Marine Inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as Modulators of Serotonin Receptors: An Example Illustrating the Power of Bromine as Part of the Uniquely Marine Chemical Space
Abstract
:1. Introduction
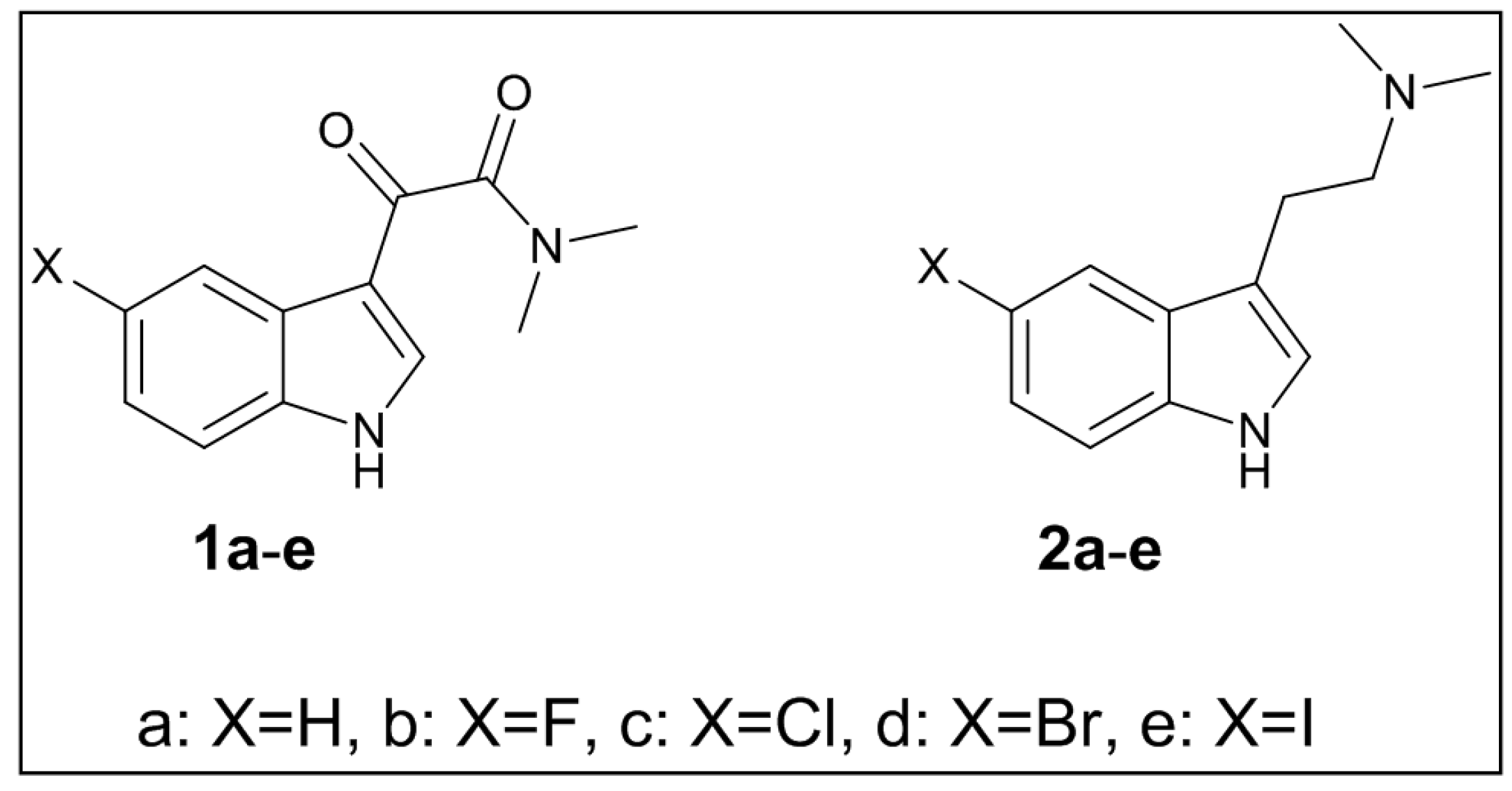
2. Results and Discussion
2.1. Assessment of the In Vitro Binding Affinity
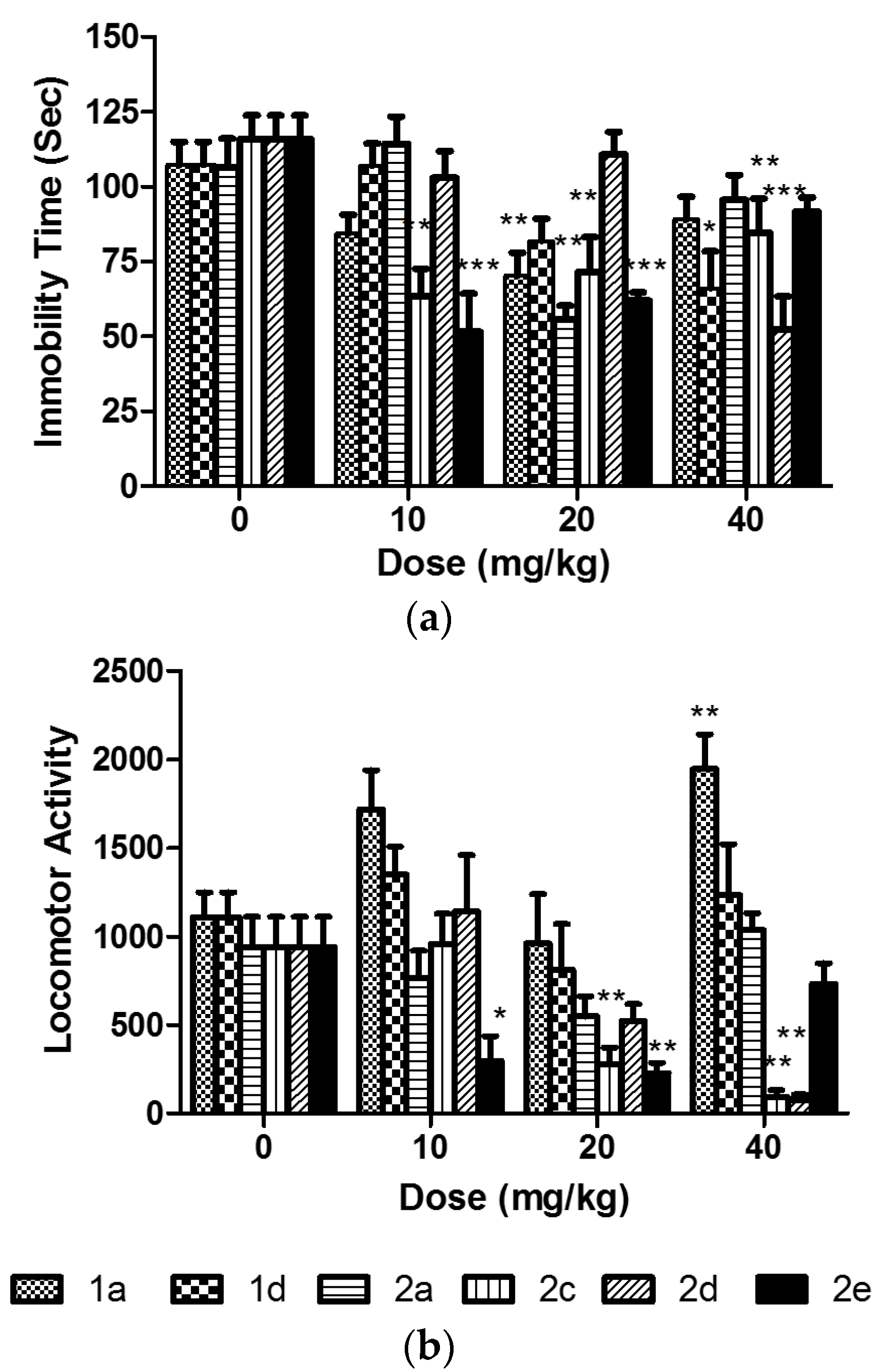
2.2. Assessment of the In Vivo Activity
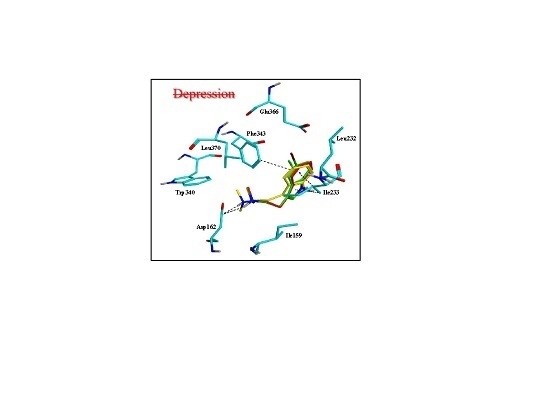
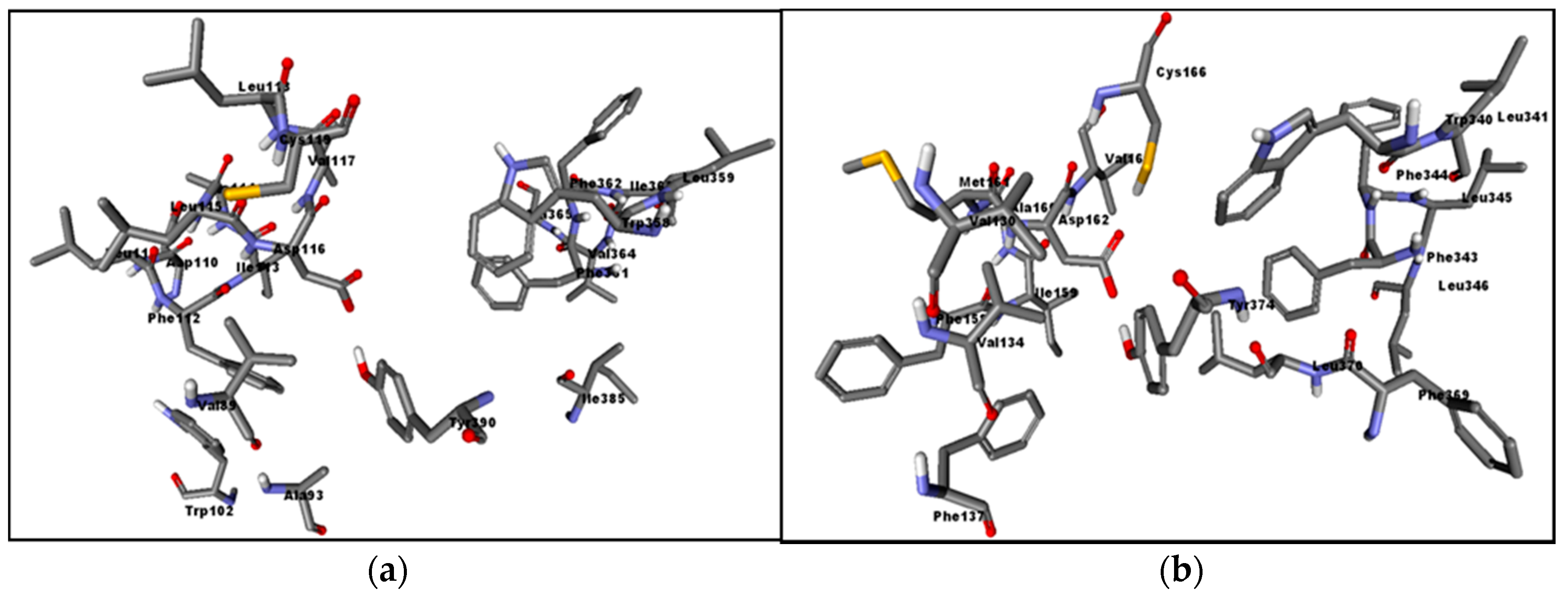
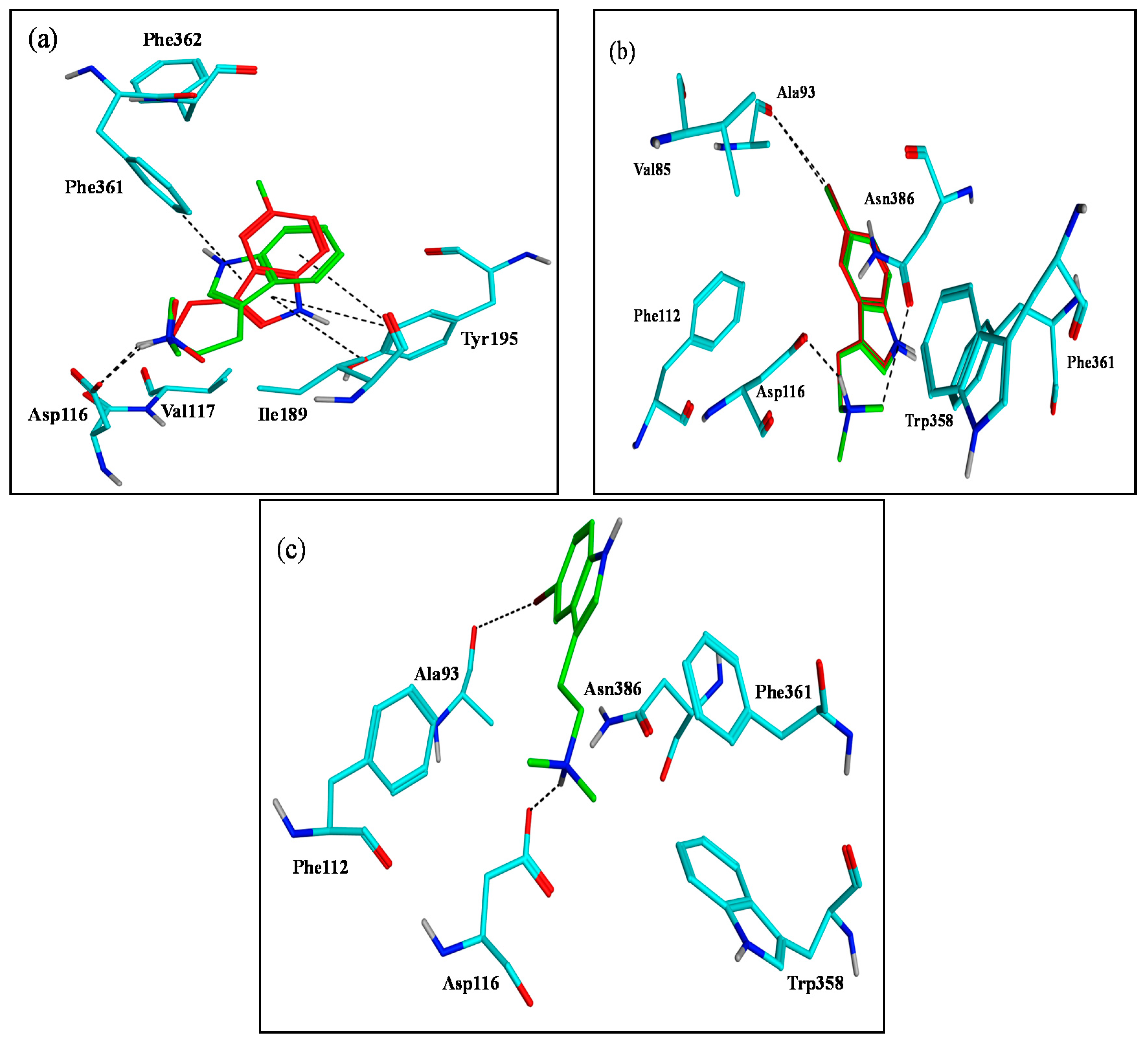
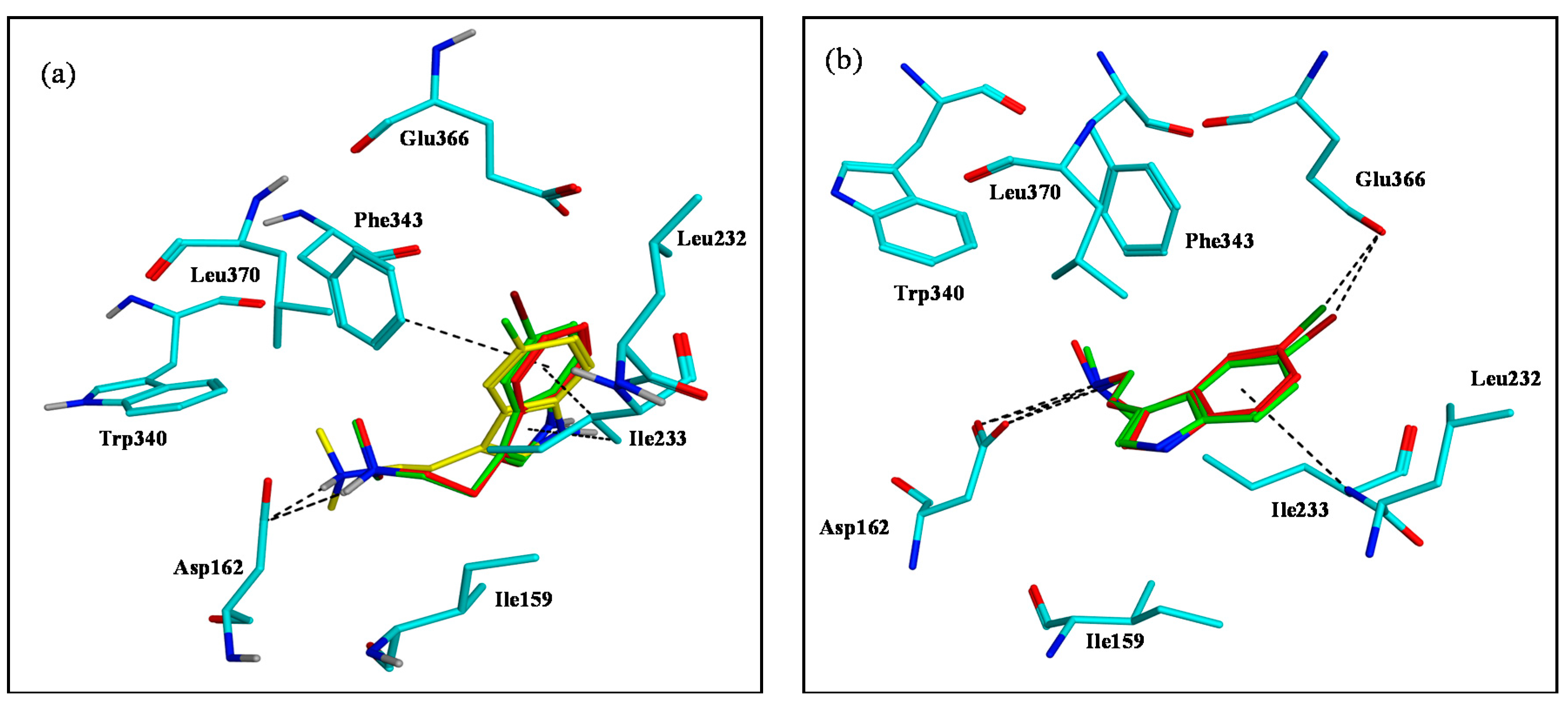
2.3. Assessment of the Docking with 5-HT7, and 5-HT1A
3. Experimental Procedures
3.1. General Procedures
3.2. Synthesis of Targeted Molecules
3.2.1. The 2-(1H-Indol-3-yl)-N,N-dimethylethanamine (2a)
3.2.2. The 2-(5-Floro-1H-indol-3-yl)-N,N-dimethylethanamine (2b)
3.2.3. The 2-(5-Chloro-1H-indol-3-yl)-N,N-dimethylethanamine (2c)
3.2.4. The 2-(5-Bromo-1H-indol-3-yl)-N,N-dimethylethanamine (2d)
3.2.5. The 2-(5-Iodo-1H-indol-3-yl)-N,N-dimethylethanamine (2e)
3.3. In Vitro Binding to Serotonin Receptors
3.4. The Forced Swim Test (FST)
3.5. The Locomotor Activity Test
3.6. Data Analysis
3.7. Homology Modeling
3.8. Preparation of Ligand Structures and Docking
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fava, M.; Kendler, K.S. Major depressive disorder. Neuron 2000, 28, 335–341. [Google Scholar] [CrossRef]
- National Survey on Drug Use and Health; 2015. Available online: http://www.nimh.nih.gov/health/publications/anxiety-disorders/introduction.shtml (accessed on 14 June 2016).
- Kochanowska, A.J.; Rao, K.V.; Childress, S.; El-Alfy, A.; Matsumoto, R.R.; Kelly, M.; Stewart, G.S.; Sufka, K.J.; Hamann, M.T. Secondary metabolites from three Florida sponges with antidepressant activity. J. Nat. Prod. 2008, 71, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Naturally occurring organohalogen compounds. Acc. Chem. Res. 1998, 31, 141–152. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.F.; Schetz, J.A.; Kelly, M.; Peng, J.N.; Ang, K.K.H.; Flotow, H.; Leong, C.Y.; Ng, S.B.; Buss, A.D.; Wilkins, S.P.; et al. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod. 2002, 65, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Canton, H.; Barrett, R.J.; Bush, E.S. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol. Biochem. Behav. 1998, 61, 323–330. [Google Scholar] [CrossRef]
- Strassman, R.J. Human psychopharmacology of N,N-dimethyltryptamine. Behav. Brain Res. 1996, 73, 121–124. [Google Scholar] [CrossRef]
- Strassman, R.J.; Qualls, C.R.; Berg, L.M. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol. Psychiatry 1996, 39, 784–795. [Google Scholar] [CrossRef]
- Ford, M.C.; Ho, P.S. Computational tools to model halogen bonds in medicinal chemistry. J. Med. Chem. 2016, 59, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Gerebtzoff, G.; Li-Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. Halogenation of drugs enhances membrane binding and permeation. ChemBioChem 2004, 5, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, H.S.; Kim, S.; Cho, S.Y.; Roth, B.L.; Chong, Y.; Choo, H. 4-Aminoethylpiperazinyl aryl ketones with 5-HT1A/5-HT7 selectivity. Bioorg. Med. Chem. 2012, 20, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An Overview of Halogen Bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Zou, J.W.; Fan, J.C.; Zhao, W.N.; Jiang, Y.J.; Yu, Q.S. Ab initio calculations on halogen bonded complexes and comparison with density functional methods. J. Comput. Chem. 2009, 30, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D. Fluorine-18 and Medical Imaging: Radiopharmaceuticals for Positron Emission Tomography. J. Fluor. Chem. 2006, 127, 1488–1493. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 2008, 47, 8998–9033. [Google Scholar] [CrossRef] [PubMed]
- Piel, M.; Vernaleken, I.; Rösch, F. Positron emission tomography in CNS drug discovery and drug monitoring. J. Med. Chem. 2014, 57, 9232–9258. [Google Scholar] [CrossRef] [PubMed]
- Diers, J.A.; Ivey, K.D.; El-Alfy, A.; Shaikh, J.; Wang, J.; Kochanowska, A.J.; Stoker, J.F.; Hamann, M.T.; Matsumoto, R.R. Identification of antidepressant drug leads through the evaluation of marine natural products with neuropsychiatric pharmacophores. Pharmacol. Biochem. Behav. 2008, 89, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Cryan, J.F.; Hoyer, D.; Marouk, A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol. Psychiatry 2003, 54, 49–58. [Google Scholar] [CrossRef]
- Cryan, J.F.; Page, M.E.; Luck, I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology 2005, 182, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.S.; Smeden, M.; Schmidt, A.W.; Sprouse, J.S.; Wikström, H.V.; Grol, C.J. Novel 5-HT7 receptor inverse agonists. Synthesis and molecular modeling of arylpiperazine- and 1,2,3,4-tetrahydroisoquinoline-based arylsulfonamides. J. Med. Chem. 2004, 47, 5451–5466. [Google Scholar] [CrossRef] [PubMed]
- Brocco, M.; Dekeyne, A.; Veiga, S.; Girardon, S.; Millan, M. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A Pharmacological characterization of diverse classes of antidepressant agents. J. Pharmacol. Biochem. Behav. 2002, 71, 667–680. [Google Scholar] [CrossRef]
- Shimamura, M.; Tobayashi, K.; Kuratani, K.; Kinoshita, M. Optimized analysis of the forced swim test using an automated experimental system: Detailed time course study in mice. J. Pharmacol. Toxicol. Methods 2008, 57, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359. [Google Scholar] [CrossRef] [PubMed]
- Kazius, J.; McGuire, R.; Bursi, R. Derivation and validation of toxicophores for mutagenicity prediction. J. Med. Chem. 2005, 48, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Blavet, N.; Daniel, M.; Jalfre, M. Immobility induced by forced swimming in rats: Effects of agents which modify central catecholamine and serotonin activity. Eur. J. Pharmacol. 1979, 57, 201–210. [Google Scholar] [CrossRef]
- Crowley, J.J.; Jones, M.D.; O’Leary, J.F.; Lucki, I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol. Biochem. Behav. 2004, 78, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.O.; Sonoda, S.; Ejima, T.; Tanaka, A.; Koga, R.; Okamoto, Y.; Fujita, M.; Otsuka, M. Zinc-mediated binding of a low-molecular-weight stabilizer of the host anti-viral factor apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G. Bioorg. Med. Chem. 2016, 24, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Heisler, L.K.; Chu, H.M.; Brennan, T.J.; Danao, J.A.; Bajwa, P.; Parsons, L.H.; Tecott, L.H. Elevated anxiety and antidepressant-like responses in serotoninn5-HT1A receptor mutant mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15049–15054. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.L.; Robinson, P.S.; Sibille, E.; Shenk, T.; Toth, M. Increased anxiety of mice lacking the serotonin1A receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 10734–10739. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Redmond, A.M.; Kelly, J.P.; Leonard, B.E. Antiemetic effects of serotonergic 5-Ht1a-receptor agonists in suncus murinus. Eur. J. Pharmacol. 1997, 7, 109–114. [Google Scholar]
- Borsini, F.; Evans, K.; Jason, K.; Rhode, F.; Alexander, B.; Pollentier, S. Pharmacology of flibanserin. CNS Drug Rev. 2002, 8, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Deakin, J. A review of clinical efficacy of 5-HT1a agonists in anxiety and depression. J. Psychopharmacol. 1993, 7, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Pecknold, J. Serotonin 5-HT: A comparative review agonists. CNS Drugs 1994, 2, 234–251. [Google Scholar] [CrossRef]
- Zhunag, X.; Gross, C.; Santarelli, L.; Compan, V.; Trillat, A.; Hen, R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 1999, 21, S52–S60. [Google Scholar] [CrossRef]
- Mayorga, A.J.; Dalvi, A.; Page, M.E.; Zimov-Levinson, S.; Hen, R.; Lucki, I. Antidepressant-Like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J. Pharmacol. Exp. Ther. 2001, 298, 1101–1107. [Google Scholar] [PubMed]
- Duxon, M.S.; Kennett, G.A.; Lightowler, S.; Blackburn, T.P.; Fone, K.C.F. Activation of 5-Ht2b receptors in the medial amygdala causes anxiolysis in the social interaction test in the rat. Neuropharmacology 1997, 36, 601–608. [Google Scholar] [CrossRef]
- Branchek, T.A.; Blackburn, T.P. 5-Ht6 receptors as emerging targets for drug discovery. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Craigo, S.C.; Choudhary, M.S.; Uluer, A.; Monsma, F.J.J.; Shen, Y.; Meltzer, H.Y.; Sibley, D.R. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994, 268, 1403–1410. [Google Scholar] [PubMed]

| Receptor | Compound 2a Ki (nM) | Compound 2c Ki (nM) | Compound 2d Ki (nM) | Compound 2e Ki (nM) | Controls | |
|---|---|---|---|---|---|---|
| Ergotamine Ki (nM) | Methysergide Ki (nM) | |||||
| 5-HT1A | 110.0 ± 17.0 | 5.5 ± 0.4 | 9.6 ± 1.1 | 130.0 ± 16.0 | 0.17 | 14.0 |
| 5-HT1B | 66.0 ± 9.0 | 66.0 ± 5.0 | 19.0 ± 2.0 | 43.0 ± 5.0 | 0.3 | 2.5 |
| 5-HT1D | 29.3 ± 3.7 | 14.0 ± 1.0 | 2.6 ± 0.32 | 8.5 ± 1.38 | 0.3 | 69.0 |
| 5-HT1E | >10,000 | 356.0 ± 34.0 | 398.0 ± 30.0 | 310.0 ± 33.0 | 19.0 | 237.0 |
| 5-HT2B | 145.0 ± 13.0 | 7.8 ± 0.7 | 27.0 ± 1.0 | 98.0 ± 4.0 | 1.9 | 0.1 |
| 5-HT3 | 5,187 ± 883 | 1,325 ± 125 | 1,374 ± 212 | 4,486 ± 804 | >10,000 | >10,000 |
| 5-HT5A | >10,000 | 408.0 ± 54.0 | 1,038 ± 110 | 1,254 ± 197 | - | >10,000 |
| 5-HT6 | 189.5 ± 32.5 | 30.0 ± 2.0.0 | 22.0 ± 2.0 | 198.0 ± 20.0 | 12.0 | 52.0 |
| 5-HT7 | 77.0 ± 16.0 | 7.2 ± 0.6 | 8.3 ± 0.9 | 116.0 ± 13.0 | 1,291 | 30.0 |
| Treatment | Immobility (sec) | Locomotor |
|---|---|---|
| Vehicle | 121 ± 7.3 | 1618 ± 142 |
| Bupropion 10 mg/kg | 101 ± 10.5 | 2746 ± 298 * |
| Bupropion 20 mg/kg | 80 ± 7.1 ** | 3564 ± 503 *** |
| Bupropion 40 mg/kg | 58 ± 8.2 *** | 5290 ± 544 *** |
| Fluoxetine 10 mg/kg | 91 ± 10 | 1898 ± 132 |
| Fluoxetine 20 mg/kg | 90 ± 6.8 | 1293 ± 243 |
| Fluoxetine 40 mg/kg | 75.8 ± 12.9 ** | 143 ± 34 *** |
| Desipramine 10 mg/kg | 112 ± 6.6 | 763 ± 112 ** |
| Desipramine 20 mg/kg | 81 ± 4.9 ** | 776 ± 265 ** |
| Desipramine 40 mg/kg | 70 ± 8.9 *** | 117 ± 43 *** |
| Compound 1a 10 mg/kg | 84.3 ± 6.3 | 1717 ± 221 |
| Compound 1a 20 mg/kg | 70.4 ± 7.8 ** | 958.6 ± 279 |
| Compound 1a 40 mg/kg | 89.1 ± 7.6 | 1945 ± 195 ** |
| Compound 1d 10 mg/kg | 106.9 ± 7.5 | 1348 ± 159 |
| Compound 1d 20 mg/kg | 81.8 ± 7.5 | 812 ± 258 |
| Compound 1d 40 mg/kg | 66 ± 12.4 * | 1233 ± 286 |
| Compound 2a 10 mg/kg | 114.3 ± 9.2 | 764 ± 155 |
| Compound 2a 20 mg/kg | 55.7 ± 4.6 ** | 549 ± 111 |
| Compound 2a 40 mg/kg | 95.7 ± 8.2 | 1036 ± 93 |
| Compound 2c 10 mg/kg | 63.4 ± 9.3 ** | 956 ± 171 |
| Compound 2c 20 mg/kg | 71.6 ± 11.7 ** | 275 ± 96 ** |
| Compound 2c 40 mg/kg | 84.6 ± 11.5 | 92 ± 38 ** |
| Compound 2d 10 mg/kg | 103 ± 8.9 | 1141 ± 317 |
| Compound 2d 20 mg/kg | 110.8 ± 7.5 | 521 ± 95 |
| Compound 2d 40 mg/kg | 52.4 ± 11.1 *** | 73 ± 34 ** |
| Compound 2e 10 mg/kg | 51.6 ± 12.8 *** | 296 ± 139* |
| Compound 2e 20 mg/kg | 62.1 ± 2.6 *** | 227 ± 59 ** |
| Compound 2e 40 mg/kg | 91.6 ± 4.9 | 729 ± 119 |
| Molecule | Molecular Weight | Log p | H-Donor | H-Acceptor | Rotatable Bonds |
|---|---|---|---|---|---|
| 2a | 188 | 1.8 | 1 | 1 | 3 |
| 2b | 206 | 1.95 | 1 | 1 | 3 |
| 2c | 222 | 2.35 | 1 | 1 | 3 |
| 2d | 266 | 2.63 | 1 | 1 | 3 |
| 2e | 314 | 3.15 | 1 | 1 | 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.A.; El-Alfy, A.T.; Ezel, K.; Radwan, M.O.; Shilabin, A.G.; Kochanowska-Karamyan, A.J.; Abd-Alla, H.I.; Otsuka, M.; Hamann, M.T. Marine Inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as Modulators of Serotonin Receptors: An Example Illustrating the Power of Bromine as Part of the Uniquely Marine Chemical Space. Mar. Drugs 2017, 15, 248. https://doi.org/10.3390/md15080248
Ibrahim MA, El-Alfy AT, Ezel K, Radwan MO, Shilabin AG, Kochanowska-Karamyan AJ, Abd-Alla HI, Otsuka M, Hamann MT. Marine Inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as Modulators of Serotonin Receptors: An Example Illustrating the Power of Bromine as Part of the Uniquely Marine Chemical Space. Marine Drugs. 2017; 15(8):248. https://doi.org/10.3390/md15080248
Chicago/Turabian StyleIbrahim, Mohamed A., Abir T. El-Alfy, Kelly Ezel, Mohamed O. Radwan, Abbas G. Shilabin, Anna J. Kochanowska-Karamyan, Howaida I. Abd-Alla, Masami Otsuka, and Mark T. Hamann. 2017. "Marine Inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as Modulators of Serotonin Receptors: An Example Illustrating the Power of Bromine as Part of the Uniquely Marine Chemical Space" Marine Drugs 15, no. 8: 248. https://doi.org/10.3390/md15080248
APA StyleIbrahim, M. A., El-Alfy, A. T., Ezel, K., Radwan, M. O., Shilabin, A. G., Kochanowska-Karamyan, A. J., Abd-Alla, H. I., Otsuka, M., & Hamann, M. T. (2017). Marine Inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as Modulators of Serotonin Receptors: An Example Illustrating the Power of Bromine as Part of the Uniquely Marine Chemical Space. Marine Drugs, 15(8), 248. https://doi.org/10.3390/md15080248