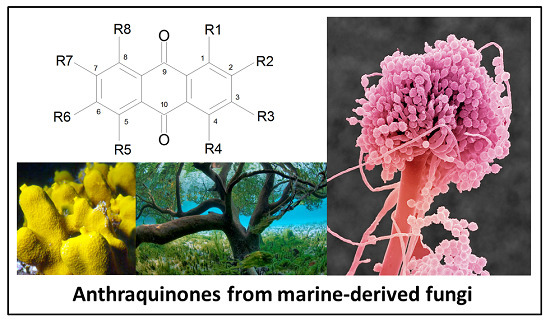
Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities
Abstract
:1. Introduction
2. Anthraquinones from Marine-Derived Fungi
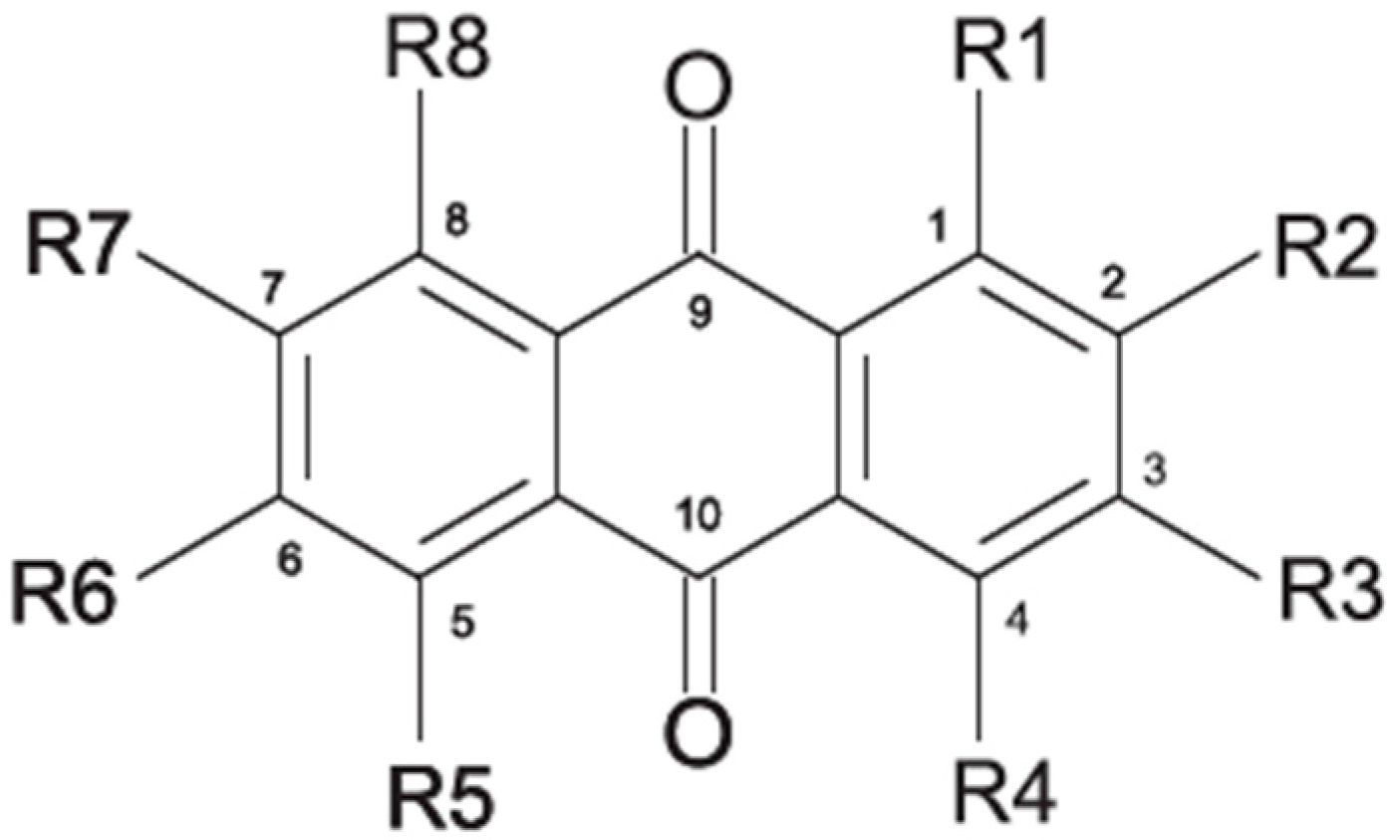
2.1. Anthraquinone′s Basic Structure
2.2. Ecology of Marine-Derived Fungal Anthraquinones Producers
- obligate marine fungi (true ones) that grow and sporulate only in seawater. Their spores are able to germinate and form new thalli in salted environment.
- transitional marine fungi (marine-derived fungi) that come from terrestrial or freshwater media and have undergone physiological adaptation to survive, grow, or reproduce in the marine environment.
2.3. Structural Diversity and Colors of Anthraquinoid Extrolites from Marine-Derived Fungi
2.3.1. Present Knowledge about Anthraquinonoid Compounds from Fungi
2.3.2. Nature and Colors of Compounds from Marine-Derived Fungi
Genera and Species
Ubiquitous Fungi
Endophytes and/or Pathogens
Lichens
3. Biosynthesis and Known Roles for Anthraquinones in Fungi
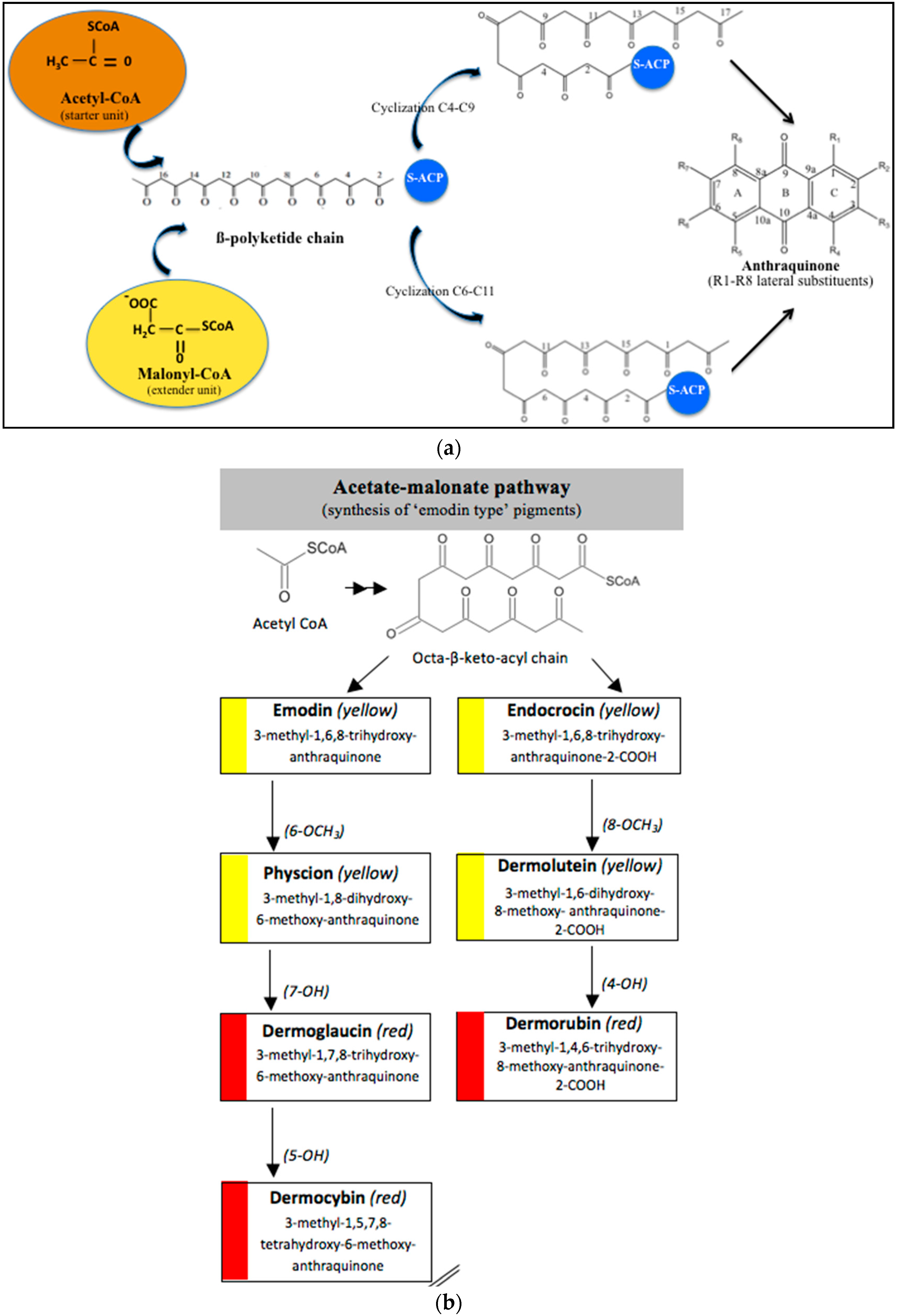
3.1. Biosynthetic Route and Genes Involved
3.2. Roles in the Biology of Fungi
3.2.1. Anti-Oxidant/Pro-oxidant Activities
3.2.2. Competition and Symbiosis
3.2.3. Chelating Properties
4. In Vitro Biological Activities of Natural Anthraquinones from Marine-Derived Filamentous Fungi
4.1. Antitumor Activity and Cytotoxicity
4.1.1. Breaking the Cell Cycle/Apoptosis Hallmarks
4.1.2. Deregulation of ALAS2/c-KIT/miR-221, miR-222, miR-200c, miR-205/Akt
4.1.3. Capsase Dependant Pathway Disturbance/Topoisomerase Inhibition
4.1.4. Cytosolic free Calcium Flux Modification/Reactive Oxygen (ROS) Formation/Mitochondria Dependant Apoptosis
4.1.5. Transport Inhibition/Synergic Ffects
4.1.6. Regulation of Fibrotic and Tumorigenic Mediators
4.1.7. Limitation of Vascularization
4.1.8. Induction of DNA Damages
4.1.9. Hydroxy Groups and Hydrogen Bonding between Biomacromolecules
4.1.10. Other Compounds
4.1.11. Carcinogenic Effects
4.2. Special Focus on Protein Kinase Inhibition
4.3. Immunomodulatory Activity
4.4. Antimicrobial, Antiviral, Antiparasitic Activities
4.4.1. Antimicrobial Activities
4.4.2. Antiviral Activity
4.4.3. Antiparasitic Activity
4.5. Other Identified Biological Activities
4.5.1. Antioxidant Activities
4.5.2. Excretion Functions
Diuretic Activity
Laxative Activity
4.5.3. Vasorelaxant or Contractile Effects
4.5.4. Effects on Lipid and Glucose Metabolism
4.5.5. Estrogenic Activity
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.-K. Marine antitumor drugs: Status, shortfalls and strategies. Mar. Drugs 2010, 8, 2702–2720. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Ireland, C.M. Marine diversity. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Damare, S. Deep-Sea Fungi: Occurrence and Adaptations. Ph.D Thesis, Goa University, Goa, India, 2006. [Google Scholar]
- Kohlmeyer, J.; Kohlmeyer, E. Marine Mycology; Elsvier: London, UK, 1979; p. 704. [Google Scholar]
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.B. G. Des champignons dans l'océan ! Biofutur 1998, 1998, 18–20. [Google Scholar] [CrossRef]
- Yu, Z.; Lang, G.; Kajahn, I.; Schmaljohann, R.; Imhoff, J.F. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008, 71, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.; Yu, Z.; Lang, G.; Wiese, J.; Kalthoff, H.; Klose, S. Production and Use of Antitumoral Cyclodepsipeptides. U.S. Patent 8,765,907 B2, 11 June 2009. [Google Scholar]
- Newman, D.; Cragg, G. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Ebel, R. Natural product diversity from marine fungi. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L., Liu, H.-W., Eds.; Elsevier: Oxford, UK, 2010; Volume 2, pp. 223–262. [Google Scholar]
- Hanson, J.R. Natural Products: The Secondary Metabolites; Royal Society of Chemistry: Cambridge, UK, 2003; p. 147. [Google Scholar]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufosse, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Gessler, N.N.; Egorova, A.S.; Belozerskaia, T.A. Fungal anthraquinones (review). Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Anslow, W.K.; Raistrick, H. Studies in the biochemistry of micro-organisms: Synthesis of cynodontin (1:4:5:8-tetrahydroxy-2-methylanthraquinone), a metabolic product of species of Helminthosporium. Biochem. J. 1940, 34, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W. Natural dyes in textile applications. Rev. Progress Color. Relat. Top. 1986, 16, 53–61. [Google Scholar] [CrossRef]
- Shore, J. Colorants and Auxiliaries; Society of Dyers and Colourists: Bradford, UK, 1990; Volume 1, p. 372. [Google Scholar]
- Stoessl, A. Some metabolites of Alternaria solani. Can. J. Chem. 1969, 47, 767–776. [Google Scholar] [CrossRef]
- Kühlwein, F.; Polborn, K.; Beck, W. Metallkomplexe von farbstoffen. VIII übergangsmetallkomplexe des curcumins und seiner derivate. Z. Anorg. Allg. Chem. 1997, 623, 1211–1219. [Google Scholar] [CrossRef]
- Yuzhen, Z.; Wenfu, H. The influence of methyl groups on the colour and dyeing properties of acid dyes derived from 1-amino-4-bromo-anthraquinone-2-sulphonic acid and arylamines. Dyes Pigm. 1996, 30, 283–289. [Google Scholar] [CrossRef]
- Yuzhen, Z.; Wenfu, H.; Yueping, T. Structure and dyeing properties of some anthraquinone violet acid dyes. Dyes Pigments 1997, 34, 25–35. [Google Scholar] [CrossRef]
- Kim, S.-K. Marine Microbiology: Bioactive Compounds and Biotechnological Applications; Wiley & Sons: Weinheim, Germany, 2013; p. 550. [Google Scholar]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L. Anthraquinones, the Dr Jekyll and Mr Hyde of the food pigment family. Food Res. Int. 2014, 65, 132–136. [Google Scholar] [CrossRef]
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. Metal complexes with 1,5- and 1,8-Dihydroxy-9,10-anthraquinones: Electronic absorption spectra and structure of ligands. Russ. J. Coord. Chem. 2004, 30, 360–364. [Google Scholar] [CrossRef]
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. Tautomerism of the natural 1,8-dihydroxy-9,10-anthraquinones chrysophanol, aloe-emodin, and rhein. Chem. Nat. Compd. 2005, 41, 146–152. [Google Scholar] [CrossRef]
- Thomson, R.H. Anthraquinones. In Naturally Occurring Quinones; Academic Press: London, UK, 1971; pp. 367–535. [Google Scholar]
- Panno, L.; Bruno, M.; Voyron, S.; Anastasi, A.; Gnavi, G.; Miserere, L.; Varese, G.C. Diversity, ecological role and potential biotechnological applications of marine fungi associated to the seagrass Posidonia oceanica. New Biotechnol. 2013, 30, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Mtui, G.; Nakamura, Y. Lignocellulosic enzymes from Flavodon flavus, a fungus isolated from Western Indian Ocean off the coast of Dar es Salaam, Tanzania. Afr. J. Biotechnol. 2008, 7, 3066–3072. [Google Scholar]
- Singh, P.; Raghukumar, C.; Meena, R.M.; Verma, P.; Shouche, Y. Fungal diversity in deep-sea sediments revealed by culture-dependent and culture-independent approaches. Fungal Ecol. 2012, 5, 543–553. [Google Scholar] [CrossRef]
- Raghukumar, C. Marine fungal biotechnology: An ecological perspective. Fungal Divers. 2008, 31, 19–35. [Google Scholar]
- Kolesnikov, M.P.; Mirchink, T.G.; Lur’ ye, N.Y. Pigments of Penicillium funiculosum strains isolated from various types of soils. Sov. Soil Sci. 1984, 16, 28–34. [Google Scholar]
- Anke, H.; Kolthoum, I.; Zähner, H.; Laatsch, H. Metabolic products of microorganisms. 185. The anthraquinones of the Aspergillus glaucus group. I. Occurrence, isolation, identification and antimicrobial activity. Arch. Microbiol. 1980, 126, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M. Bioactive natural products from marine-derived fungi: An update. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, Netherlands, 2015; Volume 45, pp. 297–361. [Google Scholar]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. In Advances in Marine Biology; Southward, A., Young, C., Fuiman, L., Tyler, P., Eds.; Academic Press: London, UK, 2001; Volume 40, pp. 81–251. [Google Scholar]
- Hawksworth, D.L. Hypocrealean fungi revisited. Mycol. Res. 2000, 104, 117–118. [Google Scholar] [CrossRef]
- Bick, I.R.C.; Rhee, C. Anthraquinone pigments from Phoma foveata Foister. Biochem. J. 1966, 98, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. Proportional response method for the assignment of π1, π* adsorption bands of ionized hydroxyanthraquinones. Žurnal fizičeskoj himii 2003, 77, 563–565. [Google Scholar]
- Fujitake, N.; Suzuki, T.; Fukumoto, M.; Oji, Y. Predomination of dimers over naturally occurring anthraquinones in soil. J. Nat. Prod. 1998, 61, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Fujitake, N.; Azuma, J.; Hamasaki, T.; Nakajima, H.; Saiki, K. Chrysotalunin, a most prominent soil anthraquinone pigment in Japanese soils. Sci. Total Environ. 1992, 117–118, 219–226. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujitake, N.; Oji, Y. Horizontal Distribution of main hydroxyanthraquinones in soil. Soil Sci. Plant Nutr. (Tokyo Jpn.) 1999, 45, 297–306. [Google Scholar] [CrossRef]
- Velmurugan, P. Studies on the Production and Dyeing Properties of Water Soluble Pigments from Filamentous Fungi. Ph.D. Thesis, Bharathiar University, Coimbatore, India, 2008. [Google Scholar]
- Charudattan, R.; Rao, K.V. Bostrycin and 4-deoxybostrycin: Two nonspecific phytotoxins produced by Alternaria eichhorniae. Appl. Environ. Microbiol. 1982, 43, 846–849. [Google Scholar] [PubMed]
- Xia, G.; Li, J.; Li, H.; Long, Y.; Lin, S.; Lu, Y.; He, L.; Lin, Y.; Liu, L.; She, Z. Alterporriol-type dimers from the mangrove endophytic fungus, Alternaria sp. (SK11), and their MptpB inhibitions. Mar. Drugs 2014, 12, 2953–2969. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Pan, J.H.; Chen, B.; Yu, M.; Huang, H.B.; Zhu, X.; Lu, Y.J.; She, Z.G.; Lin, Y.C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9–6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Teixeira, M.F.S.; de Conti, R.; Esposito, E. Ecological-friendly pigments from fungi. Crit. Rev. Food Sci. Nutr. 2002, 42, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Nielsen, K.F.; Larsen, T.O.; Frisvad, J.C.; Meyer, A.S.; Thrane, U. Exploring fungal biodiversity for the production of water-soluble pigments as potential natural food colorants. Curr. Opin. Biotechnol. 2005, 16, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Filtenborg, O. Terverticillate Penicillia: Chemotaxonomy and mycotoxin production. Mycologia 1989, 81, 837–861. [Google Scholar] [CrossRef]
- Du, L.; Zhu, T.; Liu, H.; Fang, Y.; Zhu, W.; Gu, Q. Cytotoxic Polyketides from a Marine-derived Fungus Aspergillus glaucus. J. Nat. Prod. 2008, 71, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Nakazawa, T.; Ukai, K.; Kobayashi, H.; Mangindaan, R.E.P.; Wewengkang, D.S.; Rotinsulu, H.; Namikoshi, M. Tetrahydrobostrycin and 1-deoxytetrahydrobostrycin, two new hexahydroanthrone derivatives, from a marine-derived fungus Aspergillus sp. J. Antibiot. 2008, 61, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, F.; Luo, M.; Chen, Y.; Song, Y.; Zhang, W.; Zhang, S.; Ju, J. Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J. Nat. Prod. 2012, 75, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, T.; Tao, H.; Lu, Z.; Fang, Y.; Gu, Q.; Zhu, W. Two new cytotoxic quinone type compounds from the halotolerant fungus Aspergillus variecolor. J. Antibiot. 2007, 60, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; El-Beih, A.A.; El-Halawany, A.M. Bioactive anthraquinones from endophytic fungus Aspergillus versicolor isolated from red sea algae. Arch. Pharm. Res. 2012, 35, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.M.; Wang, B.G. Anthraquinone derivatives produced by Marine-Derived Fungus Aspergillus versicolor EN-7. Biosci. Biotechnol. Biochem. 2012, 76, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, R. Dyes from lichens and mushrooms. In Handbook of Natural Colorants; Bechtold, T., Mussak, R.A.M., Eds.; John Wiley & Sons: Chippenham, Wiltshire, UK, 2009; pp. 183–200. [Google Scholar]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono, S.; Proksch, P. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.; Dethoup, T.; Singburaudom, N.; Lima, R.; Vasconcelos, M.H.; Pinto, M.; Kijjoa, A. The in vitro anticancer activity of the crude extract of the sponge-associated fungus Eurotium cristatum and its secondary metabolites. J. Nat. Pharm. 2010, 1, 25. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Kalinovskii, A.I.; Khudyakova, Y.V.; Slinkina, N.N.; Pivkin, M.V.; Kuznetsova, T.A. Metabolites from the marine fungus Eurotium repens. Chem. Nat. Compd. 2007, 43, 395–398. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Wang, B. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: Structural elucidation and DPPH radical scavenging activity. J. Microbiol. Biotechnol. 2009, 19, 675–680. [Google Scholar] [PubMed]
- Zhu, F.; Chen, G.; Chen, X.; Yuan, Y.; Huang, M.; Xiang, W.; Sun, H. Structural elucidation of three anthraquinones from a marine-derived mangrove endophytic fungus (isolate 1850). In Proceeding of the International Conference on BioMedical Engineering and Informatics, Sanya, China, 27–30 May 2008; pp. 664–667.
- Shao, C.; Wang, C.; Wei, M.; Li, S.; She, Z.; Gu, Y.; Lin, Y. Structural and spectral assignments of six anthraquinone derivatives from the mangrove fungus (ZSUH-36). Magn. Reson. Chem. 2008, 46, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.L.; Wang, C.Y.; Zheng, C.J.; She, Z.G.; Gu, Y.C.; Lin, Y.C. A new anthraquinone derivative from the marine endophytic fungus Fusarium sp. (No. b77). Nat. Prod. Res. 2010, 24, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-J.; Yang, R.-Y.; Guo, Z.-Y.; She, Z.-G.; Lin, Y.-C. New anthraquinone derivative produced by cultivation of mangrove endophytic fungus Fusarium sp. ZZF60 from the South China Sea. Chin. J. Appl. Chem. 2010, 27, 394–397. [Google Scholar]
- Chen, Y.; Cai, X.; Pan, J.; Gao, J.; Li, J.; Yuan, J.; Fu, L.; She, Z.; Lin, Y. Structure elucidation and NMR assignments for three anthraquinone derivatives from the marine fungus Fusarium sp. (No. ZH-210). Magn. Reson. Chem. 2009, 47, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Anthraquinone, cyclopentanone, and naphthoquinone derivatives from the sea fan-derived fungi Fusarium spp. PSU-F14 and PSU-F135. J. Nat. Prod. 2010, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.K.; Huang, H.R.; She, Z.G.; Shao, C.L.; Liu, F.; Cai, X.L.; Vrijmoed, L.L.P.; Lin, Y.C. 1H and 13C NMR assignments for five anthraquinones from the mangrove endophytic fungus Halorosellinia sp. (No. 1403). Magn. Reson. Chem. 2007, 45, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.S.; Zhou, W.Q.; Cai, M.H.; Zhang, Y. Method for Fermental Cultivation of Marine Fungi for Production of Anticancer Compound 1403 C. International Patent Number CN 104250657 A, 31 December 2014. [Google Scholar]
- Santesson, J. Anthraquinones in Caloplaca. Phytochemistry 1970, 9, 2149–2166. [Google Scholar] [CrossRef]
- Manojlovic, N.; Novakovic, M.; Stevovic, V.; Solujic, S. Antimicrobial metabolites from three Serbian Caloplaca. Pharm. Biol. 2005, 43, 718–722. [Google Scholar] [CrossRef]
- Manojlović, N.; Marković, Z.; Gritsanapan, W.; Boonpragob, K. High-performance liquid chromatographic analysis of anthraquinone compounds in the Laurera benguelensis. Russ. J. Phys. Chem. 2009, 83, 1554–1557. [Google Scholar] [CrossRef]
- Manojlovic, N.T.; Vasiljevic, P.J.; Markovic, Z.S. Antimicrobial activity of extracts and various fractions of chloroform extract from the lichen Laurera benguelensis. J. Biol. Res. Thessalon. 2002, 13, 27–34. [Google Scholar]
- Søchting, U.; Figueras, G. Caloplaca lenae sp. nov., and other Caloplaca species with caloploicin and vicanicin. Lichenologist 2007, 39, 7–14. [Google Scholar] [CrossRef]
- Yosioka, I.; Hino, K.; Fujio, M.; Kitagawa, I. The structure of caloploicin, a new lichen trichloro-depsidone. Chem. Pharm. Bull. 1973, 21, 1547–1553. [Google Scholar] [CrossRef]
- Ren, H.; Tian, L.; Gu, Q.; Zhu, W. Secalonic acid D; A cytotoxic constituent from marine lichen-derived fungus Gliocladium sp. T31. Arch. Pharm. Res. 2006, 29, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Elix, J. Lichen phytochemistry: Additions and amendments I John A. Elix. Australasian Lichenol. 2008, 63, 20–25. [Google Scholar]
- Johansson, S.; Søchting, U.; Elix, J.; Wardlaw, J. Chemical variation in the lichen genus Letrouitia (Ascomycota, Letrouitiaceae). Mycol. Prog. 2005, 4, 139–148. [Google Scholar] [CrossRef]
- Brauers, G.; Edrada, R.A.; Ebel, R.; Proksch, P.; Wray, V.; Berg, A.; Grafe, U.; Schachtele, C.; Totzke, F.; Finkenzeller, G.; et al. Anthraquinones and betaenone derivatives from the sponge-associated fungus Microsphaeropsis species: Novel inhibitors of protein kinases. J. Nat. Prod. 2000, 63, 739–745. [Google Scholar] [CrossRef] [PubMed]
- El-Beih, A.A.; Kawabata, T.; Koimaru, K.; Ohta, T.; Tsukamoto, S. Monodictyquinone A: A new antimicrobial anthraquinone from a sea urchin-derived fungus Monodictys sp. Chem. Pharm. Bull. 2007, 55, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Kjer, J. New Natural Products from Endophytic Fungi from Mangrove Plants: Structure Elucidation and Biological Screening; Heinrich-Heine-Universität: Düsseldorf, Germany, 2009. [Google Scholar]
- Yang, K.L.; Wei, M.Y.; Shao, C.L.; Fu, X.M.; Guo, Z.Y.; Xu, R.F.; Zheng, C.J.; She, Z.G.; Lin, Y.C.; Wang, C.Y. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J. Nat. Prod. 2012, 75, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2014, 5, 715. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, Q.; Li, J.; Shao, C.; Zhang, J.; Zhang, Y.; Liu, X.; Lin, Y.; Liu, C.; She, Z. Two new derivatives of griseofulvin from the mangrove endophytic fungus Nigrospora sp. (strain No. 1403) from Kandelia candel (L.) Druce. Planta Med. 2011, 77, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lin, Y.-C.; She, Z.-G.; Du, D.-S.; Chan, W.-L.; Zheng, Z.-H. Paeciloxanthone, a new cytotoxic xanthone from the marine mangrove fungus Paecilomyces sp. (Tree1–7). J. Asian Nat. Prod. Res. 2008, 10, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Khamthong, N.; Rukachaisirikul, V.; Tadpetch, K.; Kaewpet, M.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Tetrahydroanthraquinone and xanthone derivatives from the marine-derived fungus Trichoderma aureoviride PSU-F95. Arch. Pharm. Res. 2012, 35, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Brunati, M.; Luis Rojas, J.; Sponga, F.; Ciciliato, I.; Losi, D.; Goettlich, E.; de Hoog, S.; Genilloud, O.; Marinelli, F. Diversity and pharmaceutical screening of fungi from benthic mats of Antarctic lakes. Mar. Genom. 2009, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gu, Q.-Q.; Cui, C.-B. Anthraquinone derivatives produced by marine-derived Penicillium flavidorsum SHK1–27 and their antitumor activities. Chin. J. Med. Chem. 2007, 17, 148–154. [Google Scholar]
- Wang, P.L.; Li, D.Y.; Xie, L.R.; Wu, X.; Hua, H.M.; Li, Z.L. Two new compounds from a marine-derived fungus Penicillium oxalicum. Nat. Prod. Res. 2014, 28, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Klaiklay, S.; Rukachaisirikul, V.; Phongpaichit, S.; Pakawatchai, C.; Saithong, S.; Buatong, J.; Preedanon, S.; Sakayaroj, J. Anthraquinone derivatives from the mangrove-derived fungus Phomopsis sp. PSU-MA214. Phytochem. Lett. 2012, 5, 738–742. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Zheng, C.-J.; Chen, G.-Y.; Song, X.-P.; Han, C.-R.; Li, G.-N.; Fu, Y.-H.; Chen, W.-H.; Niu, Z.-G. Bioactive anthraquinone derivatives from the mangrove-derived fungus Stemphylium sp. 33231. J. Nat. Prod. 2014, 77, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Marmann, A.; Abdel-Aziz, M.S.; Wang, C.Y.; Müller, W.E.G.; Lin, W.H.; Mándi, A.; Kurtán, T.; Daletos, G.; Proksch, P. Tetrahydroanthraquinone Derivatives from the Endophytic Fungus Stemphylium globuliferum. Eur. J. Org. Chem. 2015, 2015, 2646–2653. [Google Scholar] [CrossRef]
- Huang, X.; Sun, X.; Lin, S.; Xiao, Z.; Li, H.; Bo, D.; She, Z. Xylanthraquinone, a new anthraquinone from the fungus Xylaria sp. 2508 from the South China Sea. Nat. Prod. Res. 2014, 28, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Elix, J.A.; Wardlaw, J.H.; SØchting, U. Two new anthraquinones from the lichen Caloplaca spitsbergensis. Herzogia 2000, 14, 27–30. [Google Scholar]
- Berger, Y. 1, 3, 6, 8-tetrahydroxyanthraquinone from Aspergillus versicolor. Phytochemistry 1980, 19, 2779–2780. [Google Scholar] [CrossRef]
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and antifungal compounds from Marine Fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Zhu, T.-J.; Tao, H.-W.; Lu, Z.-Y.; Fang, Y.-C.; Gu, Q.-Q.; Zhu, W.-M. Three novel, structurally unique spirocyclic alkaloids from the Halotolerant B-17 Fungal Strain of Aspergillus variecolor. Chem. Biodivers. 2007, 4, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Janso, J.E.; Bernan, V.S.; Greenstein, M.; Bugni, T.S.; Ireland, C.M. Penicillium dravuni, a new marine-derived species from an alga in Fiji. Mycologia 2005, 97, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, Z.G.; Song, B.B.; Chen, C.H.; Xiao, W.W.; Hu, B.; Wang, J.W.; Jiang, X.B.; Zhu, Y.H.; Wang, H.J. Advances in the study of the structures and bioactivities of metabolites isolated from mangrove-derived fungi in the South China Sea. Mar. Drugs 2013, 11, 3601–3616. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.L.; Badar Ud, D.; Ahmad, A.; Ahmad, M. The antibiotic bostrycin from Alternaria eichhorniae. Phytochemistry 1979, 18, 1579–1580. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Gao, Y.; Lin, H.; Du, L.; Yang, S.; Long, S.; She, Z.; Cai, X.; Zhou, S.; et al. The anthracenedione compound bostrycin induces mitochondria-mediated apoptosis in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhu, X.; Li, Q.; Gu, M.; He, Z.; Wu, J.; Li, J.; Lin, Y.; Li, M.; She, Z.; et al. SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br. J. Pharmacol. 2010, 159, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Cai, M.; Yu, C.; Zhang, Y.; Zhou, X. Improved production of the anticancer compound 1403C by glucose pulse feeding of marine Halorosellinia sp. (No. 1403) in submerged culture. Bioresour. Technol. 2011, 102, 10750–10753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; He, Z.; Wu, J.; Yuan, J.; Wen, W.; Hu, Y.; Jiang, Y.; Lin, C.; Zhang, Q.; Lin, M.; et al. A marine anthraquinone SZ-685C overrides adriamycin-resistance in breast cancer cells through suppressing Akt signaling. Mar. Drugs 2012, 10, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Tan, T.; Mao, Z.G.; Lei, N.; Wang, Z.M.; Hu, B.; Chen, Z.Y.; She, Z.G.; Zhu, Y.H.; Wang, H.J. The marine metabolite SZ-685C induces apoptosis in primary human nonfunctioning pituitary adenoma cells by inhibition of the Akt pathway in vitro. Mar. Drugs 2015, 13, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Wang, X.; Jiang, D.; Wang, H.; Jiao, Y.; Lou, H.; Wang, X. Metabolites from Aspergillus versicolor, an endolichenic fungus from the lichen Lobaria retigera. Drug Discov. Ther. 2014, 8, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Li, H.; Hong, J.; Cho, H.Y.; Bae, K.S.; Kim, M.A.; Kim, D.-K.; Jung, J.H. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 2010, 33, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Fouillaud, M.; Université de La Réunion, Saint Denis, France. Personal communication, 2016.
- Butinar, L.; Zalar, P.; Frisvad, J.C.; Gunde-Cimerman, N. The genus Eurotium—Members of indigenous fungal community in hypersaline waters of salterns. FEMS Microbiol. Ecol. 2005, 51, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ren-Yi, G.; Lei, X.; Yi, K.; Iii-Ming, C.; Jian-Chun, Q.; Li, L.; Sheng-Xiang, Y.; Li-Chun, Z. Chaetominine, (+)-alantrypinone, questin, isorhodoptilometrin, and 4-hydroxybenzaldehyde produced by the endophytic fungus Aspergillus sp. YL-6 inhibit wheat (Triticum aestivum) and radish (Raphanus sativus) germination. J. Plant Interact. 2015, 10, 87–92. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Mueller, W.E.G.; Totzke, F.; Zirrgiebel, U.; Schaechtele, C.; Kubbutat, M.H.G.; Lin, W.H.; et al. Bioactive metabolites from the endophytic fungus Stemphylium globuliferum isolated from Mentha pulegium. J. Nat. Prod. 2009, 72, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Yang, C.S.; Huang, C.J.; Chen, K.S.; Lin, S.F. Bostrycin, a novel coupling agent for protein immobilization and prevention of biomaterial-centered infection produced by Nigrospora sp. No. 407. Enzyme Microb. Technol. 2012, 50, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Shirasaka, M. Antibiotics from fungi. IV.production of rhodosporin (bostrycin) by Nigrospora oryzae. Sankyo Kenkyusho Nempo 1969, 21, 165–168. [Google Scholar]
- Crous, P.W.; Groenewald, J.Z. A phylogenetic re-evaluation of Athrinium. IMA Fungus 2013, 4, 133–154. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, G.W. Anthraquinones in the fungus Talaromyces stipitatus. Experientia 1973, 29, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.H. Naturally Occurring Quinones III: Recent Advances; Chapman & Hall: London, UK; New York, NY, USA, 1987. [Google Scholar]
- Noda, T.; Take, T.; Watanabe, T.; Abe, J. The structure of bostrycin. Tetrahedron 1970, 26, 1339–1346. [Google Scholar] [CrossRef]
- Noda, T.; Take, T.; Otani, M.; Miyauchi, K.; Watanabe, T.; Abe, J. Structure of bostrycin. Tetrahedron Lett. 1968, 58, 6087–6090. [Google Scholar] [CrossRef]
- Takenaka, A.; Furusaki, A.; Watanabe, T. The crystal and molecular structure of bostrycin pi-bromobenzoate, A derivative of bostrycin. Tetrahedron Lett. 1968, 6091–6094. [Google Scholar] [CrossRef]
- Kelly, T.R.; Kim, M.H.; Curtis, A.D.M. Structure correction and synthesis of the naturally occurring benzothiazinone BMY 40662. J. Org. Chem. 1993, 58, 5855–5857. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Huang, Y.; Chen, H.; Li, Y.; Zhong, L.; Chen, Y.; Chen, S.; Wang, J.; Kang, J.; et al. Anti-mycobacterial activity of marine fungus-derived 4-deoxybostrycin and nigrosporin. Molecules 2013, 18, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, E.; Heitkämper, S.; Fournier, J.; Surup, F.; Stadler, M. Hypoxyvermelhotins A–C, new pigments from Hypoxylon lechatii sp. nov. Fungal Biol. 2014, 118, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L. Freshwater and marine lichen-forming fungi. In Aquatic Mycology across the Millennium; Hyde, K.D., Ho, W.H., Pointing, S.B., Eds.; Fungal Diversity Press: Hong Kong, China, 2000; Volume 5, pp. 1–7. [Google Scholar]
- Gauslaa, Y.; McEvoy, M. Seasonal changes in solar radiation drive acclimation of the sun-screening compound parietin in the lichen Xanthoria parietina. Basic Appl. Ecol. 2005, 6, 75–82. [Google Scholar] [CrossRef]
- Larsson, P.; Večeřová, K.; Cempírková, H.; Solhaug, K.A.; Gauslaa, Y. Does UV-B influence biomass growth in lichens deficient in sun-screening pigments? Environ. Exp. Bot. 2009, 67, 215–221. [Google Scholar] [CrossRef]
- Søchting, U.; Frödén, P. Chemosyndromes in the lichen genus Teloschistes (Teloschistaceae, Lecanorales). Mycol. Progress 2002, 1, 257–266. [Google Scholar] [CrossRef]
- Søchting, U. Two major anthraquinone chemosyndromes in Teloschistaceae. Bibl. Lichenol. 1997, 68, 135–144. [Google Scholar]
- Søchting, U.; Lutzoni, F. Molecular phylogenetic study at the generic boundary between the lichen-forming fungi Caloplaca and Xanthoria (Ascomycota, Teloschistaceae). Mycol. Res. 2003, 107, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Søchting, U. Chemosyndromes with chlorinated anthraquinones in the lichen genus Caloplaca. In Lichenological Contributions in Honour of Jack Elix; McCarthy, P.M., Kantvilas, G., Louwhoff, S.H.J.J., Cramer, J., Eds.; Bibliotheca Lichenologica: Berlin, German, 2001; pp. 395–404. [Google Scholar]
- Frisvad, J.C.; Andersen, B.; Thrane, U. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol. Res. 2008, 112, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C. Taxonomy, chemodiversity and chemoconsistency of Aspergillus, Penicillium and Talaromyces species. Front. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.; Morgan, P.M. Pigments of fungi part 73—Absolute stereochemistry of fungal metabolites: Icterinoidins A1 and B1, and atrovirins B1 and B2. Arkivoc 2004, 152–165. [Google Scholar]
- Kurobane, I.; Vining, L.C.; McInnes, A.G.; Walter, J.A. Use of 13C in biosynthetic studies. The labeling pattern in dihydrofusarubin enriched from [13C]- and [13C, 2H]acetate in cultures of Fusarium solani. Can. J. Chem. 1980, 58, 1380–1385. [Google Scholar] [CrossRef]
- Julia, P.; Martinkova, L.; Lolinski, J.; Machek, F. Ethanol as substrate for pigment production by the fungus Monascus purpureus. Enzyme Microb. Tech. 1994, 16, 996–1001. [Google Scholar]
- Cho, Y.J.; Hwang, H.J.; Kim, S.W.; Song, C.H.; Yun, J.W. Effect of carbon source and aeration rate on broth rheology and fungal morphology during red pigment production by Paecilomyces sinclairii in a batch bioreactor. J. Biotech. 2002, 95, 13–23. [Google Scholar] [CrossRef]
- Cai, Y.; Din, Y.; Ta, G.; Lia, X. Production of 1,5-dihydroxy-3-methoxy-7-methylanthracene-9,10-dione by submerged culture of Shiraia bambusicola. J. Microbiol. Biotechnol. 2008, 18, 322–327. [Google Scholar] [PubMed]
- Yu, J.H.; Keller, N. Regulation of secondary metabolism in filamentous fungi. Annu. Rev. Phytopathol. 2005, 43, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.H.; Tang, Y. Navigating the fungal polyketide chemical space: From genes to molecules. J. Org. Chem. 2012, 77, 9933–9953. [Google Scholar] [CrossRef] [PubMed]
- Miethbauer, S.; Haase, S.; Schmidtke, K.; Gunther, W.; Heiser, I.; Liebermann, B. Biosynthesis of photodynamically active rubellins and structure elucidation of new anthraquinone derivatives produced by Ramularia collo-cygni. Phytochemistry 2006, 67, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Kroken, S.; Glass, N.L.; Taylor, J.W.; Yoder, O.C.; Turgeon, B.G. Phylogenomic analysis of type 1 polyketide synthase genes in pathogenic and saprobic ascomycetes. Natl. Acad. Sci. 2003, 100, 15670–15675. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Butchko, R.A.; Baker, S.E.; Proctor, R.H. Phylogenomic and functional domain analysis of polyketide synthases in Fusarium. Fungal Biol. 2012, 116, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Zabala, A.O.; Xu, W.; Chooi, Y.H.; Tang, Y. Characterization of a silent azaphilone gene cluster from Aspergillus niger ATCC 1015 reveals a hydroxylation-mediated pyran-ring formation. Chem. Biol. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Cacho, R.A.; Tang, Y.; Chooi, Y.-H. Next-generation sequencing approach for connecting secondary metabolites to biosynthetic gene clusters in fungi. Front. Microbiol. 2014, 5, 774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Javidpour, P.; Korman, T.P.; Shakya, G.; Tsai, S.C. Structural and biochemical analyses of regio- and stereospecificities observed in a type II polyketide ketoreductase. Biochemistry 2011, 50, 4638–4649. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Z.; Shao, C.-L.; Wang, J.-L.; Bai, H.; Wang, C.-Y. Bioinformatical analysis of the sequences, structures and functions of fungal polyketide synthase product template domains. Sci. Rep. 2015, 5, 10463. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Noll, T.F.; Gulder, T.A.; Grune, M.; Dreyer, M.; Wilde, C.; Pankewitz, F.; Hilker, M.; Payne, G.D.; Jones, A.L.; et al. Different polyketide folding modes converge to an identical molecular architecture. Nat. Chem. Biol. 2006, 2, 429–433. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Biosynthesis of polyketide metabolites. Nat. Prod. Rep. 1992, 9, 447–479. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, C.; Gill, M.; Gimenez, A.; Milanovic, N.M.; Raudies, E. Pigments of fungi. Part 50.1 Structure, biosynthesis and stereochemistry of new dimeric dihydroanthracenones of the phlegmacin type from Cortinarius sinapicolor Cleland. J. Chem. Soc. Perkin Trans. 1999, 1999, 119–126. [Google Scholar] [CrossRef]
- Chen, Z.G.; Fujii, I.; Ebizuka, Y.; Sankawa, U. Emodin O-methyltransferase from Aspergillus terreus. Arch. Microbiol. 1992, 158, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gill, M. Pigments of fungi (Macromycetes). Nat. Prod. Rep. 1999, 16, 301–317. [Google Scholar] [CrossRef]
- Gill, M.; Morgan, P.M. New fungal anthraquinones. Arkivoc 2001, 2001, 145–156. [Google Scholar]
- Michal, G. Lysin-Stoffwechsel. In Biochemical pathways; Michal, G., Ed.; Spektrum Akademischer Verlag Heidelberg: Berlin, German, 1999; pp. 51–54. [Google Scholar]
- Henry, K.M.; Townsend, C.A. Synthesis and fate of o-carboxybenzophenones in the biosynthesis of aflatoxin. J. Am. Chem. Soc. 2005, 127, 3300–3309. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.F.; Somoza, A.D.; Keller, N.P.; Wang, C.C. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 2012, 29, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Irmer, A.; Feineis, D.; Gulder, T.A.M.; Fiedler, H.-P. Convergence in the biosynthesis of acetogenic natural products from plants, fungi, and bacteria. Phytochemistry 2009, 70, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Kakule, T.B.; Lin, Z.; Schmidt, E.W. Combinatorialization of fungal polyketide synthase-peptide synthetase hybrid proteins. J. Am. Chem. Soc. 2014, 136, 17882–17890. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S. Chemotaxonomic Exploration of Fungal Biodiversity for Polyketide Natural Food Colorants… Discovery & Evaluation of cell Factories, and Characterization of Pigments. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2009. [Google Scholar]
- Bu’Lock, J.D.; Detroy, R.W.; Hošťálek, Z.; Munim-Al-Shakarchi, A. Regulation of secondary biosynthesis in Gibberella fujikuroi. Trans. Br. Mycol. Soc. 1974, 62, 377–389. [Google Scholar] [CrossRef]
- Pagano, M.C.; Dhar, P.P. Fungal Pigments: An Overview. In Fungal Biomolecules: Sources, Applications and Recent Developments; Gupta, V.K., Mach, R.L., Sreenivasaprasad, S., Eds.; Wiley-Blackwell: Pondichery, India, 2015; pp. 173–181. [Google Scholar]
- Hiort, J.; Maksimenka, K.; Reichert, M.; Perović-Ottstadt, S.; Lin, W.H.; Wray, V.; Steube, K.; Schaumann, K.; Weber, H.; Proksch, P.; et al. New natural products from the sponge-derived fungus Aspergillus niger. J. Nat. Prod. 2004, 67, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Margalith, P. Pigment Microbiology; Springer Netherlands: London, UK; New York, NY, USA, 1992; p. 156. [Google Scholar]
- Duran, N.; de Conti, R.; Teixeira, M.F.S. Pigments from fungi : Industrial perspective. In Advances in Fungal Biotechnology; Rai, M., Ed.; I.K. International Publishing House Pvt. Ltd.: New Delhi, India, 2009; pp. 185–225. [Google Scholar]
- Rietjens, I.M.C.M.; Boersma, M.G.; Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.P.; van Zanden, J.J.; van der Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Martin-Cordero, C.; Leon-Gonzalez, A.J.; Calderon-Montano, J.M.; Burgos-Moron, E.; Lopez-Lazaro, M. Pro-oxidant natural products as anticancer agents. Curr. Drug Targets 2012, 13, 1006–1028. [Google Scholar] [CrossRef] [PubMed]
- Kosalec, I.; Kremer, D.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Randic, M.; Zovko Koncic, M. Anthraquinone profile, antioxidant and antimicrobial activity of bark extracts of Rhamnus alaternus, R. fallax, R. intermedia and R. pumila. Food Chem. 2013, 136, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.; Chuang, D. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Choi, J.S.; Chung, H.Y.; Jung, H.A.; Park, H.J.; Yokozawa, T. Comparative evaluation of antioxidant potential of alaternin (2-Hydroxyemodin) and emodin. J. Agric. Food Chem. 2000, 48, 6347–6351. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, K.A.; Gauslaa, Y.; Nybakken, L.; Bilger, W. UV-induction of sun-screening pigments in lichens. New Phytol. 2003, 158, 91–100. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Gauslaa, Y. Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. Plant Cell Environ. 2004, 27, 167–176. [Google Scholar] [CrossRef]
- Velmurugan, P.; Lee, Y.H.; Venil, C.K.; Lakshmanaperumalsamy, P.; Chae, J.-C.; Oh, B.-T. Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J. Biosci. Bioeng. 2010, 109, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Buiak, L.I.; Landau, N.S.; Kolesnikov, M.P.; Egorov, N.S. Isolation and study of the biological properties of a protease biosynthesis stimulating factor in an associative fungal culture. Mikrobiologiia 1983, 52, 750–754. [Google Scholar] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 1997. [Google Scholar]
- Germino, M.J.; Hasselquist, N.J.; McGonigle, T.; Smith, W.K.; Sheridan, P.P. Landscape- and age-based factors affecting fungal colonization of conifer seedling roots at the alpine tree line. Can. J. For. Res. 2006, 36, 901–909. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Su, H.; Marrone, P.G.; Koivunen, M. Plant pathogen inhibitor combinations and methods of use. U.S. patent 8,889,197 B2, 30 July 2009. [Google Scholar]
- Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Mueller, W.E.G.; Kozytska, S.; Hentschel, U.; Proksch, P.; Ebel, R. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry 2008, 69, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, W. Relationship between mercury resistance and pigment production in Pyrenophora avenae. Trans. Br. Mycol. Soc. 1971, 56, 37–44. [Google Scholar] [CrossRef]
- Kühlwein, F.; Polborn, K.; Beck, W. Metallkomplexe von Farbstoffen. X. Neue Übergangsmetallkomplexe von Anthrachinonfarbstoffen. Z. Anorg. Allg. Chem. 1997, 623, 1931–1944. [Google Scholar] [CrossRef]
- Suzuki, T. Distribution and Biological Activity of Anthraquinones in Soil (PDF Document online). Available online: http://www2.kobe-u.ac.jp/~tksuzuki/kouen/aq.pdf (accessed on 26 November 2015).
- Huang, Q.; Lu, G.; Shen, H.M.; Chung, M.C.M.; Ong, C.N. Anti-cancer properties of anthraquinones from rhubarb. Med. Res. Rev. 2007, 27, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Chen, L.M.; Mi, Y.J.; Zheng, L.S.; Wang, F.; She, Z.G.; Lin, Y.C.; To, K.K.; et al. Anthracenedione Derivatives as Anticancer Agents Isolated from Secondary Metabolites of the Mangrove Endophytic Fungi. Mar. Drugs 2010, 8, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chiang, Y.M.; Kuo, P.L.; Chang, J.K.; Hsu, Y.L. Norsolorinic acid from Aspergillus nidulans inhibits the proliferation of human breast adenocarcinoma MCF-7 cells via Fas-mediated pathway. Basic Clin. Pharmacol. Toxicol. 2008, 102, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, W.W. Nidurufin as a new cell cycle inhibitor from marine-derived fungus Penicillium flavidorsum SHK1–27. Arch. Pharm. Res. 2011, 34, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.T.; Lin, S.Z.; Liu, D.L.; Wang, Z.H. The distinct mechanisms of the antitumor activity of emodin in different types of cancer (Review). Oncol. Rep. 2013, 30, 2555–2562. [Google Scholar] [PubMed]
- Tatsuda, D.; Momose, I.; Someno, T.; Sawa, R.; Kubota, Y.; Iijima, M.; Kunisada, T.; Watanabe, T.; Shibasaki, M.; Nomoto, A. Quinofuracins A–E, produced by the fungus Staphylotrichum boninense PF1444, show p53-dependent growth suppression. J. Nat. Prod. 2015, 78, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; Chen, M.T.; Wu, Z.K.; Zhao, H.L.; Yu, H.C.; Yu, J.; Zhang, J.W. Emodin can induce K562 cells to erythroid differentiation and improve the expression of globin genes. Mol. Cell. Biochem. 2013, 382, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Hu, J.; Zheng, J.; Zheng, Z.; Liu, T.; Lin, Z.; Lin, M. Emodin enhances ATRA-induced differentiation and induces apoptosis in acute myeloid leukemia cells. Int. J. Oncol. 2014, 45, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Xiao, W.W.; Jiang, X.B.; Wang, J.W.; Mao, Z.G.; Lei, N.; Fan, X.; Song, B.B.; Liao, C.X.; Wang, H.J.; et al. A novel marine drug, SZ-685C, induces apoptosis of MMQ pituitary tumor cells by downregulating miR-200c. Curr. Med. Chem. 2013, 20, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, S.; Liu, Q.; Wang, M.; Wang, C.; Yang, H. SZ-685C exhibits potent anticancer activity in both radiosensitive and radioresistant NPC cells through the miR-205-PTEN-Akt pathway. Oncol. Rep. 2013, 29, 2341–2347. [Google Scholar] [PubMed]
- Teiten, M.H.; Mack, F.; Debbab, A.; Aly, A.H.; Dicato, M.; Proksch, P.; Diederich, M. Anticancer effect of altersolanol A, a metabolite produced by the endophytic fungus Stemphylium globuliferum, mediated by its pro-apoptotic and anti-invasive potential via the inhibition of NF-kappaB activity. Bioorg. Med. Chem. 2013, 21, 3850–3858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, X.; Li, D.; Zhu, T.; Mo, X.; Li, J. Anticancer efficacy and absorption, distribution, metabolism, and toxicity studies of aspergiolide A in early drug development. Drug Des. Devel. Ther. 2014, 8, 1965–1977. [Google Scholar] [PubMed]
- Huang, L.; Zhang, T.; Li, S.; Duan, J.; Ye, F.; Li, H.; She, Z.; Gao, G.; Yang, X. Anthraquinone G503 Induces Apoptosis in Gastric Cancer Cells through the Mitochondrial Pathway. PLoS ONE 2014, 9, e108286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.; Jin, H.; Song, B.; Zhu, X.; Zhao, H.; Cai, J.; Lu, Y.; Chen, B.; Lin, Y. The cytotoxicity and anticancer mechanisms of alterporriol L, a marine bianthraquinone, against MCF-7 human breast cancer cells. Appl. Microbiol. Biotechnol. 2012, 93, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Phuwapraisirisan, P.; Rangsan, J.; Siripong, P.; Tip-pyang, S. New antitumour fungal metabolites from Alternaria porri. Nat. Prod. Res. 2009, 23, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Xing, F.; Zhu, G.; Xu, G.; Li, C.; Qu, J.; Lee, I.; Pan, L. Rhein antagonizes P2X7 receptor in rat peritoneal macrophages. Sci. Rep. 2015, 5, 14012. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhen, Y.S.; Qi, C.Q.; Chen, W.J. A fungus-derived novel nucleoside transport inhibitor potentiates the activity of antitumor drugs. Acta Pharm. Sin. 1994, 29, 656–661. [Google Scholar]
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Bellon, M.; Nicot, C. Emodin and DHA potently increase arsenic trioxide interferon-α–induced cell death of HTLV-I–transformed cells by generation of reactive oxygen species and inhibition of Akt and AP-1. Blood 2007, 109, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.C.; Bu, H.Q.; Luo, J.; Wei, W.T.; Liu, D.L.; Chen, H.; Tong, H.F.; Wang, Z.H.; Wu, H.Y.; Li, H.H.; et al. Emodin potentiates the antitumor effects of gemcitabine in PANC-1 pancreatic cancer xenograft model in vivo via inhibition of inhibitors of apoptosis. Int. J. Oncol. 2012, 40, 1849–1857. [Google Scholar] [PubMed]
- Tsang, S.W.; Bian, Z.X. Anti-fibrotic and anti-tumorigenic effects of rhein, a natural anthraquinone derivative, in mammalian stellate and carcinoma cells. Phytother. Res. 2015, 29, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.; Chen, Z. Inhibitory effect of emodin on the growth of cervical cancer in tumor-transplanted mice and underlying mechanism. China J. Cell. Mol. Immunol. 2015, 31, 350–354. [Google Scholar]
- Ma, J.; Lu, H.; Wang, S.; Chen, B.; Liu, Z.; Ke, X.; Liu, T.; Fu, J. The anthraquinone derivative Emodin inhibits angiogenesis and metastasis through downregulating Runx2 activity in breast cancer. Int. J. Oncol. 2015, 46, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, L.; Bu, H.Q.; Yu, Q.J.; Jiang, D.D.; Pan, F.P.; Wang, Y.; Liu, D.L.; Lin, S.Z. Effects of emodin on the demethylation of tumor-suppressor genes in pancreatic cancer PANC-1 cells. Oncol. Rep. 2015, 33, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Jaksic, D.; Puel, O.; Canlet, C.; Kopjar, N.; Kosalec, I.; Klaric, M.S. Cytotoxicity and genotoxicity of versicolorins and 5-methoxysterigmatocystin in A549 cells. Arch. Toxicol. 2012, 86, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jia, Q.; Ma, J.; Qin, G.; Chen, Y.; Xi, Y.; Lin, L.; Zhu, W.; Ding, J.; Jiang, H.; et al. Discovering novel quercetin-3-O-amino acid-esters as a new class of Src tyrosine kinase inhibitors. Eur. J. Med. Chem. 2009, 44, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, A.; Tanaka, Y.; Karasaki, Y.; Komaki, H.; Yazawa, K.; Mikami, Y.; Tojo, T.; Kadowaki, K.; Tsuda, M.; Kobayashi, J. Brasiliquinones A, B and C, new benz[alpha]anthraquinone antibiotics from Nocardia brasiliensis 1. Producing strain, isolation and biological activities of the antibiotics. J. Antibiot. 1997, 50, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.M.; Song, Y.C.; Shan, C.Y.; Ye, Y.H.; Tan, R.X. New and cytotoxic anthraquinones from Pleospora sp. IFB-E006, an endophytic fungus in Imperata cylindrical. Planta Med. 2005, 71, 1063–1065. [Google Scholar] [PubMed]
- Shao, C.L.; Wang, C.Y.; Zheng, D.S.; She, Z.G.; Gu, Y.C.; Lin, Y.C. Crystal Structure of a Marine Natural Compound, Anhydrofusarubin. Chin. J. Struct. Chem. 2008, 27, 824–828. [Google Scholar]
- Ueno, Y.; Sato, N.; Ito, T.; Ueno, I.; Enomoto, M.; Tsunoda, H. Chronic toxicity and hepatocarcinogenicity of (+) rugulosin, an anthraquinoid mycotoxin from penicillium species: Preliminary surveys in mice. The J. Toxicol. Sci. 1980, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luan, Y.; Qi, X.; Li, M.; Gong, L.; Xue, X.; Wu, X.; Wu, Y.; Chen, M.; Xing, G.; et al. Emodin triggers DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II. Toxicol. Sci. 2010, 118, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O.; Stopper, H. Characterization of the genotoxicity of anthraquinones in mammalian cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1428, 406–414. [Google Scholar] [CrossRef]
- Chien, S.C.; Wu, Y.C.; Chen, Z.W.; Yang, W.C. Naturally occurring anthraquinones: Chemistry and therapeutic potential in autoimmune diabetes. Evid. Based Complement. Alternat. Med. 2015, 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- De Moliner, E.; Moro, S.; Sarno, S.; Zagotto, G.; Zanotti, G.; Pinna, L.A.; Battistutta, R. Inhibition of protein kinase CK2 by anthraquinone-related compounds. A structural insight. J. Biol. Chem. 2003, 278, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.B.; Bjorling-Poulsen, M.; Guerra, B. Emodin negatively affects the phosphoinositide 3-kinase/AKT signalling pathway: A study on its mechanism of action. Int. J. Biochem. Cell Biol. 2007, 39, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Meggio, F.; Pagano, M.A.; Moro, S.; Zagotto, G.; Ruzzene, M.; Sarno, S.; Cozza, G.; Bain, J.; Elliott, M.; Deana, A.D.; et al. Inhibition of protein kinase CK2 by condensed polyphenolic derivatives. An in vitro and in vivo study. Biochemistry 2004, 43, 12931–12936. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Suh, S.J.; Li, X.; Liang, J.L.; Chi, M.; Hwangbo, K.; Kwon, O.; Chung, T.W.; Kwak, C.H.; Kwon, K.M.; et al. Citreorosein inhibits production of proinflammatory cytokines by blocking mitogen activated protein kinases, nuclear factor-kappaB and activator protein-1 activation in mouse bone marrow-derived mast cells. Biol. Pharm. Bull. 2012, 35, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.M.; Kwon, J.H.; Kim, K.; Noh, J.Y.; Kang, S.; Park, J.M.; Lee, M.Y.; Bae, O.N.; Chung, J.H. Emodin inhibits tonic tension through suppressing PKCδ-mediated inhibition of myosin phosphatase in rat isolated thoracic aorta. Br. J. Pharmacol. 2014, 171, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Nema, N.K.; Bhadra, S.; Mukherjee, D.; Braga, F.C.; Matsabisa, M.G. Immunomodulatory leads from medicinal plants. Indian J. Tradit. Knowl. 2014, 13, 235–256. [Google Scholar]
- Fujimoto, H.; Nakamura, E.; Okuyama, E.; Ishibashi, M. Six immunosuppressive features from an ascomycete, Zopfiella longicaudata, found in a screening study monitored by immunomodulatory activity. Chem. Pharm. Bull. (Tokyo) 2004, 52, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Shen, N.Y.; Liu, C.; Lv, Y. Immunosuppressive effects of emodin: An in vivo and in vitro study. Transplant. Proc. 2009, 41, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, C.; Cordella, M.; Turcano, L.; Rossi, S.; Lentini, A.; Mariotti, S.; Nisini, R.; Sette, G.; Eramo, A.; Piredda, L.; et al. Aloe-emodin exerts a potent anticancer and immunomodulatory activity on BRAF-mutated human melanoma cells. Eur. J. Pharmacol. 2015, 762, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chang, J.H.; Tung, S.F.; Wu, R.T.; Foegh, M.L.; Chu, S.H. Immunosuppressive effect of emodin, a free radical generator. Eur. J. Pharmacol. 1992, 211, 359–364. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar] [PubMed]
- Daly, S.M.; Elmore, B.O.; Kavanaugh, J.S.; Triplett, K.D.; Figueroa, M.; Raja, H.A.; El-Elimat, T.; Crosby, H.A.; Femling, J.K.; Cech, N.B.; et al. Omega-Hydroxyemodin limits Staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob. Agents Chemother. 2015, 59, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.O.; Weber, N.D.; Wood, S.G.; Hughes, B.G.; Murray, B.K.; North, J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- Nakashima, H.; Yamamoto, N.; Inouye, Y.; Nakamura, S. Inhibition by doxorubicin of human immuno-deficiency virus (HIV) infection and replication in vitro. J. Antibiot. (Tokyo) 1987, 40, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Meruelo, D.; Lavie, G.; Lavie, D. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin. Proc. Natl. Acad. Sci. USA 1988, 85, 5230–5234. [Google Scholar] [CrossRef] [PubMed]
- Schinazi, R.F.; Chu, C.K.; Babu, J.R.; Oswald, B.J.; Saalmann, V.; Cannon, D.L.; Eriksson, B.F.; Nasr, M. Anthraquinones as a new class of antiviral agents against human immunodeficiency virus. Antiviral Res. 1990, 13, 265–272. [Google Scholar] [CrossRef]
- Tang, J.; Colacino, J.M.; Larsen, S.H.; Spitzer, W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antivir. Res. 1990, 13, 313–325. [Google Scholar] [CrossRef]
- Lavie, L. Non-hygroscopic water-soluble pulverulent composition for the preparation of drinks and process for its preparation. U.S. Patent 4,769,244 A, 30 January 1987. [Google Scholar]
- Konoshima, T.; Kozuka, M.; Koyama, J.; Okatani, T.; Tagahara, K.; Tokuda, H. Studies on inhibitors of skin tumor promotion, VI. Inhibitory effects of quinones on Epstein-Barr virus activation. J. Nat. Prod. 1989, 52, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Lavie, G.; Valentine, F.; Levin, B.; Mazur, Y.; Gallo, G.; Lavie, D.; Weiner, D.; Meruelo, D. Studies of the mechanisms of action of the antiretroviral agents hypericin and pseudohypericin. Proc. Natl. Acad. Sci. USA 1989, 86, 5963–5967. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.L.; Huffman, J.H.; Morris, J.L.; Wood, S.G.; Hughes, B.G.; Sidwell, R.W. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. Antivir. Res. 1992, 17, 63–77. [Google Scholar] [CrossRef]
- Li, S.W.; Yang, T.C.; Lai, C.C.; Huang, S.H.; Liao, J.M.; Wan, L.; Lin, Y.J.; Lin, C.W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014, 738, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sydiskis, R.J.; Owen, D.G.; Lohr, J.L.; Rosler, K.H.; Blomster, R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 1991, 35, 2463–2466. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, C.F.; Hsiao, N.W.; Chang, C.Y.; Li, S.W.; Wan, L.; Lin, Y.J.; Lin, W.Y. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents 2008, 32, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.J.; Pyke, S.M.; Reynolds, G.D.; Flower, R.L. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antivir. Res. 2001, 49, 169–178. [Google Scholar] [CrossRef]
- Kubin, A.; Wierrani, F.; Burner, U.; Alth, G.; Grunberger, W. Hypericin—The facts about a controversial agent. Curr. Pharm. Des. 2005, 11, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Doong, S.L.; Tsai, C.H.; Schinazi, R.F.; Liotta, D C.; Cheng, Y.C. Inhibition of the replication of hepatitis B virus in vitro by emodin. Med. Sci. Monit. 2006, 12, 302–306. [Google Scholar]
- Awaad, A.S.; Al-Zaylaee, H.M.; Alqasoumi, S.I.; Zain, M.E.; Aloyan, E.M.; Alafeefy, A.M.; Awad, E.S.; El-Meligy, R.M. Anti-leishmanial activities of extracts and isolated compounds from Drechslera rostrata and Eurotium tonpholium. Phytother. Res. 2014, 28, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Shimoda, H.; Morikawa, T.; Yoshikawa, M. Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): Structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg. Med. Chem. Lett. 2001, 11, 1839–1842. [Google Scholar] [CrossRef]
- Riecken, E.; Zeitz, M.; Emde, C.; Hopert, R.; Witzel, L.; Hintze, R.; Marsch-Ziegler, U.; Vester, J. The effect of an anthraquinone laxative on colonic nerve tissue: A controlled trial in constipated women. Z. Gastroenterol. 1990, 28, 660–664. [Google Scholar] [PubMed]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant therapy: Current status and future prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Chen, Q.H. Biochemical study of Chinese rhubarb. XXII. Inhibitory effect of anthraquinone derivatives on Na+-K+-ATPase of the rabbit renal medulla and their diuretic action. Acta Pharma. Sin. 1988, 23, 17–20. [Google Scholar]
- Zhou, X.; Song, B.; Jin, L.; Hu, D.; Diao, C.; Xu, G.; Zou, Z.; Yang, S. Isolation and inhibitory activity against ERK phosphorylation of hydroxyanthraquinones from rhubarb. Bioorg. Med. Chem. Lett. 2006, 16, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Izhaki, I. Emodin—A secondary metabolite with multiple ecological functions in higher plants. New Phytol. 2002, 155, 205–217. [Google Scholar] [CrossRef]
- Nandani, D.; Verma, R.N.; Batra, A. Isolation and identification of quercetin and emodin from Cassia tora L. Ann. Phytomed. 2013, 2, 96–104. [Google Scholar]
- Brash, D.E.; Havre, P.A. New careers for antioxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 13969–13971. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Cefarelli, G.; Uzzo, P.; Monaco, P. Natural dibenzoxazepinones from leaves of Carex distachya: Structural elucidation and radical scavenging activity. Bioorg. Med. Chem. Lett. 2007, 17, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Kim, J.P.; Jung, W.K.; Lee, N.H.; Kang, H.S.; Jun, E.M.; Park, S.H.; Kang, S.M.; Lee, Y.J.; Park, P.J.; et al. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol. 2008, 18, 676–681. [Google Scholar] [PubMed]
- Zargar, B.A.; Masoodi, M.H.; Ahmed, B.; Ganie, S.A. Phytoconstituents and therapeutic uses of Rheum emodi wall. ex Meissn. Food Chem. 2011, 128, 585–589. [Google Scholar] [CrossRef]
- Nemeikaite-Ceniene, A.; Sergediene, E.; Nivinskas, H.; Cenas, N. Cytotoxicity of natural hydroxyanthraquinones: Role of oxidative stress. A J. Biosci. 2002, 57, 822–827. [Google Scholar]
- Evans, W.C.; Evans, D. Trease and Evans Pharmacognosy; Elsevier: London, UK, 2002. [Google Scholar]
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 4 ed.; Lavoisier: Paris, France, 2009; p. 1292. [Google Scholar]
- Van Gorkom, B.A.; de Vries, E.G.; Karrenbeld, A.; Kleibeuker, J.H. Review article: Anthranoid laxatives and their potential carcinogenic effects. Aliment. Pharmacol. Ther. 1999, 13, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Paneitz, A.; Westendorf, J. Anthranoid contents of rhubarb (Rheum undulatum L.) and other Rheum species and their toxicological relevance. Eur. Food Res. Technol. 1999, 210, 97–101. [Google Scholar] [CrossRef]
- Mueller, S.O.; Schmitt, M.; Dekant, W.; Stopper, H.; Schlatter, J.; Schreier, P.; Lutz, W.K. Occurrence of emodin, chrysophanol and physcion in vegetables, herbs and liquors. Genotoxicity and anti-genotoxicity of the anthraquinones and of the whole plants. Food Chem. Toxicol. 1999, 37, 481–491. [Google Scholar] [CrossRef]
- Rauwald, H.W. Herbal laxatives: Influence of anthrones? Anthraquinones on energy metabolism and ion transport in a model system. In Phytomedicines of Europe; American Chemical Society: Washington, DC, USA, 1998; Volume 691, pp. 97–116. [Google Scholar]
- Mobley, J.R. 1,3,6,8-trihydroxymethylanthraquinone. U.S. Patent 5039707 A. 5,039,707, 13 August 1991. [Google Scholar]
- Huang, H.C.; Chu, S.H.; Chao, P.D. Vasorelaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur. J. Pharmacol. 1991, 198, 211–213. [Google Scholar] [PubMed]
- Huang, H.C.; Lee, C.R.; Chao, P.D.; Chen, C.C.; Chu, S.H. Vasorelaxant effect of emodin, an anthraquinone from a Chinese herb. Eur. J. Pharmacol. 1991, 205, 289–294. [Google Scholar] [PubMed]
- Cheng, Y.W.; Kang, J.J. Emodin-induced muscle contraction of mouse diaphragm and the involvement of Ca2+ influx and Ca2+ release from sarcoplasmic reticulum. Br. J. Pharmacol. 1998, 123, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lin, C.C.; Yang, J.J.; Namba, T.; Hattori, M. Anti-inflammatory effects of emodin from Ventilago leiocarpa. Am. J. Chin. Med. 1996, 24, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Li, Z.F.; Liu, X.G.; Wu, Y.T.; Wang, J.X.; Wang, K.M.; Zhou, Y.F. Effects of emodin and baicalein on rats with severe acute pancreatitis. World J. Gastroenterol. 2005, 11, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Qiao, G.F.; Li, B.X.; Chai, L.M.; Li, Z.; Lu, Y.J.; Yang, B.F. Hypoglycaemic and hypolipidaemic effects of emodin and its effect on l-type calcium channels in dyslipidaemic-diabetic rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tiwari, S.; Shrivastava, A.; Srivastava, S.; Boudh, G.K.; Chourasia, S.K.; Chaturvedi, U.; Mir, S.S.; Saxena, A.K.; Bhatia, G.; et al. Antidyslipidemic effect and antioxidant activity of anthraquinone derivatives from Rheum emodi rhizomes in dyslipidemic rats. J. Nat. Med. 2014, 68, 363–371. [Google Scholar] [CrossRef] [PubMed]

| Genus | Species/Strain No | Name of Compounds Produced | Source of Isolation | Refs. |
|---|---|---|---|---|
| Alternaria | Al. eichorniae | 4-deoxyBostrycin, Bostrycin | Mar. Plant pathogen | [46] |
| Al. (SK11) | (+) α S-alterporriol C, 6-methylquinizarin, Alterporriol S, Austrocortinin | Mangrove Plant end. | [47] | |
| Al. sp. ZJ-2008003 | Alterporriol C, K–R, Altersolanol B and C, Macrosporin | Mar. Org end. | [48] | |
| Al. sp. ZJ9-6B | Alterporriols C–M, Altersolanol A, Dactylariol, Macrosporin, Physcion, TetrahydroAltersolanol B | Mar. Plant end. | [49] | |
| Aspergillus | A. glaucus | 10,10′-dimer of Emodin and Physcion, Catenarin, Cynodontin, Emodin, Erythroglaucin, Helminthosporin, Physcion, Questin, Rubrocristin, Tritisporin, Variecolorquinone A | Mangrove sed. | [50,51,52,53] |
| A. sp. 05F16 | 1-deoxytetrahydrobostrycin, Tetrahydrobostrycin | Algal end. | [54] | |
| A. sp. SCSIOF063 | (1′S)-7-chloroaverantin, 1′-O-methylaverantin 1′-O-methyl-7-chloroaverantin, 6-O-methyl-7-chloroaverantin, 6-O-methyl-7-chloroaverythrin, 6-O-methyl-7-bromoaverantin, 6,1′-O,O-dimethyl-7-chloroaverantin, 6,1′-O,O-dimethyl-7-bromoaverantin, 6,1′-O,O-dimethylaverantin, 7-chloroaverantin-1′-butyl ether, 7-chloroaverythrin | Sed. | [55] | |
| A. variecolor B-17 | (2S)-2,3-dihydroxypropyl1,6,8-trihydroxy-3-methyl-9,10-dioxoanthracene-2carboxylate, Catenarin, Emodin, Fallacinol, Physcion, Erythroglaucin, Questin, Questinol, Rubrocristin, Variecolorquinone A, | Sed. | [56] | |
| A. versicolor | 7-hydroxyemodin 6,8-methyl ether, Emodin, Isorhodoptilometrin-methyl ether, Methyl emodin | Algal end. | [57] | |
| A. versicolor EN-7 (Genbank no EU042148) | 6,8-di-O-methylversiconol 6,8-di-O-methylnidurufin 6,8-di-O-methylaverantin 6,8-di-O-methylversicolorin A, Aversin: (−)-isomer | Algal end. | [58] | |
| Curvularia | C. lunata | Cytoskyrin A, Lunatin | Mar. Org end. | [50,51,59,60] |
| Eurotium | E. cristatum (ECE) | Catenarin, Emodin, Erythroglaucyn, Physcion, Physcion anthrone, Questin, Rubrocristin | Mar. Org end. | [36,61] |
| E. repens | Catenarin, Erythroglaucyn, Physcion, Physcion anthrone | Mar. Org end. | [36,62] | |
| E. rubrum | 6,3-O-(α-d-ribofuranosyl)-questin, Questin | Mar. Plant end. | [63] | |
| Unidentified | Fungus Isolate 1850 and 2526 | Averufin, Nidurufin, versicolorin C | Mar. Plant end. | [64] |
| Fungus ZSUH-36 | 1′-O-methyl averantin, 6,8-di-O-methyl averufanin, 6,8-di-O-methyl averufin, 6,8,1′-tri-O-methyl averantin, Versicolorin C | Mar. Plant end. | [65] | |
| Fusarium | F. sp. No. B77 | 5-acetyl-2-methoxy-1,4,6-trihydroxy-anthraquinone | Mangrove Plant end. | [66] |
| F. sp. ZZF60 | 6,8-dimethoxy-1-methyl-2-(3-oxobutyl) anthraquinone | Mangrove Plant end. | [67] | |
| F. sp. No. ZH-210 | Fusaquinon A,B,C | Mangrove sed. | [68] | |
| F. sp. PSU-F14, F. sp. PSU-F135 | Austrocortirubin, Bostrycin | Mar. Org end. | [69] | |
| Halorosellinia | H. sp. (No. 1403) | 1,4,5,6,7,9-hexahydroxy-2-methoxy-7-methyl-5β,9β, 8aβ,6α,10aα—hexahydroanthracene10(10aH)-one, Austrocortirubin, Demethoxyaustrocortirubin, Hydroxy-9,10-anthraquinone, SZ-685C | Mar. Plant-derived | [70,71] |
| Lichens | Arthonia elegans, Biatorella conspersa, B. ochrophora, Pyrenula cerina, Sphaerophorus fragilis, Stereocaulon corticatulum,v. procerum, Trypethelium aeneum, T. aureomaculata, etc. | Physcion | Lichens | [72] |
| Caloplaca sp. | Phallacinol (=Teloschistin=Fallacinol)) | Lichen | [73,74] | |
| Caloplaca ehrenbergii, C. schaereri, C. spitsbergensis, etc. | 1-O-methyl-7-chloroemodin, 7-chloro-1,6,8-trihydroxy-3-methyl-10-anthrone, 7-chlorocitreorosein, 7-chloroemodic acid, 7-chloroemodin, 7-chloroemodinal, Emodin, Phallacinol, Physcion | Lichens | [75,76,77] | |
| Gliocladium sp. T 31 | Citreorosein, Emodin, Isorhodoptilometrin | Lichen | [78] | |
| Letrouitia hafellneri, L. leprolytoides | 7-chloroemodinal, 7-chloroemodin, Fragilin, Physcion | Lichens | [79,80] | |
| Microsphaeropsis | M. sp. | 1,3,6,8-tetrahydroxyanthraquinone, 1,3,6,8-tetrahydroxy-2-(1-hydroxyethyl)anthraquinone 1,3,6,8-tetrahydroxy-2-(1-methoxyethyl)anthraquinone 1,2,3,6,8-pentahydroxy-7-(1-methoxyethyl)anthraquinone | Mar. Org end. | [81] |
| Monodictys | M. sp. | Chrysophanol, Emodin, Monodictyquinone A, Pachybasin | Mar. Org end. | [14,82] |
| Nigrospora | N. spp. | 1-deoxytetrahydrobostrycin, 4-deoxybostrycin, Bostrycin, 4a-epi-9α-methoxydihydrodeoxybostrycin, 10-deoxybostrycin | Mar. Plant/Org end. | [68,83,84] |
| N. sp. MA75 | 4-deoxybostrycin, Bostrycin | Marine | [85] | |
| N. sp. 1403 | 4-deoxybostrycin, Bostrycin | Mangrove | [86] | |
| Paecilomyces | P. sp. (Tree 1-7) | Chrysophanol, Emodin | Mangrove | [87] |
| Penicillium | P. citrinum PSU-F51 (Accession no JQ66600) | Chrysophanol, Citreorosein, Emodin, Penicillanthranins A and B | Mar. Org end. | [88] |
| P. chrysogenum | Skyrin | Salt lake | [89] | |
| P. flavidorsum SHK1-27 | 6,8-O-dimethylaverufin, 8-O-methylaverufin, Averufin, Averantin, Versiconol, Versicolorin A&B, Nidurufin | Marine | [90] | |
| P. oxalicum 2-HL-M-6 | Aloe emodin, Chrysophanol, Citreorosein, Citreorosein-3-O-sulfate, Emodin, Emodin-3-O-sulfate, Isorhodoptilometrin | Mangrove sed. | [91] | |
| Phomopsis | P. sp. PSU-MA214 | Phomopsanthraquinone, 1-hydroxy-3-methoxy-6-methylanthraquinone, Ampelanol, Macrosporin | Mangrove Plant endo | [92] |
| Stemphylium | S. sp. 33231 | 2-O-acetylaltersolanol B, Alterporriol T–W, Altersolanol B&C, Auxarthrol C, Macrosporin | Mangrove Plant end. | [93] |
| S. globuliferum | 6-O-methylalaternin, Acetylalterporriol D and E, Alterporriol D and E, Altersolanol A,B and C, Dihydroaltersolanol B and C, Macrosporin, Stemphylanthranol A and B | Salt lake Plant end. | [94] | |
| Trichoderma | T. aureoviride PSU-F95 | Coniothranthraquinone 1, Trichodermaquinone | Mar. Org end. | [88] |
| Xylaria | X. sp. 2508 | Altersolanol A, Bostrycin, Deoxybostrycin, Xylanthraquinone | Marine | [95] |
| Mol. Formula/Mol. mass | Trivial name | Structure | IUPAC NAME | Source | Refs |
|---|---|---|---|---|---|
| C14H8O3/224 | Hydroxy-9,10-anthraquinone  | 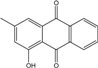 | 1-hydroxy-3-methylanthraquinone | Halorosellinia sp. No. 1403 | [70] |
| C15H10O3/238 | Pachybasin | 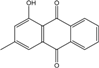 | 1-hydroxy-3-methylanthraquinone | Monodictys sp. | [14,82] |
| C15H10O4/254 | Chrysophanol | 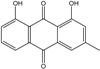 | 1,8-dihydroxy-3-methylanthraquinone | Monodictys sp. Paecilomyces sp. P. citrinum PSU-F51 P. oxalicum 2-HL-M-6 | [14,82] [87] [88] [91] |
| C15H10O4/254 | 6-Methylquinizarin | 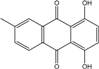 | 1,4-dihydroxy-methylanthraquinone | Al. sp. (SK11) | [47] |
| C16H12O4/268 | 1-Hydroxy-3-methoxy-6-methylanthraquinone | 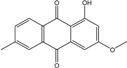 | ---- | Phomopsis sp. PSU-MA214 | [92] |
| C15H10O5/270 | Aloe emodin  | 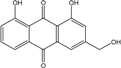 | 1,8-dihydroxy-3-(hydroxymethyl)anthraquinone | P. oxalicum 2-HL-M-6 | [91] |
| C15H10O5/270 | Emodin  | 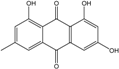 | 1,3,8-trihydroxy-6-methylanthraquinone | A. glaucus A. variecolor B-17 A. versicolor Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) Eurotium Cristatum Gliocladium sp. T31 Monodictys sp. Paecilomyces sp. P. citrinum PSU-F51 P. oxalicum 2-HL-M-6 | [91] [56] [61] [75,76,77,79,96] [36] [78] [14,82] [87] [88] [57] |
| C15H10O5/270 | Helminthosporin  | 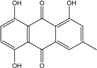 | 1,5,8-trihydroxy-3-methylanthraquinone | A. glaucus | [50,51,52,53] |
| C14H8O6/272 | 1,3,6,8-Tetrahydroxyanthraquinone | 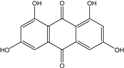 | ---- | A.versicolor Microsphaeropsis | [97] [81] |
| C15H14O5/274 | Coniothranthraquinone 1 | 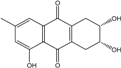 | (2S,3R)-2,3,5-trihydroxy-7-methyl-1,2,3,4-tetrahydroanthraquinone | Trichoderma aureoviride (PSU-F95) | [88,98] |
| C16H12O5/284 | 1-Methylemodin  | 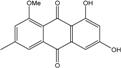 | 1,3-dihydroxy-8-methoxy-6-methylanthraquinone | A. versicolor | [57] |
| C16H12O5/284 | Austrocortinin  | 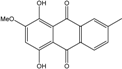 | 1,4-dihydroxy-2-methoxy-7-methylanthraquinone | Al. sp. (SK11) | [47] |
| C16H12O5/284 | Macrosporin | 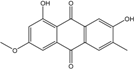 | 1,7-dihydroxy-3-methoxy-6-methylanthraquinone | Al. sp. ZJ9-6B Al. sp. ZJ-2008003 Phomopsis sp. PSU-MA214 Stemphylium globuliferum Stemphylium sp. 33231 | [49] [48] [92] [94] [93] |
| C16H12O5/284 | Marcrospin | 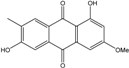 | 1,6-dihydroxy-3-methoxy-7-methylanthraquinone | Al. sp. ZJ9-6B | [49] |
| C16H12O5/284 | Monodictyquinone A  | 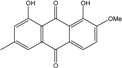 | 1,8-dihydroxy-2-methoxy-6-methylanthraquinone | Monodictys sp. | [14,82] |
| C16H12O5/284 | Phallacinol/Fallacinol | 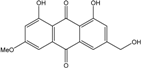 | 1,8-dihydroxy-3-(hydroxy-methyl)-6-methoxyanthraquinone | A. variecolor B-17 Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) | [99] [73,74,76,77,79,96] |
| C16H12O5/284 | Physcion  | 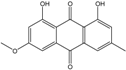 | 1,8-dihydroxy-3-methoxy-6-methylanthraquinone | Al. sp. ZJ9-6B A. glaucus A. variecolor B-17 Eurotium repens Eurotium cristatum Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) Letrouitia hafellneri, L. leprolytoides, Arthonia elegans, Biatorella conspersa, B. ochrophora, Pyrenula cerina, Sphaerophorus fragilis, Stereocaulon corticatulum, v. procerum, Trypethelium aeneum, T. aureomaculata | [49] [50,51,52,53] [56] [62] [36,61] [75,76,77,79,96] [72,80] |
| C16H12O5/284 | Questin | 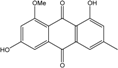 | 1,6-dihydroxy-8-methoxy-3-methylanthraquinone | A. glaucus A. variecolor B-17 Eurotium cristatum (ECE) Eurotium rubrum | [50],[51] [56] [52,53] [36,61,63] |
| C15H10O6/286 | Catenarin  | 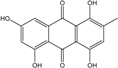 | 1,4,5,7-tetrahydroxy-2-methylanthraquinone | A. glaucus A. variecolor B-17 Eurotium cristatum (ECE) Eurotium repens | [50,51] [56] [52,53] [36,62] |
| C15H10O6/286 | Citreorosein  | 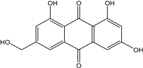 | ω-hydroxyemodin (OHM) or 1,3,8-trihydroxy-6-(hydroxymethyl) anthraquinone | Gliocladium. sp. T31 P. citrinum PSU-F51 P. oxalicum 2-HL-M-6 | [78] [88] [91] |
| C15H10O6/286 | Cynodontin  | 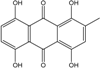 | 1,4,5,8-tetrahydroxy-2-methylanthraquinone | A. glaucus | [50,51,52,53] |
| C15H10O6/286 | Lunatin | 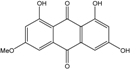 | 1,3,8-trihydroxy-6-methoxyanthraquinone | Curvularia lunata | [50,51,59,60] |
| C15H10O7/286 | Tritisporin  | 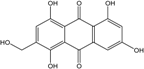 | 1,4,5,7-tetrahydroxy-2-(hydroxylmethyl) anthraquinone | A. glaucus | [50,51,52,53] |
| C15H14O6/290 | Trichodermaquinone | 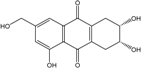 | (2S,3R)-2,3,5-trihydroxy-7-(hydroxylmethyl)-1,2,3,4-tetrahydroanthraquinone | Trichoderma aureoviride (PSU-F95) | [88,98] |
| C15H14O6/290 | Demethoxyaustrocortirubin  | 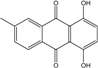 | 1,4-dihydroxy-6-methylanthraquinone | Halorosellinia sp. No. 1403 | [70,71] |
| C15H14O6/290 | 7-Hydroxyemodin 6,8-methyl ether | 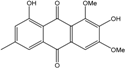 | 2,8-dihydroxy-1,3-dimethoxy-6-methyl anthraquinone | A. versicolor | [57] |
| C16H10O4/300 | Erythroglaucin  | 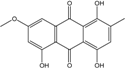 | 1, 4, 5-trihydroxy-7-methoxy-2-methylanthraquinone | A. glaucus A. variecolor B-17 Eurotium cristatum (ECE) Eurotium repens | [50,51,52,53] [56] [36,61] [36] |
| C16H12O6/300 | Carviolin | 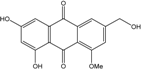 | 1,3-dihydroxy-6-(hydroxymethyl)-8-methoxyanthraquinone | P. dravuni | [100] |
| C16H12O6/300 | Questinol | 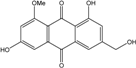 | 1,6-dihydroxy-3-(hydroxymethyl)-8-methoxyanthraquinone | A. variecolor B-17 | [56] |
| C16H12O6/300 | Rubrocristin  | 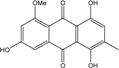 | 1,4,7-trihydroxy-5-methoxy-2-methylanthraquinone | A. glaucus A. variecolor B-17 Eurotium cristatum (ECE) | [50,51,52,53] [56] [36,61] |
| C16H12O6/300 | 6-O-methylalaternin  | 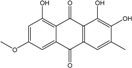 | 1,2,8-trihydroxy-6-methoxy-3-methylanthraquinone | Stemphylium globuliferum | [94] |
| C16H12O6/300 | 1,4,6-Trihydroxy-2-methoxy-7-methyl-anthraquinone  | 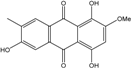 | 3,5,8-trihydroxy-7-methoxy-2-methylanthraquinone | Halorosellinia sp. No. 1403 | [70] |
| C15H9O5Cl/304 | 7-Chloroemodin | 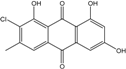 | ---- | Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) | [75,76,77,79,96] |
| C16H16O6/304 | Altersolanol B  | 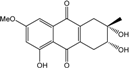 | (2S,3R)-2,3,5-trihydroxy-7-methoxy-2-methyl-1,2,3,4-tetrahydroanthraquinone | Al. sp. ZJ-2008003 Stemphylium sp. 33231 | [48] [93] |
| C16H18O6/306 | Fusaquinon A | 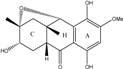 | (2R,3S,4aR,9S,9aS)-3,5,8-trihydroxy-7-methoxy-2-methyl-2,3,4,4a,9,9a-hexahydro-2,9-epoxyanthracen-10(1H)-one | Fusarium sp. No. ZH-210 | [68] |
| C16H18O6/307 | Dihydroaltersolanol B  | 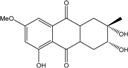 | (2S,3R)-2,3,5-trihydroxy-7-methoxy-2-methyl-1,2,3,4,4a,9a-hexahydroanthraquinone | Stemphylium globuliferum | [94] |
| C15H19O7/311 | Xylanthraquinone | 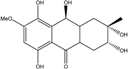 | ---- | Xylaria sp. 2508 | [95] |
| C17H14 O6/314 | Isorhodoptilometrin  | 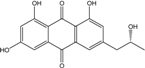 | (R)-1,3,8-trihydroxy-6-(2-hydroxypropyl)anthraquinone | Gliocladium sp. T31 P. oxalicum 2-HL-M-6 | [78] [91] |
| C16H12O7/317 | 1,3,6,8-Tetrahydroxy-2-(1-hydroxyethyl) anthraquinone  | 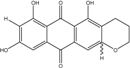 | 1,3,6,8-tetrahydroxy-2-(1-hydroxyethyl) anthracene-9,10-dione | Microsphaeropsis | [81] |
| C16H11O5Cl/318 | 1-O-Methyl-7-chloroemodin | 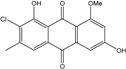 | 2-chloro-1,6-dihydroxy-8-methoxy-3-methylanthraquinone | Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) | [75,76,77,79,96] |
| C15H9O6Cl/320 | 7-Chlorocitreorosein | 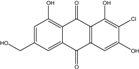 | ---- | Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) | [75,76,77,79,96] |
| C16H16O7/320 | Austrocortirubin  | 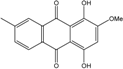 | 1,4-dihydroxy-2-methoxy-7-methylanthraquinone | Fusarium spp. PSU-F14 and PSU-F135 Halorosellinia sp. No. 1403 Nigrospora sp. ZJ-2010006 | [69] [70,71] [84] |
| C16H16O7/320 | Altersolanol C  | 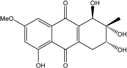 | (1R,2R,3R)-1,2,3,5-tetra-hydroxy-7-methoxy-2-methyl-1,2,3,4-tetra-hydroanthraquinone | Al. sp. ZJ9-6B Al. sp. ZJ-2008003 Stemphylium sp. 33231 | [49] [48] [93] |
| C16H16O7/320 | 4-Deoxybostrycin | 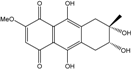 | (2R,3S,4aS,9aS,10R)-2,3,5,8,10-pentahydroxy-6-methoxy-3-methyl-1,3,4,4a,9a,10-hexahydroanthracen-9(2H)-one | Nigrospora sp. 1403 Nigrospora sp. MA75 | [68,83,84,86,101] [85] |
| C16H16O7/320 | 2,3,5,8-Tetrahydroxy-7-methoxy-2-methyl-1,2,3,4-tetrahydroanthraquinone | ---- | 2,3,5,8-tetrahydroxy-7-methoxy-2-methyl-1,2,3,4-tetrahydroanthracene-9,10-dione | Al. eichorniae | [46] |
| C16H16O7/321 | 10-Deoxybostrycin  | 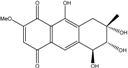 | --- | Nigrospora sp. | [84] |
| C16H18O7/323 | Dihydroaltersolanol C | 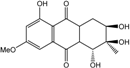 | (1R,2R,3R)-1,2,3,5-tetra-hydroxy-7-methoxy-2-methyl-1,2,3,4,4a,9a-hexahydroanthraquinone | Stemphylium globuliferum | [94] |
| C16H20O7/324 | Fusaquinon C  | 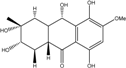 | (2S,3R,4aR,9aR,10S)-2,3,5,8,10-pentahydroxy-6-methoxy-3-methyl-1,3,4,4a,9a,10-hexahydroanthracen-9(2H)-one | Fusarium sp. No. ZH-210 | [68] |
| C16H21O7/325 | 1-Deoxytetrahydrobostrycin | 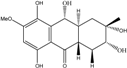 | (2R,3S)-2,3,5,8,10-pentahydroxy-6-methoxy-3-methyl-1,3,4,4a,9a,10-hexahydroanthracen-9(2H)-one | A. sp. 05F16 Nigrospora sp. | [54] [83] |
| C15H20O8/328 | Fragilin  | 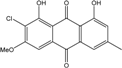 | 2-chloro-1,8-dihydroxy-3-methoxy-6-methylanthraquinone | Letrouitia hafellneri L. leprolytoides | [79,80] |
| C17H12O7/328 | 5-Acetyl-2-methoxy-1,4,6-trihydroxyanthraquinone | 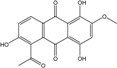 | ---- | Fuarium sp. B77 | [66] |
| C18H16O6/328 | Isorhodoptilometrin-1-methylether | 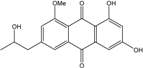 | 1,3-dihydroxy-6-2-hydroxypropyl-8-methoxyanthraquinone | A. versicolor | [57] |
| C17H14O7/329 | 1,3,6,8-Tetrahydroxy-2-(1-methoxyethyl)anthraquinone | 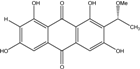 | --- | Microsphaeropsis | [81] |
| C18H20O6/332 | Phomopsanthraquinone | 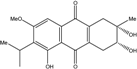 | (2R,3S)-7-ethyl-1,2,3,4-tetrahydro-2,3,8-trihydroxy-6-methoxy-3-methylanthraquinone | Phomopsis sp. PSU-MA214 | [92] |
| C16H16O8/336 | Altersolanol A | 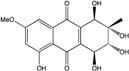 | (1R,2S,3R,4S)-1,2,3,4,5-pentahydroxy-7-methoxy-2-methyl-1,2,3,4-tetrahydroanthraquinone | Stemphylium globuliferum Xylaria sp. 2508 | [94] [95] |
| C16H16O8/336 | Bostrycin | 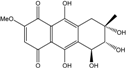 | (5S,6R,7S)-5,6,7,9,10-pentahydroxy-2-methoxy-7-methyl-5,6,7,8-tetrahydroanthracene-1,4-dione | A. sp. strain 05F16 Al. eichorniae Fusarium spp. PSU-F14/PSU-F135 Halorosellinia sp. No. 1403 Nigrospora sp. Xylaria sp. 2508 | [46,102] [69] [68,86,101,103] [84] [54] [95] |
| C16H18O8/338 | SZ-685C | 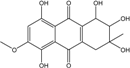 | 1,2,3,5,8-pentahydroxy-6-methoxy-3-methyl-1,2,3,4-tetrahydroanthraquinone | Halorosellinia sp. No. 1403 | [70,101,104,105,106,107] |
| C16H20O8/340 | Fusaquinon B  | 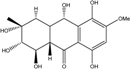 | (1R,2S,3R,4aR,9aS,10S)-1,2,3,5,8,10-hexahydroxy-6-methoxy-3-methyl-1,3,4,4a,9a,10-hexahydroanthracen-9(2H)-one | Fusarium sp. No. ZH-210 | [68] |
| C16H21O8/340 | Tetrahydroxybostrycin | 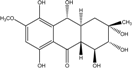 | 1,2,3,5,8,10-hexahydroxy-6-methoxy-3-methyl-1,3,4,4a,9a,10-hexahydroanthracen-9(2H)-one | A. sp. 05F16 Nigrospora sp. MA75 | [54] [85] |
| C18H12O7/340 | Versicolorin C | 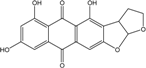 | 4,6,8-trihydroxy-3,3a-dihydroanthra[2,3-b]furo[3,2-d]furan-5,10(2H,12aH)-dione | Fungus ZSUH-36 Fungus Isolate 1850 and isolate 2526 | [65] [64] |
| C18H18O7/346 | 2-O-Acetylaltersolanol B | 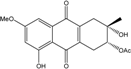 | (2R,3S)-3,8-dihydroxy-6-methoxy-3-methyl-9,10-dioxo-1,2,3,4,9,10-hexahydroanthracen-2-yl acetate | Stemphylium sp. 33231 | [93] |
| C17H14O8/347 | 1,2,3,6,8-Pentahydroxy-7-(1-methoxyethyl)anthraquinone  | 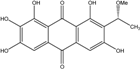 | 1,2,3,6,8-pentahydroxy-7-(1-methoxyethyl)anthracene-9,10-dione | Microsphaeropsis | [81] |
| C15H10O8S/350 | Emodin-3-O Sulfate | 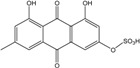 | 4,5-dihydroxy-7-methyl-9,10-dioxo-9,10-dihydroanthracen-2-yl hydrogen sulfate | P. oxalicum 2-HL-M-6 | [91] |
| C16H15O9/351 | Auxarthrol C  | 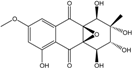 | (1S,2R,3R,4R,4aR,9aS)-1,2,3,4,5-pentahydroxy-7-methoxy-2-methyl-1,2,3,4-tetrahydro-4a,9a-epoxyanthraquinone | Stemphylium sp. 33231 | [93] |
| C19H11O7/351 | 8-O-MethylversicolorinB | 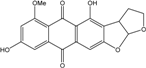 | 4,8-dihydroxy-6-methoxy-3,3a-dihydroanthra[2,3-b]furo[3,2-d]furan-5,10(2H,12aH)-dione | A. versicolor endolichenic | [108] |
| C21H20O5/352 | 6,8-Dimethoxy-1-methyl-2-(3-oxobutyl)anthraquinone | 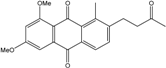 | ---- | Fusarium sp. ZZF60 | [67] |
| C19H13O7/353 | 8-O-Methylversicolorin A  | 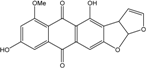 | 4,8-dihydroxy-6-methoxyanthra[2,3-b]furo[3,2-d]furan-5,10(3aH,12aH)-dione | A. versicolor endolichenic | [108] |
| C20H18O6/354 | Averythrin | 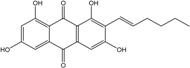 | (E)-2-(hex-1-en-1-yl)-1,3,6,8-tetrahydroxyanthraquinone | A. sp. SCSIO F063 A. versicolor endolichenic | [55] [108] |
| C30H18O10/358 | Skyrin  | 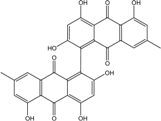 | 2,2′,4,4′,5,5′-hexahydroxy-7,7′-dimethyl-[1,1′-bianthracene]-9,9′,10,10′-tetraone | P. chrysogenum | [89] |
| C16H12O8S/364 | Macrosporin-7-O-sulfate | 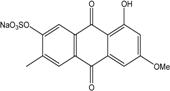 | Sodium 8-hydroxy-6-methoxy-3-methyl-9,10-dioxo-9,10-dihydroanthracen-2-yl sulfate | Stemphylium sp. 33231 | [93] |
| C15H9O9S/365 | Citreorosein-3-O-sulfate  | 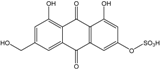 | 4,5-dihydroxy-7-(hydroxymethyl)-9,10-dioxo-9,10-dihydroanthracen-2-yl hydrogen sulfate | P. oxalicum 2-HL-M-6 | [91] |
| C20H14O7/365 | 6,8-di-O-methylversico-lorinA | 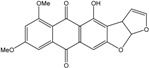 | 4-hydroxy-6,8-dimethoxyanthra[2,3-b]furo[3,2-d]furan-5,10(3aH,12aH)-dione | A. versicolor endolichenic A. versicolor EN-7 (Genbank no EU042148) | [108] [58] |
| C21H19O6/367 | 8-O-Methylaverythrin | 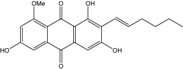 | (E)-2-(hex-1-en-1-yl)-1,3,6-trihydroxy-8-methoxyanthraquinone | A. versicolor endolichenic | [108] |
| C20H16O7/368 | Aversin | 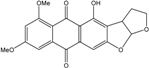 | 4-hydroxy-6,8-dimethoxy-3,3a-dihydroanthra[2,3-b]furo[3,2-d]furan-5,10(2H,12aH)-dione | A. versicolor endolichenic | [108] |
| C20H16O7/368 | Aversin : (−)-isomer | 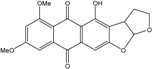 | 4-hydroxy-6,8-dimethoxy-3,3a-dihydroanthra[2,3-b]furo[3,2-d]furan-5,10(2H,12aH)-dione | A. versicolor EN-7 (Genbank no EU042148) | [58] |
| C20H20O7/372 | Averantin | 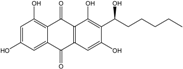 | (S)-1,3,6,8-tetrahydroxy-2-(1-hydroxyhexyl)anthraquinone | A. sp. SCSIO F063 | [55] |
| C20H20O7/372 | Averantin = (S)-(−)-averantin | 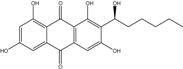 | (S)-1,3,6,8-tetrahydroxy-2-(1-hydroxyhexyl)anthraquinone | A. sp. SCSIO F063 | [55] |
| C21H18O7/382 | 6-O-Methylaverufin | 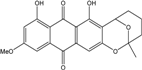 | 7,9-dihydroxy-11-methoxy-2-methyl-3,4,5,6-tetrahydro-2H-2,6-epoxyanthra[2,3-b]oxocine-8,13-dione | Fungus ZSUH-36 A. versicolor EN-7 | [65] [58] |
| C20H16O8/384 | Nidurufin | 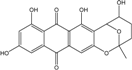 | 5,7,9,11-tetrahydroxy-2-methyl-3,4,5,6-tetrahydro-2H-2,6-epoxyanthra[2,3-b]oxocine-8,13-dione | Fungus Isolate 1850 and isolate 2526 | [64] |
| C20H15O8/386 | Averufin | 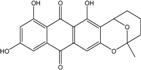 | 7,9,11-trihydroxy-2-methyl-3,4,5,6-tetrahydro-2H-2,6-epoxyanthra[2,3-b]oxocine-8,13-dione | A. versicolor Fungus ZSUH-36 Fungus Isolate 1850 and isolate 2526 | [109] [65] [64] |
| C21H22O7/386 | 1′-O-Methylaverantin  | 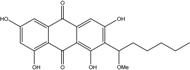 | 1,3,6,8-tetrahydroxy-2-(1-methoxyhexyl)anthraquinone | A. sp. SCSIO F063 Fungus ZSUH-36 | [55] [65] |
| C19H15O9/388 | (2S)-2,3-Dihydroxy-propyl-1,6,8-trihydroxy-3-methyl-9,10-dioxoanthracene-2-carboxylate | 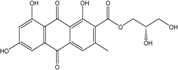 | (1S,5′S,6′R,7′S,8′R)-1′,2,5′,6′,7′,8,8′-heptahydroxy-3′,6-dimethoxy-3,6′-dimethyl-5′,6′,7′,8′,8′a,10′a-hexahydro-[1,2′-bianthracene]-9,9′,10,10′-tetraone | A. variecolor B-17 | [56] |
| C20H17ClO6/388 | 7-Chloroaverythrin | 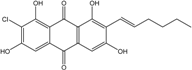 | (E)-2-chloro-7-(hex-1-en-1-yl)-1,3,6,8-tetrahydroxyanthraquinone | A. sp. SCSIO F063 | [55] |
| C20H20O8/388 | 6,8-Di-O-methylversiconol | 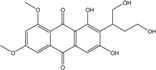 | 2-(1,4-dihydroxybutan-2-yl)-1,3-dihydroxy-6,8-dimethoxyanthraquinone | A. versicolor EN-7 (Genbank no EU042148) | [58] |
| C22H20O7/396 | 6,8-Di-O-methylaverufin | 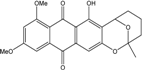 | 7-hydroxy-9,11-dimetho-xy-2-methyl-3,4,5,6-tetra-hydro-2H-2,6-epoxyanthra[2,3-b]oxocine-8,13-dione | A. versicolor endolichenic | [108] |
| C22H20O7/396 | 6,8-Di-O-methylnidurufin | 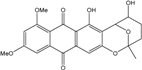 | 5,7-dihydroxy-9,11-dime-thoxy-2-methyl-3,4,5,6-tetrahydro-2H-2,6-epoxyanthra[2,3-b]oxocine-8,13-dione | A. versicolor endolichenic A. versicolor EN-7 (Genbank no EU042148) | [108] [58] |
| C22H22O7/398 | 6,8-Di-O-methylaverufanin  | 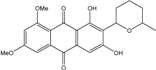 | 1,3-dihydroxy-6,8-dimethoxy-2-(6-methyltetra-hydro-2H-pyran-2-yl)anthracene-9,10-dione | Fungus ZSUH-36 | [65] |
| C22H24O7/400 | 6,1′-O,O-Dimethylaverantin | 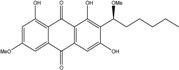 | (S)-1,3,8-trihydroxy-6-methoxy-2-(1-methoxyhexyl)anthraquinone | A. sp. SCSIO F063 | [55] |
| C20H17O9/401 | Variecolorquinone A  |  | (S)-2,3-dihydroxypropyl 1,6-dihydroxy-8-methoxy-3-methyl-9,10-dioxo-9,10-dihydroanthracene-2-carboxylate | A.glaucus A. variecolor B-17 | [50,51,52,53] [56] |
| C22H24O7/401 | 6,8-Di-O-methylaveran-tin | 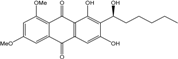 | (S)-1,3-dihydroxy-2-(1-hydroxyhexyl)-6,8-dimetho-xyanthraquinone | A. versicolor EN-7 (Genbank no EU042148) A. sp. SCSIO F063 | [58] [55] |
| C21H19ClO6/402 | 6-O-Methyl-7-chloroaverythrin  | 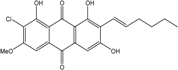 | (E)-2-chloro-7-(hex-1-en-1-yl)-1,6,8-trihydroxy-3-methoxyanthraquinone | A. sp. SCSIO F063 | [55] |
| C20H19ClO7/407 | (1′S)-7-Chloroaverantin | 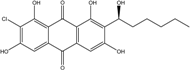 | (S)-2-chloro-1,3,6,8-tetrahydroxy-7-(1-hydroxyhexyl)anthraquinone | A. sp. SCSIO F063 | [55] |
| C22H23ClO7/407 | 6,1′-O,O-Dimethyl-7-chloroaverantin  | 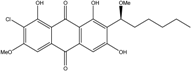 | (S)-2-chloro-1,6,8-trihydroxy-3-methoxy-7-(1-methoxyhexyl)anthraquinone | A. sp. SCSIO F063 | [55] |
| C23H26O7/414 | 6,8,1′-Tri-O-methyl-averantin | 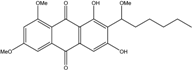 | 1,3-dihydroxy-6,8-dimethoxy-2-(1-methoxyhexyl)anthraquinone | A.versicolor endolichenic Fungus ZSUH-36 | [108] [65] |
| C21H19O9/415 | 6-3-O-(Ribofuranosyl)questin | 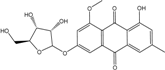 | 1,6-dihydroxy-6-O-(ribofuranosyl)-8-methoxy-3-methylanthraquinone | Eurotium rubrum | [63] |
| C21H21ClO7/420 | 1′-O-methyl-7-chloro averantin  | 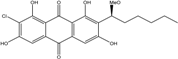 | (S)-2-chloro-1,3,6,8-tetrahydroxy-7-(1-methoxy-hexyl)anthraquinone | A. sp. SCSIO F063 | [55] |
| C21H21ClO7/421 | 6-O-methyl-7-chloro-averantin | 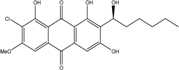 | (S)-2-chloro-1,6,8-trihydroxy-7-(1-hydroxyhexyl)-3-methoxyanthraquinone | A. sp. SCSIO F063 | [55] |
| C24H28O8/444 | Averantin-1′-butyl ether | 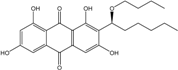 | (S)-2-(1-butoxyhexyl)-1,3,6,8-tetrahydroxy-anthraquinone | A. sp. SCSIO F063 | [55] |
| C24H27ClO7/463 | 7-Chloroaverantin-1′-butyl ether  | 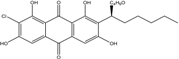 | (S)-2-(1-butoxyhexyl)-7-chloro-1,3,6,8-tetrahy-droxyanthraquinone | A. sp. SCSIO F063 | [55] |
| C21H21BrO7/465 | 6-O-Methyl-7-bromoaverantin | 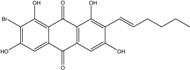 | (S)-2-bromo-1,6,8-trihydroxy-7-(1-hydroxyhexyl)-3-methoxyanthraquinone | A. sp. SCSIO F063 | [55] |
| C22H23BrO7/479 | 6,1′-O,O-Dimethyl-7-bromoaverantin  | 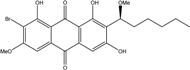 | (S)-2-bromo-1,6,8-trihydroxy-3-methoxy-7-(1-methoxyhexyl)anthraquinone | A. sp. SCSIO F063 | [55] |
| C24H23O11/487 | Macrosporin2-O-(6′-acetyl)-a-d-glucopyranoside | 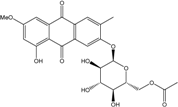 | ((2R,3S,4S,5R,6R)-3,4,5-trihydroxy-6-((8-hydroxy-6-methoxy-3-methyl-9,10-dioxo-9,10-dihydroanthracen-2-yl)oxy)tetrahydro-2H-pyran-2-yl)methyl acetate | Stemphylium sp. 33231 | [93] |
| C28H24O10/520 | Penicillanthranin A  | 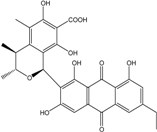 | (1S,3R,4S)-1-(6-ethyl-1,3,8-trihydroxy-9,10-dioxo-9,10-dihydroanthracen-2-yl)-6,8-dihy-droxy-3,4,5-trimethyliso-chroman-7-carboxylic acid | P. citrinum PSU-F51 | [88] |
| C28H24O11/536 | Penicillanthranin B | 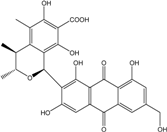 | (1S,3R,4S)-6,8-dihydroxy-3,4,5-trimethyl-1-(1,3,8-trihydroxy-6-(hydroxy-methyl)-9,10-dioxo-9,10-dihydroanthracen-2-yl)isochroman-7-carboxylic acid | P. citrinum PSU-F51 | [88] |
| C32H24O8/536 | (trans)-R (cis)-Emodin-Physcion bianthrone | 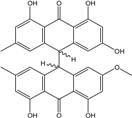 | 2,4,4′,5,5′-pentahydroxy-2′-methoxy-7,7′-dimethyl-[9,9′-bianthracene]-10,10′(9H,9′H)-dione | A. glaucus | [50,51,52,53] |
| C32H21O10/565 | Alterporriol Q  | 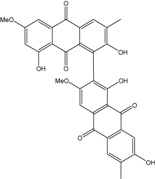 | 1′,2,7′,8-tetrahydroxy-3′,6-dimethoxy-3,6′-dimethyl-[1,2′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ-2008003 | [48] |
| C32H21O10/565 | Alterporriol R | 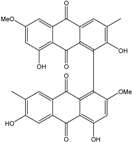 | 2,4′,6′,8-tetrahydroxy-2′,6-dimethoxy-3,7′-dimethyl-[1,1′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ-2008003 | [48] |
| C32H22O10/566 | Alterporriol V  | 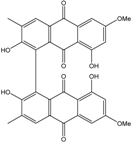 | 2,2′,8,8′-tetrahydroxy-6,6′-dimethoxy-3,3′-dimethyl-[1,1′-bianthracene]-9,9′,10,10′-tetraone | Stemphylium sp. 33231 | [93] |
| C30H22O12/574 | Cytoskyrin A | 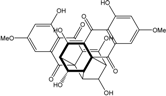 | (6R,14R,17S,18R,19R,20S)-1,7,9,15,17,20-hexahydroxy-3,11-dimethoxy-6,13a,5a,14-(epibutane[1,2,3,4]tetrayl)cycloocta[1,2-b:5,6-b′]dinaphtha-lene-5,8,13,16(6H,14H)-tetraone | Curvularia lunata | [50,51,59,60] |
| C32H26O10/586 | Alterporriol K | 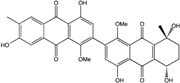 | (5S,8R)-4,4′,5,7′,8-penta-hydroxy-1,1′-dimethoxy-6′,8-dimethyl-5,6,7,8-tetrahydro-[2,2′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ9-6B Al. sp. ZJ-2008003 | [49] [48] |
| C32H30O13/590 | Alterporriol T | 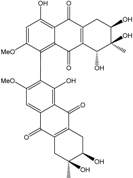 | (6R,6′S,7R,7′R,8R)-1′,4,6,6′,7,7′,8-heptahydroxy-2,3′-dimethoxy-6′,7-dime-thyl-5,5′,6,6′,7,7′,8,8′-octahydro-[1,2′-bianthracene]-9,9′,10,10′-tetraone | Stemphylium sp. 33231 | [93] |
| C32H25O12/601 | Alterporriol L | 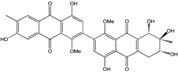 | (6S,7R,8R)-4,4′,6,7,7′,8-hexahydroxy-1,1′-dimethoxy-6′,7-dimethyl-5,6,7,8-tetrahydro-[2,2′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ9-6B Al. sp. ZJ-2008003 | [49] [48] |
| C32H25O12/601 | Alterporriol M | 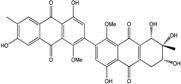 | (6S,7S,8R)-4,4′,6,7,7′,8-hexahydroxy-1,1′-dimethoxy-6′,7-dimethyl-5,6,7,8-tetrahydro-[2,2′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ9-6B Al. sp. ZJ-2008003 | [49] [48] |
| C32H25O12/601 | Alterporriol P  | 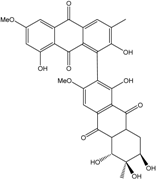 | (5′R,6′R,7′R)-1′,2,5′,6′,7′,8-hexahydroxy-3′,6-dimethoxy-3,6′-dimethyl-5′,6′,7′,8′,8′a,10′a-hexahydro-[1,2′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ-2008003 | [48] |
| C32H26O12/602 | Alterporriol W | 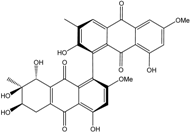 | (1′R,6R,7R,8R)-2′,4,6,7,8,8′-hexahydroxy-2,6′-dimethoxy-3′,7-dimethyl-5,6,7,8-tetrahydro-[1,1′-bianthracene]-9,9′,10,10′-tetraone | Stemphylium sp. 33231 | [93] |
| C32H30O12/606 | Alterporriol U  | 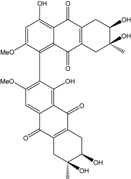 | (6R,6′S,7S,7′R)-1′,4,6,6′,7,7′-hexahydroxy-2,3′-di-methoxy-6′,7-dimethyl-5,5′,6,6′,7,7′,8,8′-octahy-dro-[1,2′-bianthracene]-9,9′,10,10′-tetraone | Stemphylium sp. 33231 | [93] |
| C31H32O13/612 | Alterporriol S | 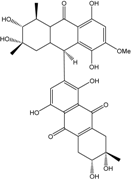 | (2′S,3′R,4′S,6R,7S,9′R)-1,2′,3′,4,5′,6,7,8′-octahy-droxy-7′-methoxy-2′,4′,7-trimethyl-2′,3′,4′,4′a,5,6,7,8,9′,9′a-decahydro-[2,9′-bianthracene]-9,10,10′(1′H)-trione | Al. sp. (SK11) | [47] |
| C32H25O13/617 | (+)-aS-alterporriol C  | 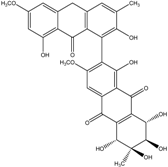 | (1S,5′S,6′R,7′S,8′R)-1′,2,5′,6′,7′,8,8′-heptahydroxy-3′,6-dimethoxy-3,6′-dime-thyl-5′,6′,7′,8′,8′a,10′a-hexahydro-[1,2′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. (SK11) | [47] |
| C32H25O13/617 | Alterporriol C | 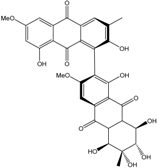 | (1S,5′R,6′S,7′R,8′S)-1′,2,5′,6′,7′,8,8′-heptahydroxy-3′,6-dimethoxy-3,6′-dime-thyl-5′,6′,7′,8′,8′a,10′a-hexahydro-[1,2′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. (SK11) Al. sp. ZJ-2008003 | [47] [48] |
| C32H29O14/637 | Alterporriol N  | 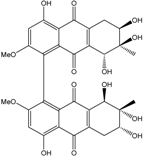 | (6R,6′R,7R,7′R,8R,8′R)-4,4′,6,6′,7,7′,8,8′-octahy-droxy-2,2′-dimethoxy-7,7′-dimethyl-5,5′,6,6′,7,7′,8,8′-octahydro-[1,1′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ-2008003 | [48] |
| C32H29O14/637 | Alterporriol O | 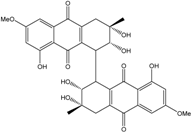 | (2R,2′R,3S,3′S)-2,2′,3,3′,8,8′-hexahydroxy-6,6′-dimethoxy-3,3′-dimethyl-1,1′,2,2′,3,3′,4,4′-octahy-dro-[1,1′-bianthracene]-9,9′,10,10′-tetraone | Al. sp. ZJ-2008003 | [48] |
| C34H33O17/713 | Acetylalterporriol D  | 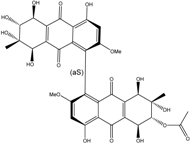 | (1′S,5S,5′S,6R,6′R,7S,7′S,8R,8′R)-4,4′,5,5′,6′,7,7′,8,8′-nonahydroxy-2,2′-dimethoxy-7,7′-dimethyl-9,9′,10,10′-tetraoxo-5,5′,6,6′,7,7′,8,8′,9,9′,10,10′-dodecahydro-[1,1′-bianthracen]-6-yl acetate | Stemphylium globuliferum | [94] |
| C34H33O17/713 | Acetylalterporriol E | 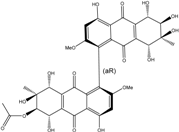 | (1′R,5S,5′S,6R,6′R,7S,7′S,8R,8′R)-4,4′,5,5′,6′,7,7′,8,8′-nonahydroxy-2,2′-dimethoxy-7,7′-dimethyl-9,9′,10,10′-tetraoxo-5,5′,6,6′,7,7′,8,8′,9,9′,10,10′-dodecahydro-[1,1′-bianthracen]-6-yl acetate | Stemphylium globuliferum | [94] |
| C34H33O17/713 | Alterporriol D  | 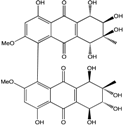 | (1S,5S,5′S,6R,6′R,7S,7′S,8R,8′R)-4,4′,5,5′,6,6′,7,7′,8,8′-decahydroxy-2,2′-dimethoxy-7,7′-dimethyl-5,5′,6,6′,7,7′,8,8′-octahy-dro-[1,1′-bianthracene]-9,9′,10,10′-tetraone | Stemphylium globuliferum | [94] |
| C34H33O17/713 | Alterporriol E | 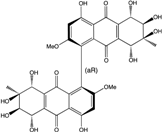 | (1R,5S,5′S,6R,6′R,7S,7′S,8R,8′R)-4,4′,5,5′,6,6′,7,7′,8,8′-decahydroxy-2,2′-dimethoxy-7,7′-dimethyl-5,5′,6,6′,7,7′,8,8′-octahy-dro-[1,1′-bianthracene]-9,9′,10,10′-tetraone | Stemphylium globuliferum | [94] |
| C48H40O21/952 | Stemphylanthranol A | 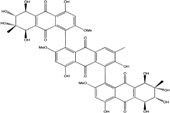 | (5S,5′′S,6R,6′′R,7S,7′′S,8R,8′′R)-2′,4,4′′,5,5′′,6,6′′,7,7′′,8,8′,8′′-dodecahydroxy-2,2′′,6′-trimethoxy-3′,7,7′′-trimethyl-5,5′′,6,6′′,7,7′′,8,8′′-octahydro-[1,1′:5′,1′′-teranthracene]-9,9′,9′′,10,10′,10′′-hexaone | Stemphylium globuliferum | [94] |
| C48H40O21/952 | Stemphylanthranol B | 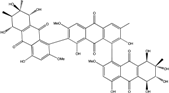 | (5S,5′′R,6R,6′′R,7S,7′′R,8R,8′′R)-2′,4,4′′,5,5′′,6,7,7′′,8,8′,8′′-undecahydroxy-2,2′′,6′-trimethoxy-3′,6′′,7,7′′-tetramethyl-5,5′′,6,6′′,7,7′′,8,8′′-octahydro-[1,1′:7′,1′′-tetranthracene]-9,9′,9′′,10,10′,10′′-hexaone | Stemphylium globuliferum | [94] |
| ---- | 7-Chloroemodic acid | ---- | ---- | Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) | [75,76,77,79,96] |
| ---- | 7-Chloroemodinal | ---- | ---- | Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.)L. hafellneri L. leprolytoides | [75,76,77,79,96] [80] |
| ---- | 7-Chloro-1,6,8-trihydroxy-3-methyl-10-anthrone | ---- | ---- | Caloplaca spp. (e.g., C. ehrenbergii, C. schaereri, C. spitsbergensis, etc.) | [75,76,77,79,96] |
 ; Orange:
; Orange:  ; Yellow:
; Yellow:  ; Red:
; Red:  ; Bronze:
; Bronze:  .
.| Name | Structure | IC50 (µM)—Ki (µM) |
|---|---|---|
| Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) | 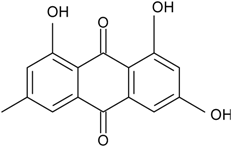 | Ki = 1.85 |
| 1,8-dihydroxy-4-nitro-anthracene-9,10-dione | 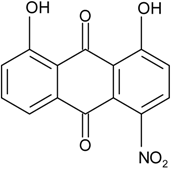 | IC50 = 0.30; Ki = 0.78 |
| 1,8-dihydroxy-3-methyl-4-nitro-anthracene-9,10-dione | 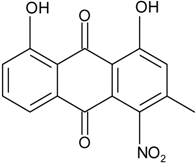 | IC50 = 0.30; Ki = 0.95 |
| 1,8-dihydroxy-4,5-dinitro-anthracene-9,10-dione | 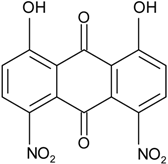 | IC50 = 0.30 |
| 1,4-dihydroxy-5,8-diamino-anthracene-9,10-dione |  | IC50 = 0.30; Ki = 0.42 |
| 1-bromo-4,5-dihydroxy-8-nitro-anthracene-9,10-dione |  | IC50 = 0.40 |
| 1,4,5-trihydroxy-8-(2-bromoacetamido)-anthracene-9,10-dione |  | IC50 = 0.70 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Mar. Drugs 2016, 14, 64. https://doi.org/10.3390/md14040064
Fouillaud M, Venkatachalam M, Girard-Valenciennes E, Caro Y, Dufossé L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Marine Drugs. 2016; 14(4):64. https://doi.org/10.3390/md14040064
Chicago/Turabian StyleFouillaud, Mireille, Mekala Venkatachalam, Emmanuelle Girard-Valenciennes, Yanis Caro, and Laurent Dufossé. 2016. "Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities" Marine Drugs 14, no. 4: 64. https://doi.org/10.3390/md14040064
APA StyleFouillaud, M., Venkatachalam, M., Girard-Valenciennes, E., Caro, Y., & Dufossé, L. (2016). Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Marine Drugs, 14(4), 64. https://doi.org/10.3390/md14040064