Bioaccessibility of Marine Carotenoids
Abstract
1. Introduction
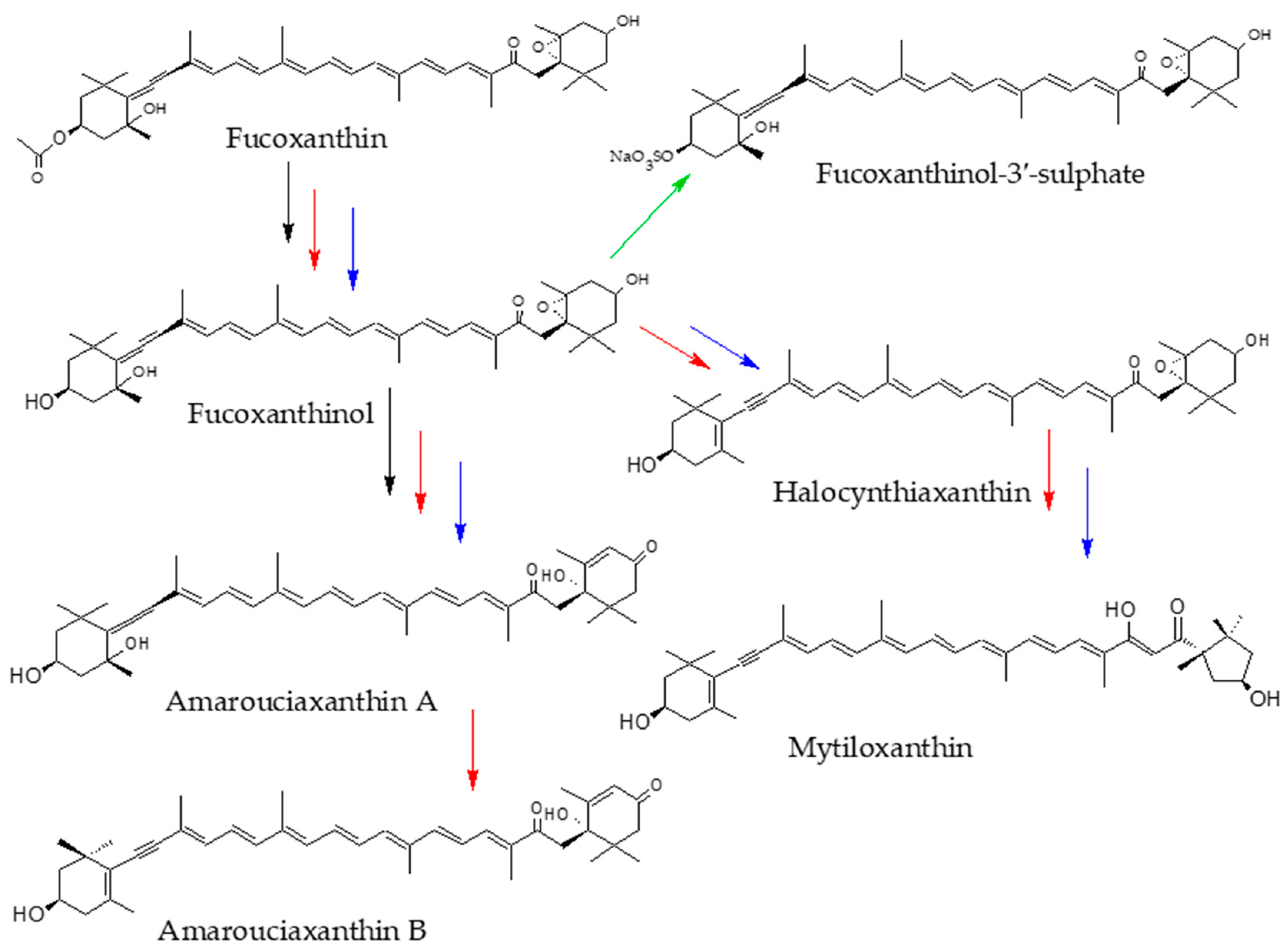
2. Bioaccessibility of Fucoxanthin
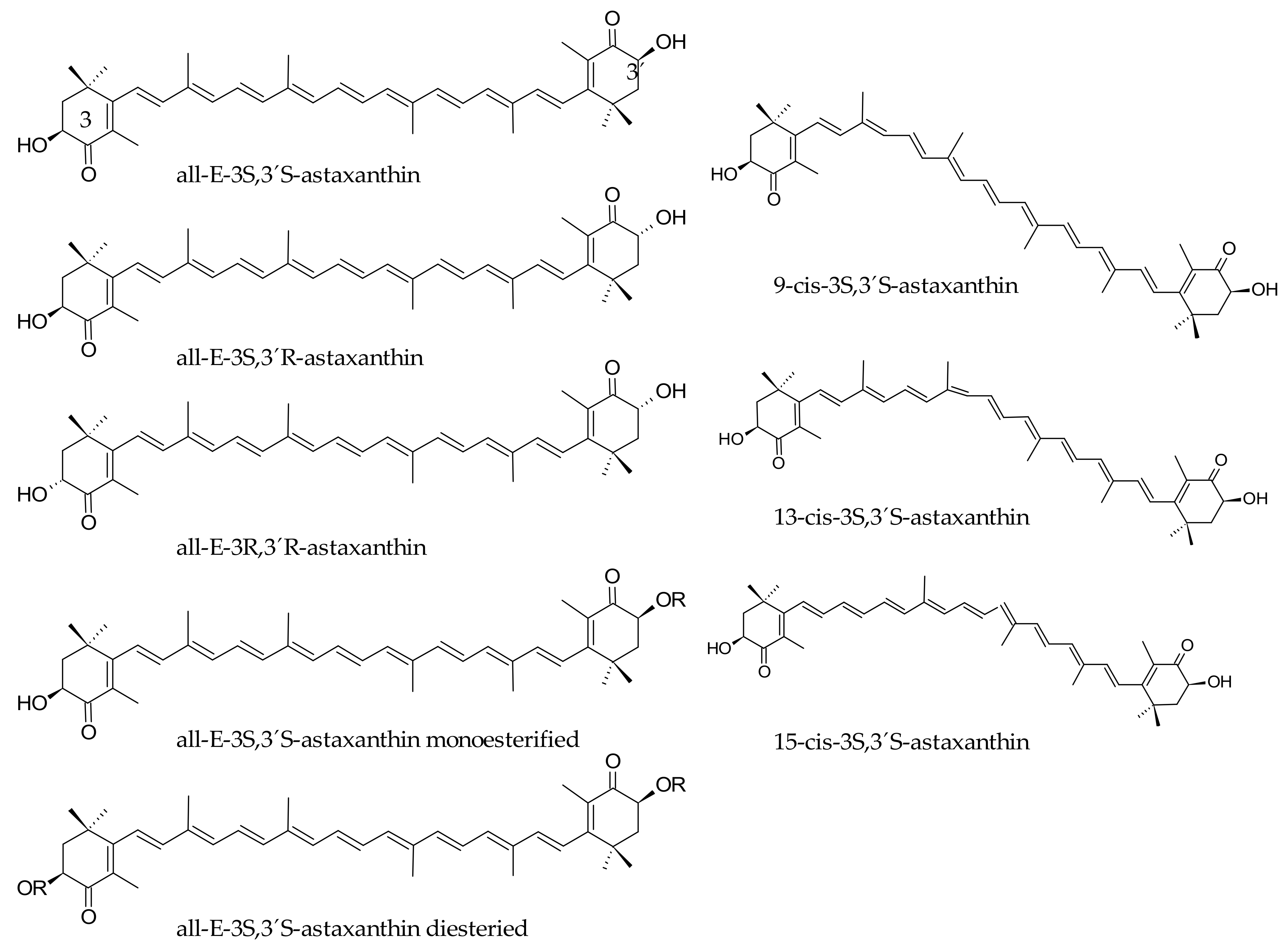
3. Bioaccessibility of Astaxanthin
4. Bioaccessibility of Other Marine Carotenoids
5. Bioaccessibility of Carotenoids for Aquaculture
Author Contributions
Funding
Conflicts of Interest
References
- Liaaen-Jensen, S. Basic carotenoid chemistry. In Carotenoids in Health and Disease; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker: New York, NY, USA, 2004; p. 1. [Google Scholar]
- Bauernfeind, J.C. Carotenoid vitamin A precursors and analogs in foods and feeds. J. Agric. Food Chem. 1972, 20, 456–473. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A. Non-provitamin A activity of carotenoids: Immunoenhancement. Trends Food Sci. Technol. 1991, 2, 127–130. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivities and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A. Carotenoids in health and disease: Recent scientific evaluations, research recommendations and the consumer. J. Nutr. 2004, 134, 221S–224S. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wang, Y.B.; Zhang, W.; Liang, J.; Lin, C.; Li, D.; Wang, F.; Pang, D.; Zhao, Y. Carotenoids and breast cancer risk: A meta-analysis and meta-regression. Breast Cancer Res. Treat. 2012, 131, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chung, S.J.; McCullough, M.L.; Song, W.O.; Fernandez, M.L.; Koo, S.I.; Chun, O.K. Dietary carotenoids are associated with cardiovascular disease risk biomarkers mediated by serum carotenoid concentrations. J. Nutr. 2014, 144, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Arathi, B.P.; Sowmya, P.R.R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Metabolomics of carotenoids: The challenges and prospects—A review. Trends Food Sci. Technol. 2015, 45, 105–117. [Google Scholar] [CrossRef]
- Liaaen-Jensen, S. Marine carotenoids: Recent progress. Pure Appl. Chem. 1991, 63, 1–12. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Hirao, S. Marine carotenoids. In Marine Biogenic Lipids, Fats and Oils; Ackman, R.G., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 251–388. [Google Scholar]
- Liaaen-Jensen, S. Marine carotenoids—Selected topics. New J. Chem. 1990, 14, 747–759. [Google Scholar]
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Desmarcherlier, C.; Borel, P. Overview of carotenoid bioavailability determinats: From dietary factors to host genetic variations. Trends. Food Sci. Nutr. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Effect of medium-chain triacylglycerols on anti-obesity effect of fucoxanthin. J. Oleo Sci. 2007, 56, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Hosokawa, M. Fucoxanthin in the management of obesity and its related disorders. J. Funct. Foods 2017, 36, 195–202. [Google Scholar] [CrossRef]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-A(y) mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017, 6, e5. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar]
- Tsu, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar]
- Asai, A.; Tsu, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Bioavailability and metabolism of fucoxanthin in rats: Structural characterization of metabolites by LC-MS (APCI). Mol. Cell. Biochem. 2010, 333, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Liaaen-Jensen, S. Carotenoids in Food Chain. In Carotenoids: Biosynthesis and Metabolism; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 359–371. ISBN 9783764358297. [Google Scholar]
- Matsuno, T.; Ookubo, M.; Komori, T. Carotenoids of tunicates, III. The structural elucidation of two new marine carotenoids, amarouciaxanthin A and B. J. Nat. Prod. 1985, 48, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Effect of glycerophospholipid class on the beta-carotene uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2010, 74, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Kobayashi, M.; Terasaki, M.; Nagao, A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr. 2010, 140, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Strand, A.; Herstad, O.; Liaaen-Jensen, S. Fucoxanthin metabolites in egg yolks of laying hens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 119, 963–974. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Airanthi, M.K.W.A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Baba, N.; Hosokawa, M.; Sashima, T.; Miyashita, K. Enhancement of hepatic docosahexaenoic acid and arachidonic acid contents in C57BL/6J mice by dietary fucoxanthin. Fish. Sci. 2009, 75, 261–263. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hosokawa, M.; Matsukawa, N.; Hagio, M.; Shinoki, A.; Nishimukai, M.; Miyashita, K.; Yajima, T.; Hara, H. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur. J. Nutr. 2010, 49, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Wu, H.; Wen, H.M.; Fang, H.; Hong, Z.; Yi, R.Z.; Liu, R. Simultaneous determination of fucoxanthin and its deacetylated metabolite fucoxanthinol in rat plasma by liquid chromatography-tandem mass spectrometry. Mar. Drugs 2015, 13, 6521–6536. [Google Scholar] [CrossRef] [PubMed]
- Mok, I.K.; Lee, J.K.; Kim, J.H.; Pan, C.H.; Kim, S.M. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 2018, 258, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Odeberg, J.M.; Lignell, A.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Baskaran, V.; Tsu, T.; Nagao, A. Phospholipids affect the intestinal absorption of carotenoids in mice. Lipids 2003, 38, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Nagao, A. Absorption and metabolism of xanthophylls. Mar. Drugs 2011, 9, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Yonekura, L.; Nagao, A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr. 2008, 100, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.B.; Olson, J.A. Xanthophyll epoxides, unlike beta-carotene monoepoxides, are not detectibly absorbed by humans. J. Nutr. 2001, 131, 3212–3215. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Martin, H.D.; Sies, H.; Stahl, W. Incorporation of carotenoids from paprika oleoresin into human chylomicrons. Br. J. Nutr. 2003, 89, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Nagao, A. Soluble fibers inhibit carotenoid micellization in vitro and uptake by Caco-2 cells. Biosci. Biotechnol. Biochem. 2009, 73, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Mizuno, M.; Yoshida, M.; Nishitani, Y.; Azuma, T.; Komoto, A.; Maoka, T.; Tanino, Y.; Kanazawa, K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br. J. Nutr. 2012, 107, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Chou, H.N.; Huang, C.J. Dietary fucoxanthin increases metabolic rate and upregulated mRNA expressions of the PGC-1alpha network, mitochondrial biogenesis and fusion genes in white adipose tissues of mice. Mar. Drugs 2014, 12, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.; Baskaran, V. Chitosan-glycolipid nanocarriers improve the bioavailability of fucoxanthin via up-regulation of PPAR gamma and SRB1 and antioxidant activity in rat model. J. Funct. Foods 2017, 28, 215–226. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, H.Y.; Wang, F.Q.; Xia, G.X.; Liu, H.J.; Cheng, X.J.; Kong, M.; Liu, Y.; Feng, C.; Chen, X.G.; et al. Isolation of fucoxanthin from Sargassum thunbergii and preparation of microcapsules based on palm stearin solid lipid core. Front. Mater. Sci. 2017, 11, 66–74. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.H.; Wang, J.W.; Zhu, J.X.; Wang, D.F.; Zhao, L.L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocolloids 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Ravi, H.; Arunkumar, R.; Baskaran, V. Chitosan-glycolipid nanogels loaded with anti-obese marine carotenoid fucoxanthn: Acute and sub-acute toxicity evaluation in rodent model. J. Biomater. Appl. 2015, 30, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Sun, Q.; Urn, B.H.; Park, Y.; McClements, D.J. In vitro and in vivo study of fucoxanthin bioavailability from nanoemulsion-based delivery systems: Impact of lipid carrier type. J. Funct. Foods 2015, 17, 293–304. [Google Scholar] [CrossRef]
- Huang, Z.H.; Xu, L.Q.; Zhu, X.M.; Hu, J.N.; Peng, H.L.; Zeng, Z.L.; Xiong, H. Stability and bioaccessibility of fucoxanthin in nanoemulsions prepared from pinolenic acid-contained structured lipid. Int. J. Food Eng. 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Lysoglyceroglycolipids improve the intestinal absorption of micellar fucoxanthin by Caco-2 cells. J. Oleo Sci. 2015, 64, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Lee, J.Y. Astaxanthin structure, metabolism, and health benefits. J. Hum. Nutr. Food Sci. 2013, 1, 1–11. [Google Scholar]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food. Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Buesen, R.; Schulte, S.; Strauss, V.; Treumann, S.; Becker, M.; Gröters, S.; Carvalho, S.; Van Ravenzwaay, B. Safety assessment of [3S, 3′S]-astaxanthin—Subchronic toxicity study in rats. Food Chem. Toxicol. 2015, 81, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Paterson, E.; Gordon, M.H.; Niwat, C.; George, T.W.; Parr, L.; Waroonphan, S.; Lovegrove, J.A. Supplementation with fruit and vegetable soups and beverages increases plasma carotenoid concentrations but does not alter marker of oxidative stress or cardiovascular risk factors. J. Nutr. 2006, 136, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S.; Hayek, M.G.; Massimino, S.; Reinhart, G.A. Dietary astaxanthin stimulates cell-mediated and humoral immune response in cats. Nut. Metab. 2004, 18, 533. [Google Scholar]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Yamashita, E.; Miki, W. Comparison of Astaxanthin’s singlet oxygen quenching activity with common fat and water soluble antioxidants. In Proceedings of the 21st Annual Meeting on Carotenoid Research, Osaka, Japan, 6–7 September 2007. [Google Scholar]
- Kamath, B.S.; Srikanta, B.M.; Dharmesh, S.M.; Sarada, R.; Ravishankar, G.A. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur. J. Pharmacol. 2008, 590, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Reddy, A.H.; Aradhya, S.M. Antibacterial properties of Spirulina platensis, Haematococcus pluvialis, Botryococcus braunii micro algal extracts. Curr. Trends Biotechnol. Pharm. 2010, 4, 809–819. [Google Scholar]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Quatrin, A.; Somacal, S.; Conterato, G.M.; Sobieskim, R.; Ruviaro, A.R.; Maurer, L.H.; Duarte, M.M.; Roehrs, M.; Emanuelli, T. Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. J. Clin. Biochem. Nutr. 2012, 51, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M.; Obara, Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Lee, Y.K. Effect of total secondary carotenoids extracts from Chlorococcum sp on Helicobacter pylori-infected BALB/c mice. Int. Immunopharmacol. 2003, 3, 979–986. [Google Scholar] [CrossRef]
- Andersen, L.P.; Holck, S.; Kupcinskas, L.; Kiudelis, G.; Jonaitis, L.; Janciauskas, D.; Permin, H.; Wadström, T. Gastric inflmmatory markers and interleukins in patients with functional dyspepsia treated with astaxanthin. FEMS Immunol. Med. Microbiol. 2007, 50, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kupcinskas, L.; Lafolie, P.; Lignell, A.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Minenaka, Y.; Ichimura, M.; Tatsumi, K.; Nadamoto, T.; Urabe, K. Effects of astaxanthin and vitamin C on the prevention of gastric ulcerations in stressed rats. J. Nutr. Sci. Vitaminol. 2005, 51, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of astaxanthin supplementation on inflammation and cardiac function in BALB/c mice. Anticancer Res. 2010, 30, 2721–2725. [Google Scholar] [PubMed]
- Chew, W.; Mathison, B.D.; Kimble, L.L.; Mixter, P.F.; Chew, B.P. Astaxanthin decreases inflammatory biomarkers associated with cardiovascular disease in human umbilical vein endothelial cells. Am. J. Adv. Food Sci. Technol. 2013, 1, 1–17. [Google Scholar] [CrossRef]
- Iizuka, M.; Ayaori, M.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Sasaki, M.; Komatsu, T.; Yogo, M.; et al. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol. 2012, 58, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, K.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef]
- Karppi, J.; Rissanen, T.H.; Nyyssonen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, H.; Takahashi, J.; Tsukahara, H.; Takehara, I. Effects of astaxanthin on human blood rheology. J. Clin. Biochem. Nutr. 2008, 43, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, S.K.; Choi, S.Y.; Kim, H.K.; Chang, H.I. Suppressive effect of astaxanthin isolated from the Xanthophyllomyces dendrorhous mutant on ethanolinduced gastric mucosal injury in rats. Biosci. Biotechnol. Biochem. 2005, 69, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, Y.; Hayasaka, S.; Yamada, T.; Hayasaka, Y.; Sanada, M.; Uonomi, T. Effects of astaxanthin on accommodation, critical flicker fusion, and pattern visual evoked potential in visual display terminal workers. J. Trad. Med. 2002, 19, 170–173. [Google Scholar]
- Nagaki, Y.; Mihara, M.; Tsukuhara, H.; Ono, S. The supplementation effect of astaxanthin on accommodation and asthenopia. J. Clin. Ther. Med. 2006, 22, 41–54. [Google Scholar]
- Iwasaki, T.; Tawara, A. Effects of astaxanthin on eyestrain induced by accommodative dysfunction. J. Eye 2006, 6, 829–834. [Google Scholar]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on human subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [PubMed]
- Andrisani, A.; Donà, G.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Alvisi, G.; Gizzo, S.; Ambrosini, G.; Ragazzi, E. Astaxanthin improves human sperm capacitation by inducing lyn displacement and activation. Mar. Drugs 2015, 13, 5533–5551. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Yamaoka, K.; Kaibara, A.; Suzuki, S.; Terakawa, M.; Hata, T. Moment analysis program available on Microsoft Excel. Metabol. Dispos. 1999, 14, 286–293. [Google Scholar]
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Arakane, K. Superior skin protection via astaxanthin. Carotenoid Res. 2002, 5, 21–24. [Google Scholar]
- Mimoun-Benarroch, M.; Hugot, C.; Rhazi, L.; Niamba, C.N.; Depeint, F. The bioavailability of astaxanthin is dependent on both the source and the isomeric variants of the molecule. Bulletin UASVM Food Sci. Technol. 2016, 73, 61–69. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Moeseneder, J.; Briviba, K.; Rechkemmer, G.; Bub, A. Bioavailability of astaxanthin stereoisomers from wild (Oncorhynchus Spp.) and aquacultured (Salmo salar) salmon in healthy men: A randomised, double-blind study. Br. J. Nutr. 2008, 99, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z isomers in plasma lipoproteins of after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.N.; Ytrestøyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in Haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Reddy, R.L.R.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. In vivo bioavailability and antioxidant activity of carotenoids from micro algal biomass—A repeated dose study. Food Res. Int. 2013, 54, 711–717. [Google Scholar] [CrossRef]
- Ranga Rao, A.; Siew-Moi, P.; Sarada, R.; Ravishankar, G.A. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar]
- Olson, J.A. Absorption, transport, and metabolism of carotenoids in humans. Pure Appl. Chem. 1994, 66, 1011–1116. [Google Scholar] [CrossRef]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 1993, 686, 12–27. [Google Scholar] [PubMed]
- Wolz, E.; Liechti, H.; Notter, B.; Oesterhelt, G.; Kistler, A. Characterization of metabolites of astaxanthin in primary cultures of rat hepatocytes. Drug Metab. Dispos. 1999, 27, 456–462. [Google Scholar] [PubMed]
- Kistler, A.; Liechti, H.; Pichard, L.; Wolz, E.; Oesterhelt, G.; Hayes, A.; Maurel, P. Metabolism and CYP-inducer properties of astaxanthin in man and primary human hepatocytes. Arch. Toxicol. 2002, 75, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Kocis, J.A. Separation of paprika pigments by HPLC. J. Agric. Food Chem. 1987, 35, 55–57. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Bamedi, A. Carotenoid esters in vegetables and fruits: A screening with emphasis on beta-cryptoxanthin esters. J. Agric. Food Chem. 2001, 49, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A. Vitamin A and carotenoids. In Modern Nutrition in Health and Disease; Shils, M.E., Shike, M., Eds.; Lippincott Wiiliams & Wilkins: Philadelphia, PA, USA, 2006; pp. 351–375. [Google Scholar]
- Sugawara, T.; Yamashita, K.; Asai, A.; Nagao, A.; Shiraishi, T.; Imai, I.; Hirata, T. Esterification of xanthophylls by human intestinal Caco-2 cells. Arch. Biochem. Biophys. 2009, 483, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.E.; Herbst-Espinosa, S.M.; Hussain, E.A.; Stacewicz-Sapuntzakis, M. Esterification does not impair lutein bioavailability in humans. J. Nutr. 2002, 132, 3668–3673. [Google Scholar] [CrossRef] [PubMed]
- Granado, F.; Olmedilla, B.; Gil-Martinez, E.; Blanco, I. Lutein ester in serum after lutein supplementation in human subjects. Br. J. Nutr. 1998, 80, 445–449. [Google Scholar] [PubMed]
- Lombardo, D.; Guy, O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim. Biophys. Acta Enzymol. 1980, 611, 147–155. [Google Scholar] [CrossRef]
- Call for Data Relevant to the Safety Assessment of Astaxanthin in the Framework of Regulation 2283/2015. Available online: https://www.efsa.europa.eu/en/consultations/call/180725 (accessed on 28 September 2018).
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Pressman, P.; Clemens, R.; Hayes, W.; Reddy, C. Food additive safety. A review of toxicologic and regulatory issues. Toxicol. Res. Appl. 2017, 1, 1–22. [Google Scholar]
- Fuller, C.J.; Butterfoss, D.N.; Failla, M.L. Relative bioavailability of β-carotene from supplement sources. Nutr. Res. 2001, 21, 1209–1215. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Acién-Fernández, G.; Molina-Grima, E.; Fernández-Sevilla, J.M.; Pérez-Sacristán, B.; Blanco-Navarro, I. In vitro bioaccessibility of lutein and zeaxanthin from the microalgae Scenedesmus almeriensis. Food Chem. 2009, 114, 747–752. [Google Scholar] [CrossRef]
- Kläui, H.; Bauernfeind, J.C. Carotenoids as food colors. In Carotenoids as Colorants and Vitamin A Precursors: Technological and Nutritional Applications; Bauernfeind, J.C., Ed.; Academic Press: New York, NY, USA, 1981; pp. 47–317. [Google Scholar]
- Bjerkeng, B. Carotenoids in aquaculture: Fish and crustaceans. In Carotenoids: Natural functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 237–254. [Google Scholar]
- Meyers, S.P. Developments in world aquaculture, feed formulations, and role of carotenoids. Pure Appl. Chem. 1994, 66, 1069–1076. [Google Scholar] [CrossRef]
- Torrissen, O.J.; Christiansen, R. Requirements for carotenoids in fish diets. J. Appl. Ichthyol. 1995, 11, 225–230. [Google Scholar] [CrossRef]
- Storebakken, T.; No, H.K. Pigmentation of rainbow trout. Aquaculture 1992, 100, 209–229. [Google Scholar] [CrossRef]
- Nickell, D.C.; Bromage, N.R. The effect of timing and duration of feeding astaxanthin on the development and variation of fillet colour and efficiency of pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture 1998, 169, 233–246. [Google Scholar] [CrossRef]
- Torrissen, O.J. Pigmentation of salmonids: Interactions of astaxanthin and canthaxanthin on pigment deposition in rainbow trout. Aquaculture 1989, 79, 363–374. [Google Scholar] [CrossRef]
- Clydesdale, F.M. Color as a factor in food choice. Crit. Rev. Food Sci. Nutr. 1993, 33, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Torrissen, O.J.; Naevdal, G. Pigmentation of salmonids—Genetical variation in carotenoid deposition in rainbow trout. Aquaculture 1984, 38, 59–66. [Google Scholar] [CrossRef]
- Nakano, T.; Tosa, M.; Takeuchi, M. Improvement of biochemical features in fish health by red yeast and synthetic astaxanthin. J. Agric. Food Chem. 1995, 43, 1570–1573. [Google Scholar] [CrossRef]
- Christiansen, R.; Torrissen, O.J. Effects of dietary astaxanthin supplementation on fertilization and eff survival in Atlantic salmon (Salmo salar L.). Aquaculture 1997, 153, 51–62. [Google Scholar] [CrossRef]
- Rehulka, J. Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout, Oncorhynchus mykiss. Aquaculture 2000, 190, 27–47. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Følling, M.; Lagocki, S.; Storebakken, T.; Olli, J.J.; Alsted, N. Biavailability of all-E-astaxanthin and Z-isomers of astaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 1997, 157, 63–82. [Google Scholar] [CrossRef]
- Torrissen, O.J.; Hardy, R.W.; Shearer, K.D. Pigmentation of salmonids—Carotenoid deposition and metabolism. CRC Crit. Rev. Aquat. Sci. 1989, 1, 209–225. [Google Scholar]
- Torrissen, O.J. Pigmentation of salmonids: Factors affecting carotenoid deposition in rainbow trout (Salmo gairdneri). Aquaculture 1985, 46, 133–142. [Google Scholar] [CrossRef]
- Choubert, G.; Heinrich, O. Carotenoid pigments of the green alga Haematococcus pluvialis: Assay on rainbow trout, Onchorhynchus mykiss, pigmentation in comparison with synthetic astaxanthin and canthaxanthin. Aquaculture 1993, 112, 217–226. [Google Scholar] [CrossRef]
- White, D.A.; Moody, A.J.; Serwata, R.D.; Bowen, J.; Soutar, C.; Young, A.J.; Davies, S.J. The degree of carotenoid esterification influences the absorption of astaxanthin in rainbow trout, Onchorhynchus mykiss (Walbaum). Aquacult. Nutr. 2003, 9, 247–251. [Google Scholar] [CrossRef]
- Hardy, R.W.; Torrissen, O.J.; Scott, T.M. Absorption and distribution of 14C-labelled canthaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 1990, 87, 331–340. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, Z. Traced studies on metabolism of astaxanthin in Atlantic salmon (Salmo salar). J. Exp. Zool. 2004, 301, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalifa, A.S.; Simpson, K.L. Metabolism of astaxanthin in the rainbow trout (Salmo gairdneri). Comp. Biochem. Physiol. 1988, 91, 563–568. [Google Scholar]
- Schiedt, K. Absorption and metabolism of carotenoids in bird, fish and crustaceans. In Carotenoids: Biosynthesis and Metabolism; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 285–358. [Google Scholar]
- Aas, G.H.; Bjerkeng, B.; Hatlen, B.; Storebakken, T. Idoxanthin, a major carotenoid in the flesh of Arctic charr (Salvelinus alpinus) fed diets containing astaxanthin. Aquaculture 1997, 150, 135–142. [Google Scholar] [CrossRef]
- Maltby, J.B.; Albright, L.J.; Kennedy, C.J.; Higgs, D.A. Effect of route of administration and carrier on bioavailability and kinetics of astaxanthin in Atlantic salmon Salmo salar L. Aquacult. Res. 2003, 34, 829–838. [Google Scholar] [CrossRef]
- Choubert, G.; Storebakken, T. Dose response to astaxanthin and canthaxanthin pigmentation of rainbow trout fed various dietary carotenoid concentrations. Aquaculture 1989, 81, 69–77. [Google Scholar] [CrossRef]
- Yamada, S.; Tanaka, Y.; Sameshima, M.; Ito, Y. Pigmentation of prawn (Penaeus japonicus) with carotenoids I. Effect of dietary astaxanthin, β-carotene and canthaxanthin on pigmentation. Aquaculture 1990, 87, 323–330. [Google Scholar] [CrossRef]
- Torrissen, O.J.; Hardy, R.W.; Shearer, K.D.; Scott, T.M.; Stone, F.E. Effects of dietary canthaxanthin level and lipid level on apparent digestibility coefficients for canthaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 1990, 88, 351–362. [Google Scholar] [CrossRef]
- Hildingstam, J. The economics of research and development in the sea farming of salmonid species. Fish Farming Int. 1976, 3, 16–19. [Google Scholar]
- Mori, T.; Makabe, K.; Yamaguchi, K.; Konosu, S.; Arai, S. Comparison between krill astaxanthin diester and synthesized free astaxanthin supplemented to diets in their absorption and deposition by juvenile coho salmon (Oncorhynchus kisutch). Comp. Biochem. Physiol. 1989, 93, 255–258. [Google Scholar] [CrossRef]
- Peterson, D.H.; Jäger, H.K.; Savage, G.M.; Washburn, G.N.; Westers, H. Natural coloration of trout using xanthophylls. Trans. Am. Fish. Soc. 1966, 95, 408–414. [Google Scholar] [CrossRef]
- Spinelli, J.; Mahnken, C. Carotenoid deposition in pen-reared salmonids fed diets containing oil extracts of red crab (Pleuroncodes planipes). Aquaculture 1978, 13, 213–223. [Google Scholar] [CrossRef]
- Supamattaya, K.; Kiriratnikom, S.; Boonyaratpalin, M.; Borowitzka, L. Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon). Aquaculture 2005, 248, 207–216. [Google Scholar] [CrossRef]
- Chien, Y.H.; Jeng, S.C. Pigmentation of kuruma prawn, Penaeus japonicus Bate, by various pigment sources and levels and feeding regimes. Aquaculture 1992, 102, 333–346. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Choubert, G.; Morais, R. Effect of dietary bile extracts on serum response of astaxanthin in rainbow trout (Oncorhynchus mykiss): A preliminary study. Aquacult. Nutr. 2004, 10, 353–357. [Google Scholar] [CrossRef]
- Johnson, E.A.; Villa, T.G.; Lewis, M.J. Phaffia rhodozyma as an astaxanthin source in salmonid diets. Aquaculture 1980, 20, 123–134. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Peisker, M.; von Schwartzenberg, K.; Ytrestoyl, T.; Asgard, T. Digestibility and muscle retention of astaxanthin in Atlantic salmon, Salmo salar, fed diets with the red yeast Phaffia rhodozyma in comparison with synthetic formulated astaxanthin. Aquaculture 2007, 269, 476–489. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green algae Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Sommer, T.R.; Potts, W.T.; Morrissy, N.M. Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 1991, 94, 79–88. [Google Scholar] [CrossRef]
- Sommer, T.R.; D’Souza, F.M.L.; Morrissy, N.M. Pigmentation of adult rainbow trout, Oncorhynchus mykiss, using the green alga Haematococcus pluvialis. Aquaculture 1992, 106, 63–74. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Morais, R.; Choubert, G. Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 176, 331–341. [Google Scholar] [CrossRef]
- White, D.A.; Page, G.I.; Swaile, J.; Moody, A.J.; Davies, S.J. Effect of esterification on the absorption of astaxanthin in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquacult. Res. 2002, 33, 343–350. [Google Scholar] [CrossRef]
- León, R.; Martín, M.; Vigara, J.; Vilchez, C.; Vega, J.M. Microalgae mediated photoproduction of beta-carotene in aqueous-organic two phase systems. Biomol. Eng. 2003, 20, 177–182. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Technological aspects of β-carotene production. Food Bioprocess Technol. 2011, 4, 693–701. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Dunaliella: Biology, production, and markets. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons: Chichester, UK, 2013; pp. 359–368. [Google Scholar]
- García-González, M.; Moreno, J.; Manzano, J.C.; Florencio, F.J.; Guerrero, M.G. Production of Dunaliella salina biomass rich in 9-cis-beta-carotene and lutein in a closed tubular photobioreactor. J. Biotechnol. 2005, 115, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Dufosse, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as source of pigments for use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravi-Kumar, R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Beneman, J.R.; Zhang, X.; Hu, H.; Qin, S. Microalgal industry in China: Challenges and prospects. J. Appl. Phycol. 2016, 28, 715–725. [Google Scholar] [CrossRef]


| Dose | Plasma | Liver | Adipose Tissue | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F 7 | FxOH | AxA | F | FxOH | AxA | F | FxOH | AxA | ||
| 1.5 nmol FxOH s.d. 1 i.v. 2 | 29.1 pmol/mL | 17.2 pmol/mL | 108.9 pmol/g | 18.2 pmol/g | [29] | |||||
| 160 nmol F s.d. i.g. 3 | 132 nmol/L | 230 nmol/L | 584 nmol/g | 190 nmol/g | 39 nmol/g | 84 nmol/g | [36] | |||
| 160 nmol/d F 7 d i.g. | 45 nmol/L | 82 nmol/L | 15 nmol/g | 83 nmol/g | 40 nmol/g | 23.1 nmol/g | 60 nmol/g | 97 nmol/g | [36] | |
| 40 nmol F s.d. i.s. 4 | 0.4 nmol/L | [28] | ||||||||
| 0.05% F 5 w diet 5 | 78.1 µg/g protein | 64.7 µg/g protein | [38] | |||||||
| 0.128 mmol/d F 14 d diet 5 | 0.34 µmol/L | 0.95 µmol/L | 0.85 µmol/kg | 0.96 µmol/kg | 2.14 µmol/kg | 7.85 µmol/kg | [34] | |||
| 3.1 µmol F s.d. d.i. 6 | 0.33 µmol 8 | [39] | ||||||||
| 3.2 µmol FxOH s.d. d.i. | 0.44 µmol 8 | [39] | ||||||||
| 2 mg/kg F s.d. i.v. | 14,000 µg/L | 598.2 µg/L | [40] | |||||||
| 65 mg/kg F s.d. i.g. | 29.1 µg/L | 263.3 µg/L | [40] | |||||||
| 7 mg/kg F s.d. | 18.8 nmol/L | 68.6 nmol/L | [41] | |||||||
| Doses of F 1 Administered | FxOH in Plasma | Reference |
|---|---|---|
| 6.1 mg 1 week | 0.8 nmol/l after 1 week | [45] |
| 31 mg one dose | 44 nmol/L at 4 hours | [49] |
| 2 mg/d 8 weeks | 2.7 nmol/L after 8 weeks | [26] |
| Source | Carotenoid Composition | Pigmentation Species | Reference |
|---|---|---|---|
| Marine and freshwater sources | |||
| krill | astaxanthin diester (200 mg/100 g oil) | Coho salmon (Oncorhynchus kisutch) | [148] |
| shrimp wastes | astaxanthin (3–12 mg/kg) | Rainbow trout (Salmo gairdneri) | [135] |
| crayfish oil extracts | astaxanthin | Rainbow trout (Salmo gairdneri) | [149] |
| red crab wastes and oil extracts | astaxanthin diester (155 mg/100 g oil) | Coho salmon (Oncorhynchus kisutch) | [150] |
| Dunaliella salina | β-carotene (200–300 mg/kg) | Black tiger shrimp (Penaeus monodon) | [151] |
| Dunaliella salina | β-carotene (50–200 mg/100 g) | Kuruma prawn (Penaeus japonicus, Bate) | [152] |
| Haematococcus pluvialis | astaxanthin (90 mg/kg) | Rainbow trout (Oncorhynchus mykiss, Walbaum) | [153] |
| Haematococcus pluvialis | astaxanthin (30 mg/kg) | Rainbow trout (Oncorhynchus mykiss, Walbaum) | [137] |
| Yeast | |||
| Phaffia rhodozima | astaxanthin (55–80 mg/kg) | Rainbow trout (Salmo gairdneri) | [154] |
| Phaffia rhodozima | astaxanthin (40 mg/kg) | Atlantic salmon (Salmo salar) | [155] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of Marine Carotenoids. Mar. Drugs 2018, 16, 397. https://doi.org/10.3390/md16100397
Viera I, Pérez-Gálvez A, Roca M. Bioaccessibility of Marine Carotenoids. Marine Drugs. 2018; 16(10):397. https://doi.org/10.3390/md16100397
Chicago/Turabian StyleViera, Isabel, Antonio Pérez-Gálvez, and María Roca. 2018. "Bioaccessibility of Marine Carotenoids" Marine Drugs 16, no. 10: 397. https://doi.org/10.3390/md16100397
APA StyleViera, I., Pérez-Gálvez, A., & Roca, M. (2018). Bioaccessibility of Marine Carotenoids. Marine Drugs, 16(10), 397. https://doi.org/10.3390/md16100397







