Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics
Abstract
:1. Introduction
2. Results and Discussion
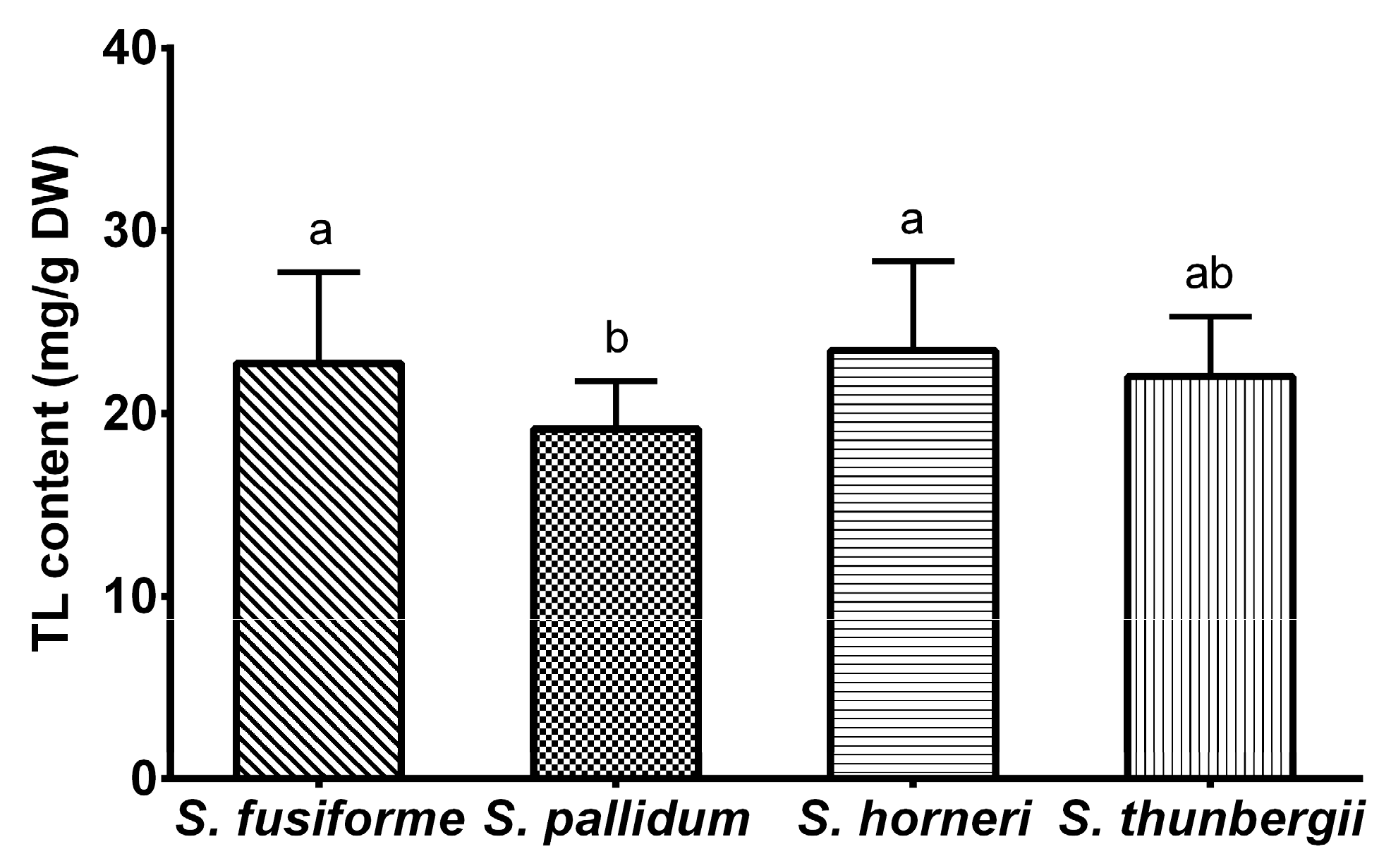
2.1. Total Lipids
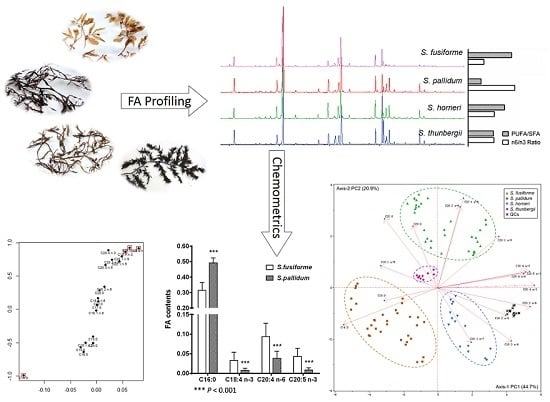
2.2. Fatty Acid Profiles
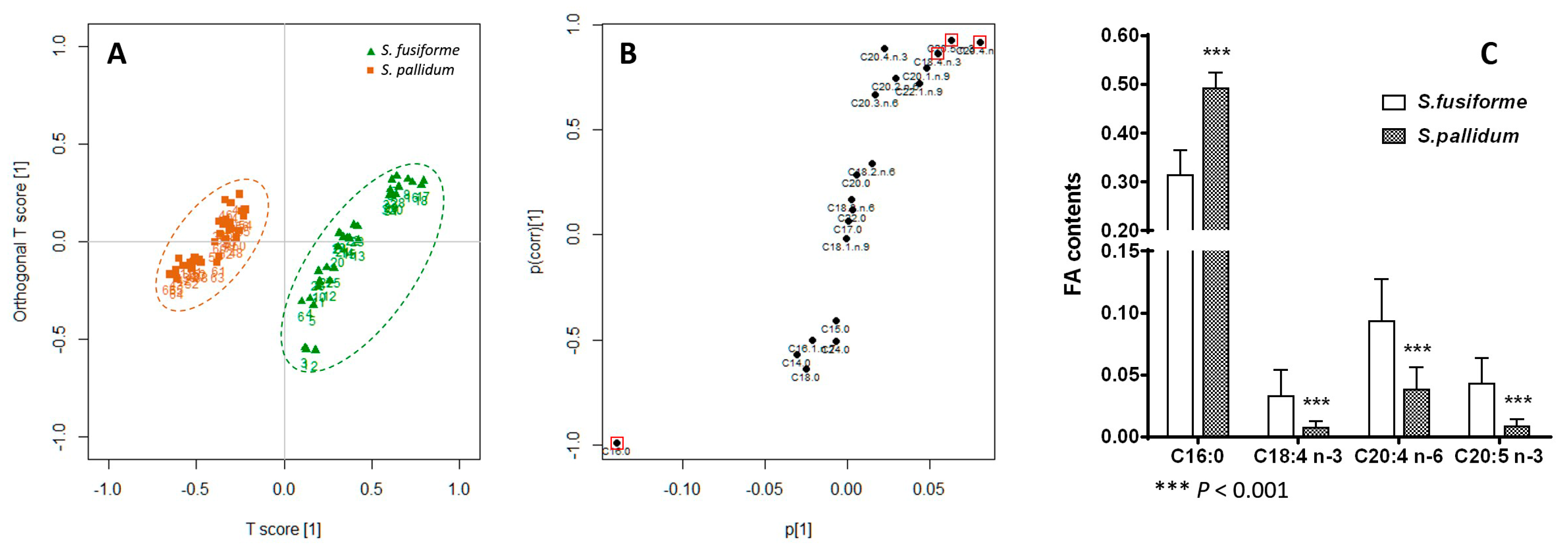
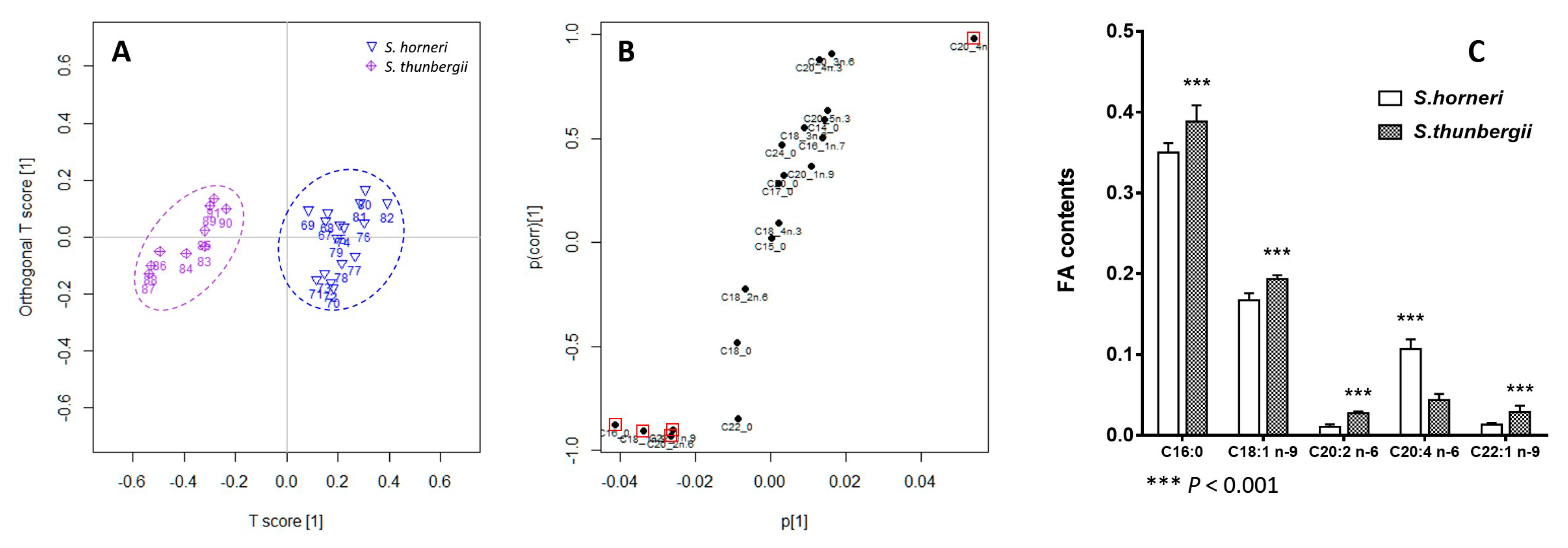
2.3. Principal Component Analysis
2.4. Orthogonal Projection to Latent Structures Discriminant Analysis
3. Experimental Section
3.1. Sampling and Processing of Algal Material
3.2. Lipid Extraction
3.3. Fatty Acid Esterification
3.4. Fatty Acid Analysis
3.5. Quality Control
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TCM | traditional Chinese medicine |
| CVH | cardiovascular health |
| FA | fatty acid |
| FAME | fatty acid methyl ester |
| SFA | saturated fatty acid |
| UFA | unsaturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
| UI | unsaturation index |
| AI | atherogenic index |
| TI | thrombogenic index |
| PCA | principal component analysis |
| OPLS-DA | orthogonal projection to latent structures discriminant analysis |
| VIP | variable importance in the projection |
| QC | quality control |
References
- Kris-Etherton, P.M.; Hecker, K.D.; Binkoski, A.E. Polyunsaturated fatty acids and cardiovascular health. Nutr. Rev. 2004, 62, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A.; Dhillon, S. Omega-3 carboxylic acids (epanova®): A review of its use in patients with severe hypertriglyceridemia. Am. J. Cardiovasc. Drugs 2014, 14, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, J.C.; Hilleman, D.E. Overview of omega-3 Fatty Acid therapies. P. T. 2013, 38, 681–691. [Google Scholar] [PubMed]
- Jeon, Y.-J.; Samarakoon, K.W.; Elvitigala, D.A.S. Marine-Derived Pharmaceuticals and Future Prospects. In Hb25_Springer Handbook of Marine Biotechnology; Springer: Berlin, Germany; Heidelberg, Germany, 2015. [Google Scholar]
- De Caterina, R. n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 2011, 364, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Chen, Z.; Wang, T.; Song, N.; Yan, Q.; Fang, Y.; Guan, H.; Liu, H. Isolation of the molecular species of monogalactosyldiacylglycerols from brown edible seaweed Sargassum horneri and their inhibitory effects on triglyceride accumulation in 3T3-L1 adipocytes. J. Agric. Food Chem. 2014, 62, 11157–11162. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, H. Research progress in chemical components and biological activity of Sargassum. Chin. J. Mar. Drugs 2012, 31, 41–51. [Google Scholar]
- World Health Organization. Population nutrient intake goals for preventing diet-related chronic diseases. Recommendations for preventing dental diseases. In Diet, Nutrition and the Prevention of Chronic Diseases; Report of a Joint WHO/FAO Expert Consulta; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Department of Health. Nutritional Aspects of Cardiovascular Disease; Report on Health and Social Subjects No.46; Her Majesty’s Stationery Office (HMSO): London, UK, 1994. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Sanina, N.M.; Goncharova, S.N.; Kostetsky, E.Y. Seasonal changes of fatty acid composition and thermotropic behavior of polar lipids from marine macrophytes. Phytochemistry 2008, 69, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseirahakodatensis (Yendo) from the northern seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef]
- Murakami, K.; Yamaguchi, Y.; Noda, K.; Fujii, T.; Shinohara, N.; Ushirokawa, T.; Sugawa-Katayama, Y.; Katayama, M. Seasonal variation in the chemical composition of a marine brown alga, Sargassum horneri (Turner) C. Agardh. J. Food Compos. Anal. 2011, 24, 231–236. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, C.C.; Yang, J.Y.; Liu, Q.G.; Li, J. Le Hijiki Seaweed (Hizikia fusiformis): Nutritional Value, Safety Concern and Arsenic Removal Method. Adv. Mater. Res. 2013, 634–638, 1247–1252. [Google Scholar] [CrossRef]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, S.V. Fatty acid composition of seven Sargassum species. Phytochemistry 1991, 30, 2639–2641. [Google Scholar] [CrossRef]
- Wu, X.; Lu, B.; Tseng, C.K. Comparative fatty acid composition of four Sargassum species (Fucales, Phaeophyta). Chin. J. Oceanol. Limnol. 1995, 13, 370–373. [Google Scholar]
- Sanina, N.M.; Goncharova, S.N.; Kostetsky, E.Y. Fatty acid composition of individual polar lipid classes from marine macrophytes. Phytochemistry 2004, 65, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Airanthi, M.K.W.-A.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, X.; Han, L.; Lou, Q. Fatty acids of some algae from the Bohai Sea. Phytochemistry 2002, 59, 157–161. [Google Scholar] [CrossRef]
- Narayan, B.; Miyashita, K.; Hosakawa, M. Comparative evaluation of fatty acid composition of different Sargassum (Fucales, Phaeophyta) species harvested from temperate and tropical waters. J. Aquat. Food Prod. Technol. 2004, 13, 53–70. [Google Scholar] [CrossRef]
- Gerasimenko, N.I.; Martyyas, E.A.; Logvinov, S.V.; Busarova, N.G. Biological activity of lipids and photosynthetic pigments of Sargassum pallidum C. Agardh. Appl. Biochem. Microbiol. 2013, 50, 73–81. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Svetashev, V.I. A high level of dihomogammalinolenic acid in brown alga Sargassum pallidum (Turn.). Phytochemistry 1999, 50, 1209–1211. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Sun, L. Analysis of fatty acids in haihaozi (Sargassum pallidum). Chin. J. Mar. Drugs 2001, 40–41. [Google Scholar]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Hong, Y.P.; Zhang, Y.P.; Zhang, H. Evaluation on nutritional components of Sargassum fusiforme. J. Fish. China 2002, 26, 382–384. [Google Scholar]
- Xia, J.F.; Wang, C.S.; Qian, G.Y. Comparative analysis of fatty acids between Sargassum fusiforme and Laminaria japonica. Food Res. Dev. 2009, 12, 5–8. [Google Scholar]
- Lin, J.; Lin, T.; Lin, L.P.; Chen, W.F.; He, Q. Nutrition and feed safety of seaweed from Fujian coastal waters. Fujian J. Agric. Sci. 2011, 26, 997–1002. [Google Scholar]
- Tsai, C.J.; Sun Pan, B. Identification of sulfoglycolipid bioactivities and characteristic fatty acids of marine macroalgae. J. Agric. Food Chem. 2012, 60, 8404–8410. [Google Scholar] [CrossRef] [PubMed]
- Jeromson, S.; Gallagher, I.; Galloway, S.; Hamilton, D. Omega-3 fatty acids and skeletal muscle health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef] [PubMed]
- Alles, M.S.; Eussen, S.R.B.M.; van der Beek, E.M. Nutritional challenges and opportunities during the weaning period and in young childhood. Ann. Nutr. Metab. 2014, 64, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Yoon, M.H.; Kang, Y.P.; Yu, J.; Park, J.H.; Lee, J.; Kwon, S.W. Comparison of primary and secondary metabolites for suitability to discriminate the origins of Schisandra chinensis by GC/MS and LC/MS. Food Chem. 2013, 141, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, G.; Gika, H.G.; Wilson, I.D. LC-MS-based methodology for global metabolite profiling in metabonomics/metabolomics. TrAC Trends Anal. Chem. 2008, 27, 251–260. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Carreau, J.; Dubacq, J. Adaptation of a macro-scale method to the micro-scale for fatty acid methyl transesterification of biological lipid extracts. J. Chromatogr. A 1978, 151, 384–390. [Google Scholar] [CrossRef]
- Sangster, T.; Major, H.; Plumb, R.; Wilson, A.J.; Wilson, I.D. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis. Analyst 2006, 131, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Gaude, E.; Chignola, F.; Spiliotopoulos, D.; Mari, S.; Spitaleri, A.; Ghitti, M. Muma: Metabolomics Univariate and Multivariate Analysis. R Package Version 1.4, 2012. Available online: https://CRAN.R-project.org/package=muma (accessed on 26 March 2016).


| Fatty Acid (%) | S. fusiforme | S. pallidum | S. horneri | S. thunbergii |
|---|---|---|---|---|
| C14:0 | 5.33 ± 1.22 b | 6.70 ± 1.73 a | 5.90 ± 0.55 a,b | 5.23 ± 0.53 a,b |
| C15:0 | 0.51 ± 0.14 a,b | 0.58 ± 0.11 a | 0.42 ± 0.11 b | 0.41 ± 0.08 b |
| C16:0 | 31.44 ± 5.07 c | 48.66 ± 3.69 a | 35.02 ± 1.17 b | 38.86 ± 1.99 b |
| C17:0 | 0.28 ± 0.10 a | 0.25 ± 0.05 b | 0.20 ± 0.05 b | 0.16 ± 0.07 b |
| C18:0 | 1.95 ± 0.68 b | 2.79 ± 0.69 a | 1.65 ± 0.32 b | 2.02 ± 0.33 b |
| C20:0 | 0.86 ± 0.17 a | 0.80 ± 0.10 b | 0.71 ± 0.08 b | 0.68 ± 0.18 b |
| C22:0 | 1.15 ± 0.30 a | 1.07 ± 0.21 b | 0.64 ± 0.07 c | 0.84 ± 0.05 b,c |
| C24:0 | 0.18 ± 0.09 b | 0.28 ± 0.05 a | 0.17 ± 0.05 b,c | 0.11 ± 0.01 c |
| ∑SFA | 41.70 ± 6.88 c | 61.12 ± 4.80 a | 44.71 ± 1.41 b,c | 48.30 ± 1.83 b |
| C16:1 n-7 | 4.34 ± 0.98 c | 5.19 ± 0.70 b | 7.15 ± 0.88 a | 6.41 ± 0.37 a |
| C18:1 n-9 | 18.91 ± 1.49 a | 19.57 ± 1.38 a | 16.71 ± 0.88 b | 19.36 ± 0.47 a |
| C20:1 n-9 | 4.57 ± 0.82 a,b | 1.19 ± 0.31 c | 5.06 ± 1.04 a | 4.21 ± 0.20 b |
| C22:1 n-9 | 4.39 ± 1.13 a | 1.15 ± 0.26 c | 1.35 ± 0.19 c | 2.92 ± 0.75 b |
| ∑MUFA | 32.21 ± 1.91 a | 27.09 ± 1.18 c | 30.27 ± 1.44 b | 32.89 ± 1.07 a |
| C18:2 n-6 | 4.89 ± 0.63 b | 5.03 ± 1.66 b | 6.59 ± 0.99 a | 7.01 ± 0.97 a |
| C18:3 n-6 | 0.26 ± 0.08 b | 0.26 ± 0.14 b | 0.57 ± 0.31 a | 0.29 ± 0.09 b |
| C18:4 n-3 | 3.33 ± 2.11 a | 0.52 ± 0.33 b | 1.52 ± 0.64 b | 1.50 ± 0.53 b |
| C20:2 n-6 | 2.19 ± 0.49 b | 0.91 ± 0.23 c | 1.07 ± 0.30 c | 2.75 ± 0.20 a |
| C20:3 n-6 | 0.81 ± 0.31 a | 0.55 ± 0.31 b | 0.98 ± 0.21 a | 0.38 ± 0.15 b |
| C20:4 n-6 | 9.37 ± 3.38 a | 3.33 ± 1.53 b | 10.70 ± 1.19 a | 4.37 ± 0.77 b |
| C20:4 n-3 | 0.96 ± 0.28 a | 0.49 ± 0.22 b | 0.93 ± 0.07 a | 0.55 ± 0.24 b |
| C20:5 n-3 | 4.29 ± 2.10 a | 0.71 ± 0.43 c | 2.66 ± 0.53 b | 1.94 ± 0.46 b |
| ∑PUFA | 26.09 ± 7.97 a | 11.79 ± 4.62 c | 25.03 ± 2.10 a | 18.81 ± 1.88 b |
| PUFA/SFA | 0.67 ± 0.31 a | 0.20 ± 0.09 c | 0.56 ± 0.06 a,b | 0.39 ± 0.05 b,c |
| ∑n-6 FA | 17.51 ± 3.72 a,b | 10.07 ± 3.70 c | 19.91 ± 1.78 a | 14.81 ± 0.88 b |
| ∑n-3 FA | 8.58 ± 4.37 a | 1.71 ± 0.95 c | 5.11 ± 0.99 b | 4.00 ± 1.20 b,c |
| n-6/n-3 | 2.43 ± 0.83 c | 7.17 ± 2.75 a | 4.03 ± 0.80 b | 3.98 ± 1.11 b,c |
| UI A | 125.65 ± 32.25 a | 62.27 ± 15.05 c | 116.16 ± 5.77 a | 89.87 ± 7.44 b |
| AI B | 0.94 ± 0.28 b | 1.99 ± 0.45 a | 1.06 ± 0.06 b | 1.16 ± 0.10 b |
| TI C | 0.46 ± 0.21 b | 1.60 ± 0.56 a | 0.65 ± 0.07 b | 0.76 ± 0.14 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Xu, Y.; Liu, T.; Zhang, L.; Liu, H.; Guan, H. Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics. Mar. Drugs 2016, 14, 68. https://doi.org/10.3390/md14040068
Chen Z, Xu Y, Liu T, Zhang L, Liu H, Guan H. Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics. Marine Drugs. 2016; 14(4):68. https://doi.org/10.3390/md14040068
Chicago/Turabian StyleChen, Zhen, Yibing Xu, Tao Liu, Lining Zhang, Hongbing Liu, and Huashi Guan. 2016. "Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics" Marine Drugs 14, no. 4: 68. https://doi.org/10.3390/md14040068
APA StyleChen, Z., Xu, Y., Liu, T., Zhang, L., Liu, H., & Guan, H. (2016). Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics. Marine Drugs, 14(4), 68. https://doi.org/10.3390/md14040068