Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites
Abstract
1. Introduction
2. Alkaloids
3. Terpenes, Meroterpenes, and Steroids
4. Polyketides
5. Lipopeptides
6. Miscellaneous Compounds
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Metabolites | Producing Stain | Environment Source | Type | Cell Lines/Brine Shrimp | IC50, LD50, or IR (%) | Target | Reference |
|---|---|---|---|---|---|---|---|
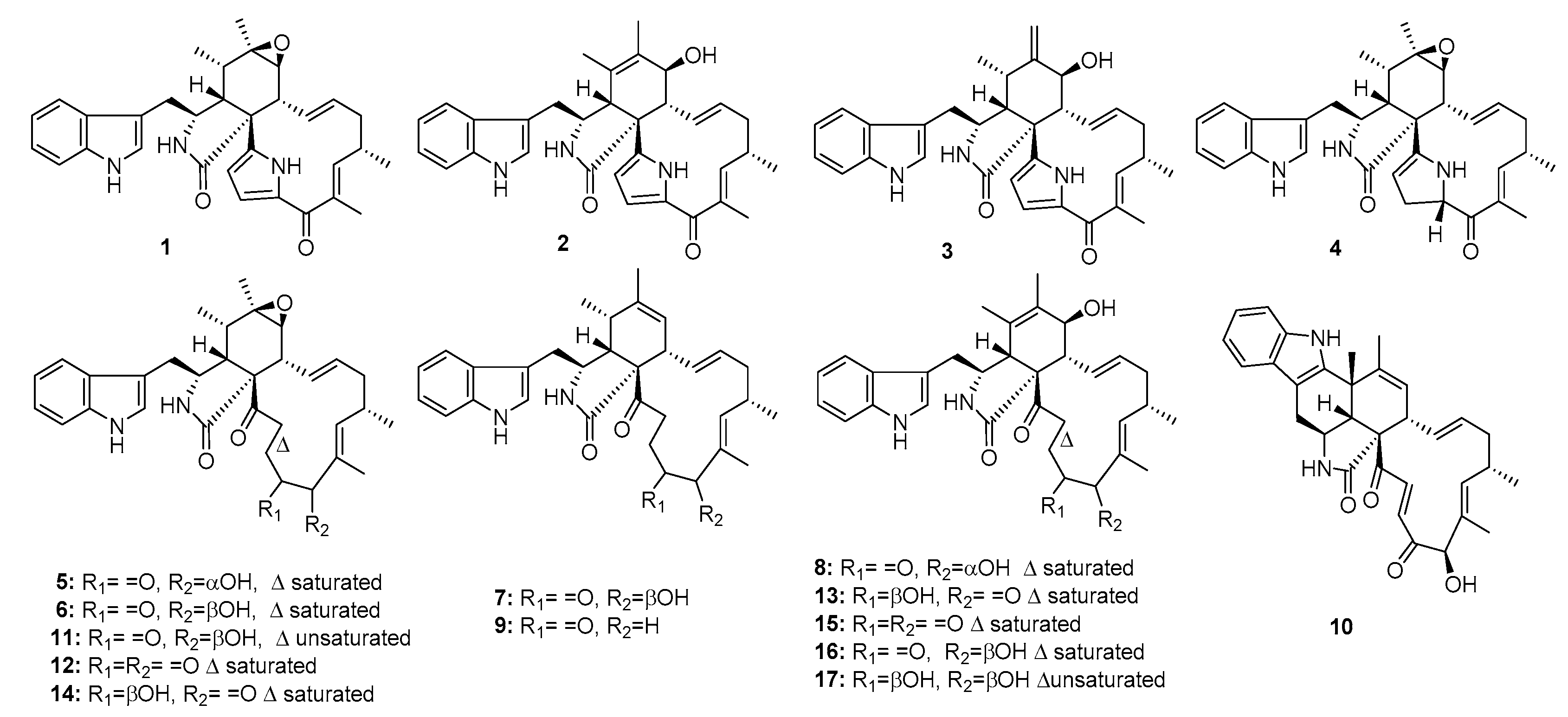
| Penochalasin A (1) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 0.4 μg/mL | [8] | |
| Penochalasin B (2) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 0.3 μg/mL | [8] | |
| Penochalasin C (3) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 0.5 μg/mL | [8] | |
| Penochalasin D (4) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 3.2 μg/mL | [7] | |
| Penochalasin E (5) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 2.1 μg/mL | [7] | |
| Penochalasin F (6) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 1.8 μg/mL | [7] | |
| Penochalasin G (7) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 1.9 μg/mL | [7] | |
| Penochalasin H (8) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 2.8 μg/mL | [7] | |
| Penochalasin I (9) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (7.55, 7.32, 16.13) μM | [6] | |
| Penochalasin J (10) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (36.68, 37.70, 35.93) μM | [6] | |
| Chaetoglobosin A (11) | P. chrysogenum V11 | Mangrove | Indole alkaloid | P388, MDA-MB-435, SGC-7901, A549 | 0.6 μg/mL (37.56, 7.84, 6.56) μM | Cell-cycle arrest induction, membrane ruffling inhibition, and cell migration | [6,8,9] |
| Chaetoglobosin C (12) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (19.97, 15.36, 17.82) μM | [6] | |
| Chaetoglobosin E (13) | P. chrysogenum V11 | Mangrove | Indole alkaloid | A549 | 36.63 μM | [6] | |
| Chaetoglobosin F (14) | P. chrysogenum V11 | Mangrove | Indole alkaloid | P388, MDA-MB-435, SGC-7901, A549 | 0.9 μg/mL, (37.77, 26.53, 27.72) μM | [6,8] | |
| Chaetoglobosin G (15) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (38.77, 25.86, 27.63) μM | [6] | |
| Chaetoglobosin O (16) | Penicillium sp. | Marine alga | Indole alkaloid | P388 | 2.4 μg/mL | [7] | |
| Cytoglobosin C (17) | P. chrysogenum V11 | Mangrove | Indole alkaloid | MDA-MB-435, SGC-7901, A549 | (12.58, 8.15, 3.35) μM | [6] | |
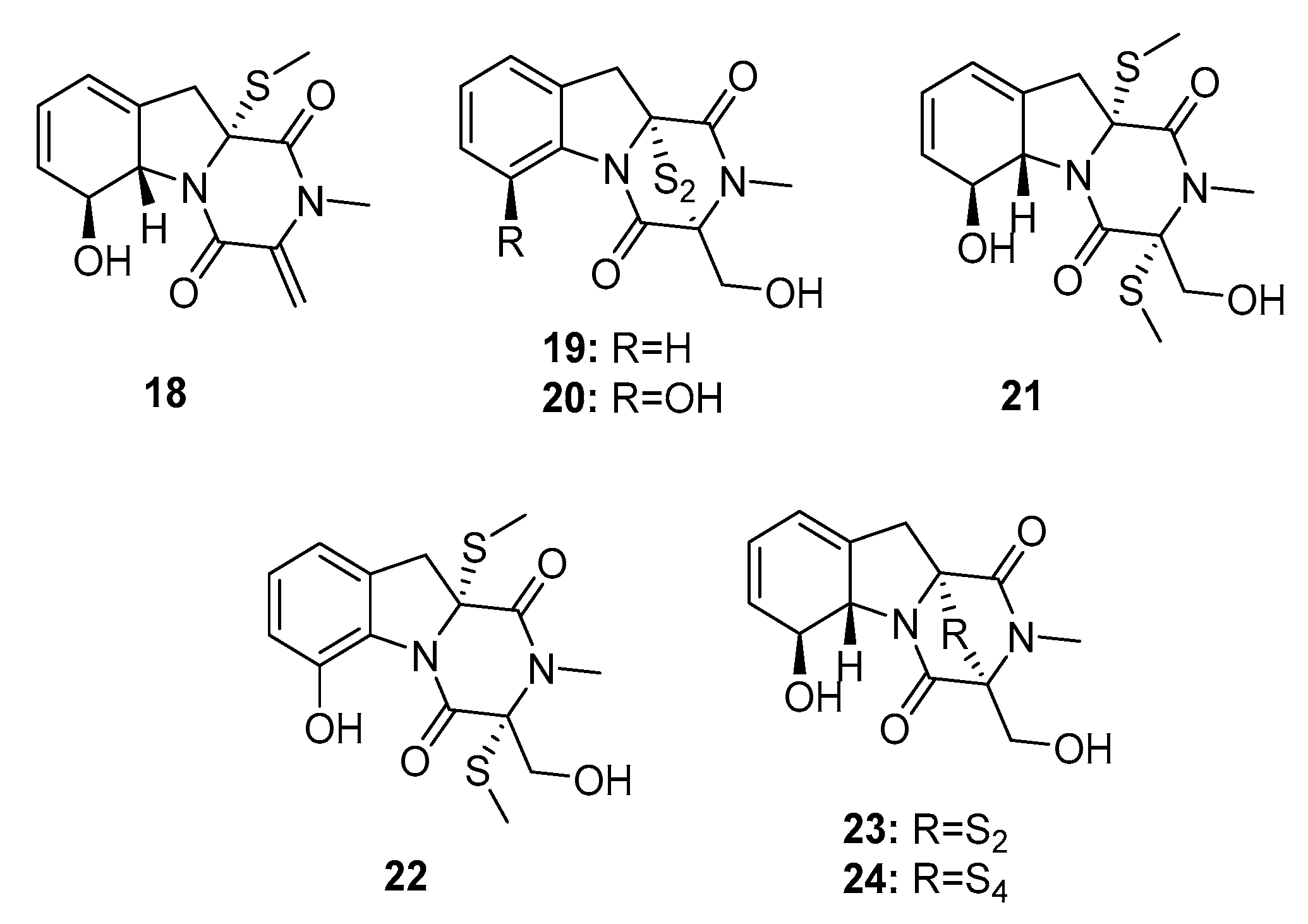
| 18 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 3.4 μM | [12] | |
| 19 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.058 μM | HMT G9a (IC50 = 55 μM) | [12] |
| 20 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.11 μM | [12] | |
| 21 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.11 μM | HMT G9a (IC50 = 58 μM) | [12] |
| 22 | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.056 μM | HMT G9a (IC50 = 2.6 μM) | [12] |
| Gliotoxin (23) | Penicillium sp. JMF034 | Deep sea sediment | Diketopiperazine | P388 | 0.024 μM | HMT G9a (IC50 = 6.4 μM) Dual inhibitor of farnesyltransferase and geranylgeranyltransferase I | [12,13] |
| Gliotoxin G (24) | Penicillium sp. JMF034 P. brocae MA-231 | Deep sea sediment Mangrove | Diketopiperazine | P388 A2780, A2780 CisR | 0.02 μM (0.664, 0.661) μM | HMT G9a (IC50 = 2.1 μM) | [12] [14] |
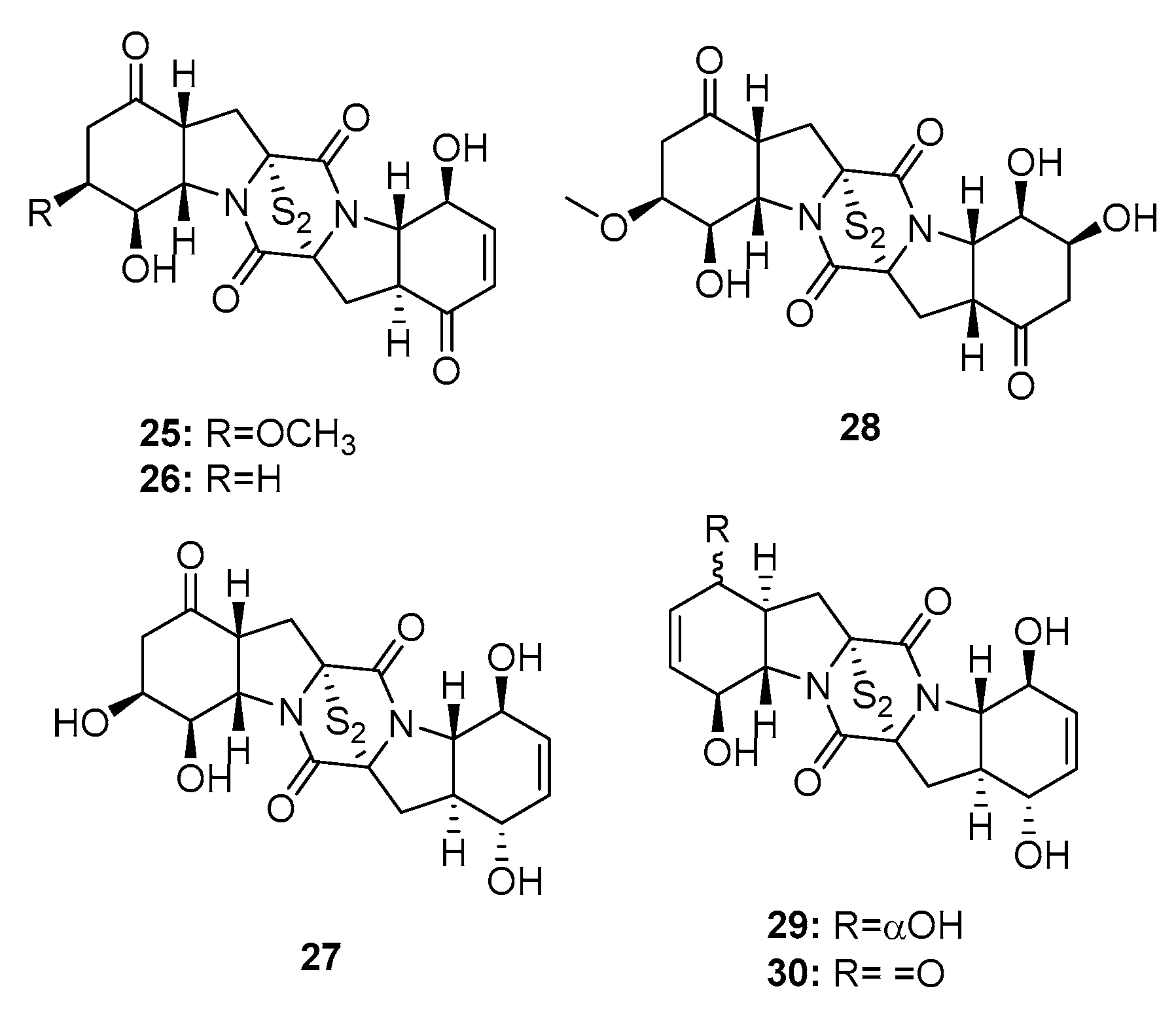
| Brozazine A (25) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, SW480, U251 | (4.2, 6.8, 6.4, 5.5, 4.9, 2.6, 6.0, 2.0, 5.2) μM | [16] | |
| Brozazine B (26) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, SW480, U251 | (3.6, 5.3, 5.5, 6.1, 4.0, 2.4, 6.4, 1.2, 3.5) μM | [16] | |
| Brozazine E (29) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, U251 | (11.2, 4.3, 5.6, 9.0, 12.4, 3.3, 2.1, 6.1) μM | [16] | |
| Brozazine F (30) | P. brocae MA-231 | Mangrove | Diketopiperazine | Du145, Hela, HepG2, MCF-7, NCI-H460, SGC-7901, SW1990, U251 | (1.7, 6.9, 2.9, 3.0, 0.89, 8.0, 5.9, 5.3) μM | [16] | |
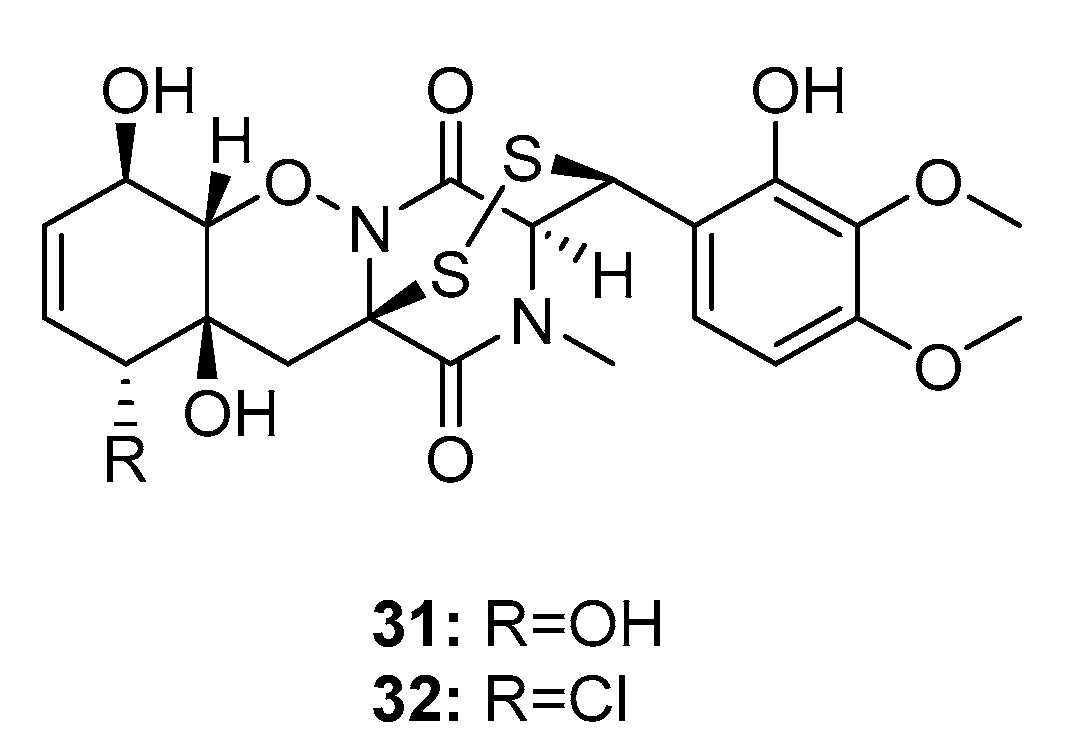
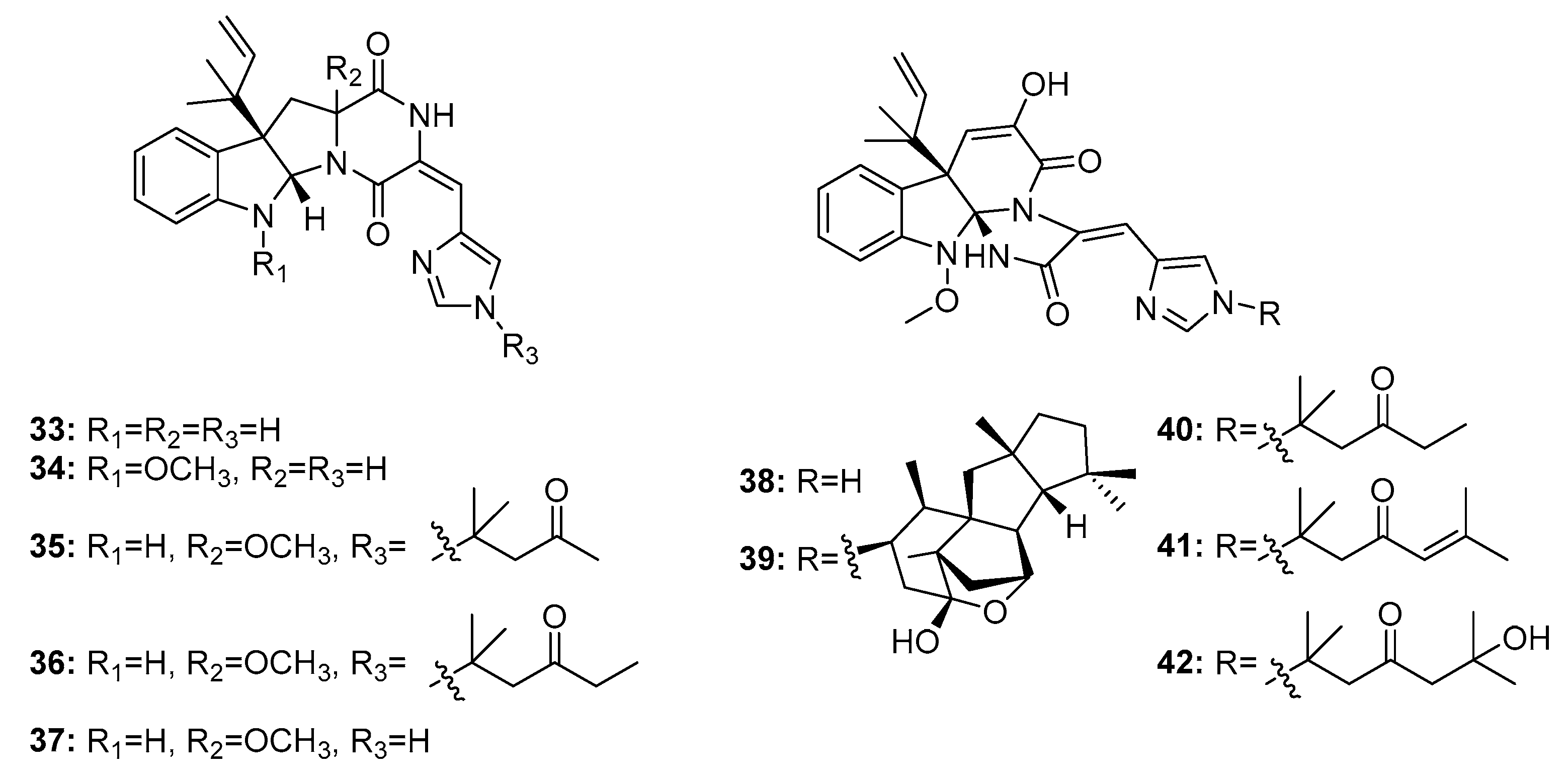
| N-methylpretrichodermam ide B/adametizines A (32) | P. adametzioides AS-53 Penicillium sp. | Marine sponge/sediment/alga | Diketopiperazine | Artemia salina | 4.8 μM | [17,18,19] | |
| L5178Y, 22Rv1, PC-3, LNCaP | (2, 0.51, 5.11, 1.76) μM | ||||||
| Roquefortine C (33) | Penicillium sp. | Deep sea sediment | Diketopiperazine | Activate P-glycoprotein and inhibit P450-3A and other haemoproteins | [20,22] | ||
| Roquefortine F (34) | Penicillium sp. | Deep sea sediment | Diketopiperazine | A549, HL-60, BEL-7402, MOLT-4 | (14.0, 33.6, 13.0, 21.2) μM | [22] | |
| Roquefortine G (35) | Penicillium sp. | Deep sea sediment | Diketopiperazine | A549, HL-60 | (42.5, 36.6) μM | [22] | |
| Meleagrin (38) | Penicillium sp. P. commune SD-118 | Deep sea sediment | Indole alkaloid | A549, HL-60 HepG2, NCI-H460, Hela, DU145, MDA-MB-231, | (19.9, 7.4) μM (12.0, 22.0, 20.0, 11.0, 5.0) μg/mL | Arrest the cell cycle through G2/M phase Inhibitor of tubulin polymerization | [21,22,23] |
| Meleagrin B (39) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549, HL-60, BEL-7402, MOLT-4 | (2.7, 6.7, 1.8, 2.9) μM | [21,22] | |
| Meleagrin C (40) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549, BEL-7402, MOLT-4 | (9.9, 10.0, 4.7) μM | [22] | |
| Meleagrin D (41) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549 | 32.2 μM | [21] | |
| Meleagrin E (42) | Penicillium sp. | Deep sea sediment | Indole alkaloid | A549 | 55.9 μM | [21] | |
| Penicimutanin (43) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, Hela, MCF-7 | IR% (100 μg/mL): 22.6%, 17.9%, 26.5% | [24] | |
| Penicimutanin A (44) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, HL-60, Hela, BGC-823, MCF-7 | (11.4, 5.4, 9.5, 8.0, 5.4) μM | [24] | |
| Penicimutanin B (45) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, HL-60, Hela, BGC-823, MCF-7 | (19.9, 12.1, 17.7, 16.6, 8.0) μM | [24] | |
| Fructigenine A (46) | Mutant P. purpurogenum G59 | Marine soil | Diketopiperazine | K562, Hela, MCF-7, BGC-823 | IR% (100μg/mL): 20.8%, 55.3%, 65.6%, 34.8% | [24,25] | |
| 11,11′-dideoxyverticillin A (49) | Penicillium sp. | Marine alga | Diketopiperazine | HCT-116 | 30 ng/mL | Induce G2/M arrest through p38 MAPK pathway; Epidermal growth factor receptor tyrosine kinase inhibitor | [28,30,31] |
| 11′-deoxyverticillin A (50) | Penicillium sp. | Marine alga | Diketopiperazine | HCT-116 | 30 ng/mL | [28] | |
| Penitrem A (51) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) MDA-MB-231 (anti-invasion) | (11.9, 9.8) μM 8.7 μM IR% (15 μM)> 75% | BK channel inhibitor | [34] |
| Penitrem B (52) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) | (5.5, 13.7) μM 10.3 μM | [34] | |
| Penitrem D (53) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) | (8.3, 29.7) μM 9.2 μM | [34] | |
| Penitrem E (54) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) | (17.5, 25.4) μM 20.3 μM | [34] | |
| Penitrem F (55) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) | (15.0, 13.8) μM 35.0 μM | [34] | |
| 6-bromopenitrem B (56) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) MDA-MB-231 (anti-invasion) | (19.3, 18.8) μM 30.3 μM IR%(15 μM) > 40% | [34] | |
| 6-bromopenitrem E (57) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) | (16.7, 8.5) μM 9.6 μM | BK channel inhibitor | [34] |
| Paspaline (58) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) | (12.8, 12.4) μM 7.6 μM | [34] | |
| Emnidole SB (59) | P. commune isolate GS20 | Sponge/Sediment | Indole alkaloid | MCF-7, MDA-MB-231 (antiproliferative) MDA-MB-231 (antimigratory) | (10.1, 21.3) μM 19.0 μM | [34,37] | |
| Communesin A (60) | Penicillium sp. | Marine alga/Sediment | Indole alkaloid | P388 | 3.5 μg/mL | [38,40] | |
| Communesin B (61) | Penicillium sp. | Marine alga/Sponge/Sediment | Indole alkaloid | P388, U-937, THP-1, NAMALWA, MOLT-3, SUP-B15 | (0.45, 10.4, 11.4, 9.9, 8.1, 7.2) μg/mL | [38,39,40] | |
| Communesin C (62) | Penicillium sp. | Marine sponge | Indole alkaloid | U-937, THP-1, NAMALWA, MOLT-3, SUP-B15 | (11.3, 13.1, 8.2, 8.6, 10.8) μg/mL | [39] | |
| Communesin D (63) | Penicillium sp. | Marine sponge | Indole alkaloid | U-937, THP-1, NAMALWA, MOLT-3, SUP-B15 | (13.1, 16.2, 14.6, 9.9, 9.0) μg/mL | [39] | |
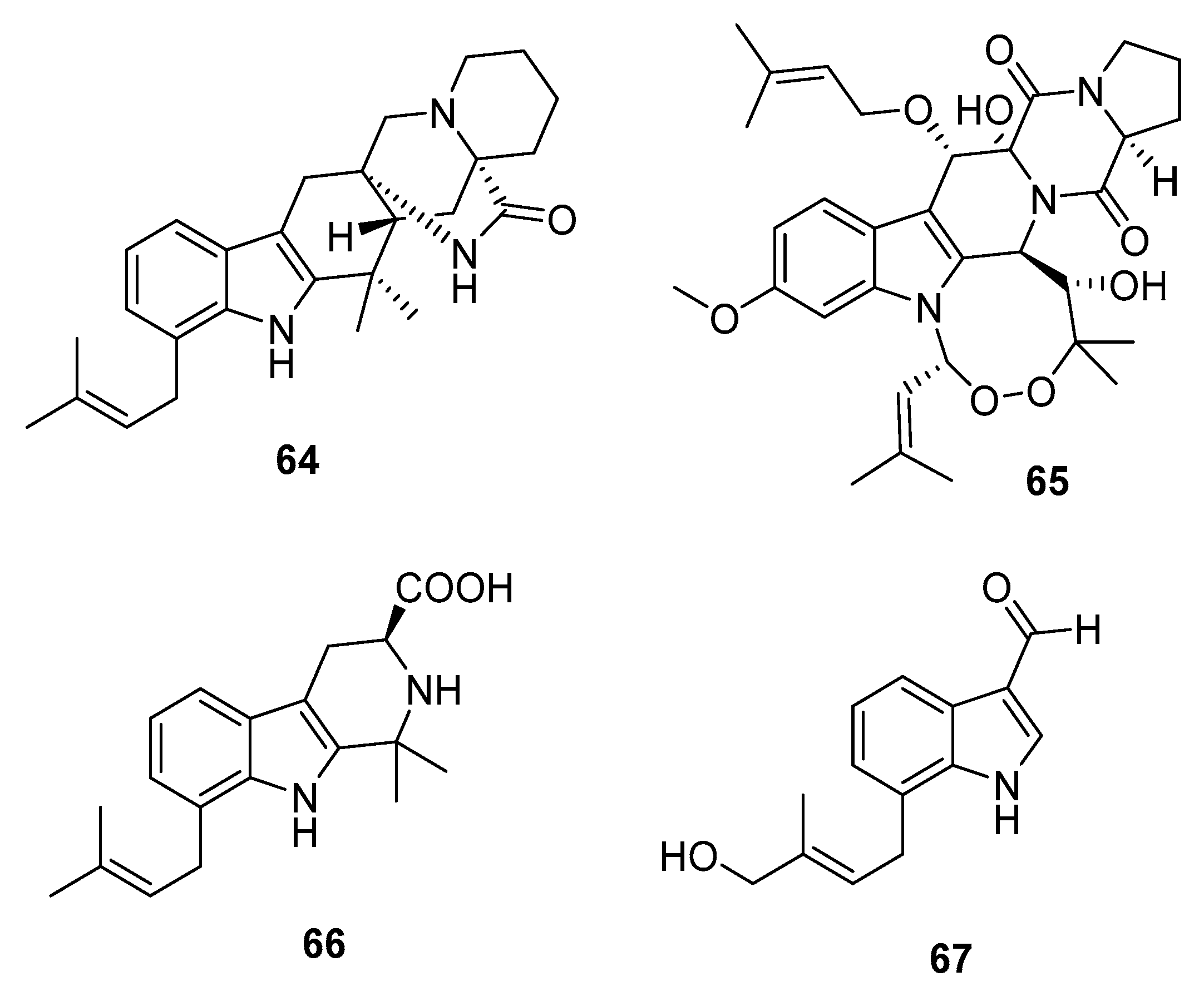
| Penioxamide (64) | P. oxalicum EN-201 | Mangrove | Indole alkaloid | A. salina | 5.6 μM | [42] | |
| 65 | P. brefeldianum SD-273 | Marine sediment | Indole alkaloid | A. salina | 9.4 μM | [43] | |
| Penipaline B (66) | P. paneum SD-44 | Marine sediment | Indole alkaloid | A549, HCT-116 | (20.44, 14.88) μM | [44] | |
| Penipaline C (67) | P. paneum SD-44 | Marine sediment | Indole alkaloid | A549, HCT-116 | (21.54, 18.54) μM | [44] | |
| Terretrione A (68) | P. vinaceum | Marine sponge | 1,4-diazepane alkaloid | MDA-MB-231 | 17.7 μM | [45] | |
| Terretrione C (69) | Penicillium sp.CYE-87 | Marine tunicate | 1,4-diazepane alkaloid | MDA-MB-231 | 17.6 μM | [46] | |
| Terretrione D (70) | Penicillium sp.CYE-87 | Marine tunicate | 1,4-diazepane alkaloid | MDA-MB-231 | 16.5 μM | [46] | |
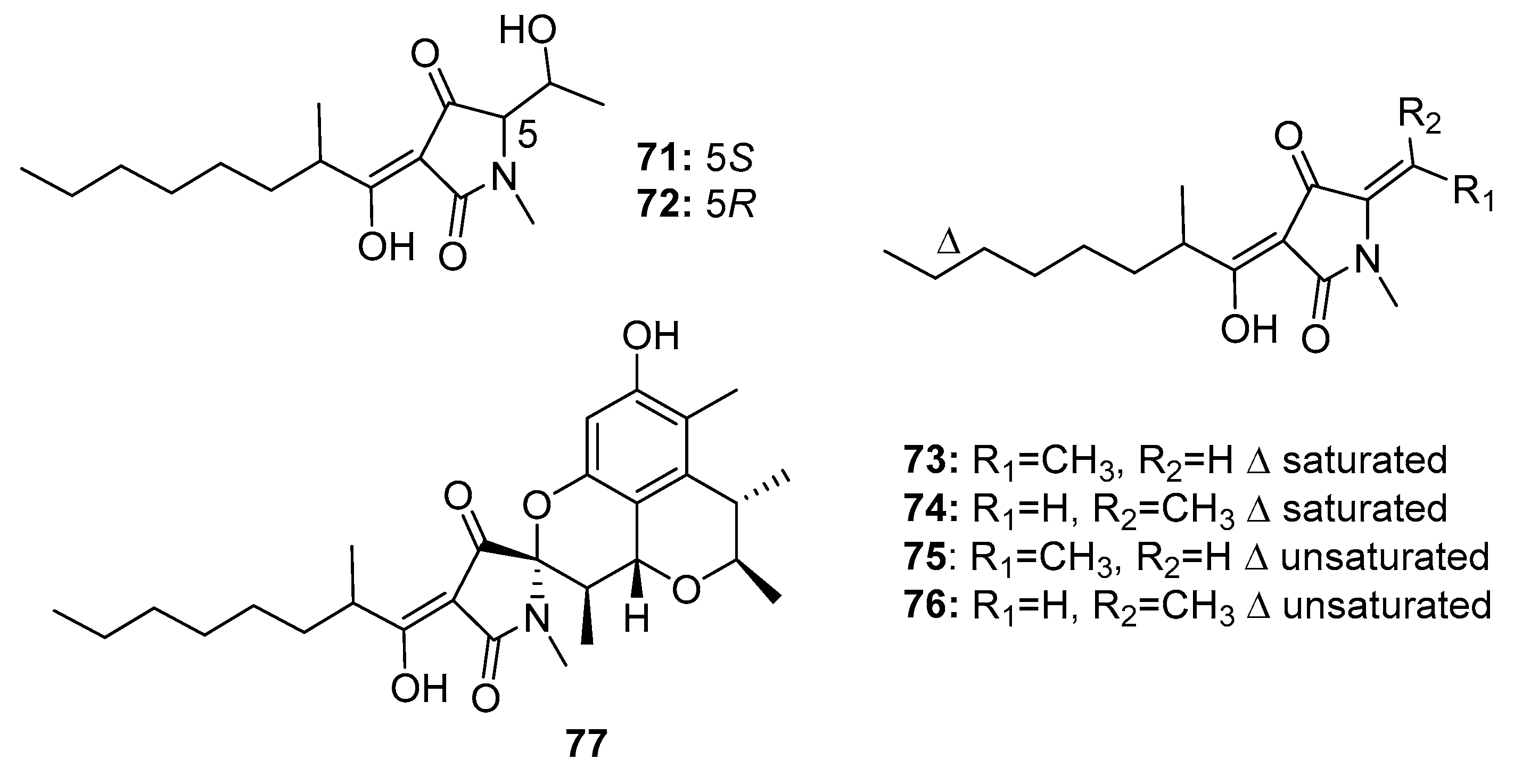
| Penicillenol A1 (71) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375, HL-60, A549, BEL-7402, P388 | 3.2 μg/mL (0.76, 23.8, 13.03, 8.85) μM | [47,49] | |
| Penicillenol A2 (72) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375 HL-60 | 13.8 μg/mL 16.26 μM | [47,49] | |
| Penicillenol B1 (73) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375 HL-60 | 2.8 μg/mL 3.2 μM | [47,49] | |
| Penicillenol B2 (74) | Penicillium sp. GQ-7/P. citrinum | Mangrove/Marine sediment | Pyrrolidinone alkaloid | A-375 HL-60 | 0.97 μg/mL 7.65 μM | [47,49] | |
| Penicillenol D1 (75) | P. citrinum | Marine sediment | Pyrrolidinone alkaloid | A549, HL-60 | (17.2, 18.5) μg/mL | [48] | |
| Penicillenol D2 (76) | P. citrinum | Marine sediment | Pyrrolidinone alkaloid | A549, HL-60 | (12.1, 14.5) μg/mL | [48] | |
| Penitrinine A (77) | P. citrinum | Marine sediment | Pyrrolidinone alkaloid | A-375, SPC-A1, HGC-27 | (20.12, 28.67, 29.49) μM | Upregulate Bax, downregulate Bcl-2, suppress MMP-9 and TIMP-1 | [50] |
| 78 | P. janczewskii | Sea water | Quinolinone | MDA-MB-231, DU-145, SKOV-3, HT-29, A549, CAKI-1, SK-MEL-2, K562 | IR % (10 μg/mL) = 20~50% | [52] | |
| 79 | P. janczewskii | Sea water | Quinolinone | MDA-MB-231, DU-145, SKOV-3, HT-29, A549, CAKI-1, SK-MEL-2, K562 | IR % (10 μg/mL) = 30~90% | [52] | |
| 80 | P. janczewskii | Sea water | Quinolinone | MDA-MB-231, DU-145, SKOV-3, HT-29 | IR % (10 μg/mL) = 91.6%, 69.2%, 79.8%, 96.0% | [52] | |
| 81 | Penicillium sp. ghq208/Penicillium sp. | Marine sediment/Mangrove | Quinolinone | 95-D, HepG2 | (0.57, 6.5) μg/mL | [53,54] | |
| 82 | Penicillium sp. ghq208 | Marine sediment | Quinolinone | HepG2 | 13.2 μM | [53] | |
| 83 | P. commune SD-118 | Deep sea sediment | Quinazolinone | SW1990 | 20 μg/mL | [23] | |
| 84 | P. chrysogenum EN-118 | Marine alga | Quinazolinone | DU145, A549, Hela | 8 μg/mL | [56] | |
| 85 | P. oxalicum 0312F1 | Marine (not clear) | Quinazolinone | SGC-7901, BEL-7404 | IR % (200 μg/mL) = 30~40% | [55] | |
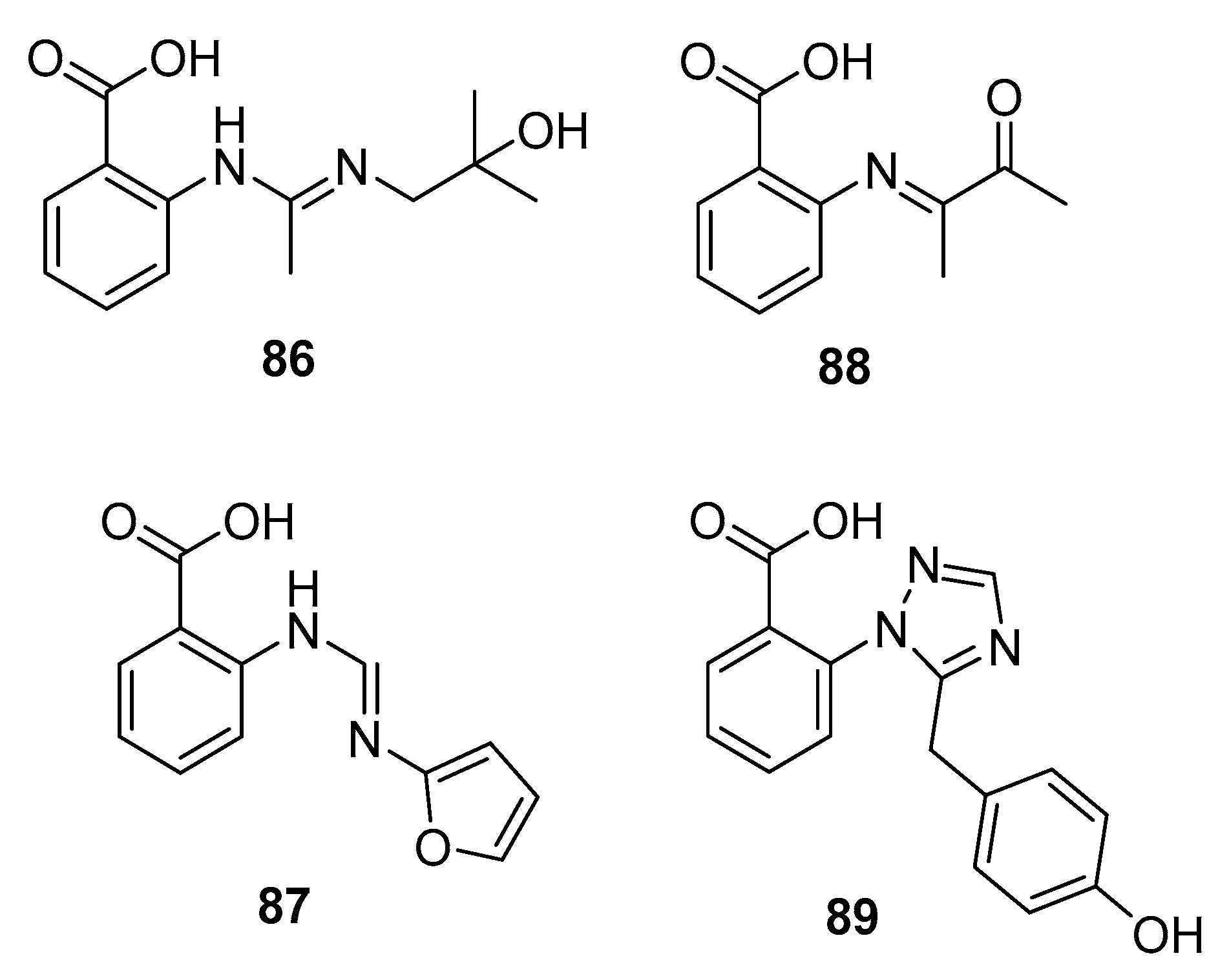
| Penipacid A (86) | P. paneum SD-44 | Deep sea sediment | Amidine alkaloid | RKO | 8.4 μM | [57] | |
| Penipacid E (87) | P. paneum SD-44 | Deep sea sediment | Amidine alkaloid | RKO | 9.7 μM | [57] | |
| 88 | P. paneum SD-44 | Deep sea sediment | Imine alkaloid | Hela | 6.6 μM | [57] | |
| Penipanoid A (89) | P. paneum SD-44 | Deep sea sediment | Triazole alkaloid | SMMC-7721 | 54.2 μM | [58] | |
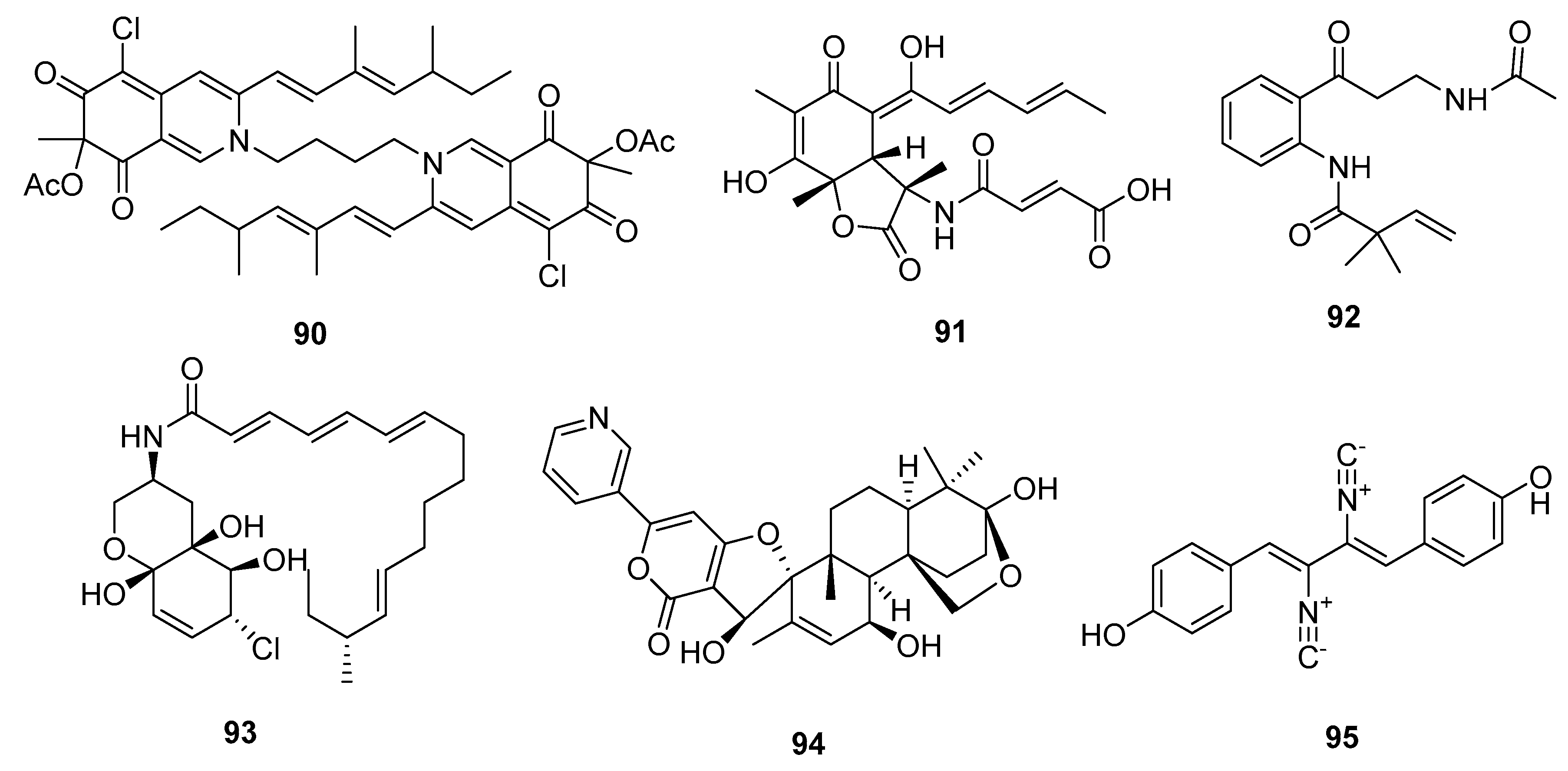
| Bis-sclerotioramin (90) | Penicillium 303# | Mangrove | Azaphilone alkaloid | MDA-MB-231 | 7.13 μM | [59] | |
| Sorbicillactone (91) | P. chrysogenum | Marine sponge | Miscellaneous Alkaloid | L5178Y | 2.2 μg/mL | Selective anti-leukemic | [60] |
| Brocaeloid B (92) | P. brocae | Mangrove | Miscellaneous Alkaloid | A. salina | 36.7 μM | [62] | |
| Varitatin (93) | Mutant P. varibile | Mangrove | Amide alkaloid | HCT-116 | 2.8 μM | IR%(1μM) = 50% and 40% (PDGFR-βand ErbB4) | [63] |
| 18-hydroxydecaturin B (94) | P. oxalicum EN-201 | Mangrove | Pyridinyl-α-pyrone alkaloid | A. salina | 2.3 μM | [42] | |
| Xantocillin X (95) | P. commune SD-118 | Deep sea sediment | Isocyanide alkaloid | MCF-7, HepG2, NCI-H460, Hela, DU145, MDA-MB-231 | (12, 7, 10, 10, 8, 8) μg/mL | Inhibit MEK/EPK pathway and activate class III PI3K/Beclin 1 pathway | [23,67] |
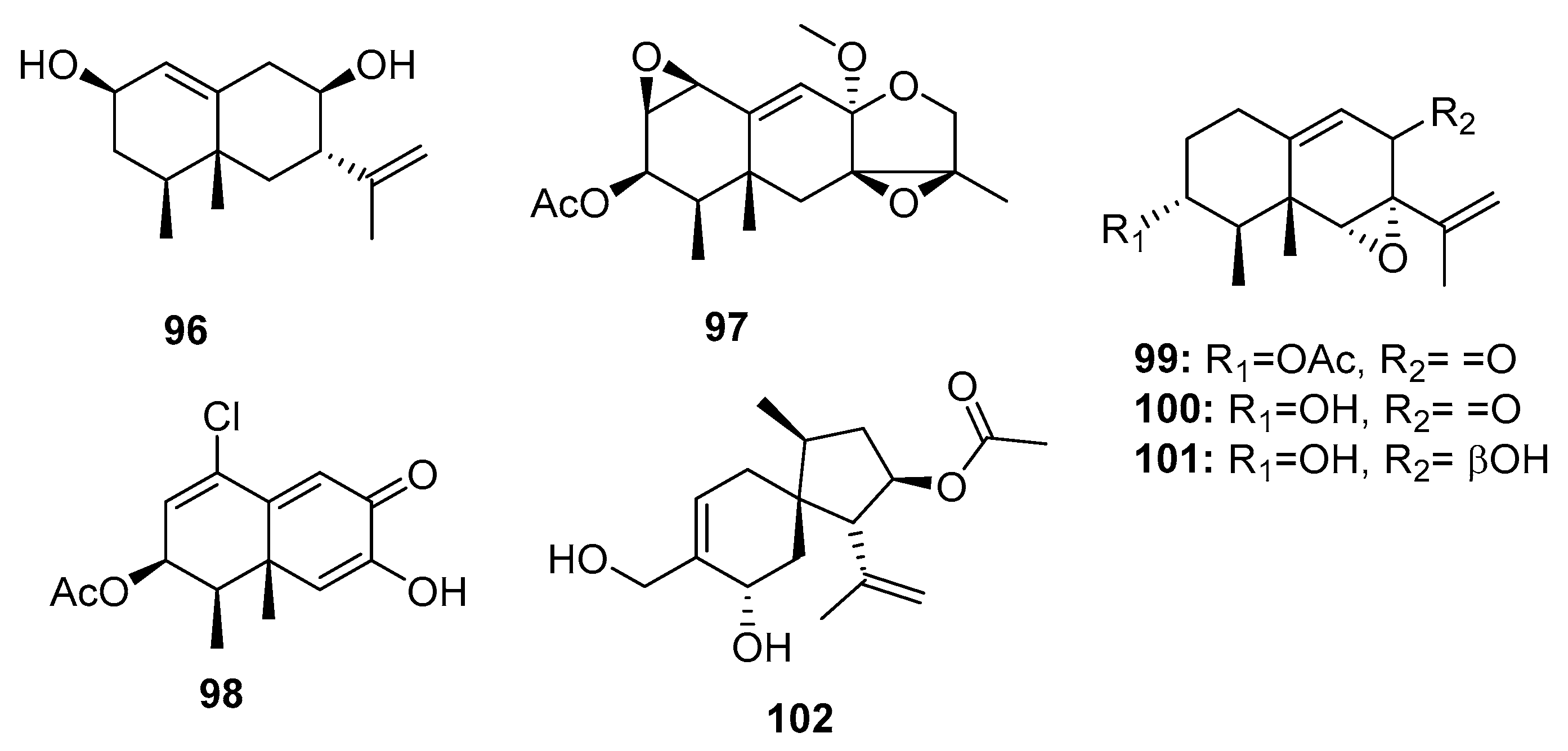
| 96 | Penicillium sp. PR19 N-1 | Marine sludge | Sesquiterpene | HL-60, A549 | (45.8, 82.8) μM | [70] | |
| 97 | Penicillium sp. PR19 N-1 | Marine sludge | Sesquiterpene | HL-60, A549 | (28.3, 5.2) μM | [70] | |
| 98 | Penicillium sp. PR19 N-1 | Marine sludge | Sesquiterpene | HL-60, A549 | (11.8, 12.2) μM | [71] | |
| 99 | Penicillium sp. BL 27-2 | Sea mud | Sesquiterpene | P388, A549, HL-60, BEL-7402 | (0.073, 0.096, 0.065, 4.59) μM | [72] | |
| Sporogen-AO 1 (100) | Penicillium sp. BL 27-2 | Sea mud | Sesquiterpene | P388, A549, HL-60, BEL-7402 | (10.1, 8.81, 10.4, 5.7) μM | [72] | |
| 101 | Penicillium sp. BL 27-2 | Sea mud | Sesquiterpene | P388, A549, HL-60, BEL-7402 | (8.71, 3.51, 7.75, 11.8) μM | [72] | |
| Adametacorenol B (102) | P. adametzioides AS-53 | Marine sponge | Diterpene | NCI-H446 | 5.0 μM | [17] | |
| Conidiogenone B (103) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60 | (40.3, 28.2) μM | [22] | |
| Conidiogenone C (104) | Penicillium sp. | Deep sea sediment | Diterpene | HL-60, BEL-7402 | (0.038, 0.97) μM | [22] | |
| Conidiogenone D (105) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, BEL-7402, MOLT-4 | (9.3, 5.3, 11.7, 21.1) μM | [22] | |
| Conidiogenone E (106) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, MOLT-4 | (15.1, 8.5, 25.8) μM | [22] | |
| Conidiogenone F (107) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, BEL-7402 | (42.2,17.8, 17.1) μM | [22] | |
| Conidiogenone G (108) | Penicillium sp. | Deep sea sediment | Diterpene | A549, HL-60, BEL-7402, MOLT-4 | (8.3, 1.1, 43.2, 4.7) μM | [22] | |
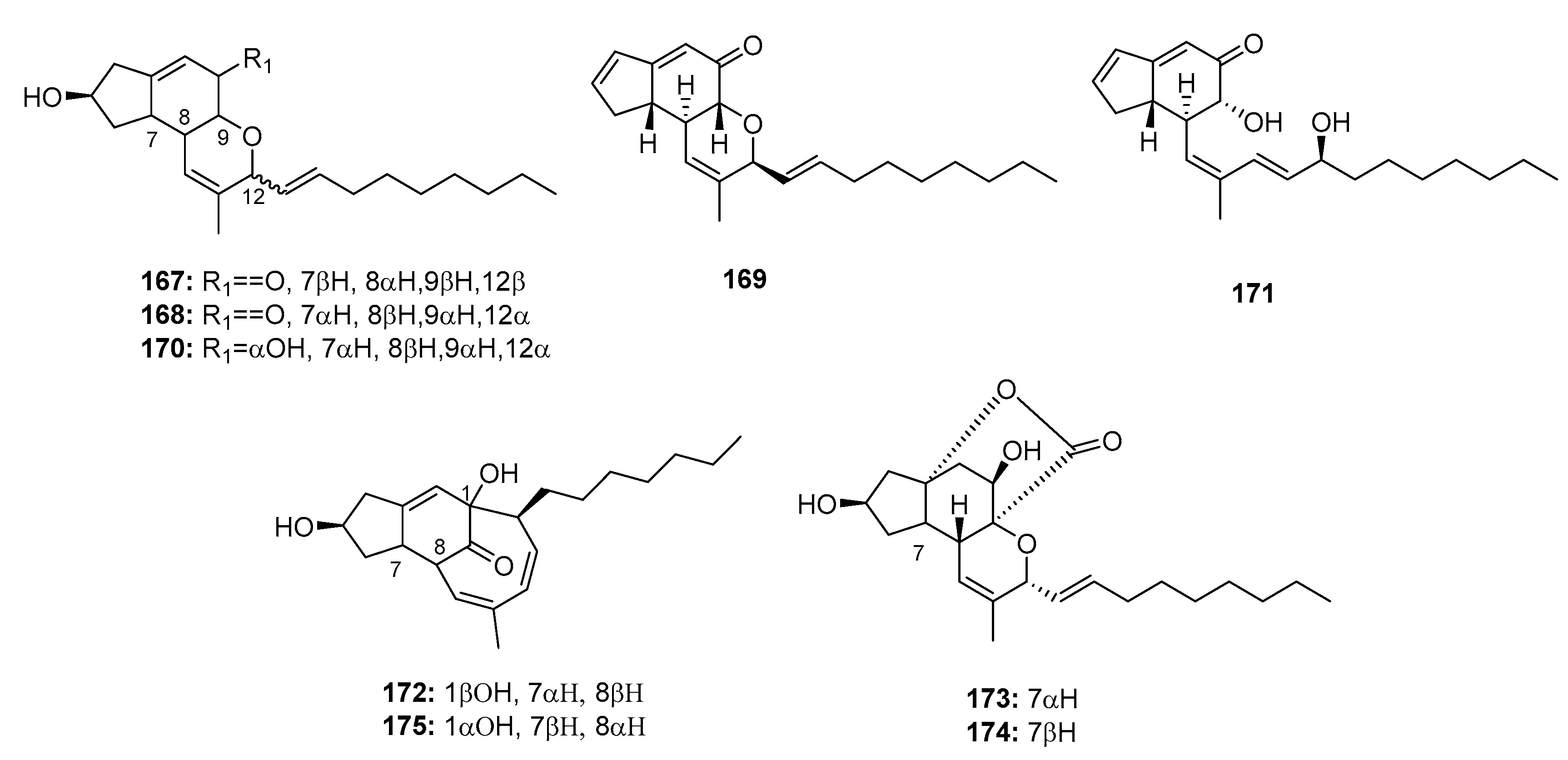
| Brevione I (109) | Penicillium sp. | Deep sea sediment | Diterpene | MCF-7 | 7.44 μM | [73] | |
| Brevione A (110) | Penicillium sp. | Deep sea sediment | Diterpene | MCF-7 | 28.4 μM | [73] | |
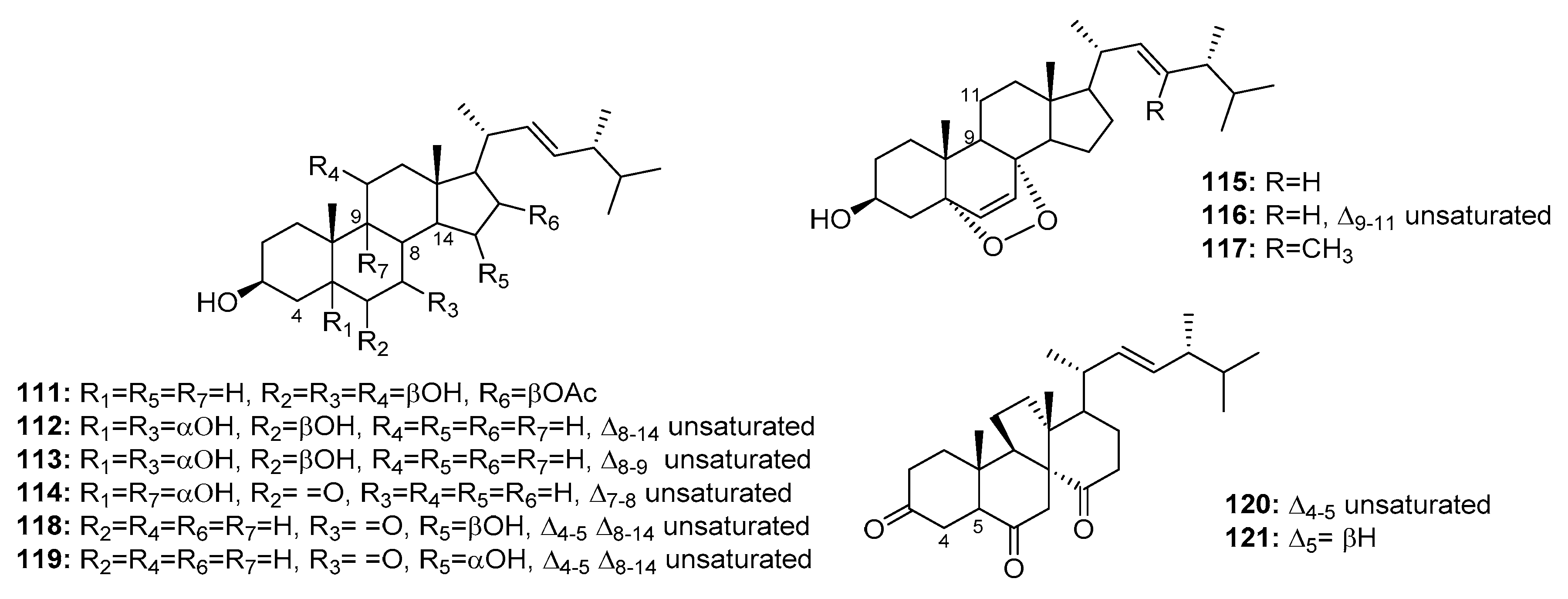
| Penicisteroid A (111) | P. chrysogenum QEN-24S | Marine alga | Steroid | Hela, SW1990, NCI-H460 | (15, 31, 40) μg/mL | [74] | |
| 112 | Penicillium sp. | Marine moss | Steroid | HepG2 | 10.4 μg/mL | [75] | |
| 113 | Penicillium sp. | Marine moss | Steroid | HepG2 | 15.6 μg/mL | [75] | |
| 114 | Penicillium sp. | Marine moss | Steroid | HepG2 | 20.7 μg/mL | [75] | |
| 115 | Penicillium sp. | Marine moss | Steroid | HepG2 | 16.8 μg/mL | [75] | |
| 116 | Penicillium sp. | Marine moss | Steroid | HepG2 | 21.3 μg/mL | [75] | |
| 117 | P. stoloniferum QY2-10 | Sea squirt | Steroid | P388 | 4.07 μM | [76] | |
| 118 | Penicillium sp. | Marine sponge | Steroid | K562 | 5.54 μM | [77] | |
| 119 | Penicillium sp. | Marine sponge | Steroid | K562 | 4.38 μM | [77] | |
| Dankasterone A (120) | Penicillium sp. | Marine sponge | Steroid | HL-60, Hela, K562 | (0.78, 4.11, 7.57) μM | [77] | |
| Dankasterone B (121) | Penicillium sp. | Marine sponge | Steroid | HL-60, Hela, K562 | (3.25, 4.74, 7.89) μM | [77] | |
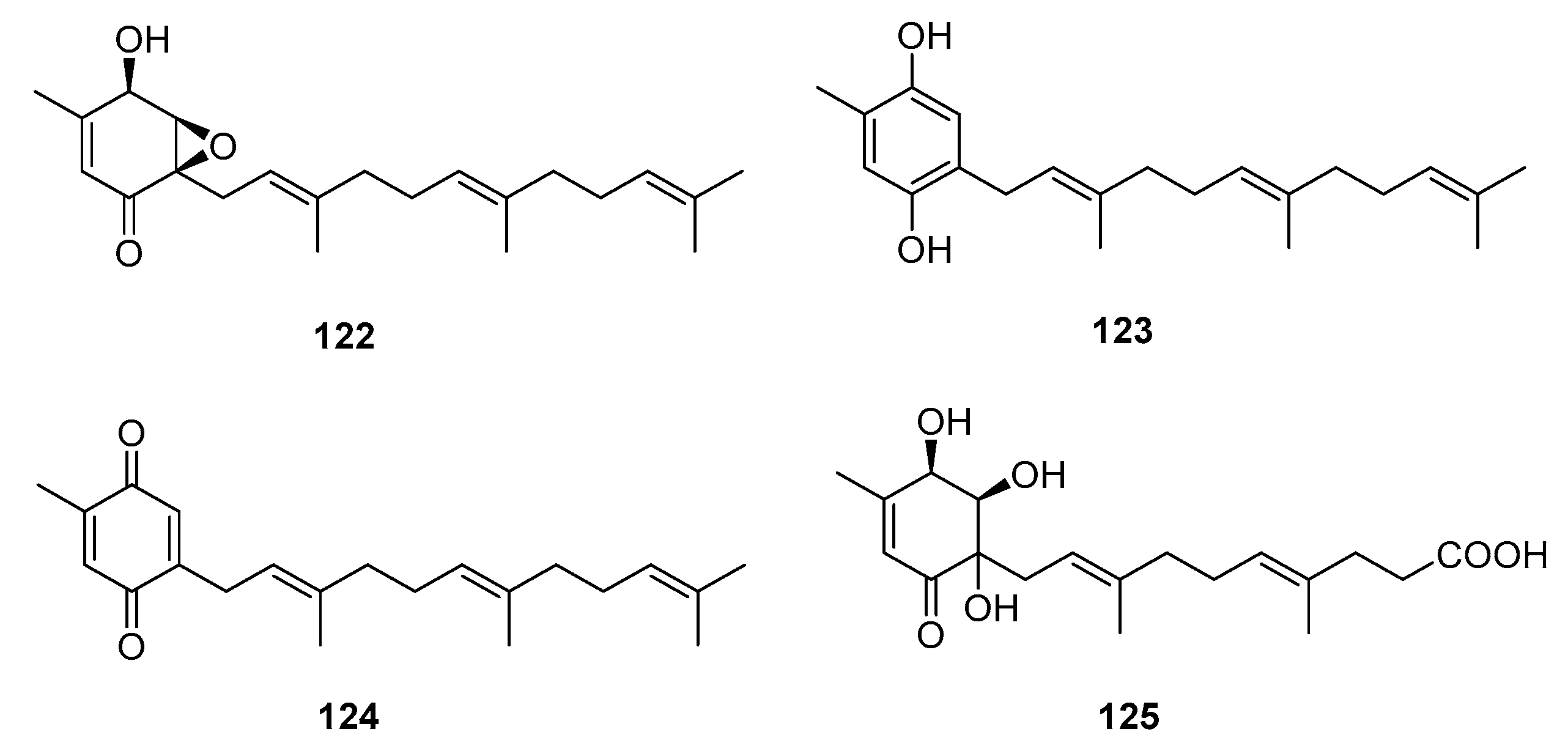
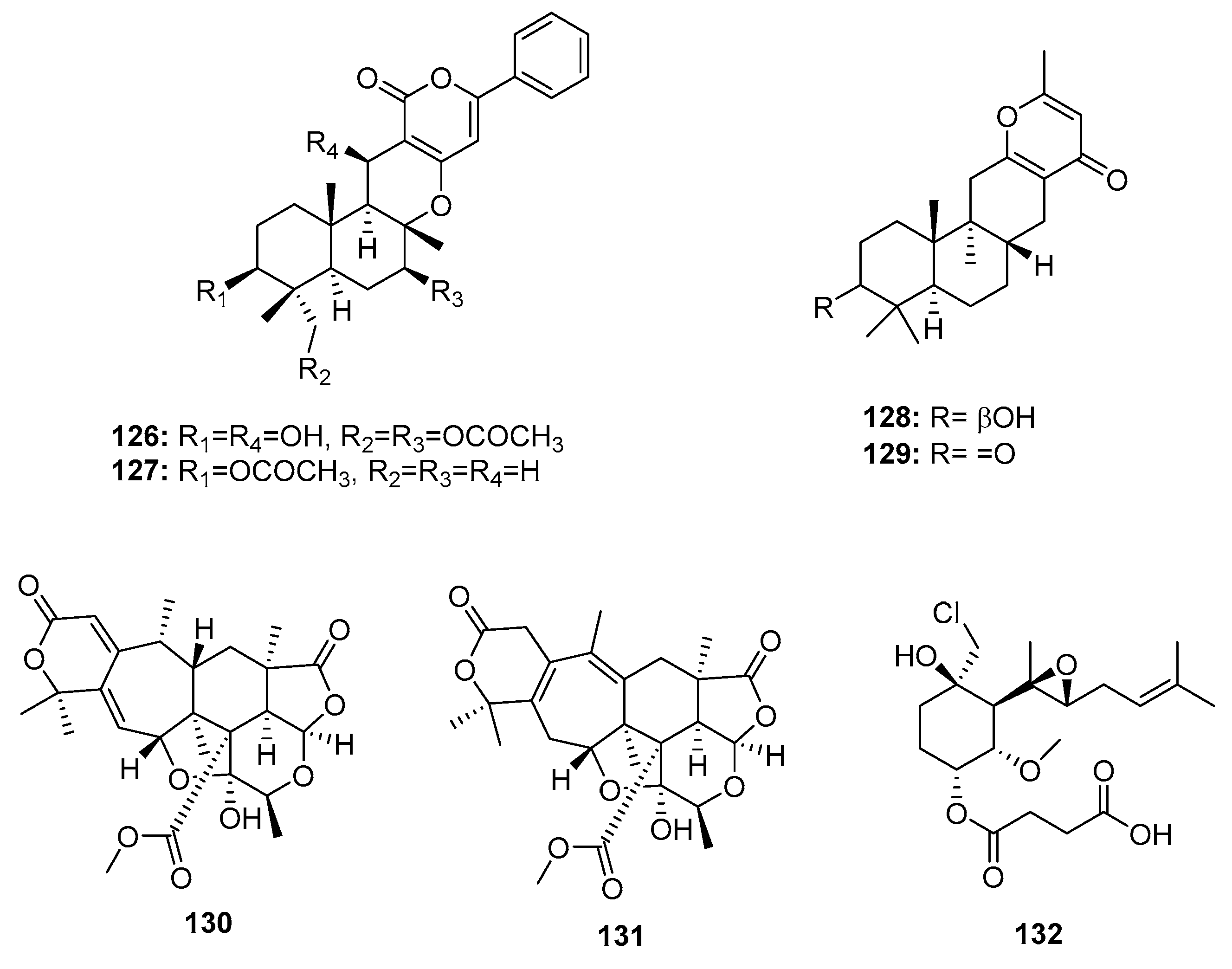
| 7-deacetoxyyanuthone (122) | Penicillium sp. | Marine (not clear) | Meroterpene | A549, SKOV-3, SKMEL-2, XF498, HCT-15 | (7.74, 6.35, 3.86, 10.04, 10.07) μg/mL | [79] | |
| Farnesylbenzenediol (123) | Penicillium sp. | Marine (not clear) | Meroterpene | A549, SKOV-3, SKMEL-2, XF498, HCT-15 | (4.73, 5.31, 4.80, 5.94, 6.11) μg/mL | [79] | |
| Farnesylquinone (124) | Penicillium sp. | Marine (not clear) | Meroterpene | A549, SKOV-3, SKMEL-2, XF498, HCT-15 | (25.44, 37.29, 18.41, 38.07, 42.56) μg/mL | [79] | |
| Penicillone A (125) | Penicillium sp. F11 | Marine (not clear) | Meroterpene | HT1080, Cne2 | (45.8, 46.2) μM | [80] | |
| Phenylpyropene E (126) | P. concentricum ZLQ-69 | Sea water | Sesquiterpene | MGC-803 | 19.1 μM | [81] | |
| Phenylpyropene F (127) | P. concentricum ZLQ-69 | Sea water | Sesquiterpene | MGC-803 | 13.6 μM | [81] | |
| Penicillipyrone A (128) | Penicillium sp. F446 | Marine sediment | Sesquiterpene | K562, A549 | (28, 15) μM | [82] | |
| Penicillipyrone B (129) | Penicillium sp. F446 | Marine sediment | Sesquiterpene | K562, A549 | (50, 17) μM | [82] | |
| 130 | Penicillium 303# | Mangrove | Meroterpene | MDA-MB-435, HepG2, HCT-116, A549 | (34.25, 24.56, 33.72, 37.82) μg/mL | [59] | |
| 131 | Penicillium 303# | Mangrove | Meroterpene | MDA-MB-435, HepG2, HCT-116, A549 | (31.32, 23.87, 29.19, 34.06) μg/mL | [59] | |
| Ligerin (132) | Penicillium sp. | Sea water | Merosesquiterpene | POS1, SaOS2, MG63 | (117/78, 137, 1459) nM | [84,85] | |
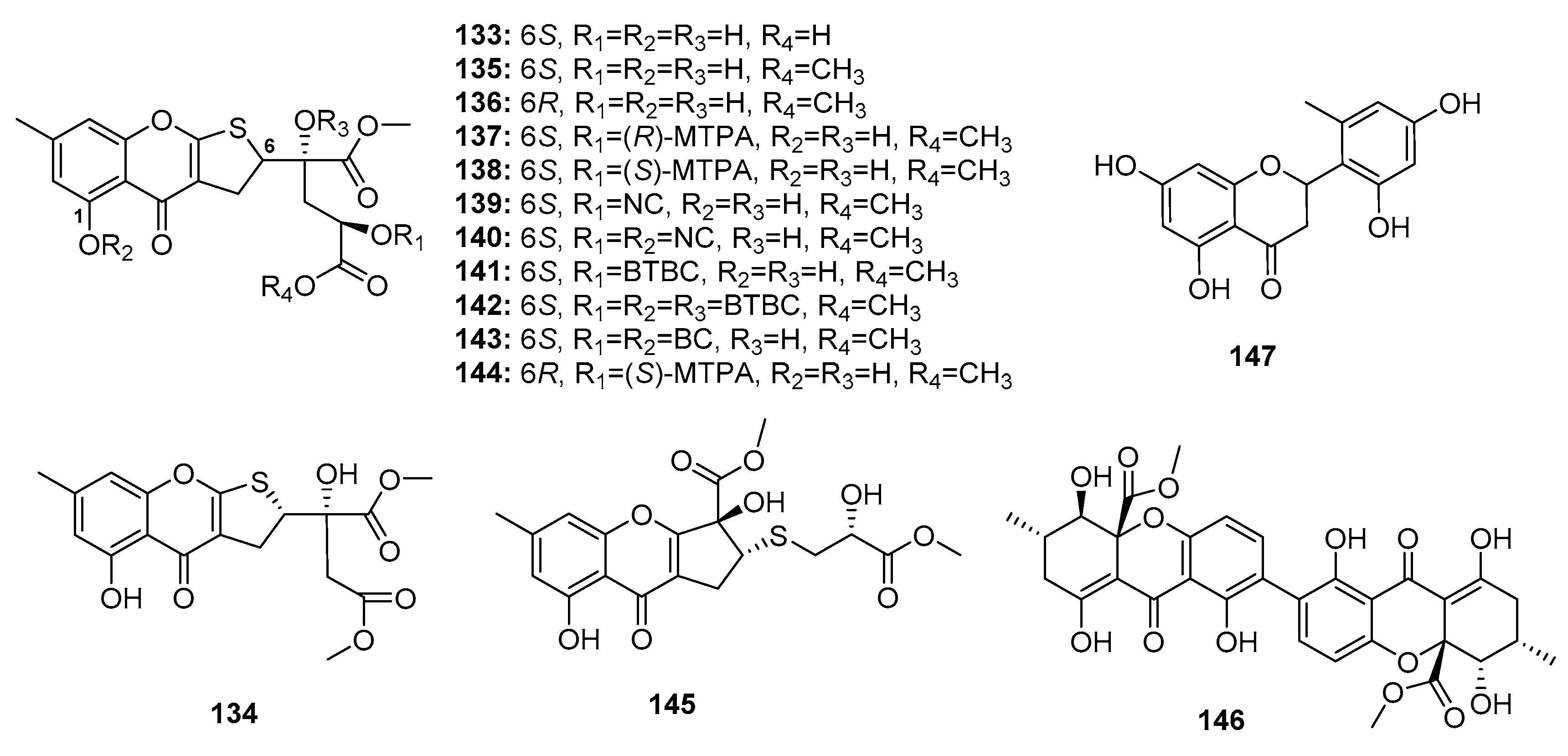
| Oxalicumone D (133) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | BGC823, MOLT-4 | (10.10, 5.74) μM | [87] | |
| Oxalicumone E (134) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | H1975, U937, K5652, BGC823, MOLT-4, MCF-7, HL-60, Huh-7 | (5.45, 4.16, 8.80, 1.96, 1.36, 4.32, 2.96, 6.33) μM | [87] | |
| Oxalicumone A (135) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | H1975, U937, K5652, BGC823, MOLT-4, MCF-7, HL-60, Huh-7, A375, A549, Hela, HepG2, SW-620, L-02 | (10.38, 2.35, 4.53, 4.89, 0.30, 11.30, 2.55, 9.49, 11.7, 41.9, 46.2, 77.8, 22.6, 99.0) μM | [87,88] | |
| Oxalicumone B (136) | P. oxalicum SCSGAF 0023 | Marine gorgonian | Chromone | U937, MOLT-4, HL-60, A375, Hela, SW-620 | (5.00, 2.30, 6.41, 27.8, 60.9, 40.6) μM | [87,88] | |
| Chromosulfine (145) | Mutant P. purpurogenum G59 | Marine (not clear) | Chromone | K562, HL-60, BGC-823, Hela, MCF-7 | (60.8, 16.7, 73.8, 75.4, 59.2) μM | [91] | |
| Secalonic acid F (146) | Penicillium sp. | Deep sea sediment | Xanthone | HL-60 | Modulate Rho GDP dissociation inhibitor 2 Activate caspase 3 and caspase 9 | [92,93] | |
| Penimethavone A (147) | P. chrysogenum | Marine gorgonian | Flavone | Hela, rhabdomyosarcoma | (8.41, 8.18) μM | [94] | |
| Bipenicillisorin (148) | P. chrysogenum SCSIO 41001 | Deep sea sediment | Coumarin | K562, A549, Huh-7 | (6.78, 6.94, 2.59) μM | [95] | |
| Monocerin (149) | Penicillium sp. | Marine sponge | Coumarin | L5178Y | 8.4 μM | [96] | |
| 150 | Penicillium sp. ZH16 | Mangrove | Coumarin | KB, KBv200 | (5, 10) μg/mL | [97] | |
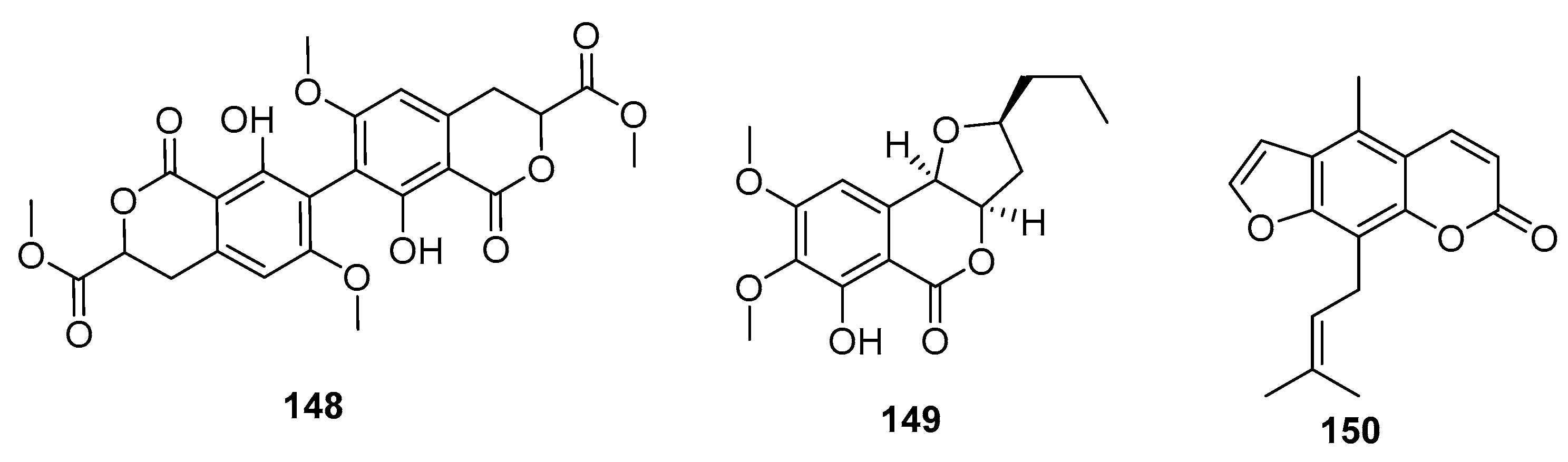
| Citrinin (151) | Penicillium sp FF001 | Marine sponge | Azaphilone polyketide | Inhibit respiration complex III | [99,101] | ||
| Penicitrinol L (152) | P. citrinum | Marine sediment | Azaphilone polyketide | SW-620 | 25.6 μM | [48] | |
| Penicitrinol M (153) | P. citrinum | Marine sediment | Azaphilone polyketide | SW-620 | 20.9 μM | [48] | |
| Berkelic acid (154) | Penicillium sp. | Acid mine lake | Azaphilone polyketide | OVCAR-3 (in NCI60) | 91 nM | Inhibit MMP-3 (GI50 = 1.87 μM) Inhibit caspase 1 (GI50 = 98 μM) | [102] |
| Sargassopenilline C (155) | P. thomii | Marine alga | Azaphilone polyketide | Inhibit the oncogenic nuclear factor AP-1 (IC50 = 15 μM) | [104] | ||
| Sculezonone A (156) | Penicillium sp. | Marine sponge | Azaphilone polyketide | Inhibit both DNA polymerases (α and β) | [105] | ||
| Sculezonone B (157) | Penicillium sp. | Marine sponge | Azaphilone polyketide | Inhibit both DNA polymerases (α and β) | [105] | ||
| Dicitrinone B (158) | P. citrinum | Marine sediment | Azaphilone polyketide | Induce apoptosis through ROS-related caspase pathway | [106] | ||
| Perinadine A (159) | P. citrinum | Marine fish | Azaphilone polyketide | L1210 | 20 μg/mL | [107] | |
| Herqueiazole (160) | Penicillium sp F011 | Marine sediment | Azaphilone polyketide | A549 | 67.3 μM | [108] | |
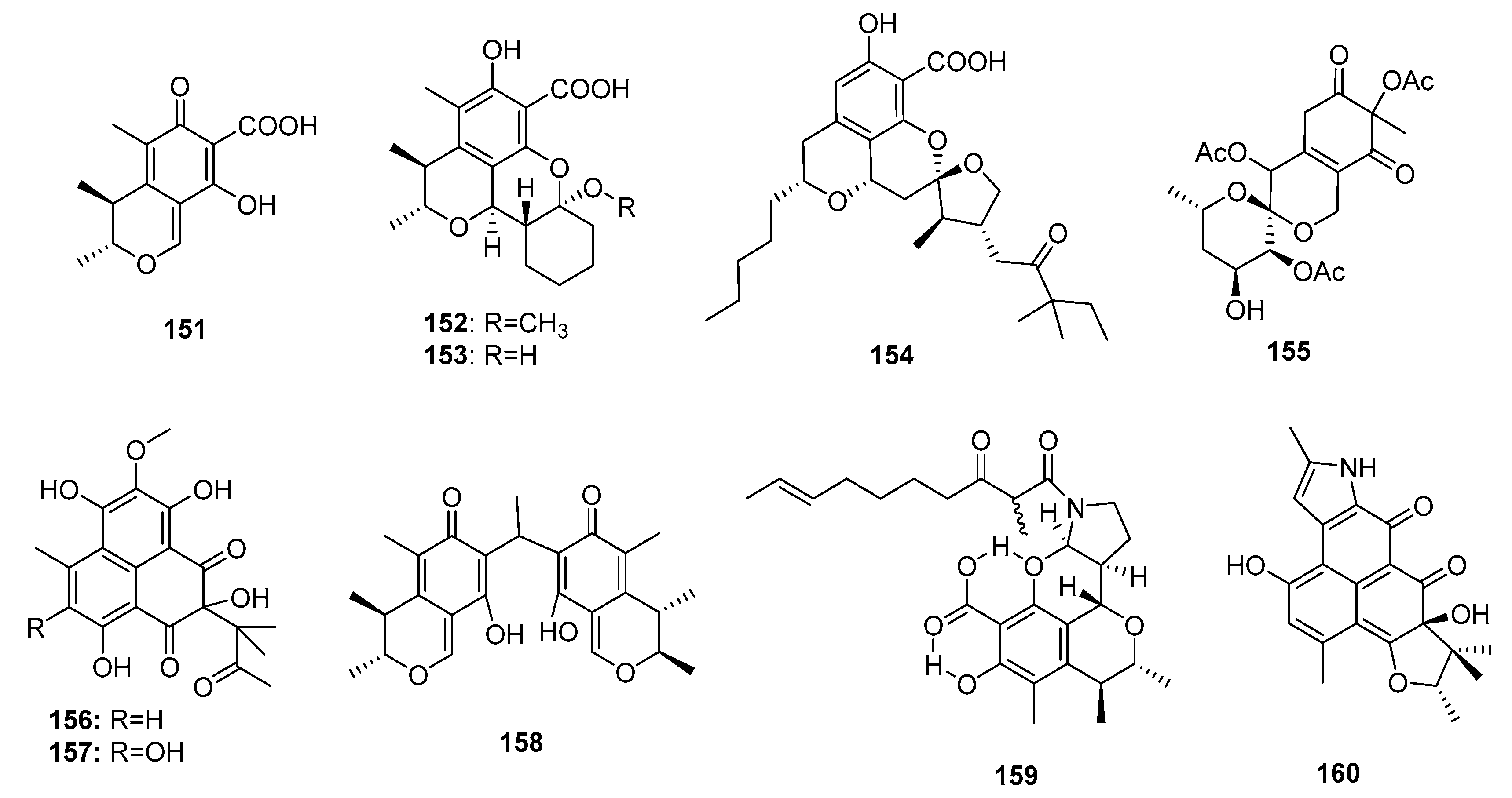
| Comazaphilone D (161) | P. commune QSD-17 | Marine sediment | Azaphilone polyketide | SW1990 | 51 μM | [109] | |
| Comazaphilone E (162) | P. commune QSD-17 | Marine sediment | Azaphilone polyketide | SW1990 | 26 μM | [109] | |
| Comazaphilone F (163) | P. commune QSD-17 | Marine sediment | Azaphilone polyketide | SW1990 | 53 μM | [109] | |
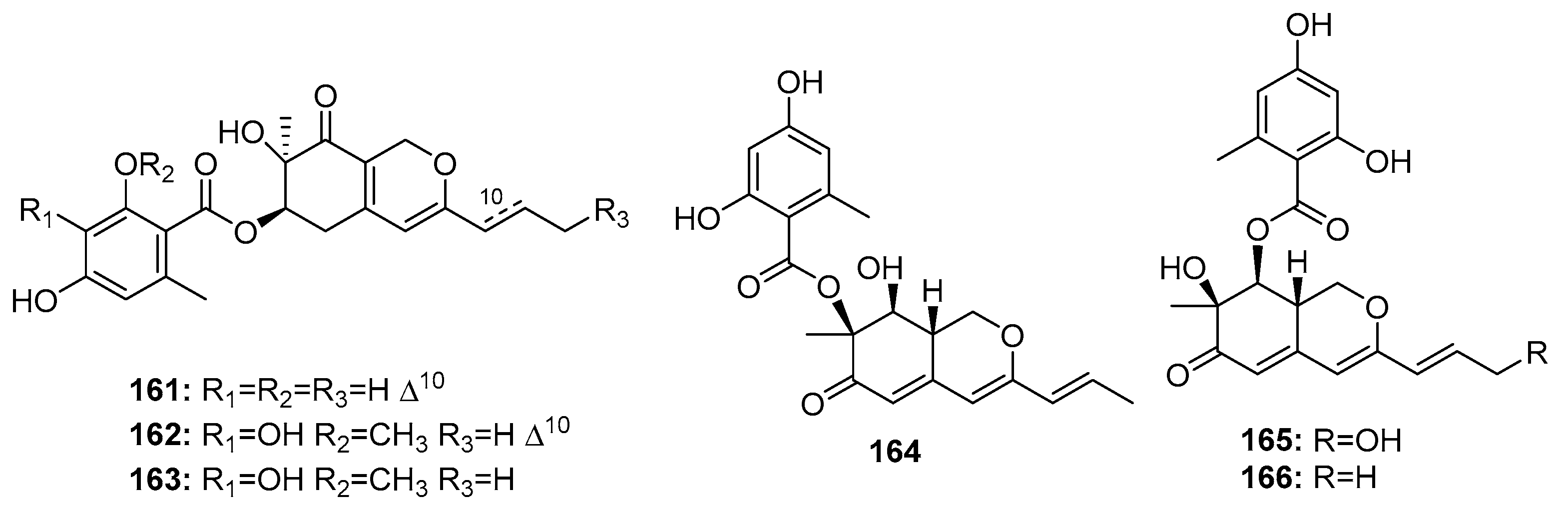
| Pinophilin A (164) | P. pinophilum Hedgcok | Marine seaweed | Azaphilone polyketide | A549, BALL-1, HCT-116, Hela, NUGC-3 | (52.5, 50.2, 51.3, 55.6, 54.7) μM | Inhibit the mammalian DNA polymerases A, B, Y family | [110] |
| Pinophilin B (165) | P. pinophilum Hedgcok | Marine seaweed | Azaphilone polyketide | A549, BALL-1, HCT-116, Hela, NUGC-3 | (93.1, 90.4, 92.5, 99.0, 96.8) μM | Inhibit the mammalian DNA polymerases A, B, Y family | [110] |
| Sch 725680 (166) | P. pinophilum Hedgcok | Marine seaweed | Azaphilone polyketide | A549, BALL-1, HCT-116, Hela, NUGC-3 | (65.7, 62.0, 64.6, 68.8, 66.4) μM | Inhibit the mammalian DNA polymerases A, B, Y family | [110] |
| Penostatin A (167) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.8 μg/mL | PTP1B inhibitor (IC50 = 15.87 μM) | [111,113] |
| Penostatin B (168) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 1.2 μg/mL | PTP1B inhibitor (IC50 = 33.65 μM) | [111,113] |
| Penostatin C (169) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388, BSY-1, MCF-7, HCC2998, NCI-H522, DMS114, OVCAR-3, MKN1 | (1.0, 2.0, 1.6, 2.0, 2.5, 1.9, 2.4, 1.7) μg/mL | PTP1B inhibitor (IC50 = 0.37 μM) | [111,113] |
| Penostatin D (170) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 11.0 μg/mL | [111] | |
| Penostatin E (171) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.9 μg/mL | [111] | |
| Penostatin F (172) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 1.4 μg/mL | [112] | |
| Penostatin G (173) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.5 μg/mL | [112] | |
| Penostatin H (174) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 0.8 μg/mL | [112] | |
| Penostatin I (175) | Penicillium sp. OUPS-79 | Marine alga | Polyketide | P388 | 1.2 μg/mL | [112] | |
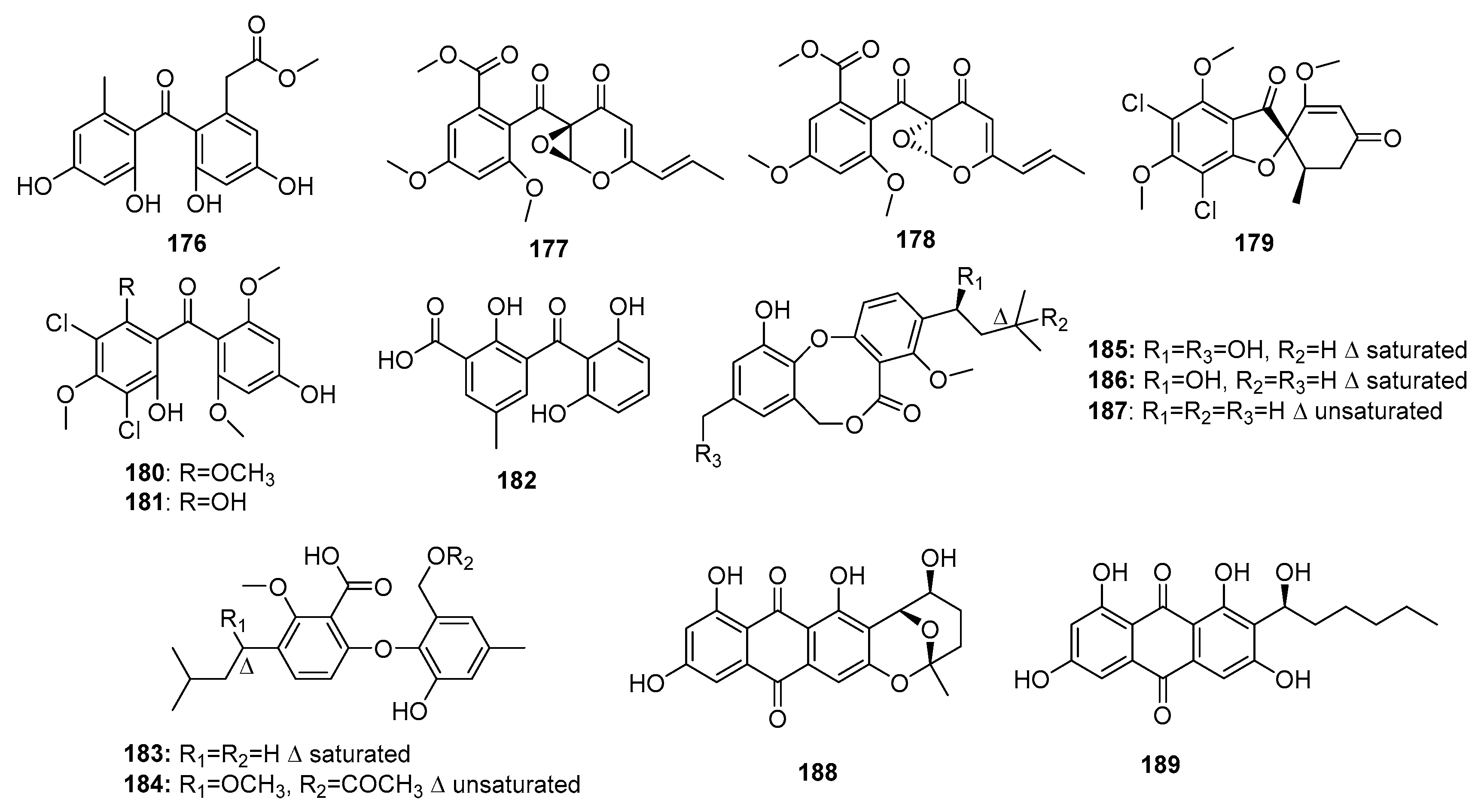
| 176 | P. oxalicum HSY05 | Marine sediment | Phenolic polyketide | DNA topoisomerase I inhibitor | [117] | ||
| 177 | Co-cultured Penicillium sp. WC-29-5 | Mangrove | Phenolic polyketide | H1975 | 3.97 μM | [118] | |
| 178 | Co-cultured Penicillium sp. WC-29-5 | Mangrove | Phenolic polyketide | H1975, HL-60 | (5.73, 3.73) μM | [118] | |
| (+)-5-chlorogriseofulvin (179) | P. canescens MMS460 | Sea water | Phenolic polyketide | KB | IR% (0.6 μM) = 49% | [119] | |
| Griseophenone I (180) | P. canescens MMS460 | Sea water | Phenolic polyketide | KB | IR% (0.6 μM) = 58% | [119] | |
| Griseophenone G (181) | P. canescens MMS460 | Sea water | Phenolic polyketide | KB | IR% (0.6 μM) = 47% | [119] | |
| Iso-monodictyphenone (182) | Penicillium sp. MA-37 | Mangrove | Phenolic polyketide | A. salina | 25.3 μM | [120] | |
| Penikellide A (183) | Penicillium sp. MA-37 | Mangrove | Phenolic polyketide | A. salina | 14.2 μM | [120] | |
| Penikellide B (184) | Penicillium sp. MA-37 | Mangrove | Phenolic polyketide | A. salina | 39.2 μM | [120] | |
| Penicillide (185) | Penicillium sp. ZLN29 | Marine sediment | Phenolic polyketide | HepG2 | (6.7/9.7, 7.8) μM | ACAT and nonpeptide calpain inhibitor | [121,122,124,125] |
| Prepenicillide (186) | Penicillium sp. ZLN29 | Marine sediment | Phenolic polyketide | HepG2, RD | 9.9 μM | [124] | |
| Hydroxypenicillide (187) | P. pinophilum | Marine gorgonian | Phenolic polyketide | Hela | 6.1 μM | [125] | |
| Nidurufin (188) | P. flavidorsum SHK1-27 | Marine sediment | Anthraquinone | K562 | 12.6 μM | Induce cell cycle arrest at G2/M transition | [126] |
| Averantin (189) | P. flavidorsum SHK1-27 | Marine sediment | Anthraquinone | K562 | 12.6 μM | [126] | |
| 190 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (5.3, 15.7) μM | [128] | |
| 191 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (7.6, 10.5) μM | [128] | |
| Dihydrobisvertinolone (192) | P. terrestre | Marine sediment | Polyketide | A549, P388 | (0.52, 1.7) μM | [128] | |
| 193 | P. terrestre | Marine sediment | Polyketide | A549 | 1.4 μM | [128] | |
| 194 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (2.1, 2.8) μM | [129] | |
| 195 | P. terrestre | Marine sediment | Polyketide | A549, P388 | (4.3, 8.8) μM | [129] | |
| Trichodimerol (196) | P. terrestre | Marine sediment | Polyketide | A549, P388 | (4.7, 0.33) μM | [129] | |
| Chloctanspirone A (197) | P. terrestre | Marine sediment | Polyketide | HL-60, A549 | (9.2, 39.7) μM | [131] | |
| Chloctanspirone B (198) | P. terrestre | Marine sediment | Polyketide | HL-60 | 37.8 μM | [131] | |
| (10E,15S)-10,11-Dehydrocurvularin (199) | Penicillium sp. DRF2 | Marine sponge | Macrolide | 36 tumor cell lines | 0.28~6 μM | [133,134] | |
| Curvularin (200) | Penicillium sp. DRF2 | Marine sponge | Macrolide | HSP90 inhibitor | [132] | ||
| Tanzawaic acid P (201) | Penicillium sp. CF07370 | Marine sediment | Polyketide | Jurkat, K562, Raji | (28.6, 30.2, 20.3) μM | Active the mitochondrial apoptotic pathway | [137] |
| Tanzawaic acid D (202) | P. steckii | Marine (not clear) | Polyketide | Bind to the FOXO1 which regulates EFGR signaling and stabilizes the FOXO1-DNA conformation | [138] | ||
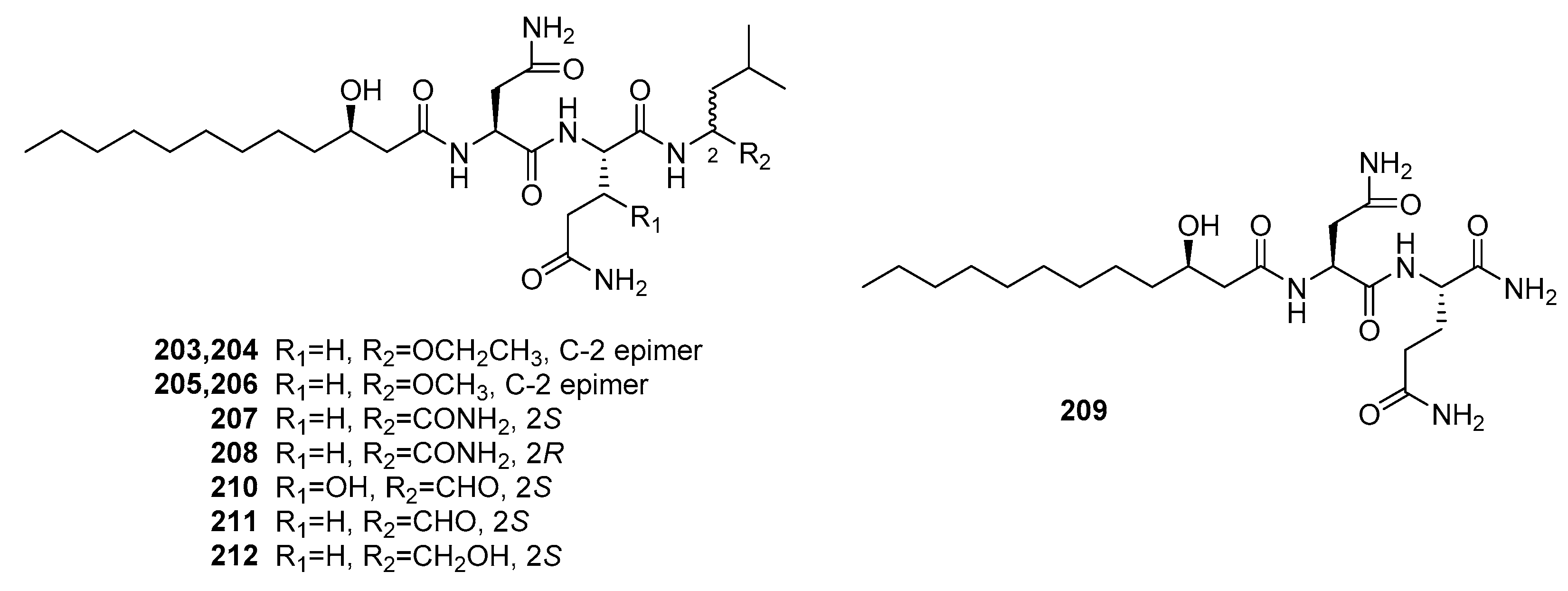
| Penicimutamide A (203) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~40% | [140] | |
| Penicimutamide B (204) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 25~40% | [140] | |
| Penicimutamide C (205) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~40% | [140] | |
| Penicimutamide D (206) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 20~40% | [140] | |
| Penicimutamide E (207) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~45% | [140] | |
| Penicimutamide F (208) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~50% | [140] | |
| Penicimutamide G (209) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 10~20% | [140] | |
| Fellutamide A (210) | P. fellutanum | Marine fish | Lipopepetide | P388, L1210 | (0.2, 0.8) μg/mL | [139] | |
| Fellutamide B (211) | P. fellutanum | Marine fish | Lipopepetide | P388, L1210 | (0.1, 0.7) μg/mL | [139] | |
| Fellutamide C (212) | Mutant P. purpurogenum G59 | Marine soil | Lipopepetide | K562, HL-60, Hela, BGC-823, MCF-7 | IR% (100 μg/mL) = 30~50% | [140] | |
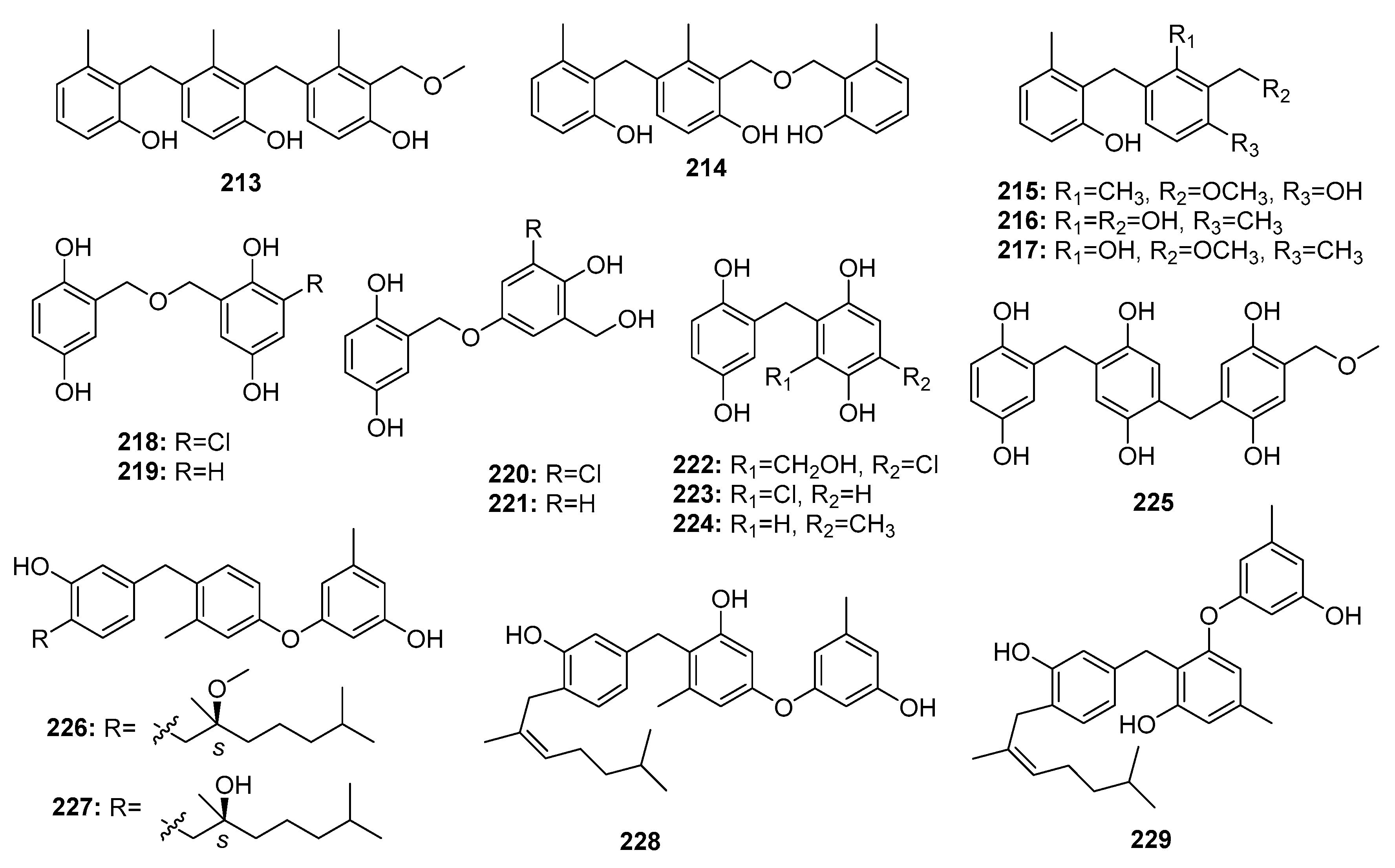
| Peniphenylane A (213) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela | 14.5 μM | [141] | |
| Peniphenylane B (214) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela, HCT-116 | (11.4, 15.8) μM | [141] | |
| Peniphenylane D (215) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela, HL-60, HCT-116 | (9.3, 18.2, 31.7) μM | [141] | |
| Peniphenylane F (216) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela | 29.3 μM | [141] | |
| Peniphenylane G (217) | P. fellutanum HDN14-323 | Deep sea sediment | Polyphenol | Hela, HL-60, HCT-116 | (16.6, 23.2, 24.7) μM | [141] | |
| Terrestol B (218) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (6.1, 5.8, 18.3, 62.3) μM | [142] | |
| Terrestol C (219) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (5.5, 5.6, 18.2, 57.3) μM | [142] | |
| Terrestol D (220) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (5.3, 5.5, 14.3, 38.5) μM | [142] | |
| Terrestol E (221) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (54.7, 6.4, 9.6, 59.0) μM | [142] | |
| Terrestol F (222) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (55.0, 58.1, 13.8, 63.2) μM | [142] | |
| Terrestol G (223) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (5.1, 6.5, 5.7, 6.0) μM | [142] | |
| Terrestol H (224) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (6.3, 5.8, 33.8, 61.9) μM | [142] | |
| Terrestol A (225) | P. terrestre | Marine sediment | Polyphenol | HL-60, MOLT-4, A549, BEL-7402 | (33.3, 5.5, 23.5, 57.0) μM | [142] | |
| Expansol A (226) | P. expansum 091006 | Mangrove | Polyphenol | HL-60 | 15.7 μM | [143] | |
| Expansol B (227) | P. expansum 091006 | Mangrove | Polyphenol | HL-60, A549 | (5.4, 1.9) μM | [143,144] | |
| Expansol C (228) | P. expansum 091006 | Mangrove | Polyphenol | HL-60 | 18.2 μM | [143] | |
| Expansol E (229) | P. expansum 091006 | Mangrove | Polyphenol | HL-60 | 20.8 μM | [143] | |
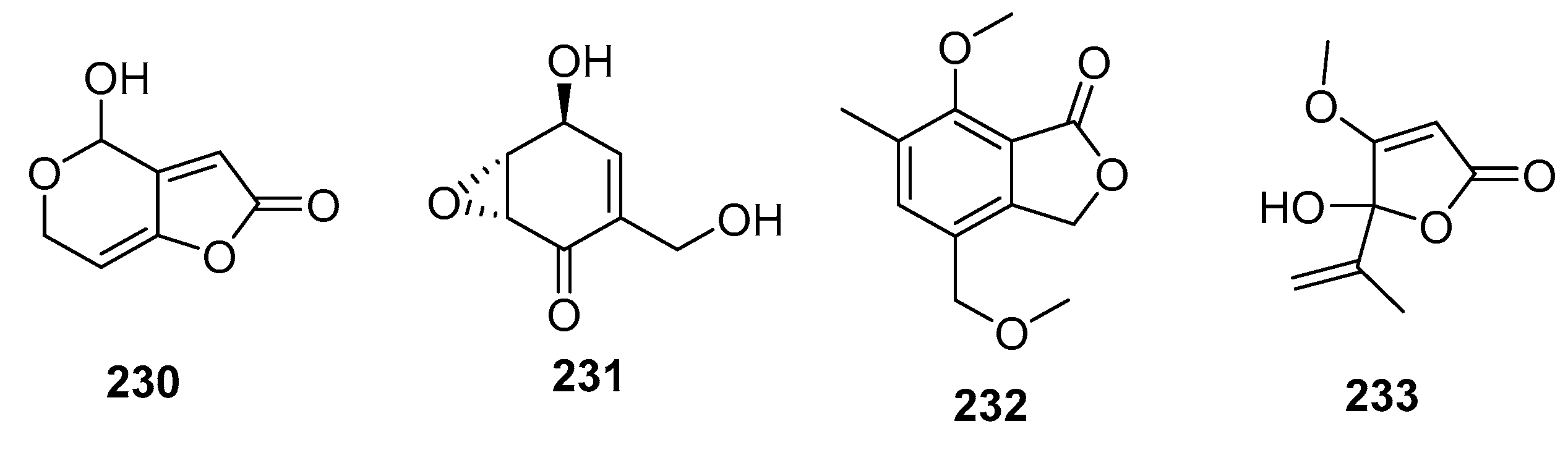
| Patulin (230) | Penicillium sp. | Marine alga | Other | P388, BSY-1, MCF-7, HCC2998, NCI-H522, DMS114, OVCAR-3, MKN1 | (0.06, 0.34, 0.65, 1.54, 0.30, 0.57, 0.37, 0.39) μg/mL | Potassium-uptake inhibitor Ion flux across cell membranes inducer | [111] |
| (+)-Epiepoxydon (231) | Penicillium sp. | Marine alga | Other | P388 | 0.2 μg/mL | [111] | |
| 232 | Penicillium sp. | Mangrove | Other | KB, KBv200 | (6, 10) μg/mL | [145] | |
| Penicillic acid (233) | Penicillium sp. | Sea water | Other | POS1, AT6-1, L299 | (7.8, 29.4, 12.9) μM | [84] |
References
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hong, J.; Yin, J.; Moon, H.R.; Liu, Y.; Wei, X.; Oh, D.-C.; Jung, J.H. Dimeric Octaketide Spiroketals from the Jellyfish-Derived Fungus Paecilomyces variotii J08NF-1. J. Nat. Prod. 2015, 78, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, M.J.; Li, H.; Zhang, P.; Bao, B.; Lee, K.J.; Jung, J.H. Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar. Biotechnol. 2013, 15, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Song, Y.C.; Chen, J.R.; Xu, C.; Ge, H.M.; Wang, X.T.; Tan, R.X. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J. Nat. Prod. 2006, 69, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ge, H.M.; Jiao, R.H.; Li, J.; Peng, H.; Wang, Y.R.; Wu, J.H.; Song, Y.C.; Tan, R.X. Cytotoxic chaetoglobosins from the endophyte Chaetomium globosum. Planta Med. 2010, 76, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, H.; Li, W.; Zhu, X.; Ding, W.; Li, C. Bioactive Chaetoglobosins from the Mangrove Endophytic Fungus Penicillium chrysogenum. Mar. Drugs 2016, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, C.; Yamada, T.; Ito, Y.; Minoura, K.; Numata, A. Cytotoxic cytochalasans from a Penicillium species separated from a marine alga. Tetrahedron 2001, 57, 2997–3004. [Google Scholar] [CrossRef]
- Numata, A.; Takahashi, C.; Ito, Y.; Minoura, K.; Yamada, T.; Matsuda, C.; Nomoto, K. Penochalasins, a novel class of cytotoxic cytochalasans from a Penicillium species separated from a marine alga: Structure determination and solution conformation. J. Chem. Soc. Perkin 1 1996, 3, 239–245. [Google Scholar] [CrossRef]
- Knudsen, P.B.; Hanna, B.; Ohl, S.; Sellner, L.; Zenz, T.; Dohner, H.; Stilgenbauer, S.; Larsen, T.O.; Lichter, P.; Seiffert, M. Chaetoglobosin A preferentially induces apoptosis in chronic lymphocytic leukemia cells by targeting the cytoskeleton. Leukemia 2014, 28, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Schümann, J.; Hertweck, C. Molecular basis of cytochalasan biosynthesis in fungi: Gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J. Am. Chem. Soc. 2007, 129, 9564–9565. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J. Antiviral action of gliotoxin. Arch. Virol. 1969, 27, 255–267. [Google Scholar] [CrossRef]
- Sun, Y.; Takada, K.; Takemoto, Y.; Yoshida, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 2011, 75, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Vigushin, D.; Mirsaidi, N.; Brooke, G.; Sun, C.; Pace, P.; Inman, L.; Moody, C.; Coombes, R. Gliotoxin is a dual inhibitor of farnesyltransferase and geranylgeranyltransferase I with antitumor activity against breast cancer in vivo. Med. Oncol. 2004, 21, 21–30. [Google Scholar] [CrossRef]
- Meng, L.-H.; Wang, C.-Y.; Mándi, A.; Li, X.-M.; Hu, X.-Y.; Kassack, M.U.; Kurtán, T.; Wang, B.-G. Three Diketopiperazine Alkaloids with Spirocyclic Skeletons and One Bisthiodiketopiperazine Derivative from the Mangrove-Derived Endophytic Fungus Penicillium brocae MA-231. Org. Lett. 2016, 18, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-S.; Guo, Y.-W. Epipolythiodioxopiperazines from fungi: Chemistry and bioactivities. Mini Rev. Med. Chem. 2011, 11, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Li, X.-M.; Lv, C.-T.; Huang, C.-G.; Wang, B.-G. Brocazines A–F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.-M.; Meng, L.-H.; Jiang, W.-L.; Xu, G.-M.; Huang, C.-G.; Wang, B.-G. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Orfali, R.S.; Aly, A.H.; Ebrahim, W.; Abdel-Aziz, M.S.; Müller, W.E.; Lin, W.; Daletos, G.; Proksch, P. Pretrichodermamide C and N-methylpretrichodermamide B, two new cytotoxic epidithiodiketopiperazines from hyper saline lake derived Penicillium sp. Phytochem. Lett. 2015, 11, 168–172. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Kalinovsky, A.I.; Khudyakova, Y.V.; Kirichuk, N.N.; Popov, R.S.; Bokemeyer, C.; Von Amsberg, G.; Chingizova, E.A. Pretrichodermamides D–F from a Marine Algicolous Fungus Penicillium sp. KMM 4672. Mar. Drugs 2016, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Aninat, C.; Andre, F.; Delaforge, M. Oxidative metabolism by P450 and function coupling to efflux systems: Modulation of mycotoxin toxicity. Food Addit. Contam. 2005, 22, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Feng, T.; Zhao, B.; Li, D.; Cai, S.; Zhu, T.; Wang, F.; Xiao, X.; Gu, Q. Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities. J. Antibiot. 2010, 63, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, D.; Zhu, T.; Cai, S.; Wang, F.; Xiao, X.; Gu, Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Shang, Z.; Li, X.; Meng, L.; Li, C.; Gao, S.; Huang, C.; Wang, B. Chemical profile of the secondary metabolites produced by a deep sea sediment-derived fungus Penicillium commune SD-118. Chin. J. Oceanol. Limnol. 2012, 30, 305–314. [Google Scholar] [CrossRef]
- Fang, S.-M.; Wu, C.-J.; Li, C.-W.; Cui, C.-B. A practical strategy to discover new antitumor compounds by activating silent metabolite production in fungi by diethyl sulphate mutagenesis. Mar. Drugs 2014, 12, 1788–1814. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.-J.; Cui, C.-B.; Li, C.-W.; Wu, C.-J.; Tian, C.-K.; Hua, W. Activation of the dormant secondary metabolite production by introducing gentamicin-resistance in a marine-derived Penicillium purpurogenum G59. Mar. Drugs 2012, 10, 559–582. [Google Scholar] [CrossRef] [PubMed]
- Hauser, D.; Weber, H.; Sigg, H. Isolation and configuration of Chaetocin. Helv. Chim. Acta 1970, 53, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Sato, K.; Hayakawa, S.; Matsushima, T.; Minato, H. Verticillin A, a new anti-biotic from Verticillium sp. J. Antibiot. 1970, 23, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Jensen, P.; Kauffman, C.; Fenical, W. New Cytotoxic Epidithiodioxopiperazines Related to Verticillin A From A Marine Isolate of the Fungus Penicillium. Nat. Prod. Lett. 1999, 13, 213–222. [Google Scholar] [CrossRef]
- Greiner, D.; Bonaldi, T.; Eskeland, R.; Roemer, E.; Imhof, A. Identification of a specific inhibitor of the histone methyltransferase SU (VAR) 3-9. Nat. Chem. Biol. 2005, 1, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miao, Z.-H.; Zhao, W.-M.; Ding, J. The p53 pathway is synergized by p38 MAPK signaling to mediate 11,11′-dideoxyverticillin-induced G2/M arrest. FEBS Lett. 2005, 579, 3683–3690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Chen, Y.; Guo, X.-N.; Zhang, X.-W.; Zhao, W.-M.; Zhong, L.; Zhou, J.; Xi, Y.; Lin, L.-P.; Ding, J. 11,11′-dideoxy-verticillin: A natural compound possessing growth factor receptor tyrosine kinase-inhibitory effect with anti-tumor activity. Anti-Cancer Drugs 2005, 16, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ashenhurst, J.A.; Movassaghi, M. Total synthesis of (+)-11,11′-dideoxyverticillin A. Science 2009, 324, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Movassaghi, M. Biogenetically-inspired total synthesis of epidithiodiketopiperazines and related alkaloids. Acc. Chem. Res. 2015, 48, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudah, A.I.; El Sayed, K.A. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. MedChemComm 2013, 4, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Sings, H.; Singh, S. Tremorgenic and nontremorgenic 2,3-fused indole diterpenoids. Alkaloids Chem. Biol. 2003, 60, 51–163. [Google Scholar] [PubMed]
- Saikia, S.; Nicholson, M.J.; Young, C.; Parker, E.J.; Scott, B. The genetic basis for indole-diterpene chemical diversity in filamentous fungi. Mycol. Res. 2008, 112, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.; Holton, J.; Nolan, C.; Ray, D.; Naik, J.; Mantle, P. The effects of the tremorgenic mycotoxin penitrem A on the rat cerebellum. Vet. Pathol. 1998, 35, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Numata, A.; Takahashi, C.; Ito, Y.; Takada, T.; Kawai, K.; Usami, Y.; Matsumura, E.; Imachi, M.; Ito, T.; Hasegawa, T. Communesins, cytotoxic metabolites of a fungus isolated from a marine alga. Tetrahedron Lett. 1993, 34, 2355–2358. [Google Scholar] [CrossRef]
- Jadulco, R.; Edrada, R.A.; Ebel, R.; Berg, A.; Schaumann, K.; Wray, V.; Steube, K.; Proksch, P. New Communesin Derivatives from the Fungus Penicillium sp. Derived from the Mediterranean Sponge Axinella v errucosa. J. Nat. Prod. 2004, 67, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Vansteelandt, M.; Kerzaon, I.; Blanchet, E.; Tankoua, O.F.; Du Pont, T.R.; Joubert, Y.; Monteau, F.; Le Bizec, B.; Frisvad, J.C.; Pouchus, Y.F. Patulin and secondary metabolite production by marine-derived Penicillium strains. Fungal Biol. 2012, 116, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Crawley, S.L.; Funk, R.L. A synthetic approach to nomofungin/communesin B. Org. Lett. 2003, 5, 3169–3171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.-M.; Liu, H.; Li, X.; Wang, B.-G. Two new alkaloids from Penicillium oxalicum EN-201, an endophytic fungus derived from the marine mangrove plant Rhizophora stylosa. Phytochem. Lett. 2015, 13, 160–164. [Google Scholar] [CrossRef]
- An, C.-Y.; Li, X.-M.; Li, C.-S.; Xu, G.-M.; Wang, B.-G. Prenylated indolediketopiperazine peroxides and related homologues from the marine sediment-derived fungus Penicillium brefeldianum SD-273. Mar. Drugs 2014, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Li, X.M.; An, C.Y.; Wang, B.G. Prenylated Indole Alkaloid Derivatives from Marine Sediment-Derived Fungus Penicillium paneum SD-44. Helv. Chim. Acta 2014, 97, 1440–1444. [Google Scholar] [CrossRef]
- Asiri, I.A.; Badr, J.M.; Youssef, D.T. Penicillivinacine, antimigratory diketopiperazine alkaloid from the marine-derived fungus Penicillium vinaceum. Phytochem. Lett. 2015, 13, 53–58. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T. Identification and bioactivity of compounds from the fungus Penicillium sp. CYE-87 isolated from a marine tunicate. Mar. Drugs 2015, 13, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, K.; Zhong, P. Tumonoic Acids K and L, Novel Metabolites from the Marine-Derived Fungus Penicillium citrinum. Heterocycles 2012, 85, 413–419. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, T.; Zhao, Y.-Y. Four new penicitrinols and two new penicillenols from the marine-derived fungus Penicillium citrinum. Heterocycles 2015, 91, 1007–1016. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Lu, Z.-Y.; Zhu, T.-J.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Penicillenols from Penicillium sp. GQ-7, an endophytic fungus associated with Aegiceras corniculatum. Chem. Pharm. Bull. 2008, 56, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-Y.; Zhou, T.; Zhao, Y.-Y.; Chen, L.; Gong, M.-W.; Xia, Q.-W.; Ying, M.-G.; Zheng, Q.-H.; Zhang, Q.-Q. Antitumor effects and related mechanisms of penicitrinine A, a novel alkaloid with a unique spiro skeleton from the marine fungus Penicillium citrinum. Mar. Drugs 2015, 13, 4733–4753. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Khajouei, M.R.; Hassanzadeh, F.; Hakimelahi, G.H.; Khodarahmi, G.A. Quinazolinone and quinazoline derivatives: Recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci. 2016, 11, 1–14. [Google Scholar] [PubMed]
- He, J.; Lion, U.; Sattler, I.; Gollmick, F.A.; Grabley, S.; Cai, J.; Meiners, M.; Schünke, H.; Schaumann, K.; Dechert, U. Diastereomeric Quinolinone Alkaloids from the Marine-Derived Fungus Penicillium j anczewskii. J. Nat. Prod. 2005, 68, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, L.; Zhu, T.; Gu, Q.; Li, D. Unusual pyrrolyl 4-quinolinone alkaloids from the marine-derived fungus Penicillium sp. ghq208. Chem. Pharm. Bull. 2012, 60, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.-L.; Wang, C.-Y.; Gu, Y.-C.; Wei, M.-Y.; Pan, J.-H.; Deng, D.-S.; She, Z.-G.; Lin, Y.-C. Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorg. Med. Chem. Lett. 2010, 20, 3284–3286. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, W.; Wang, J. A novel and other bioactive secondary metabolites from a marine fungus Penicillium oxalicum 0312f1. Nat. Prod. Rep. 2013, 27, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- An, C.Y.; Li, X.M.; Li, C.S.; Gao, S.S.; Shang, Z.; Wang, B.G. Triazoles and Other N-Containing Metabolites from the Marine-Derived Endophytic Fungus Penicillium chrysogenum EN-118. Helv. Chim. Acta 2013, 96, 682–687. [Google Scholar] [CrossRef]
- Li, C.-S.; Li, X.-M.; Gao, S.-S.; Lu, Y.-H.; Wang, B.-G. Cytotoxic anthranilic acid derivatives from deep sea sediment-derived fungus Penicillium paneum SD-44. Mar. Drugs 2013, 11, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-S.; An, C.-Y.; Li, X.-M.; Gao, S.-S.; Cui, C.-M.; Sun, H.-F.; Wang, B.-G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Lin, Y.; Yuan, J.; Lu, Y.; Zhu, X.; Li, J.; Li, M.; Lin, Y.; He, J. Meroterpenes and azaphilones from marine mangrove endophytic fungus Penicillium 303. Fitoterapia 2014, 97, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Gulder, T.A.; Tsuruta, H.; Mühlbacher, J.; Maksimenka, K.; Steffens, S.; Schaumann, K.; Stöhr, R.; Wiese, J. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.A.; Lang, G.; Schmitt, S.; Stöhr, R.; Wiese, J.; Nagel, K.; Imhoff, J.F. Large-scale biotechnological production of the antileukemic marine natural product sorbicillactone A. Mar. Drugs 2007, 5, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, L.H.; Mándi, A.; Kurtán, T.; Li, X.M.; Liu, Y.; Li, X.; Li, C.S.; Wang, B.G. Brocaeloids A–C, 4-Oxoquinoline and Indole Alkaloids with C-2 Reversed Prenylation from the Mangrove-Derived Endophytic Fungus Penicillium brocae. Eur. J. Org. Chem. 2014, 2014, 4029–4036. [Google Scholar] [CrossRef]
- He, X.; Zhang, Z.; Chen, Y.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Varitatin A, a Highly Modified Fatty Acid Amide from Penicillium variabile Cultured with a DNA Methyltransferase Inhibitor. J. Nat. Prod. 2015, 78, 2841–2845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Swenson, D.C.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Novel antiinsectan oxalicine alkaloids from two undescribed fungicolous Penicillium spp. Org. Lett. 2003, 5, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, A.; Suzuki, S.; Ando, K.; Tamura, G.; Arima, K. New antiviral antibiotics; xanthocillin X mono-and dimethylether, and methoxy-xanthocillin X dimethylether. I: Isolation and characterization. J. Antibiot. 1968, 21, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rothe, W. Vorläufige Mitteilung über eine neues Antibiotikum. Pharmazie 1950, 5, 190. [Google Scholar]
- Zhao, Y.; Chen, H.; Shang, Z.; Jiao, B.; Yuan, B.; Sun, W.; Wang, B.; Miao, M.; Huang, C. SD118-xanthocillin X (1), a novel marine agent extracted from Penicillium commune, induces autophagy through the inhibition of the MEK/ERK pathway. Mar. Drugs 2012, 10, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, F.; Hallock, Y.; Bunkers, G.; Kenfield, D.; Strobel, G.; Yoshida, S. Phytoactive eremophilanes produced by the weed pathogen Drechslera gigantea. Biosci. Biotechnol. Biochem. 1993, 57, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Tirilly, Y.; Kloosterman, J.; Sipma, G.; Kettenes-Van den Bosch, J.J. A fungitoxic sesquiterpene from Hansfordia pulvinata. Phytochemistry 1983, 22, 2082–2083. [Google Scholar] [CrossRef]
- Lin, A.; Wu, G.; Gu, Q.; Zhu, T.; Li, D. New eremophilane-type sesquiterpenes from an Antarctic deep sea derived fungus, Penicillium sp. PR19 N-1. Arch. Pharm. Res. 2014, 37, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lin, A.; Gu, Q.; Zhu, T.; Li, D. Four new chloro-eremophilane sesquiterpenes from an Antarctic deep sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs 2013, 11, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Qiao, L.; Lv, A.L.; Pei, Y.H.; Tian, L. Eremophilane sesquiterenes from the marine fungus Penicillium sp. BL27–2. Chin. Chem. Lett. 2008, 19, 562–564. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D.; Shao, Z.; Cui, C.; Che, Y. A sterol and spiroditerpenoids from a Penicillium sp. isolated from a deep sea sediment sample. Mar. Drugs 2012, 10, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Li, X.-M.; Li, C.-S.; Proksch, P.; Wang, B.-G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 2011, 21, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, L.; Huang, J.; Li, W.; Pei, Y.-H. Cytotoxic sterols from marine-derived fungus Pennicillium sp. Nat. Prod. Rep. 2006, 20, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.-H.; Zhu, T.-J.; Wang, W.-L.; Du, L.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Isocoumarin derivatives from the sea squirt-derived fungus Penicillium stoloniferum QY2-10 and the halotolerant fungus Penicillium notatum B-52. Arch. Pharm. Res. 2007, 30, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shao, C.-L.; Liu, M.; Qi, X.; Wang, C.-Y. Bioactive steroids from a marine-derived fungus Penicillium sp. from the South China Sea. Chem. Nat. Compd. 2014, 3, 568–570. [Google Scholar] [CrossRef]
- Menna, M.; Imperatore, C.; D’Aniello, F.; Aiello, A. Meroterpenes from marine invertebrates: Structures, occurrence, and ecological implications. Mar. Drugs 2013, 11, 1602–1643. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choi, H.D.; Kang, J.S.; Lee, C.-O.; Son, B.W. New polyoxygenated farnesylcyclohexenones, deacetoxyyanuthone A and its hydro derivative from the marine-derived fungus Penicillium sp. J. Nat. Prod. 2003, 66, 1499–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Tang, X.-X.; Yi, Z.-W.; Qiu, Y.-K.; Wu, Z. Two new compounds from marine-derived fungus Penicillium sp. F11. J. Asian Nat. Prod. Res. 2012, 14, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, L.; Fu, J.; Che, Q.; Li, D.; Gu, Q.; Zhu, T. Phenylpyropenes E and F: New meroterpenes from the marine-derived fungus Penicillium concentricum ZLQ-69. J. Antibiot. 2015, 68, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Lee, J.-H.; You, M.; Choi, T.J.; Park, W.; Lee, S.K.; Oh, D.-C.; Oh, K.-B.; Shin, J. Penicillipyrones A and B, meroterpenoids from a marine-derived Penicillium sp. fungus. J. Nat. Prod. 2014, 77, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Hanson, F.; Eble, T. An antiphage agent isolated from Aspergillus sp. J. Bacteriol. 1949, 58, 527–529. [Google Scholar] [PubMed]
- Vansteelandt, M.; Blanchet, E.; Egorov, M.; Petit, F.; Toupet, L.; Bondon, A.; Monteau, F.; Le Bizec, B.; Thomas, O.P.; Pouchus, Y.F. Ligerin, an antiproliferative chlorinated sesquiterpenoid from a marine-derived Penicillium strain. J. Nat. Prod. 2013, 76, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, E.; Vansteelandt, M.; Bot, R.L.; Egorov, M.; Guitton, Y.; Pouchus, Y.F.; Grovel, O. Synthesis and antiproliferative activity of ligerin and new fumagillin analogs against osteosarcoma. Eur. J. Med. Chem. 2014, 79, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Luo, J.-F.; Qin, X.-C.; Xu, X.-Y.; Zhang, X.-Y.; Tu, Z.-C.; Qi, S.-H. Dihydrothiophene-condensed chromones from a marine-derived fungus Penicillium oxalicum and their structure-bioactivity relationship. Bioorg. Med. Chem. Lett. 2014, 24, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Bao, J.; Liu, K.-S.; Zhang, X.-Y.; He, F.; Wang, Y.-F.; Nong, X.-H.; Qi, S.-H. Cytotoxic dihydrothiophene-condensed chromones from the marine-derived fungus Penicillium oxalicum. Planta Med. 2013, 79, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.-L.; Nong, X.-H.; Zhang, X.-Y.; Xu, X.-Y.; Qi, S.-H.; Wang, Y.-F. Oxalicumone A, a new dihydrothiophene-condensed sulfur chromone induces apoptosis in leukemia cells through endoplasmic reticulum stress pathway. Eur. J. Pharmacol. 2016, 783, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Guo, K.; Wang, X.; Chen, H.; Min, J.; Qi, S.; Zhao, W.; Li, W. Toxicity study of oxalicumone A, derived from a marine-derived fungus Penicillium oxalicum, in cultured renal epithelial cells. Mol. Med. Rep. 2017, 15, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cui, C.-B.; Li, C.-W.; Peng, J.-X.; Gu, Q.-Q. Chromosulfine, a novel cyclopentachromone sulfide produced by a marine-derived fungus after introduction of neomycin resistance. RSC Adv. 2016, 6, 43975–43979. [Google Scholar] [CrossRef]
- Li, N.; Yi, Z.; Wang, Y.; Zhang, Q.; Zhong, T.; Qiu, Y.; Wu, Z.; Tang, X. Differential proteomic analysis of HL60 cells treated with secalonic acid F reveals caspase 3-induced cleavage of Rho GDP dissociation inhibitor 2. Oncol. Rep. 2012, 28, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, H.; Liu, D.; Zhang, J.; Zhang, J.; Yan, M.; Pan, X. Secalonic acid-F inhibited cell growth more effectively than 5-fluorouracil on hepatocellular carcinoma in vitro and in vivo. Neoplasma 2017, 64, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-M.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. Penimethavone A, a flavone from a gorgonian-derived fungus Penicillium chrysogenum. Nat. Prod. Rep. 2016, 30, 2274–2277. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Wang, Z.; Lin, X.; Zhao, B.; Kaliaperumal, K.; Liao, X.; Tu, Z.; Li, J.; Xu, S. Structurally diverse secondary metabolites from a deep sea-derived fungus Penicillium chrysogenum SCSIO 41001 and their biological evaluation. Fitoterapia 2017, 117, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Aktas, N.; Konuklugil, B.; Mandi, A.; Daletos, G.; Lin, W.; Dai, H.; Kurtan, T.; Proksch, P. A new fusarielin analogue from Penicillium sp. isolated from the Mediterranean sponge Ircinia oros. Tetrahedron Lett. 2015, 56, 5317–5320. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J.; Cai, X.; She, Z.; Lin, Y. A new furanocoumarin from the mangrove endophytic fungus Penicillium sp.(ZH16). Nat. Prod. Rep. 2012, 26, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.C.; Raistrick, H. On the production and chemical constitution of a new yellow colouring matter, citrinin, produced from glucose by Penicillium citrinum Thom. Philos. Trans. R. Soc. Lond. 1931, 220, 269–295. [Google Scholar] [CrossRef]
- Chagas, G.M.; Campello, A.P.; Klüppel, M.; Lúcia, W. Mechanism of citrinin-induced dysfunction of mitochondria. I. Effects on respiration, enzyme activities and membrane potential of renal cortical mitochondria. J. Appl. Toxicol. 1992, 12, 123–129. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef]
- Subramani, R.; Kumar, R.; Prasad, P.; Aalbersberg, W. Cytotoxic and antibacterial substances against multi-drug resistant pathogens from marine sponge symbiont: Citrinin, a secondary metabolite of Penicillium sp. Asian Pac. J. Trop. Biomed. 2013, 3, 291–296. [Google Scholar] [CrossRef]
- Stierle, A.A.; Stierle, D.B.; Kelly, K. Berkelic acid, a novel spiroketal with selective anticancer activity from an acid mine waste fungal extremophile. J. Org. Chem. 2006, 71, 5357–5360. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, J.; Snider, B.B. Synthesis of (−)-Berkelic Acid. Angew. Chem. Int. Ed. 2009, 48, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Sobolevskaya, M.P.; Afiyatullov, S.S.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Dyshlovoy, S.A. Sargassopenillines A–G, 6,6-spiroketals from the alga-derived fungi Penicillium thomii and Penicillium lividum. Mar. Drugs 2014, 12, 5930–5943. [Google Scholar] [CrossRef] [PubMed]
- Perpelescu, M.; Kobayashi, J.I.; Furuta, M.; Ito, Y.; Izuta, S.; Takemura, M.; Suzuki, M.; Yoshida, S. Novel phenalenone derivatives from a marine-derived fungus exhibit distinct inhibition spectra against eukaryotic DNA polymerases. Biochemistry 2002, 41, 7610–7616. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gong, M.-W.; Peng, Z.-F.; Zhou, T.; Ying, M.-G.; Zheng, Q.-H.; Liu, Q.-Y.; Zhang, Q.-Q. The marine fungal metabolite, dicitrinone B, induces A375 cell apoptosis through the ROS-related caspase pathway. Mar. Drugs 2014, 12, 1939–1958. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Tsuda, M.; Sekiguchi, M.; Mikami, Y.; Kobayashi, J.I. Perinadine A, a Novel Tetracyclic Alkaloid from Marine-Derived Fungus Penicillium citrinum. Org. Lett. 2005, 7, 4261–4264. [Google Scholar] [CrossRef] [PubMed]
- Julianti, E.; Lee, J.-H.; Liao, L.; Park, W.; Park, S.; Oh, D.-C.; Oh, K.-B.; Shin, J. New polyaromatic metabolites from a marine-derived fungus Penicillium sp. Org. Lett. 2013, 15, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Li, X.-M.; Zhang, Y.; Li, C.-S.; Cui, C.-M.; Wang, B.-G. Comazaphilones A–F, azaphilone derivatives from the marine sediment-derived fungus Penicillium commune QSD-17. J. Nat. Prod. 2011, 74, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Myobatake, Y.; Takeuchi, T.; Kuramochi, K.; Kuriyama, I.; Ishido, T.; Hirano, K.; Sugawara, F.; Yoshida, H.; Mizushina, Y. Pinophilins A and B, inhibitors of mammalian A-, B-, and Y-family DNA polymerases and human cancer cell proliferation. J. Nat. Prod. 2012, 75, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, C.; Minoura, K.; Oka, T.; Ohta, T.; Hagishita, S.; Numata, A. Absolute stereostructures of novel cytotoxic metabolites, penostatins A–E, from a Penicillium species separated from an Enteromorpha alga. Tetrahedron 1999, 55, 14353–14368. [Google Scholar] [CrossRef]
- Iwamoto, C.; Minoura, K.; Hagishita, S.; Nomoto, K.; Numata, A. Penostatins F–I, novel cytotoxic metabolites from a Penicillium species separated from an Enteromorpha marine alga. J. Chem. Soc. Perkin 1 1998, 3, 449–456. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Yang, C.-G.; Wei, P.-Y.; Li, L.; Luo, D.-Q.; Zheng, Z.-H.; Lu, X.-H. Penostatin derivatives, a novel kind of protein phosphatase 1B inhibitors isolated from solid cultures of the entomogenous fungus Isaria tenuipes. Molecules 2014, 19, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Liu, T. Total Synthesis of (±)-Deoxypenostatin A. Approaches to the Syntheses of Penostatins A and B. J. Org. Chem. 2000, 65, 8490–8498. [Google Scholar] [CrossRef] [PubMed]
- Barriault, L.; Ang, P.J.; Lavigne, R.M. Rapid Assembly of the Bicyclo [5.3.1] undecenone Core of Penostatin F: A Successive Diels-Alder/Claisen Reaction Strategy with an Efficient Stereochemical Relay. Org. Lett. 2004, 6, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.M.; Townsend, C.A. New insights into the formation of fungal aromatic polyketides. Nat. Rev. Microbiol. 2010, 8, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, H.-F.; Zhang, L.-H.; Liu, F.; He, F.-J.; Bai, J.; Hua, H.-M.; Chen, G.; Pei, Y.-H. New compound with DNA Topo I inhibitory activity purified from Penicillium oxalicum HSY05. Nat. Prod. Rep. 2015, 29, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zhuang, Y.; Kong, F.; Zhang, C.; Zhu, W. Phenolic polyketides from the co-cultivation of marine-derived Penicillium sp. WC-29–5 and Streptomyces fradiae 007. Mar. Drugs 2014, 12, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Roullier, C.; Guitton, Y.; Valery, M.; Amand, S.; Prado, S.; Robiou du Pont, T.; Grovel, O.; Pouchus, Y.F. Automated detection of natural halogenated compounds from LC-MS profiles–Application to the isolation of bioactive chlorinated compounds from marine-derived fungi. Anal. Chem. 2016, 88, 9143–9150. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, X.-M.; Li, C.-S.; Wang, B.-G. Diphenyl ether and benzophenone derivatives from the marine mangrove-derived fungus Penicillium sp. MA-37. Phytochem. Lett. 2014, 9, 22–25. [Google Scholar] [CrossRef]
- Nishida, H.; Tomoda, H.; Cao, J.; Araki, S.; Okuda, S.; Omura, S. Purpactins, new inhibitors of Acyl-CoA: Cholesterol acyltransferase produced by Penicillium purpurogenum. J. Antibiot. 1991, 44, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.-C.; Lee, H.; Chun, H.; Kho, Y. Penicillide, a nonpeptide calpain inhibitor, produced by Penicillium sp. J. Microbiol. Biotechnol. 1998, 8, 188–190. [Google Scholar]
- Salituro, G.M.; Pettibone, D.J.; Clineschmidt, B.V.; Williamson, J.M.; Zink, D.L. Potent, non-peptidic oxytocin receptor antagonists from a natural source. Bioorg. Med. Chem. Lett. 1993, 3, 337–340. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, L.; Li, D.; Gu, Q.; Zhu, T.J. New Cytotoxic Metabolites from the Marine-Derived Fungus Penicillium sp. ZLN29. Helv. Chim. Acta 2013, 96, 514–519. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Shao, C.-L.; Zhang, Q.; Wang, K.-L.; Guan, F.-F.; Shi, T.; Wang, C.-Y. Azaphilone and diphenyl ether derivatives from a gorgonian-derived strain of the fungus Penicillium pinophilum. J. Nat. Prod. 2015, 78, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, W.-W. Nidurufin as a new cell cycle inhibitor from marine-derived fungus Penicillium flavidorsum SHK1-27. Arch. Pharm. Res. 2011, 34, 901–905. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, G.J.; Murphy, R.A., Jr.; Cava, M.P. Aflatoxin precursors: Total synthesis of (.+−.)-averufin and (.+−.)-nidurufin. J. Org. Chem. 1985, 50, 5533–5537. [Google Scholar] [CrossRef]
- Liu, W.; Gu, Q.; Zhu, W.; Cui, C.; Fan, G. Two new benzoquinone derivatives and two new bisorbicillinoids were isolated from a marine-derived fungus Penicillium terrestre. J. Antibiot. 2005, 58, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, Q.; Zhu, W.; Cui, C.; Fan, G. Dihydrotrichodimerol and tetrahydrotrichodimerol, two new bisorbicillinoids, from a marine-derived Penicillium terrestre. J. Antibiot. 2005, 58, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Harned, A.M.; Volp, K.A. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 2011, 28, 1790–1810. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, L.; Zhu, T.; Kurtán, T.; Mándi, A.; Zhao, Z.; Li, J.; Gu, Q. Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron 2011, 67, 7913–7918. [Google Scholar] [CrossRef]
- Janin, Y.L. Heat shock protein 90 inhibitors. A text book example of medicinal chemistry? J. Med. Chem. 2005, 48, 7503–7512. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.V.; Ióca, L.P.; Williams, D.E.; Costa, B.Z.; Mizuno, C.M.; Santos, M.F.; de Jesus, K.; Ferreira, E.V.L.; Seleghim, M.H.; Sette, L.D. Condensation of Macrocyclic Polyketides Produced by Penicillium sp. DRF2 with Mercaptopyruvate Represents a New Fungal Detoxification Pathway. J. Nat. Prod. 2016, 79, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Greve, H.; Schupp, P.J.; Eguereva, E.; Kehraus, S.; Kelter, G.; Maier, A.; Fiebig, H.H.; König, G.M. Apralactone A and a New Stereochemical Class of Curvularins from the Marine Fungus Curvularia sp. Eur. J. Org. Chem. 2008, 30, 5085–5092. [Google Scholar] [CrossRef] [PubMed]
- Getino, M.; Fernández-López, R.; Palencia-Gándara, C.; Campos-Gómez, J.; Sánchez-López, J.M.; Martínez, M.; Fernández, A.; de la Cruz, F. Tanzawaic acids, a chemically novel set of bacterial conjugation inhibitors. PLoS ONE 2016, 11, e0148098. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Pil, G.B.; Heo, S.-J.; Lee, H.-S.; Lee, J.S.; Lee, Y.-J.; Lee, J.; Won, H.S. Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar. Drugs 2016, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Martínez, F.; José, M.; Díaz-Marrero, A.R.; Darias, J.; Cerella, C.; Diederich, M.; Cueto, M. Tanzawaic acids isolated from a marine-derived fungus of the genus Penicillium with cytotoxic activities. Org. Biomol. Chem. 2015, 13, 7248–7256. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ai, X.; Hou, J.; Ye, X.; Liu, R.; Shen, S.; Li, Z.; Lu, S. Integrated discovery of FOXO1–DNA stabilizers from marine natural products to restore chemosensitivity to anti-EGFR-based therapy for metastatic lung cancer. Mol. Biosyst. 2017, 13, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, H.; Wakuri, S.; Yazawa, K.; Nakamura, T.; Sasaki, T.; Kobayashi, J.I. Fellutamides A and B, cytotoxic peptides from a marine fish-possessing fungus Penicillium fellutanum. Tetrahedron 1991, 47, 8529–8534. [Google Scholar] [CrossRef]
- Wu, C.-J.; Li, C.-W.; Cui, C.-B. Seven new and two known lipopeptides as well as five known polyketides: The activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1815–1838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, W.; He, X.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Peniphenylanes A–G from the Deep sea-Derived Fungus Penicillium fellutanum HDN14-323. Planta Med. 2016, 82, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W. Gentisyl alcohol derivatives from the marine-derived fungus Penicillium terrestre. J. Nat. Prod. 2007, 71, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Liu, P.; Wang, Y.; Li, J.; Hong, K.; Zhu, W. Cytotoxic Polyphenols from the Fungus Penicillium expansum 091 006 Endogenous with the Mangrove Plant Excoecaria agallocha. Planta Med. 2012, 78, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhu, H.; Fu, P.; Wang, Y.; Zhang, Z.; Lin, H.; Liu, P.; Zhuang, Y.; Hong, K.; Zhu, W. Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum. J. Nat. Prod. 2010, 73, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, R.; Qiu, S.X.; She, Z.; Lin, Y. A new isobenzofuranone from the mangrove endophytic fungus Penicillium sp.(ZH58). Nat. Prod. Rep. 2013, 27, 1902–1905. [Google Scholar] [CrossRef] [PubMed]
- Kornprobst, J.-M.; La Barre, S. New Trends in Marine Natural Products. J. Oceanogr. Mar. Res. 2014, 2, 1000e109. [Google Scholar]
- Zeng, Y.; Wang, H.; Kamdem, R.S.; Orfali, R.S.; Dai, H.; Makhloufi, G.; Janiak, C.; Liu, Z.; Proksch, P. A new cyclohexapeptide, penitropeptide and a new polyketide, penitropone from the endophytic fungus Penicillium tropicum. Tetrahedron Lett. 2016, 57, 2998–3001. [Google Scholar] [CrossRef]
- Koul, M.; Singh, S. Penicillium spp.: Prolific producer for harnessing cytotoxic secondary metabolites. Anti-Cancer Drugs 2017, 28, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.F.; Simmons, L.; Kim, J.H.; Schmidt, E.W. Metagenomic approaches to natural products from free-living and symbiotic organisms. Nat. Prod. Rep. 2009, 26, 1488–1503. [Google Scholar] [CrossRef] [PubMed]



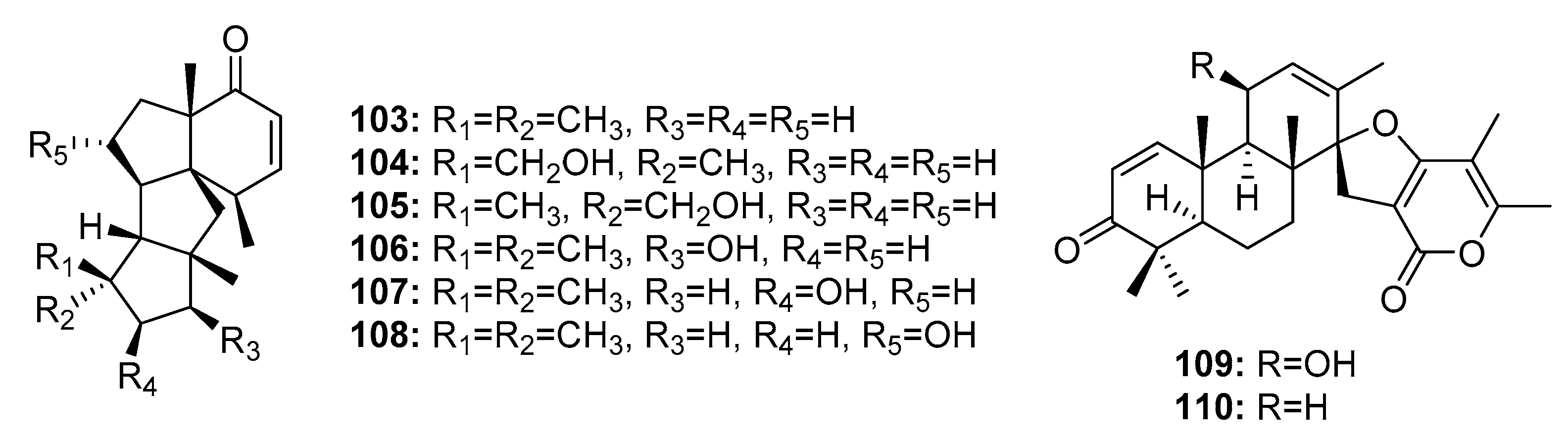

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Su, M.; Song, S.-J.; Jung, J.H. Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites. Mar. Drugs 2017, 15, 329. https://doi.org/10.3390/md15100329
Liu S, Su M, Song S-J, Jung JH. Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites. Marine Drugs. 2017; 15(10):329. https://doi.org/10.3390/md15100329
Chicago/Turabian StyleLiu, Sen, Mingzhi Su, Shao-Jiang Song, and Jee H. Jung. 2017. "Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites" Marine Drugs 15, no. 10: 329. https://doi.org/10.3390/md15100329
APA StyleLiu, S., Su, M., Song, S.-J., & Jung, J. H. (2017). Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites. Marine Drugs, 15(10), 329. https://doi.org/10.3390/md15100329





