Potential Application of Eicosapentaenoic Acid Monoacylglyceride in the Management of Colorectal Cancer
Abstract
:1. Introduction
2. Results
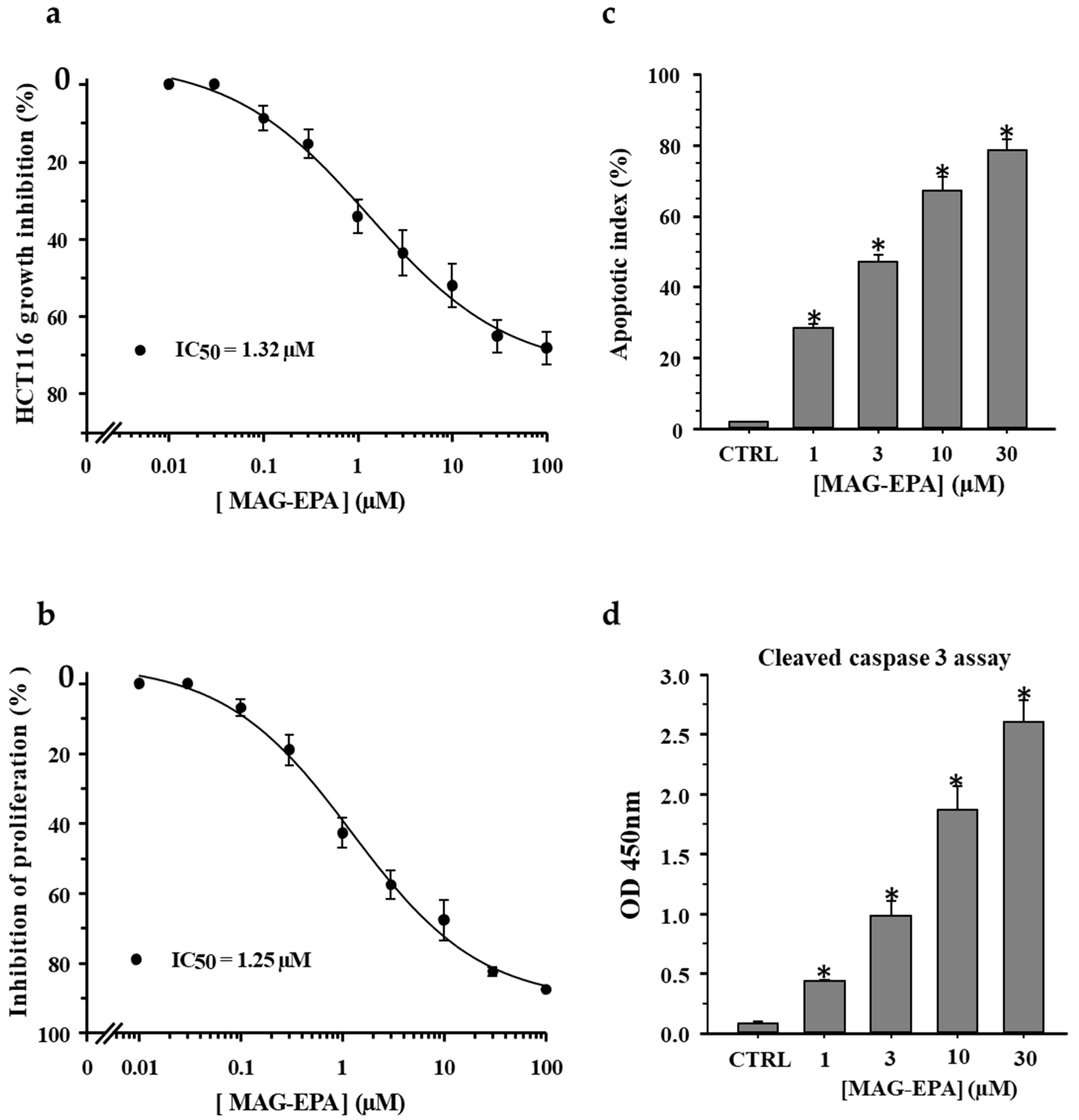
2.1. Effect of n-3 PUFA Monoglyceride Treatments on Cell Proliferation and Apoptosis in HCT116 Cells
2.2. Effect of MAG-EPA on HCT116 Spheroids Proliferation and Apoptosis
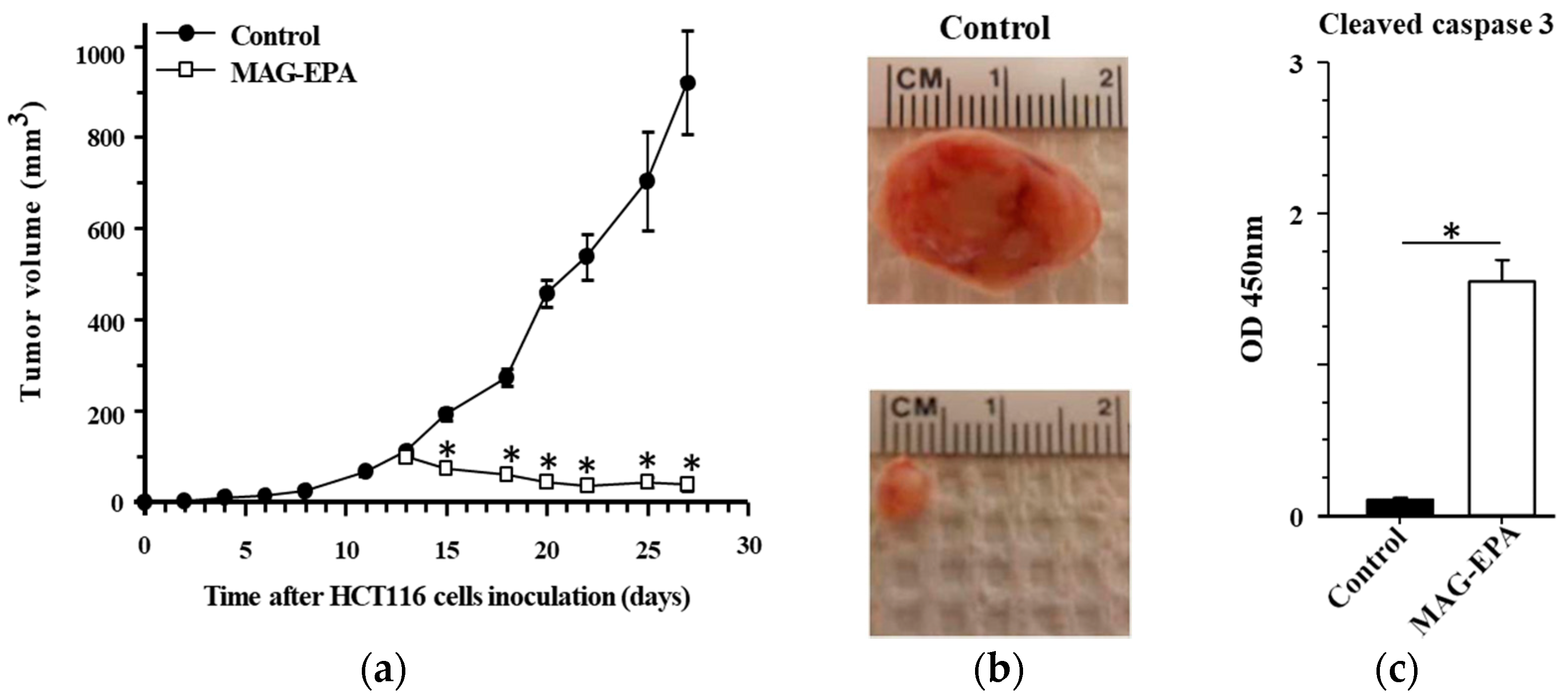
2.3. MAG-EPA Treatment Reduced HCT116-Induced Tumor Formation
2.4. Effect of MAG-EPA on VEGF, HIF1α and VEGFR Phosphorylation Level
2.5. MAG-EPA Reduced EGFR and AKT Phosphorylation Levels in Tumor Tissues
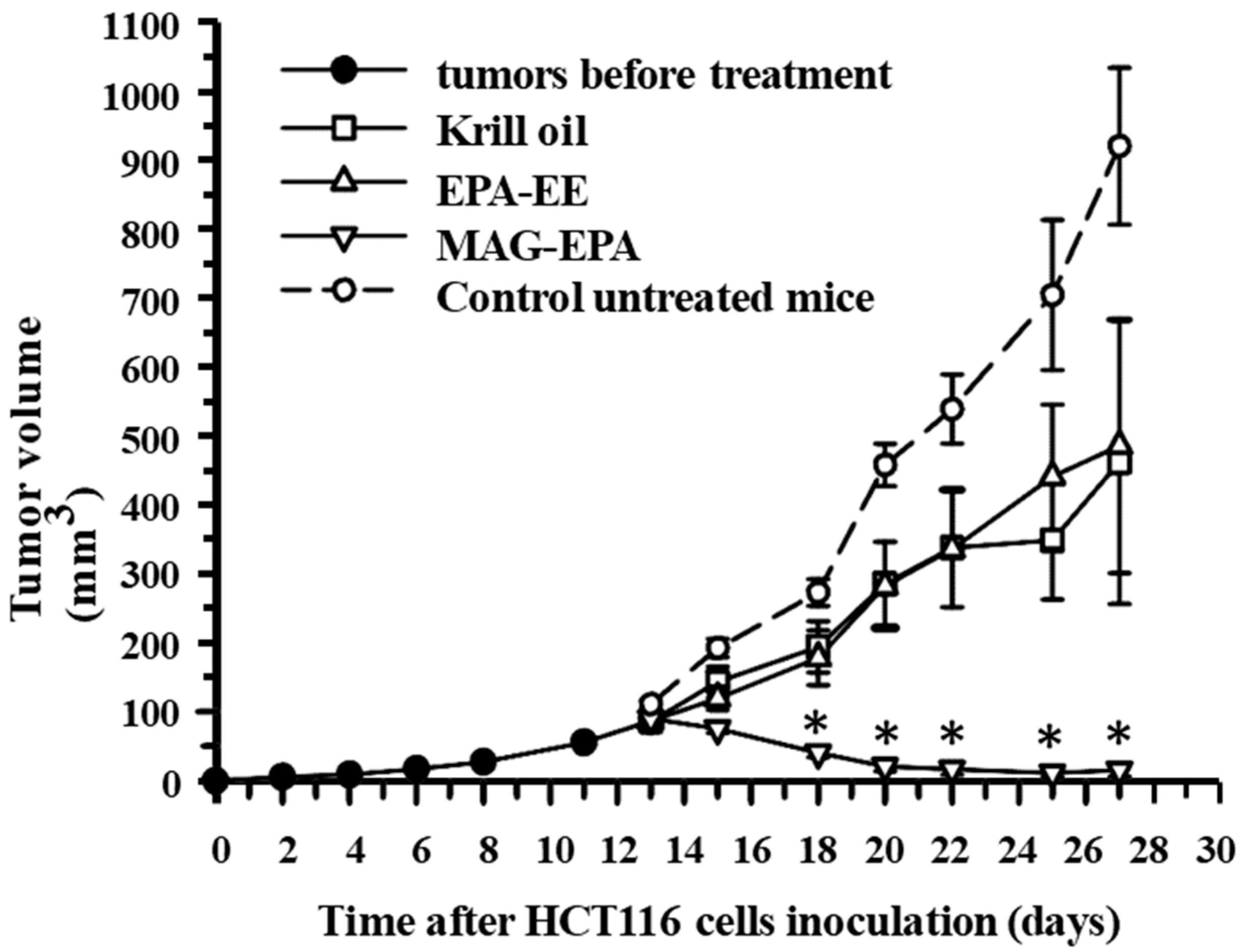
2.6. Comparative Effects of Krill Oil, EPA-EE and MAG-EPA Treatment on HCT116-Induced Tumor Formation
3. Discussion
3.1. MAG-EPA Decreases Cell Proliferation and Induces Apoptosis of Colorectal Carcinoma Cells
3.2. MAG-EPA Decreases VEGFR and EGFR Activation Pathways in Colorectal Carcinoma Cells
4. Conclusions
5. Materials and Methods
5.1. Marine Omega-3 Oils
5.2. Cell Line and Culture
5.3. Spheroids
5.4. In Vivo Tumor Xenograft Experiments
5.5. Data Analysis and Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dyerberg, J.; Madsen, P.; Møller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Geelen, A.; Schouten, J.M.; Kamphuis, C.; Stam, B.E.; Burema, J.; Renkema, J.M. Fish consumption, n-3 fatty acids, and colorectal cancer: A meta-analysis of prospective cohort studies. Am. J. Epidemiol. 2007, 166, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.N.; Campos, H.; Li, H.; Sesso, H.D.; Stampfer, M.J.; Willett, W.C. Blood levels of long-chain polyunsaturated fatty acids, aspirin, and the risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hernandez, C.; Thakkar, S.K.; Moulin, J.; Oliveira, M.; Masserey-Elmelegy, I.; Dionisi, F.; Destaillats, F. Benefits of structured and free monoacylglycerols to deliver eicosapentaenoic (EPA) in a model of lipid malabsorption. Nutrients 2012, 4, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S. Polyunsaturated Fatty Acid Monoglycerides, Derivatives, and Uses Thereof. U.S. Patent 8,119,690 B2, 21 February 2012. [Google Scholar]
- Fortin, S. Compositions Comprising Polyunsaturated Fatty Acid Monoglycerides or Derivatives Thereof and Uses Thereof. U.S. Patent 8,222,295 B2, 17 July 2012. [Google Scholar]
- Morin, C.; Rousseau, É.; Fortin, S. Anti-proliferative effects of a new docosapentaenoic acid monoacylglyceride in colorectal carcinoma cells. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Olsen, A.H.; Sasieni, P. The potential for prevention of colorectal cancer in the UK. Eur. J. Cancer Prev. 2009, 18, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Istfan, N.W. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot. Essent. Fat. Acids 2000, 63, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.F.; Shen, J.H.; Pan, W.S.; Shen, S.R.; Das, U.N. Effects of polyunsaturated fatty acids on the growth of gastric cancer cells in vitro. Lipids Health Dis. 2013, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Serini, S.; Piccioni, E.; Toesca, A.; Monego, G.; Cittadini, A.R.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis by altering the expression and cellular location of GRP78 in colon cancer cell lines. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, H.; Ma, Q.; Shen, S.; Das, U.N. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Fortin, S.; Cantin, A.M.; Sirois, M.; Sirois, C.; Rizcallah, E.; Rousseau, É. Anti-cancer effects of a new docosahexaenoic acid monoacylglyceride in lung adenocarcinoma. Recent Pat. Anticancer Drug Discov. 2013, 8, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids enhance free radical generation and lipid peroxidation to induce apoptosis of tumor cells. Clin. Lipidol. 2011, 6, 463–489. [Google Scholar] [CrossRef]
- Chen, E.P.; Smyth, E.M. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins Other Lipid Mediat. 2011, 96, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Qualtrough, D.; Kaidi, A.; Chell, S.; Jabbour, H.N.; Williams, A.C.; Paraskeva, C. Prostaglandin F(2α) stimulates motility and invasion in colorectal tumor cells. Int. J. Cancer 2007, 121, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Lipoxins, resolvins, protectins, maresins and nitrolipids, and their clinical implications with specific reference to cancer: part I. Clin. Lipidol. 2013, 8, 437–463. [Google Scholar] [CrossRef]
- Serhan, C.N.; Arita, M.; Hong, S.; Gotlinger, K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids 2004, 39, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, P.; Mani, A.M.; Naveen, K.V.G.; Anasuya, D.H.; Siresha, B.; Das, U.N. Effect of polyunsaturated fatty acids and their metabolites on bleomycin-induced cytotoxic action on human neuroblastoma cells in vitro. PLoS ONE 2014, 9, e114766. [Google Scholar]
- Okuyama, H.; Kobayashi, T.; Watanabe, S. Dietary fatty acids—The n-6/n-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog. Lipid Res. 1996, 35, 409–457. [Google Scholar] [CrossRef]
- Latham, P.; Lund, E.K.; Johnson, I.T. Dietary n-3 PUFA increases the apoptotic response to 1,2-dimethylhydrazine, reduces mitosis and suppresses the induction of carcinogenesis in the rat colon. Carcinogenesis 1999, 20, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kwon, S.H.; Han, Y.M.; Hahm, K.B.; Kim, E.H. Omega-3 Polyunsaturated Fatty Acids as Potential Chemopreventive Agent for Gastrointestinal Cancer. J. Cancer Prev. 2013, 18, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Uccella, S.; Finzi, G.; Albarello, L.; Sessa, F.; Capella, C. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: Correlation with microvessel density and clinicopathologic features. Hum. Pathol. 2003, 34, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Masood, R.; Cai, J.; Zheng, T.; Smith, D.L.; Hinton, D.R.; Gill, P.S. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood 2001, 98, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Von Marschall, Z.; Cramer, T.; Höcker, M.; Burde, R.; Plath, T.; Schirner, M.; Heidenreich, R.; Breier, G.; Riecken, E.O.; Wiedenmann, B.; et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: Evidence for an autocrine mitogenic loop. Gastroenterology 2000, 119, 1358–1372. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin. Colorectal Cancer 2003, 2, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Wells, A. EGF receptor. Int. J. Biochem. Cell Biol. 1999, 31, 637–643. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Jovenitti, I.E.; Cremona, A.; Berra, B.; Rizzo, A.M. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2011, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Yam, D.; Peled, A.; Shinitzky, M. Suppression of tumor growth and metastasis by dietary fish oil combined with vitamins E and C and cisplatin. Cancer Chemother. Pharmacol. 2001, 47, 34–40. [Google Scholar] [CrossRef] [PubMed]
- De Bravo, M.G.; De Antueno, R.J.; Toledo, J.; De Tomas, M.E.; Mercuri, O.F.; Quintans, C. Effects of an eicosapentaenoic and docosahexaenoic acid concentrate on a human lung carcinoma grown in nude mice. Lipids 1991, 26, 866–870. [Google Scholar] [CrossRef] [PubMed]



| 3.0 g/Day Total Omega-3 Human Equivalent | EPA (mg/g) | DHA (mg/g) | Total Omega-3 (mg/g) |
|---|---|---|---|
| MAG-EPA | 819 | 39 | 888 |
| EPA-EE | 724 | 104 | 930 |
| Krill Oil (3X volume) | 150 | 90 | 300 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, C.; Rodríguez, E.; Blier, P.U.; Fortin, S. Potential Application of Eicosapentaenoic Acid Monoacylglyceride in the Management of Colorectal Cancer. Mar. Drugs 2017, 15, 283. https://doi.org/10.3390/md15090283
Morin C, Rodríguez E, Blier PU, Fortin S. Potential Application of Eicosapentaenoic Acid Monoacylglyceride in the Management of Colorectal Cancer. Marine Drugs. 2017; 15(9):283. https://doi.org/10.3390/md15090283
Chicago/Turabian StyleMorin, Caroline, Enrique Rodríguez, Pierre U. Blier, and Samuel Fortin. 2017. "Potential Application of Eicosapentaenoic Acid Monoacylglyceride in the Management of Colorectal Cancer" Marine Drugs 15, no. 9: 283. https://doi.org/10.3390/md15090283
APA StyleMorin, C., Rodríguez, E., Blier, P. U., & Fortin, S. (2017). Potential Application of Eicosapentaenoic Acid Monoacylglyceride in the Management of Colorectal Cancer. Marine Drugs, 15(9), 283. https://doi.org/10.3390/md15090283





