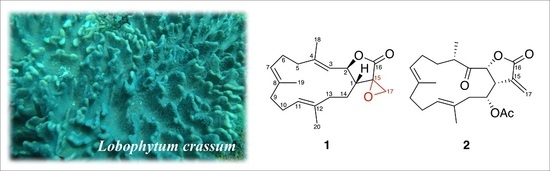
Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum
Abstract
:1. Introduction
2. Results
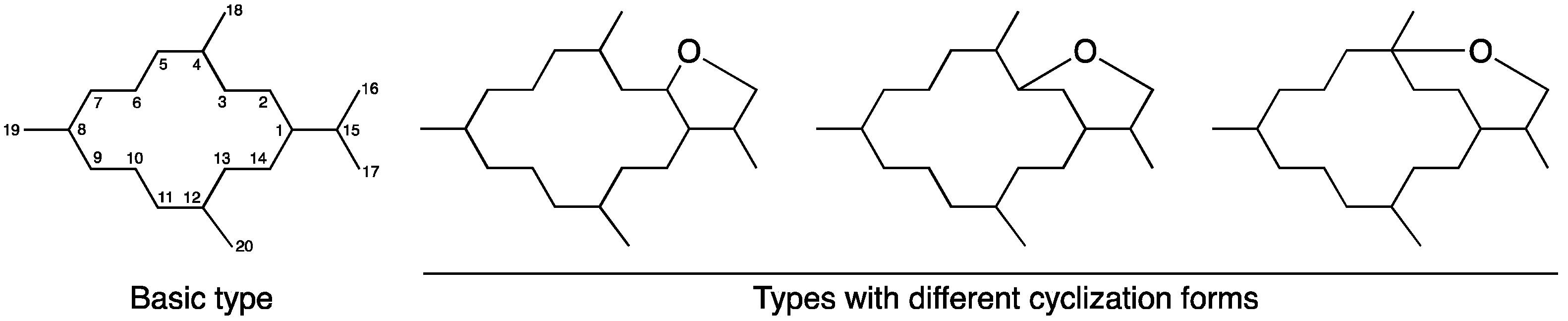
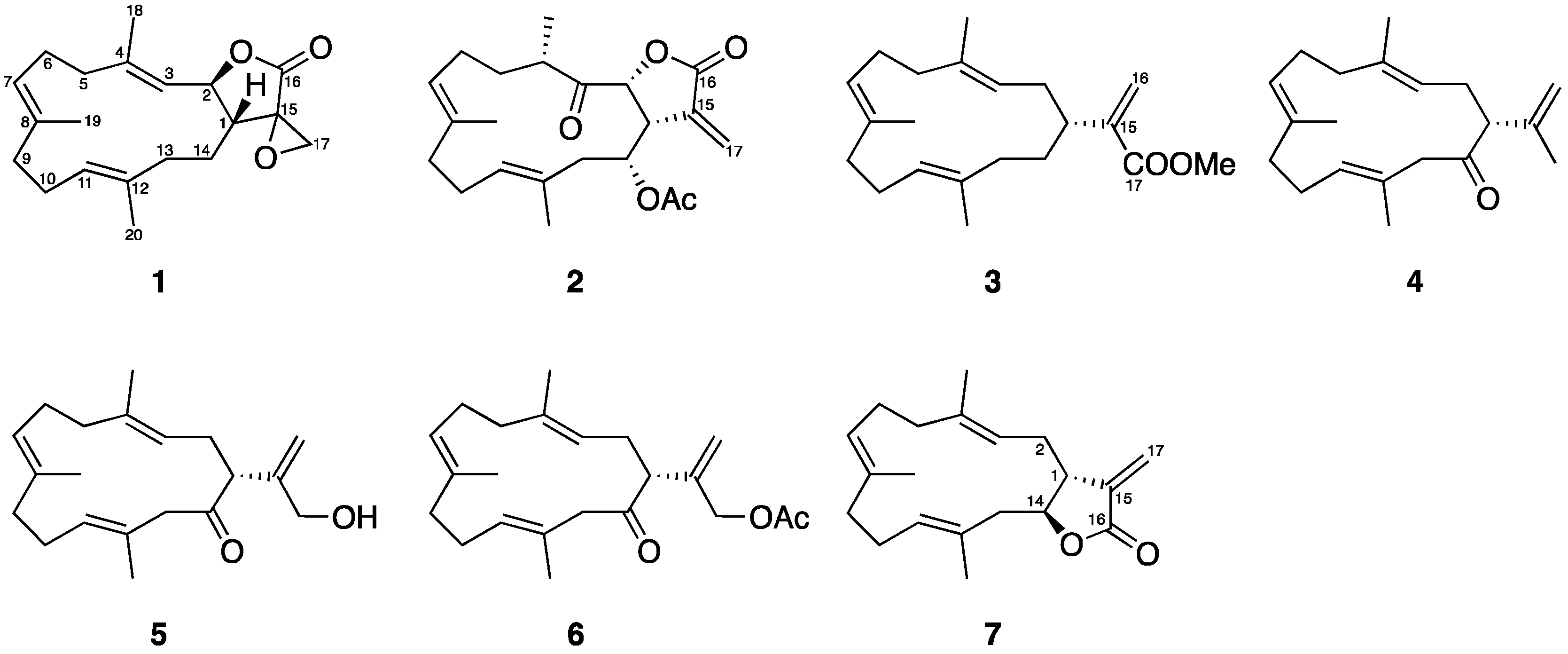
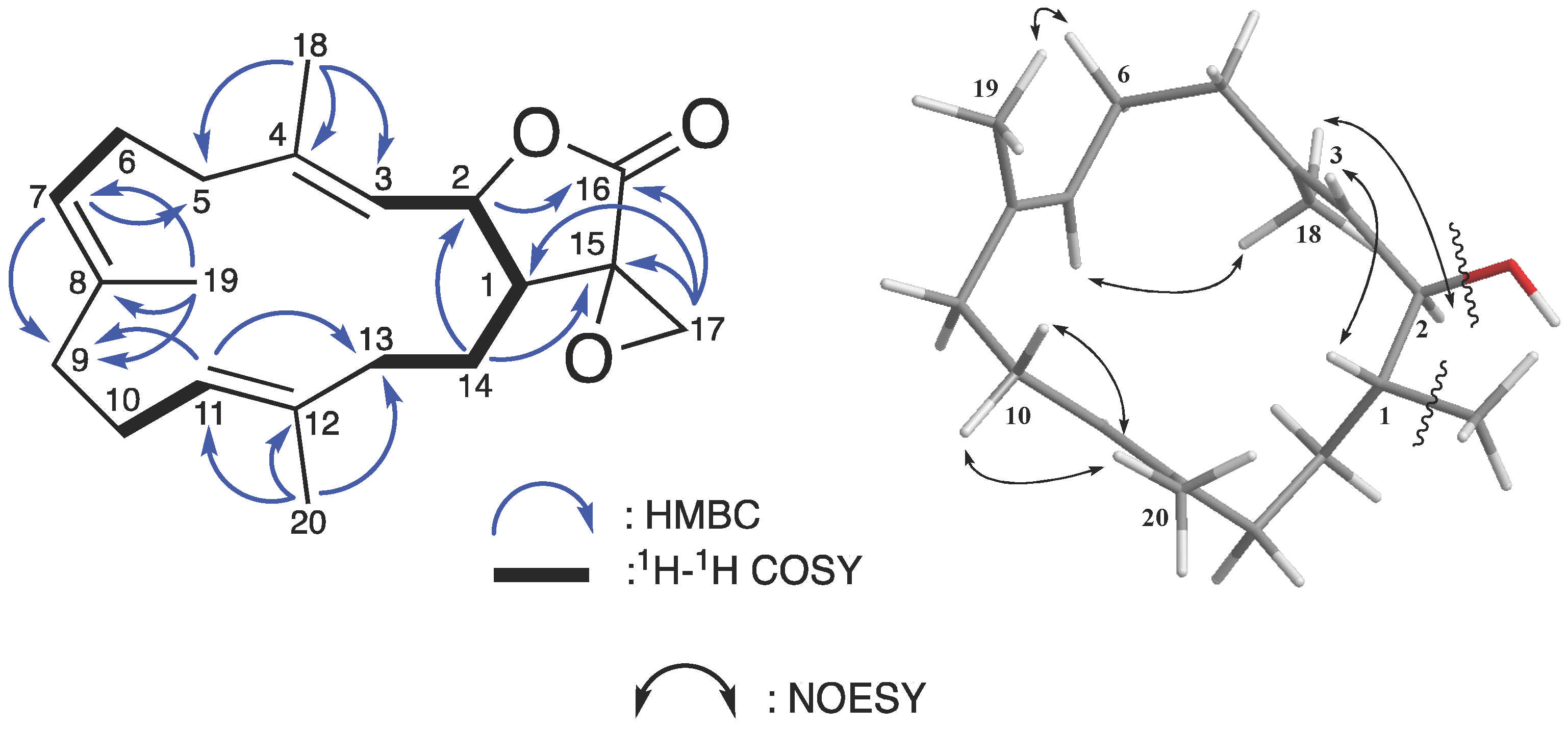
2.1. Chemical Identification of Cembranoids
2.2. Anti-Inflammatory Activity of the Isolated Cembranoids
3. Material and Methods
3.1. General Experimental Procedure
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Preparation of Mouse Bone Marrow-Derived Dendritic Cells (DCs)
3.5. Measurement of Cytokines Production by DCs
3.6. Measurement of Nitric Oxide (NO) Production by DCs
3.7. Cell Viability Assay
3.8. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coll, J.C.; La Barre, S.; Sammarco, P.W.; Williams, W.T.; Bakus, G.J. Chemical defences in soft corals (coelenterata: Octocorallia) of the great barrier reef: A study of comparative toxicities. Mar. Ecol. Prog. Ser. 1982, 8, 271–278. [Google Scholar] [CrossRef]
- Tursch, B.; Braekman, J.C.; Daloze, D.; Hérin, M.; Karlsson, R. Chemical studies of marine invertebrates. X. Lobophytolide, a new cembranolide diterpene from the soft coral lobophytum cristagalli (coelenterata, octocorallia, alcyonacea). Tetrahedron Lett. 1974, 15, 3769–3772. [Google Scholar] [CrossRef]
- Ahond, A.; Bowden, B.; Coll, J.; Fourneron, J.; Mitchell, S. Further cembranolide diterpenes from lobophytum crassospiculatum and a correction of a previous stereochemical assignment. Aust. J. Chem. 1979, 32, 1273–1280. [Google Scholar] [CrossRef]
- Bowden, B.; Brittle, J.; Coll, J.; Liyanage, N.; Mitchell, S.; Stokie, G. A new cembranolide diterpene from the soft coral lobophytum crassum. Tetrahedron Lett. 1977, 18, 3661–3662. [Google Scholar] [CrossRef]
- Kashman, Y.; Carmely, S.; Groweiss, A. Further cembranoid derivatives from the red sea soft corals alcyonium flaccidum and lobophytum crassum. J. Org. Chem. 1981, 46, 3592–3596. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Huang, B.-T.; Dai, C.-F. Cytotoxic cembrenolide diterpenes from the formosan soft coral lobophytum crassum. J. Nat. Prod. 2000, 63, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-W.; Shi, Y.-P.; Li, X.-M.; Wang, B.-G. A novel hydroperoxyl substituted cembranolide diterpene from marine soft coral lobophytum crassum. Chin. Chem. Lett. 2005, 16, 1489–1491. [Google Scholar]
- Yin, S.W.; Shi, Y.P.; Li, X.M.; Wang, B.G. A new cembranoid diterpene and other related metabolites from the south china sea soft coral lobophytum crassum. Helv. Chim. Acta 2006, 89, 567–572. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Ding, J.; Miao, Z.-H.; Zhou, X.-H.; Chen, S.-H.; Pescitelli, G.; Salvadori, P.; Kurtan, T.; Guo, Y.-W. Structural and stereochemical studies of α-methylene-γ-lactone-bearing cembrane diterpenoids from a south china sea soft coral lobophytum crassm. J. Nat. Prod. 2008, 71, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Yeh, H.-C.; Sheu, J.-H. Cytotoxic and anti-inflammatory cembranoids from the soft coral lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-T.; Wang, S.-K.; Cheng, S.-Y.; Duh, C.-Y. Lobocrasol, a new diterpenoid from the soft coral lobophytum crassum. Org. Lett. 2009, 11, 3012. [Google Scholar] [CrossRef] [PubMed]
- Wanzola, M.; Furuta, T.; Kohno, Y.; Fukumitsu, S.; Yasukochi, S.; Watari, K.; Tanaka, C.; Higuchi, R.; Miyamoto, T. Four new cembrane diterpenes isolated from an okinawan soft coral lobophytum crassum with inhibitory effects on nitric oxide production. Chem. Pharm. Bull. 2010, 58, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-J.; Su, H.-J.; Shyue, Y.-C.; Wen, Z.-H.; Sheu, J.-H.; Su, J.-H. Two new cembranoids from the soft coral lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 653–655. [Google Scholar] [CrossRef]
- Tseng, Y.-J.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive cembranoids from the dongsha atoll soft coral lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 1102–1106. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Su, J.-H.; Lu, M.-C.; Hwang, T.-L.; Wang, W.-H.; Chen, J.-J.; Sheu, J.-H.; Kuo, Y.-H.; Weng, C.-F.; Fang, L.-S. Lobocrassins a–e: New cembrane-type diterpenoids from the soft coral lobophytum crassum. Mar. Drugs 2011, 9, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-L.; Su, J.-H. Tetrahydrofuran cembranoids from the cultured soft coral lobophytum crassum. Mar. Drugs 2011, 9, 2526–2536. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-T.; Wang, S.-K.; Duh, C.-Y. Cembranoids from the dongsha atoll soft coral lobophytum crassum. Mar. Drugs 2011, 9, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Kao, C.-Y.; Kao, S.-Y.; Chang, C.-H.; Su, J.-H.; Hwang, T.-L.; Kuo, Y.-H.; Wen, Z.-H.; Sung, P.-J. Terpenoids from the octocorals menella sp.(plexauridae) and lobophytum crassum (alcyonacea). Mar. Drugs 2012, 10, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.T.; Ngan, N.T.T.; Song, S.B.; Cuong, N.X.; Nam, N.H.; Van Kiem, P.; Kim, Y.H.; Van Minh, C. New anti-inflammatory cembranoid diterpenoids from the vietnamese soft coral lobophytum crassum. Bioorg. Med. Chem. Lett. 2014, 24, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.X.; Thao, N.P.; Luyen, B.T.T.; Ngan, N.T.T.; Thuy, D.T.T.; Song, S.B.; Nam, N.H.; Van Kiem, P.; Kim, Y.H.; Van Minh, C. Cembranoid diterpenes from the soft coral lobophytum crassum and their anti-inflammatory activities. Chem. Pharm. Bull. 2014, 62, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Wang, S.-K.; Duh, C.-Y. Secocrassumol, a seco-cembranoid from the dongsha atoll soft coral lobophytum crassum. Mar. Drugs 2014, 12, 6028–6037. [Google Scholar] [CrossRef] [PubMed]
- Van Minh, C.; Xuan Cuong, N.; Phuong Thao, N.; Thu Thuy, D.T.; Ngoc, N.T.; Van Thanh, N.; Hoai Nam, N.; Young Ho, K.; Van Kiem, P. Cembranoid constituents from lobophytum crassum. Vietnam J. Chem. 2015, 53, 5–8. [Google Scholar]
- Zhao, M.; Cheng, S.; Yuan, W.; Xi, Y.; Li, X.; Dong, J.; Huang, K.; Gustafson, K.R.; Yan, P. Cembranoids from a chinese collection of the soft coral lobophytum crassum. Mar. Drugs 2016, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.A.; Elshamy, A.I.; Hussien, T.A.; Su, J.-H.; Sheu, J.-H.; Hegazy, M.E.F. Lobophylins f-h: Three new cembrene diterpenoids from soft coral lobophytum crassum. J. Asian Nat. Prod. Res. 2017, 19, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.; De Costa, M.; Mackay, M.; Mahendran, M.; De Silva, E.; Willis, R. The structure determination of a new cembranolide diterpene from the soft coral lobophytum cristigalli (coelenterata, octocorallia, alcyonacea). Aust. J. Chem. 1984, 37, 545–552. [Google Scholar] [CrossRef]
- Uchio, Y.; Eguchi, S.; Kuramoto, J.; Nakayama, M.; Hase, T. Denticulatolide, an ichthyotoxic peroxide-containing cembranolide from the soft coral lobophytum denticulatum. Tetrahedron Lett. 1985, 26, 4487–4490. [Google Scholar] [CrossRef]
- Uchio, Y.; Eguchi, S.; Fukazawa, Y.; Kodama, M. 7-epidenticulatolide, a new cembranolide with a cyclic peroxide function from the soft coral lobophytum denticulatum. Bull. Chem. Soc. Jpn. 1992, 65, 1182–1184. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wen, Z.-H.; Chiou, S.-F.; Hsu, C.-H.; Wang, S.-K.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Durumolides a-e, anti-inflammatory and antibacterial cembranolides from the soft coral lobophytum durum. Tetrahedron 2008, 64, 9698–9704. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Duh, C.-Y. Anti-inflammatory cembranolides from the soft coral lobophytum durum. Bioorg. Med. Chem. 2009, 17, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral lobophytum durum. J. Nat. Prod. 2008, 72, 152–155. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chen, P.-W.; Chen, H.-P.; Wang, S.-K.; Duh, C.-Y. New cembranolides from the dongsha atoll soft coral lobophytum durum. Mar. Drugs 2011, 9, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Toyota, J.; Nozaki, H.; Nakayama, M.; Nishizono, Y.; Hase, T. Lobohedleolide and (7z)-lobohedleolide, new cembranolides from the soft coral lobophytum hedleyi whitelegge. Tetrahedron Lett. 1981, 22, 4089–4092. [Google Scholar] [CrossRef]
- Quang, T.H.; Ha, T.T.; Van Minh, C.; Van Kiem, P.; Huong, H.T.; Ngan, N.T.T.; Nhiem, N.X.; Tung, N.H.; Tai, B.H.; Thuy, D.T.T. Cytotoxic and anti-inflammatory cembranoids from the vietnamese soft coral lobophytum laevigatum. Bioorg. Med. Chem. 2011, 19, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.; Mitchell, S.J.; Stokie, G.J. A novel cembrenoid diterpene from lobophytum michaelae. Aust. J. Chem. 1977, 30, 1859–1863. [Google Scholar] [CrossRef]
- Wang, S.-K.; Duh, C.-Y.; Wu, Y.-C.; Wang, Y.; Cheng, M.-C.; Soong, K.; Fang, L.-S. Studies on formosan soft corals. Ii: Cytotoxic cembranolides from the soft coral lobphytum michaelae. J. Nat. Prod. 1992, 55, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-T.; Wang, S.-K.; Soong, K.; Duh, C.-Y. New cytotoxic cembranolides from the soft coral lobophytum michaelae. Chem. Pharm. Bull. 2007, 55, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Duh, C.-Y. New cytotoxic cembranolides from the soft coral lobophytum michaelae. Mar. Drugs 2012, 10, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Suzuki, S.; Iguchi, K.; Kikuchi, H.; Tsukitani, Y.; Horiai, H. Two new cembranolides from the soft coral lobophytum pauciflorum (ehrenberg). Chem. Pharm. Bull. 1980, 28, 2035–2038. [Google Scholar] [CrossRef]
- Yamada, Y.; Suzuki, S.; Iguchi, K.; Kikuchi, H.; Horiai, H.; Shibayama, F. The stereochemistry of 13-membered carbocyclic cembranolide diterpenes from the soft coral lobophytum pauciflorum (ehrenberg). Tetrahedron Lett. 1980, 21, 3911–3914. [Google Scholar] [CrossRef]
- Yan, P.; Deng, Z.; Ofwegen, L.V.; Proksch, P.; Lin, W. Lobophytones o-t, new biscembranoids and cembranoid from soft coral lobophytum pauciflorum. Mar. Drugs 2010, 8, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Deng, Z.; Van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones h-n, biscembranoids from the chinese soft coral lobophytum pauciflorum. Chem. Pharm. Bull. 2010, 58, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Lv, Y.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones a-g, new isobiscembranoids from the soft coral lobophytum pauciflorum. Org. Lett. 2010, 12, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Deng, Z.; Van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones u–z1, biscembranoids from the chinese soft coral lobophytum pauciflorum. Chem. Biodivers. 2011, 8, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lin, Y.-C.; Wen, Z.-H.; Su, J.-H.; Sung, P.-J.; Hsu, C.-H.; Kuo, Y.-H.; Chiang, M.Y.; Dai, C.-F.; Sheu, J.-H. Steroid and cembranoids from the dongsha atoll soft coral lobophytum sarcophytoides. Tetrahedron 2010, 66, 7129–7135. [Google Scholar] [CrossRef]
- Yamada, K.; Ryu, K.; Miyamoto, T.; Higuchi, R. Three new cembrane-type diterpenoids from the soft coral lobophytum schoedei. J. Nat. Prod. 1997, 60, 798–801. [Google Scholar] [CrossRef]
- Bowden, B.; Coll, J.; Mitchell, S.; Stokie, G. Two new diterpenes from an unknown species of soft coral (genus lobophytum). Aust. J. Chem. 1978, 31, 1303–1312. [Google Scholar] [CrossRef]
- Bowden, B.; Coll, J.; Tapiolas, D. New cembranoid diterpenes from a lobophytum species. Aust. J. Chem. 1983, 36, 2289–2295. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Rao, C.V.; Anjaneyulu, V.; Satyanarayana, P.; Rao, P.S.; Ward, R.S.; Pelter, A. New diterpenes from a new species of lobophytum soft coral of the south andaman coast. Tetrahedron 1992, 48, 3111–3120. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. Hiv-inhibitory cembrane derivatives from a philippines collection of the soft coral lobophytum species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Huang, H.; Guo, Y.W. Four new cembrane diterpenes from the hainan soft coral lobophytum sp. Chin. J. Chem. 2008, 26, 2223–2227. [Google Scholar] [CrossRef]
- Chen, S.H.; Guo, Y.W.; Huang, H.; Cimino, G. Six new cembranolides from the hainan soft coral lobophytum sp. Helv. Chim. Acta 2008, 91, 873–880. [Google Scholar] [CrossRef]
- Chen, S.-H.; Huang, H.; Guo, Y.-W. A new diterpenoid from the south china sea soft coral lobophytum sp. J. Asian Nat. Prod. Res. 2008, 10, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Irace, C.; Maffettone, C.; Bavestrello, G.; Cerrano, C. Oxygenated cembranoids of the decaryiol type from the indonesian soft coral lobophytum sp. Tetrahedron 2009, 65, 2898–2904. [Google Scholar] [CrossRef]
- Bonnard, I.; Jhaumeer-Laulloo, S.B.; Bontemps, N.; Banaigs, B.; Aknin, M. New lobane and cembrane diterpenes from two comorian soft corals. Mar. Drugs 2010, 8, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Su, J.-H.; Sung, P.-J.; Sheu, J.-H. Cembranoids with 3, 14-ether linkage and a secocembrane with bistetrahydrofuran from the dongsha atoll soft coral lobophytum sp. Mar. Drugs 2011, 9, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, X.; Zhao, F.; Cheng, S.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Four new 7, 8-epoxycembranoids from a chinese soft coral lobophytum sp. Chem. Pharm. Bull. 2013, 61, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yin, J.; Jiang, W.; Ma, M.; Lei, X.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Cytotoxic and antibacterial cembranoids from a south china sea soft coral, lobophytum sp. Mar. Drugs 2013, 11, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Ashimine, R.; Miyazato, H.; Taira, J.; Ueda, K. New casbane and cembrane diterpenoids from an okinawan soft coral, lobophytum sp. Molecules 2016, 21, 679. [Google Scholar] [CrossRef] [PubMed]
- Chau, V.M.; Phan, V.K.; Nguyen, X.; Nguyen, X.C.; Nguyen, P.T.; Nguyen, H.N.; Hoang le, T.A.; Do, C.T.; Thuy, D.T.; Kang, H.K.; et al. Cytotoxic and antioxidant activities of diterpenes and sterols from the vietnamese soft coral lobophytum compactum. Bioorg. Med. Chem. Lett. 2011, 21, 2155–2159. [Google Scholar] [PubMed]
- Matthée, G.F.; König, G.M.; Wright, A.D. Three new diterpenes from the marine soft coral lobophytum crassum. J. Nat. Prod. 1998, 61, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheng, L.; Wang, C.-Y.; Zhou, Y.-B.; Huang, H.; Li, X.-B.; Li, J.; Mollo, E.; Gavagnin, M.; Guo, Y.-W. Diterpenes from the hainan soft coral lobophytum cristatum tixier-durivault. J. Nat. Prod. 2011, 74, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.; Coll, J.; Liyanage, N.; Mitchell, S.; Stokie, G.; Altena, I. A novel bicyclic diterpene from lobophytum hedleyi. Aust. J. Chem. 1978, 31, 163–170. [Google Scholar] [CrossRef]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; Van Ofwegen, L. Four new bioactive lobane diterpenes of the soft coral lobophytum pauciflorum from mindoro, philippines. J. Nat. Prod. 1998, 61, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Govindam, S.V.; Yoshioka, Y.; Kanamoto, A.; Fujiwara, T.; Okamoto, T.; Ojika, M. Cyclolobatriene, a novel prenylated germacrene diterpene, from the soft coral lobophytum pauciflorum. Bioorg. Med. Chem. 2012, 20, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Lakshmana Raju, B.; Subbaraju, G.V.; Bheemasankara Rao, C.; Trimurtulu, G. Two new oxygenated lobanes from a soft coral of lobophytum species of the andaman and nicobar coasts. J. Nat. Prod. 1993, 56, 961–966. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y.; Loya, Y.; Benayahu, Y. New prostaglandin (pgf) derivatives from the soft coral lobophyton depressum. Tetrahedron Lett. 1980, 21, 875–878. [Google Scholar] [CrossRef]
- Chao, C.-H.; Huang, H.-C.; Wu, Y.-C.; Lu, C.-K.; Dai, C.-F.; Sheu, J.-H. Glycolipids from the formosan soft coral lobophytum crassum. Chem. Pharm. Bull. 2007, 55, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Radhika, P.; Rao, V.L.; Laatsch, H. Chemical constituents of a marine soft coral of the genus lobophytum. Chem. Pharm. Bull. 2004, 52, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, P.; Kumar, M.M.; Krishna, N.; Rao, C.B.; Rao, D.V. New sphingolipids and a sterol from a lobophytum species of the indian ocean. Chem. Pharm. Bull. 2005, 53, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, Y.; Rao, M.R.; Ramesh, P. A new polyhydroxy sterol from the soft coral lobophytum crassum. J. Nat. Prod. 1997, 60, 1301–1302. [Google Scholar] [CrossRef]
- Rama Rao, M.; Venkatesham, U.; Rami Reddy, M.V.; Venkateswarlu, Y. An unusual novel c29 steroid from the soft coral lobophytum crassum. J. Nat. Prod. 1999, 62, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.T.; Van Kiem, P.; Van Minh, C.; Kim, Y.H. A new sterol from the soft coral lobophytum crassum. Notes 2013, 34, 249. [Google Scholar]
- Carmely, S.; Kashman, Y. Isolation and structure elucidation of lobophytosterol, depresosterol and three other closely related sterols: Five new c28 polyoxygenated sterols from the red sea soft coral lobophytum depressum. Tetrahedron 1981, 37, 2397–2403. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, T.A.; Elshamy, A.I.; Hassanien, A.A.; Abdel-Azim, N.S.; Shreadah, M.A.; Abdelgawad, I.I.; Elkady, E.M.; Paré, P.W. A new steroid from the red sea soft coral lobophytum lobophytum. Nat. Prod. Res. 2016, 30, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Tursch, B.; Hootele, C.; Kaisin, M.; Losman, D.; Karlsson, R. Structure and absolute configuration of lobosterol, a novel polyoxygenated sterol from the alcyonacean lobophytum pauciflorum. Steroids 1976, 27, 137–142. [Google Scholar] [CrossRef]
- Yamada, Y.; Suzuki, S.; Iguchi, K.; Kikuchi, H.; Tsukitani, Y.; Horiai, H.; Nakanishi, H. New polyhydroxylated sterols from the soft coral lobophytum pauciflorum (ehrenberg). Chem. Pharm. Bull. 1980, 28, 473–478. [Google Scholar] [CrossRef]
- Lu, Q.; Faulkner, D.J. Two 11α-acetoxysterols from the palauan soft coral lobophytum cf. Pauciflorum. Nat. Prod. Lett. 1997, 10, 231–237. [Google Scholar] [CrossRef]
- Morris, L.A.; Christie, E.M.; Jaspars, M.; van Ofwegen, L.P. A bioactive secosterol with an unusual a and b ring oxygenation pattern isolated from an indonesian soft coral lobophytum sp. J. Nat. Prod. 1998, 61, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Lin, S.-T.; Wang, S.-K.; Duh, C.-Y. Α-tocopherols from the formosan soft coral lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 783–787. [Google Scholar] [CrossRef]
- Tung, N.H.; Minh, C.; Kiem, P.; Huong, H.T.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Nhiem, N.X.; Hyun, J.-H.; Kang, H.-K. Chemical components from the vietnamese soft coral lobophytum sp. Arch. Pharm. Res. 2010, 33, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Achmad, M.J.; Bavestrello, G.; Cerrano, C. Lobozoanthamine, a new zoanthamine-type alkaloid from the indonesian soft coral lobophytum sp. Tetrahedron Lett. 2008, 49, 2189–2192. [Google Scholar] [CrossRef]
- Tsai, T.C.; Chen, H.Y.; Sheu, J.H.; Chiang, M.Y.; Wen, Z.H.; Dai, C.F.; Su, J.H. Structural elucidation and structure-anti-inflammatory activity relationships of cembranoids from cultured soft corals sinularia sandensis and sinularia flexibilis. J. Agric Food Chem. 2015, 63, 7211–7218. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hamaguchi, T. Marine terpenes and terpenoids. Vi. Isolation of several plausible precursors of marine cembranolides, from the soft coral, sinularia mayi. Chem. Pharm. Bull. 1988, 36, 3780–3786. [Google Scholar] [CrossRef]
- Hsiao, T.H.; Sung, C.S.; Lan, Y.H.; Wang, Y.C.; Lu, M.C.; Wen, Z.H.; Wu, Y.C.; Sung, P.J. New anti-inflammatory cembranes from the cultured soft coral nephthea columnaris. Mar. Drugs 2015, 13, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W.; Reid, R.G. Convenient synthesis of α-epoxylactones (4-oxo-1,5-dioxaspiro[2.4]heptanes and -[2.5]octanes). Synthesis 1985, 1, 35–38. [Google Scholar] [CrossRef]
- Evtushenk, Y.M.; Lvanov, V.M.; Zaitsev, B.E. Determination of epoxide and hydroxyl groups in epoxide resins by ir spectrometry. J. Anal. Chem. 2003, 58, 347–350. [Google Scholar] [CrossRef]
- Shriner, R.L. The Systematic Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2004; p. 723. [Google Scholar]
- Marshall, J.A.; Crooks, S.L.; DeHoff, B.S. Cembranolide total synthesis. Macrocyclization of (alpha-alkoxyallyl)stannane-acetylenic aldehydes as a route to cembrane lactones. J. Org. Chem. 1988, 53, 1616–1623. [Google Scholar] [CrossRef]
- Lin, M.K.; Yu, Y.L.; Chen, K.C.; Chang, W.T.; Lee, M.S.; Yang, M.J.; Cheng, H.C.; Liu, C.H.; Chen Dz, C.; Chu, C.L. Kaempferol from semen cuscutae attenuates the immune function of dendritic cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Y.; Zhang, M.; Ikeda, H.; Deng, Z.; Cane, D.E. Product-mediated regulation of pentalenolactone biosynthesis in Streptomyces species by the MarR/SlyA family activators PenR and PntR. J. Bacteriol. 2013, 195, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Eguchi, S.; Nakayama, M.; Hase, T. The isolation of two simple .GAMMA.-lactonic cembranolides from the soft coral Sinularia mayi. Chem. Lett. 1982, 11, 277–278. [Google Scholar] [CrossRef]
- McAulay, K.; Clark, J.S. Total Synthesis of 7-epi-Pukalide and 7-Acetylsinumaximol B. Chemistry 2017, 23, 9761–9765. [Google Scholar] [CrossRef] [PubMed]

| Position | δH (J in Hz) a | δC (Mult.) b | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.26 m | 39.0 (CH) | H-2, H-14 | |
| 2 | 5.06 dd (10.0, 4.5) | 79.4 (CH) | H-1, H-3 | C-16 |
| 3 | 5.17 d (10.0) | 122.7 (CH) | C-5 | |
| 4 | 142.5 (C) | |||
| 5 | 2.22 m | 38.4 (CH2) | ||
| 6 | 2.21 m; 2.30 m | 24.1 (CH2) | H-7 | |
| 7 | 4.89 t (5.0) | 125.1 (CH) | H-6 | C-5, C-9 |
| 8 | 133.8 (C) | |||
| 9 | 1.99 m; 2.12 m | 39.0 (CH2) | ||
| 10 | 2.07 m; 2.20 m | 23.9 (CH2) | H-11 | |
| 11 | 4.93 t (5.0) | 125.2 (CH) | H-10 | C-9, C-13 |
| 12 | 131.4 (C) | |||
| 13 | 2.03 m | 35.1 (CH2) | H-14 | |
| 14 | 1.55 m; 1.77 m | 24.2 (CH2) | H-13, H-1 | C-2, C-15 |
| 15 | 57.9 (C) | |||
| 16 | 173.8 (C) | |||
| 17 | 2.96 d (6.0); 3.30 d (6.0) | 52.2 (CH2) | C-1, C-15, C-16 | |
| 18 | 1.74 s | 16.4 (CH3) | C-3, C-4, C-5 | |
| 19 | 1.59 s | 15.2 (CH3) | C-7, C-8, C-9 | |
| 20 | 1.52 s | 15.9 (CH3) | C-11, C-12, C-13 |
| Position | δH (J in Hz) a | δC (Mult.) b | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 3.26 dd (3.0, 2.0) | 41.9 (CH) | H-2 | |
| 2 | 4.97 d (3.5) | 80.2 (CH) | H-1 | C-3, C-14 |
| 3 | 209.9 (C) | |||
| 4 | 2.66 m | 41.8 (CH) | H-5, H-18 | |
| 5 | 1.46 m; 1.95 m | 31.3 (CH2) | H-4, H-6 | |
| 6 | 1.83 m; 2.20 m | 26.0 (CH2) | H-5, H-7 | |
| 7 | 4.98 m | 125.7 (CH) | H-6 | C-9 |
| 8 | 135.2 (C) | |||
| 9 | 2.04 m; 2.18 m | 39.3 (CH2) | H-9, H-11 | |
| 10 | 2.13 m; 2.24 m | 24.4 (CH2) | H-11 | |
| 11 | 5.20 t (6.3) | 130.3 (CH) | H-10 | C-13 |
| 12 | 129.3 (C) | |||
| 13 | 2.31 d (13.0); 2.45 dd (15.0, 11.0) | 41.1 (CH2) | H-14 | C-1 |
| 14 | 5.09 dt (11.0, 2.5) | 75.6 (CH) | H-13 | |
| 15 | 135.1 (C) | |||
| 16 | 169.5 (C) | |||
| 17 | 5.73 d (2.5); 6.42 d (2.5) | 124.8 (CH2) | C-1, C-15, C-16 | |
| 18 | 1.14 d (7.0) | 17.7 (CH3) | C-3, C-4, C-5 | |
| 19 | 1.49 s | 15.0 (CH3) | C-7, C-8, C-9 | |
| 20 | 1.72 s | 16.1 (CH3) | C-11, C-12, C-13 | |
| 14-OAC | 2.02 s | 21.0 (CH3) | C-14-OAc | |
| 170.0 (C) |
| Compounds | LPS-Induced IL-12 Release | LPS-Induced NO Production | Survivals of DCs |
|---|---|---|---|
| (Inh%) a | (Inh%) a | (Survival%) b | |
| 1 | 93.4 ± 0.5 | 93.5 ± 6.5 | 76.0 ± 0.01 |
| 2 | 93.6 ± 0.0 | 95.9 ± 3.2 | 52.0 ± 0.04 |
| 3 | 86.3 ± 1.1 | 86.1 ± 2.2 | 75.0 ± 0.01 |
| 4 | 77.0 ± 1.5 | 54.9 ± 0.0 | 85.0 ± 0.08 |
| 5 | 92.6 ± 0.6 | 96.2 ± 2.2 | 51.0 ± 0.01 |
| Quercetin c | 86.4 ± 0.0 | 86.1 ± 3.0 | 85.0 ± 5.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, K.-H.; You, W.-J.; Lin, C.-C.; El-Shazly, M.; Liao, Z.-J.; Su, J.-H. Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. https://doi.org/10.3390/md15100327
Lai K-H, You W-J, Lin C-C, El-Shazly M, Liao Z-J, Su J-H. Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum. Marine Drugs. 2017; 15(10):327. https://doi.org/10.3390/md15100327
Chicago/Turabian StyleLai, Kuei-Hung, Wan-Jing You, Chi-Chen Lin, Mohamed El-Shazly, Zuo-Jian Liao, and Jui-Hsin Su. 2017. "Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum" Marine Drugs 15, no. 10: 327. https://doi.org/10.3390/md15100327
APA StyleLai, K.-H., You, W.-J., Lin, C.-C., El-Shazly, M., Liao, Z.-J., & Su, J.-H. (2017). Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum. Marine Drugs, 15(10), 327. https://doi.org/10.3390/md15100327