Components from the Leaves and Twigs of Mangrove Lumnitzera racemosa with Anti-Angiogenic and Anti-Inflammatory Effects
Abstract
:1. Introduction
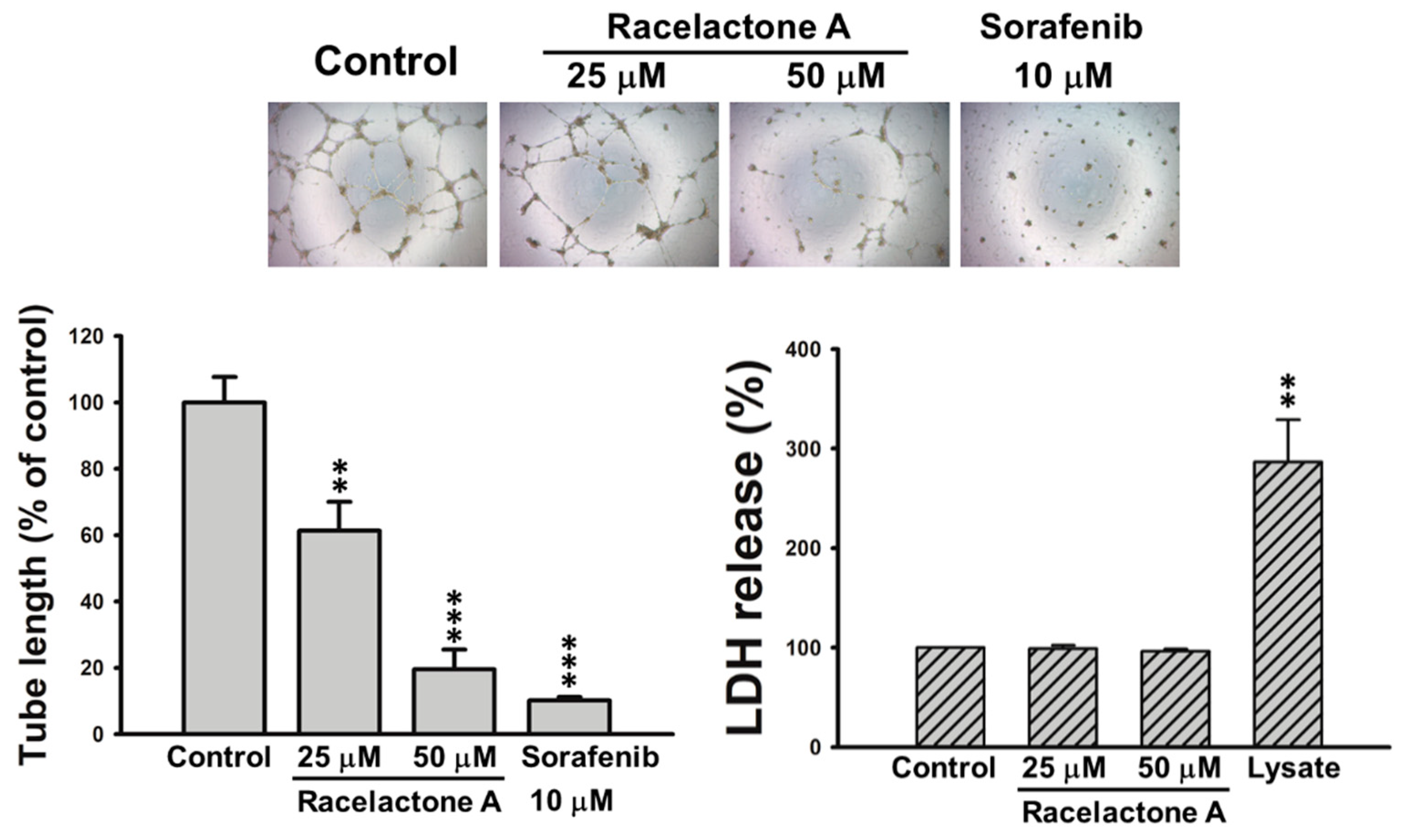
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Material
3.3. Extraction and Isolation
3.4. Preparation of Human EPCs
3.5. Tube Formation Assay
3.6. Cytotoxicity Assay
3.7. Superoxide Anion and Elastase Release Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parida, A.L.; Jha, B. Salt tolerance mechanisms in mangroves: A review. Trees 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Chen, J. Flora of China; Missouri Botanical Garden: St. Louis, MO, USA; Harvard University Herbaria: Cambridge, MA, USA, 2007; Volume 13, p. 310. [Google Scholar]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 2016; p. 236. ISBN 9781107080676. [Google Scholar]
- Chong, K.Y.; Tan, H.T.W.; Corlett, R.T. A Checklist of the Total Vascular Plant Flora of Singapore Native, Naturalized and Cultivated Species; National University of Singapore: Singpore, 2009; p. 273. [Google Scholar]
- Anjaneyulu, A.S.R.; Murthy, Y.L.N.; Rao, V.L.; Sreedhar, K. A new aromatic ester from the mangrove plant Lumnitzera racemosa willd+. ARKIVOC 2003, 3, 25–30. [Google Scholar] [CrossRef]
- Lin, T.C.; Hsu, F.L.; Cheng, J.T. Antihypertensive activity of corilagin and chebulinic acid, tannins from Lumnitzera racemosa. J. Nat. Prod. 1993, 56, 629–632. [Google Scholar] [CrossRef]
- D’Souza, L.; Wahidulla, S.; Devi, P. Antibacterial phenolics from the mangrove Lumnitzera racemosa. Indian J. Mar. Sci. 2010, 39, 294–298. [Google Scholar]
- Ravikumar, S.; Gnanadesigan, M. Hepatoprotective and antioxidant activity of a mangrove plant Lumnitzera racemosa. Asian Pac. J. Trop. Biomed. 2011, 1, 348–352. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.T.; Diep, C.N.; Tai, B.H.; Kim, E.J.; Kang, H.K.; Lee, S.H.; Jang, H.D.; Cuong, N.T.; Thanh, N.V.; et al. In vitro evaluation of the antioxidant and cytotoxic activities of constituents of the mangrove Lumnitzera racemosa Willd. Arch. Pharm. Res. 2015, 38, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. At the crossroads of inflammation and cancer. Cell 2004, 118, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Lee, J.; Ferrara, N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat. Rev. Cancer 2010, 10, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Yegnasubramanian, V.; Nelson, W.G.; Isaacs, W.B.; Marzo, A.M.D. Cyclooxygenases in cancer: Progress and perspective. Cancer Lett. 2004, 215, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-R.; Kuo, Y.-H.; Ho, Y.-L.; Wang, C.-Y.; Yang, C.-S.; Lin, C.-W.; Chang, Y.-S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 Cells. Molecules 2014, 19, 9515–9534. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Himmelreich, U.; Adam, G. Brassinosteroids, sterols and lup-20(29)-en-2α,3β,28-triol from Rheum Rhabarbarum. Phytochemistry 1995, 40, 527–531. [Google Scholar] [CrossRef]
- Begum, S.S.; Tauseef, S.; Siddiqui, B.S.; Nizami, S.S.; Ghulam, H.; Ahmad, A. In vitro antibacterial and antifungal activity of flower buds (clove) of Syzygium aromaticum. J. Chem. Soc. Pak. 2014, 36, 723–728. [Google Scholar]
- Madikizela, B.; Aderogba, M.A.; Finnie, J.F.; Staden, J.V. Isolation and characterization of antimicrobial compounds from Terminalia phanerophlebia Engl. & Diels leaf extracts. J. Ethnopharmacol. 2014, 156, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella Cambess. using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Forgo, P.; Kövér, K.E. Gradient enhanced selective experiments in the 1H NMR chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids 2004, 69, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.S.; Donza, M.R.H.; Rodrigues, A.P.D.; Silva, B.J.M.; Brasil, A.S.B.; Zoghbi, M.G.B.; Andrade, E.H.A.; Guilhon, G.M.S.P.; Silva, E.O. Leishmanicidal activity of (+)-phyllanthidine and the phytochemical profile of Margaritaria nobilis (Phyllanthaceae). Molecules 2015, 20, 22157–22169. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-G.; Hong, X.; Li, L.; Zhou, J.; Hao, Z.-J. Chemical constituents of two Chinese Magnoliaceae plants, Tsoongiodendron odorum and Manglietiastrum sinicum, and their inhibition of platelet aggregation. Planta Med. 2000, 66, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Vongvanich, N.; Kittakoop, P.; Charoenchai, P.; Intamas, S.; Danwisetkanjana, K.; Thebtaranonth, Y. Combretastatins D-3 and D-4, new macrocyclic lactones from Getonia floribunda. Planta Med. 2005, 71, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Quistorf, P.D.; Fry, J.A.; Herald, D.L.; Hamel, E.; Chapuis, J.-C. Antineoplastic agents. 565. synthesis of combretastatin D-2 phosphate and dihydro-combretastatin D-21. J. Nat. Prod. 2009, 72, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Dimmeler, S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ. Res. 2004, 95, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, M.; Mishima, Y.; Sahin, I.; Manier, S.; Glavey, S.; Vacca, A.; Roccaro, A.M.; Ghobrial, I.M. Role of endothelial progenitor cells in cancer progression. BBA-Rev. Cancer 2014, 1846, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-H.; Chang, C.-H.; Chen, S.-S.; Wang, H.-H.; Yen, J.-Y.; Hsiao, C.-J.; Wu, N.-L.; Chen, Y.-L.; Huang, T.-F.; Wang, P.-C.; et al. Butein inhibits angiogenesis of human endothelial progenitor cells via the translation dependent signaling pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, e943187. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Wang, S.-W.; Wang, G.-J.; Pang, K.-L.; Lee, C.-K.; Kuo, Y.-H.; Cha, H.-J.; Lin, R.-K.; Lee, T.-H. Angiogenesis inhibitors and anti-inflammatory agents from Phoma sp. NTOU4195. J. Nat. Prod. 2016, 79, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; Hwang, T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide–activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
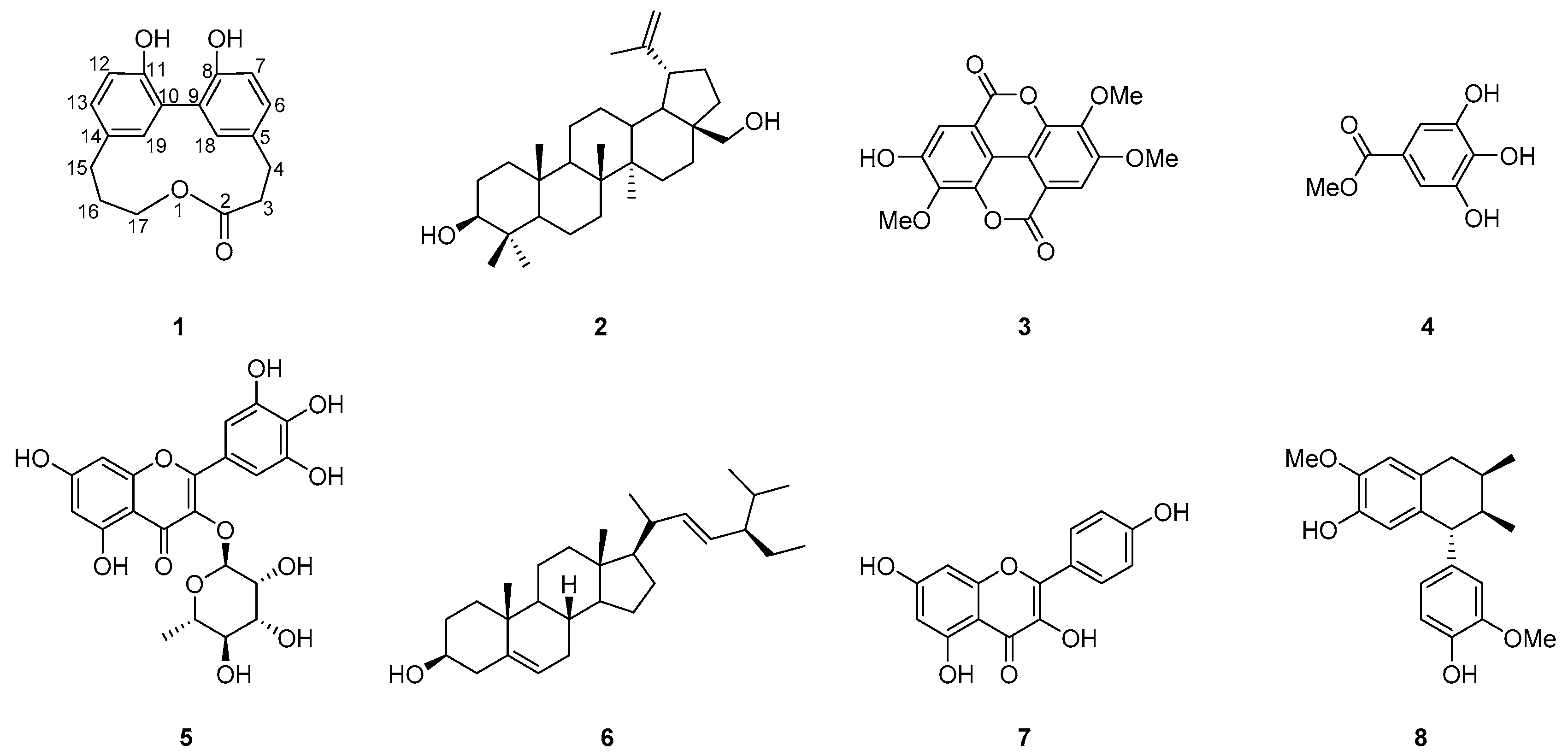
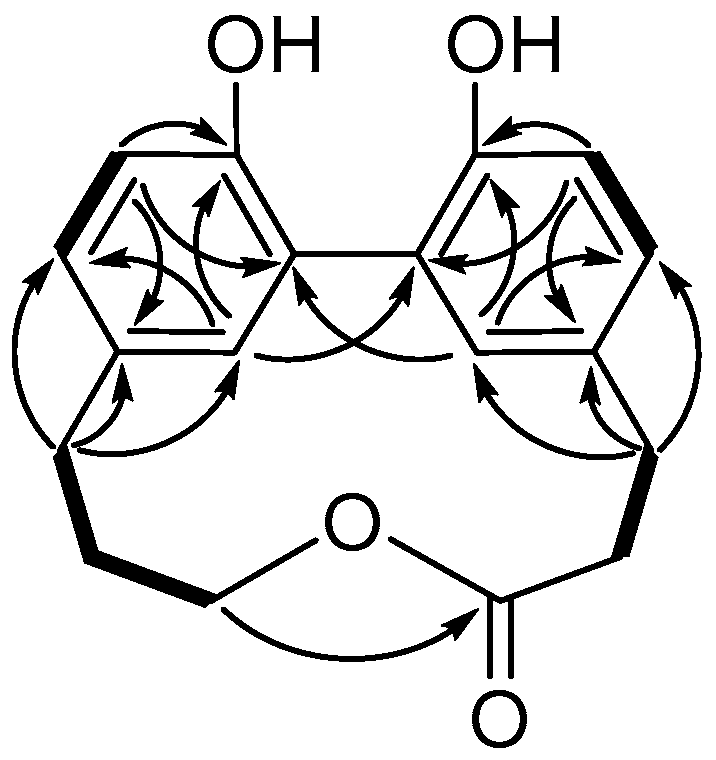
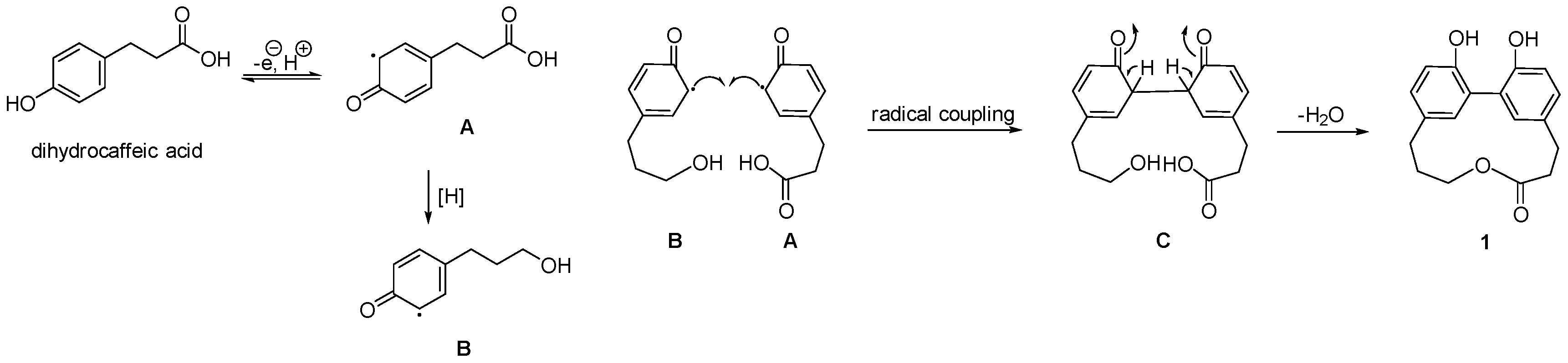
| Position | δH, mult (J in Hz) | δC, Type | HMBC (1H-13C) |
|---|---|---|---|
| 2 | - | 175.0, C | - |
| 3 | 2.58, m | 35.7, CH2 | 2, 4, 5 |
| 4 | 2.98, m | 29.7, CH2 | 2, 3, 5, 6, 18 |
| 5 | - | 132.7, C | - |
| 6 | 7.01, dd (8.1, 2.5) | 128.3, CH | 7, 8, 18 |
| 7 | 6.81, d (8.1) | 115.9, CH | 5, 6, 8, 9 |
| 8 | - | 152.7, C | - |
| 9 | - | 127.2, C | - |
| 10 | - | 126.6, C | - |
| 11 | - | 151.9, C | - |
| 12 | 6.87, d (8.2) | 116.4, CH | 10, 11, 14 |
| 13 | 7.03, dd (8.2, 2.5) | 129.2, CH | 11, 12, 15, 19 |
| 14 | - | 131.4, C | - |
| 15 | 2.82, m | 30.7, CH2 | 13, 14, 16, 17, 19 |
| 16 | 2.25, m | 25.2, CH2 | 14, 15, 17 |
| 17 | 4.29, t (5.0) | 65.7, CH2 | 2, 15, 16 |
| 18 | 7.06, d (2.5) | 133.1, CH | 4, 6, 8, 10 |
| 19 | 7.19, d (2.5) | 133.3, CH | 9, 11, 13 |
| Compound | Percentage of IC50 (μM) a | |||
|---|---|---|---|---|
| Superoxide Anion | Elastase Release | |||
| 1 | 4.95 ± 0.89 | ** | >10 | |
| 4 | 1.95 ± 0.40 | *** | >10 | |
| 5 | 2.57 ± 0.23 | *** | >10 | |
| genisteinb | 1.54 ± 0.37 | *** | 17.47 ± 2.80 | *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-Y.; Wang, S.-W.; Hwang, T.-L.; Wei, B.-L.; Su, C.-J.; Chang, F.-R.; Cheng, Y.-B. Components from the Leaves and Twigs of Mangrove Lumnitzera racemosa with Anti-Angiogenic and Anti-Inflammatory Effects. Mar. Drugs 2018, 16, 404. https://doi.org/10.3390/md16110404
Yu S-Y, Wang S-W, Hwang T-L, Wei B-L, Su C-J, Chang F-R, Cheng Y-B. Components from the Leaves and Twigs of Mangrove Lumnitzera racemosa with Anti-Angiogenic and Anti-Inflammatory Effects. Marine Drugs. 2018; 16(11):404. https://doi.org/10.3390/md16110404
Chicago/Turabian StyleYu, Szu-Yin, Shih-Wei Wang, Tsong-Long Hwang, Bai-Luh Wei, Chien-Jung Su, Fang-Rong Chang, and Yuan-Bin Cheng. 2018. "Components from the Leaves and Twigs of Mangrove Lumnitzera racemosa with Anti-Angiogenic and Anti-Inflammatory Effects" Marine Drugs 16, no. 11: 404. https://doi.org/10.3390/md16110404
APA StyleYu, S.-Y., Wang, S.-W., Hwang, T.-L., Wei, B.-L., Su, C.-J., Chang, F.-R., & Cheng, Y.-B. (2018). Components from the Leaves and Twigs of Mangrove Lumnitzera racemosa with Anti-Angiogenic and Anti-Inflammatory Effects. Marine Drugs, 16(11), 404. https://doi.org/10.3390/md16110404







