |
| Asta+L1 10 | 74 ± 6 | 77.7 ± 11.3 a | 67.0 ± 7.5 a | 4.9 ± 0.7 a |
| L1 (%) | CTB (%) | Lyn (%) | Tyr-P (%) | ARC (%) | NVC (%) | ||
|---|---|---|---|---|---|---|---|
| Tot. Stained | Head | Head | Head | Head | |||
| T0 | ND | ND | 13.4 ± 1.7 | 9.2 ± 1.2 | 15.3 ± 2.1 | 8.1 ± 1.3 | 7.1 ± 1.2 |
| C | ND | ND | 72.0 ± 3.6 | 63.1 ± 2.5 | 68.4 ± 2.9 | 65.7 ± 4.2 | 10.1 ± 0.9 |
| L1 1 µg/mL | 55.3 ± 3.6 a | 17.6 ± 7.0 a | 25.5 ± 3.5 a | 20.5 ± 2.7 a | 19.7 ± 2.3 a | 20.9 ± 3.2 a | 10.3 ± 1.1 |
| L1 10 µg/mL | 98.1 ± 1.6 a | 59.9 ± 3.9 a | 21.3 ± 4.2 a | 16.3 ± 3.5 a | 11.3 ± 1.7 a | 19.4 ± 2.8 a | 10.6 ± 1.3 |
| L1 13 µg/mL | 99.1 ± 0.7 a | 62.1 ± 4.8 a | 15.8 ± 3.5 a | 13.9 ± 2.2 a | 10.2 ± 1.3 a | 14.6 ± 3.3 a | 11.4 ± 1.0 b |
| Asta | ND | ND | 72.9 ± 2.8 | 65.4 ± 2.8 | 71.3 ± 1.8 b | 67.9 ± 3.5 | 9.3 ± 1.5 |
| Asta+L1 10 | 45.5 ± 4.2 a,c | 43.8 ± 9.0 a,c | 50.2 ± 2.7 a,c | 43.2 ± 3.0 a,c | 43.6 ± 3.9 a,c | 40.8 ± 2.6 a,c | 9.6 ± 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donà, G.; Andrisani, A.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Ambrosini, G.; Ragazzi, E.; Bordin, L. Astaxanthin Prevents Human Papillomavirus L1 Protein Binding in Human Sperm Membranes. Mar. Drugs 2018, 16, 427. https://doi.org/10.3390/md16110427
Donà G, Andrisani A, Tibaldi E, Brunati AM, Sabbadin C, Armanini D, Ambrosini G, Ragazzi E, Bordin L. Astaxanthin Prevents Human Papillomavirus L1 Protein Binding in Human Sperm Membranes. Marine Drugs. 2018; 16(11):427. https://doi.org/10.3390/md16110427
Chicago/Turabian StyleDonà, Gabriella, Alessandra Andrisani, Elena Tibaldi, Anna Maria Brunati, Chiara Sabbadin, Decio Armanini, Guido Ambrosini, Eugenio Ragazzi, and Luciana Bordin. 2018. "Astaxanthin Prevents Human Papillomavirus L1 Protein Binding in Human Sperm Membranes" Marine Drugs 16, no. 11: 427. https://doi.org/10.3390/md16110427
APA StyleDonà, G., Andrisani, A., Tibaldi, E., Brunati, A. M., Sabbadin, C., Armanini, D., Ambrosini, G., Ragazzi, E., & Bordin, L. (2018). Astaxanthin Prevents Human Papillomavirus L1 Protein Binding in Human Sperm Membranes. Marine Drugs, 16(11), 427. https://doi.org/10.3390/md16110427







