Marine Isonitriles and Their Related Compounds
Abstract
:1. Introduction
2. Marine Isonitriles and Related Compounds
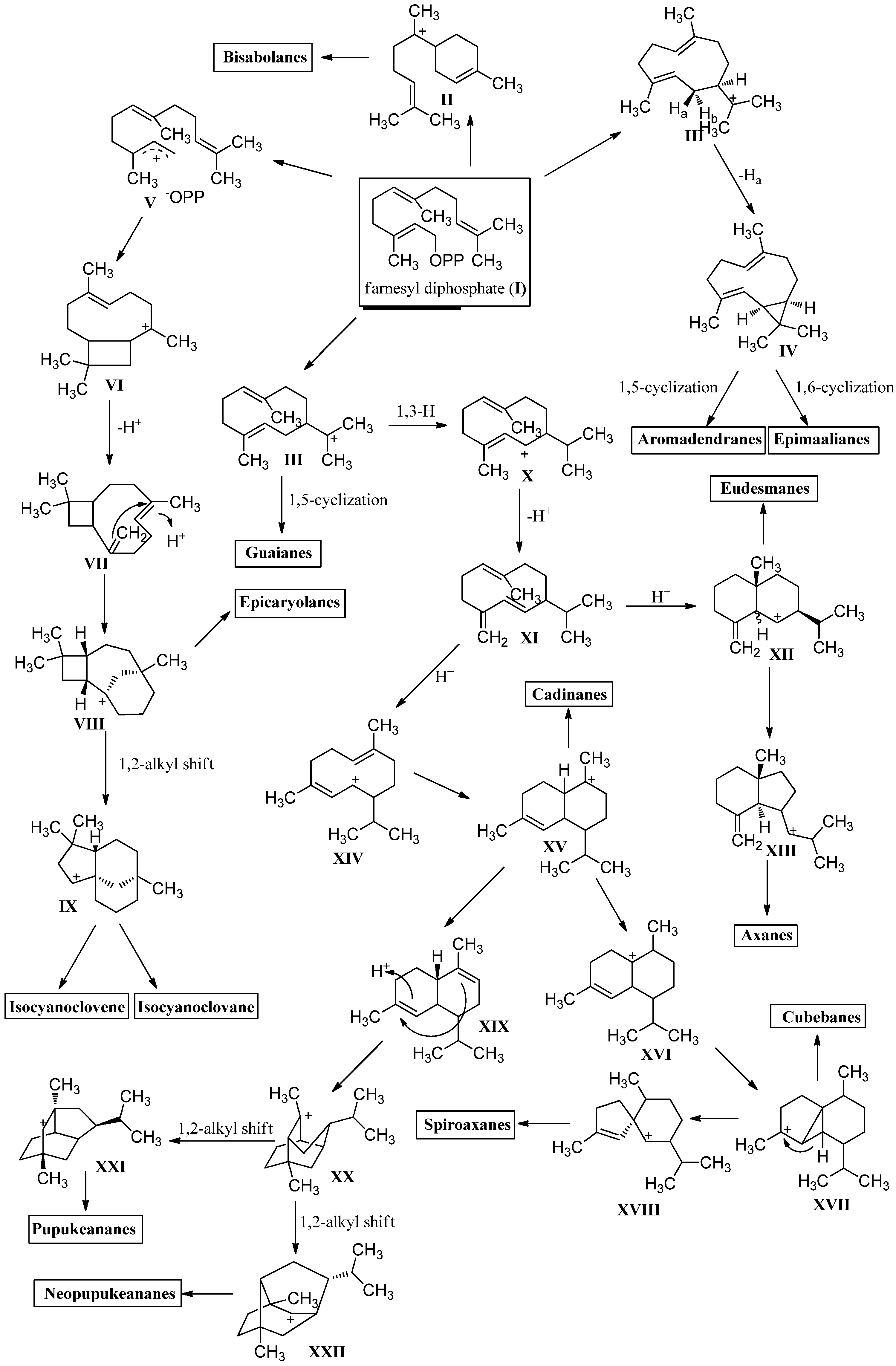
2.1. Sesquiterpenoids
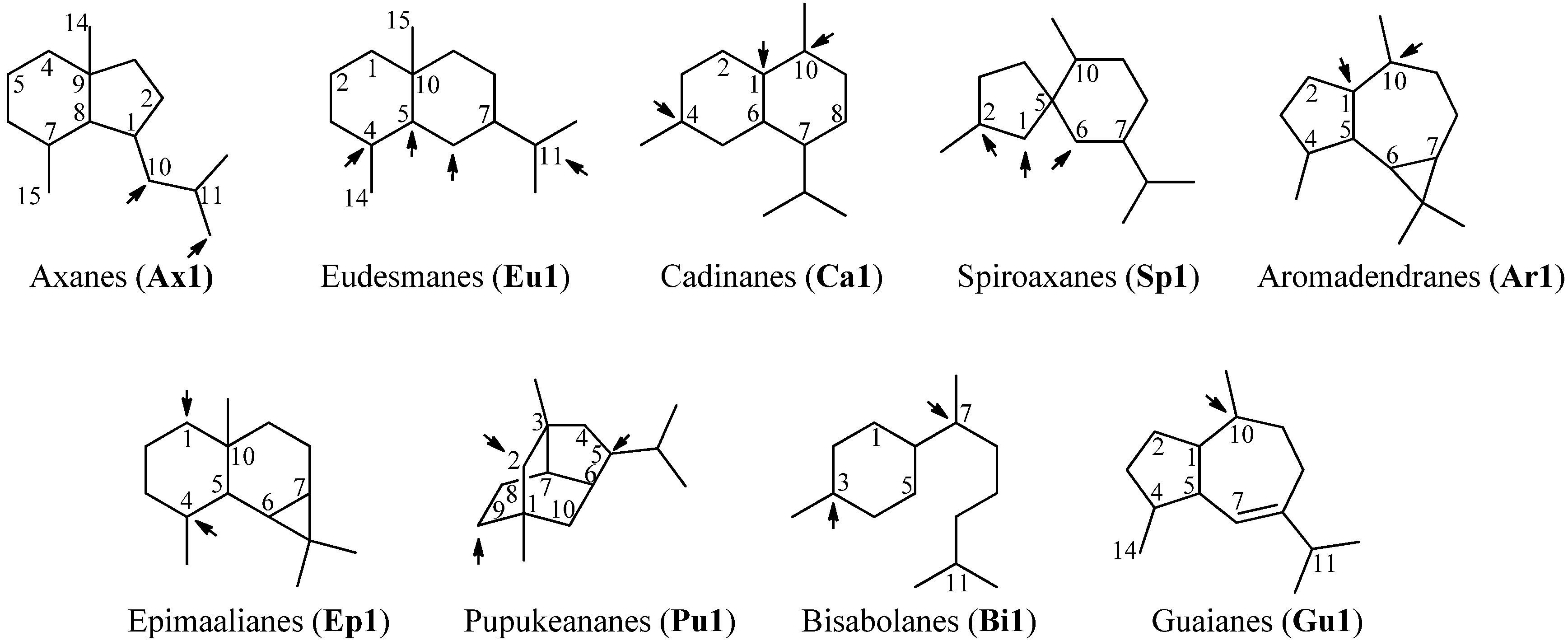
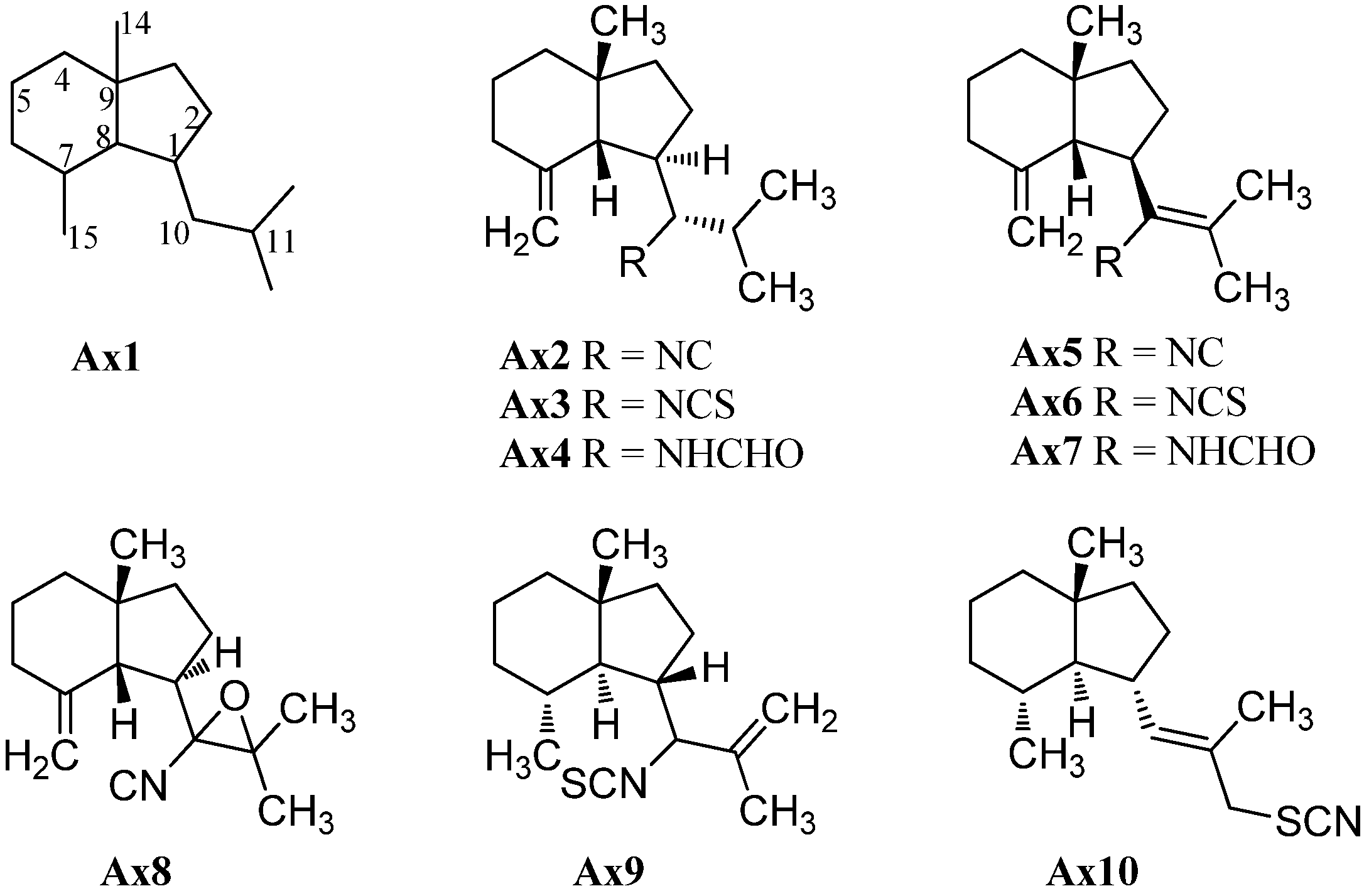
2.1.1. Axanes
2.1.2. Eudesmanes
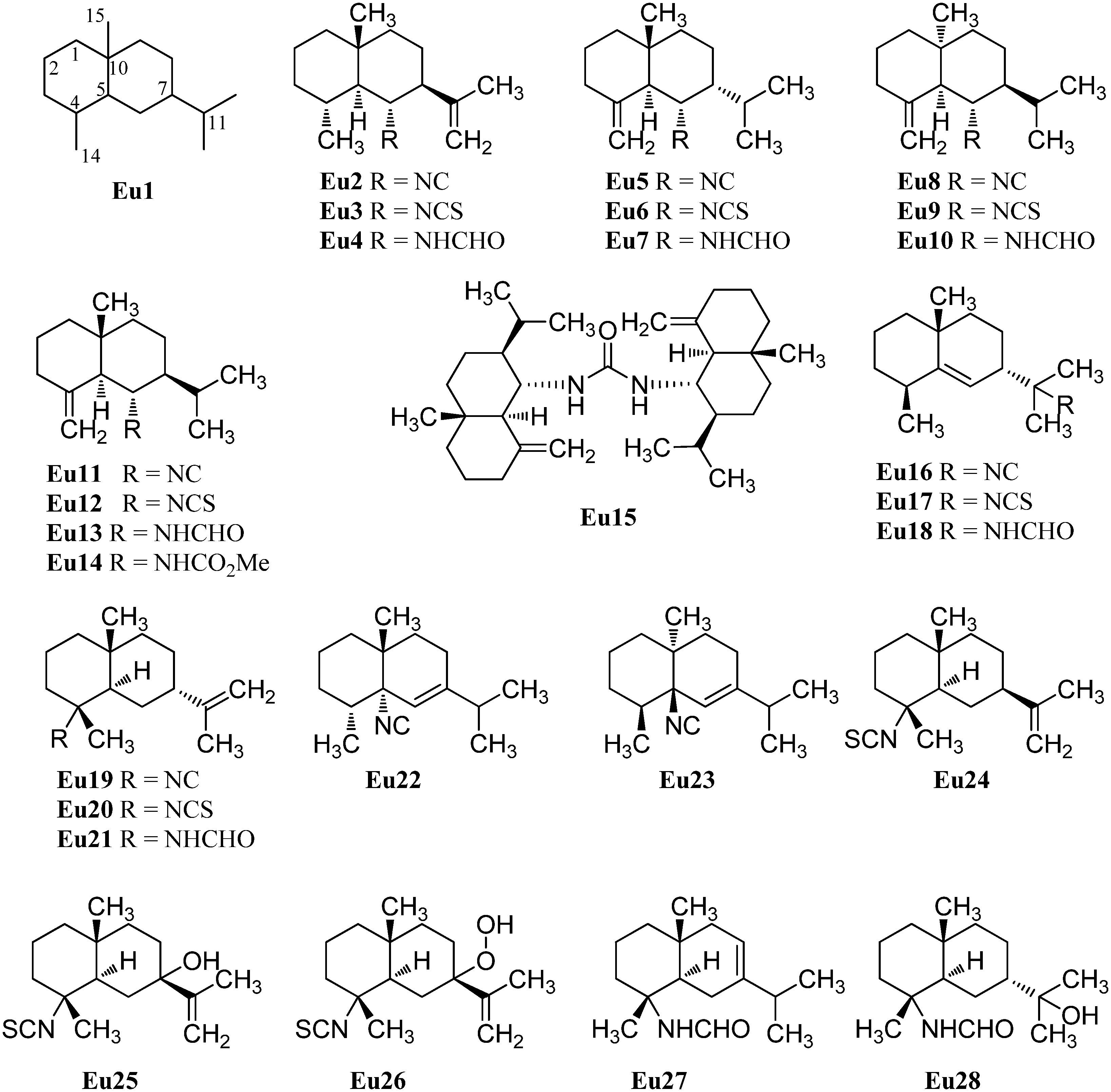
2.1.3. Cadinanes
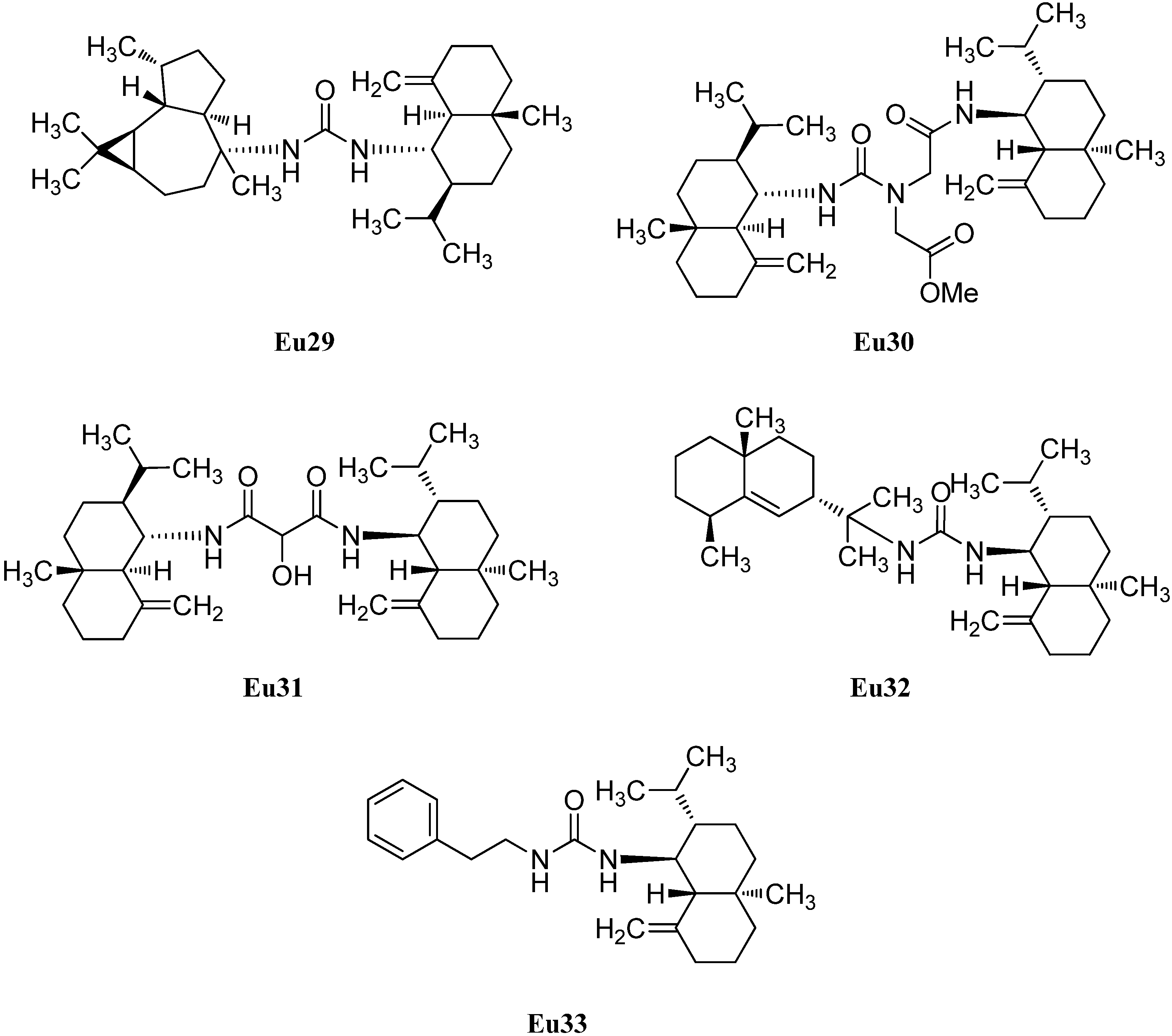
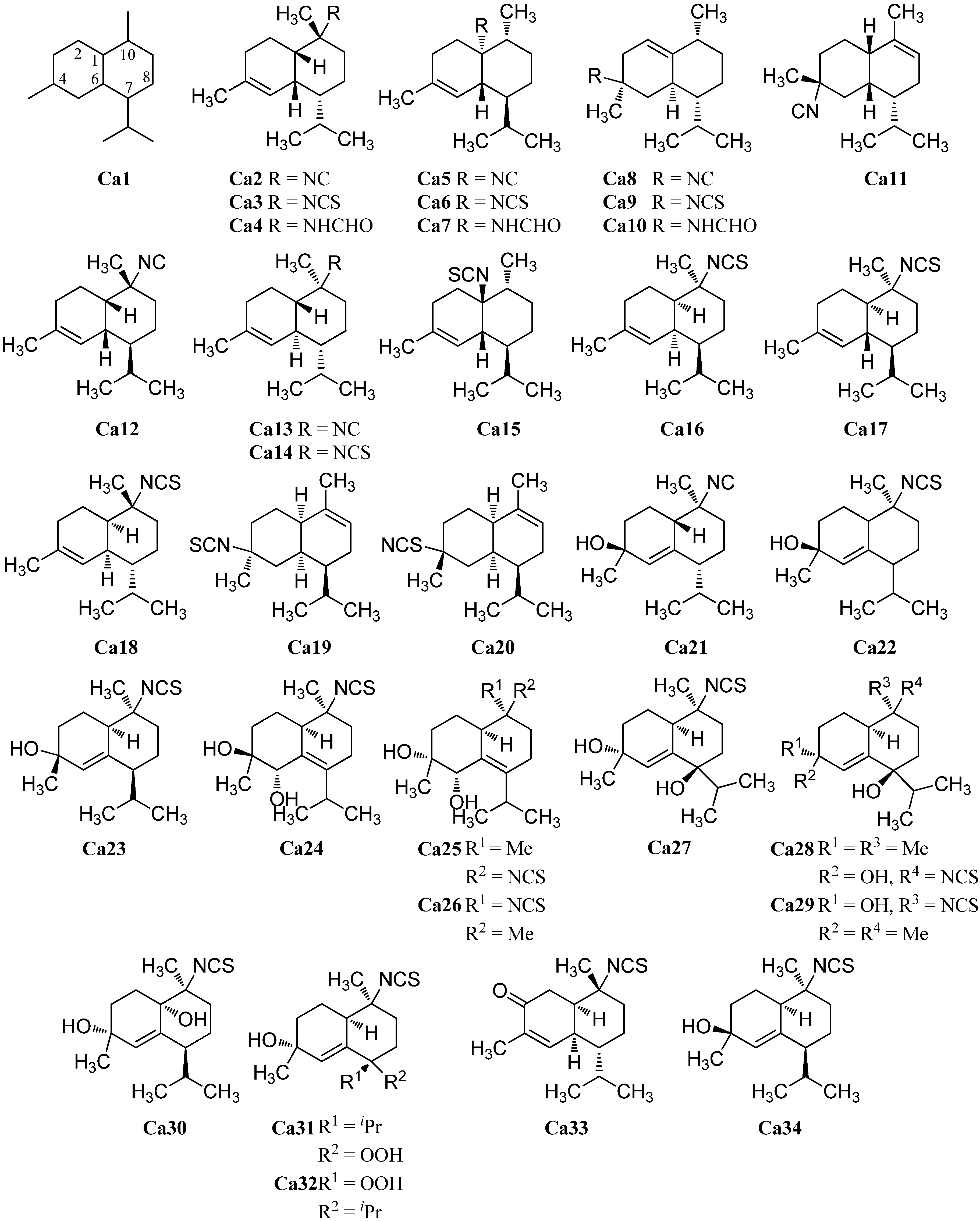
2.1.4. Spiroaxane
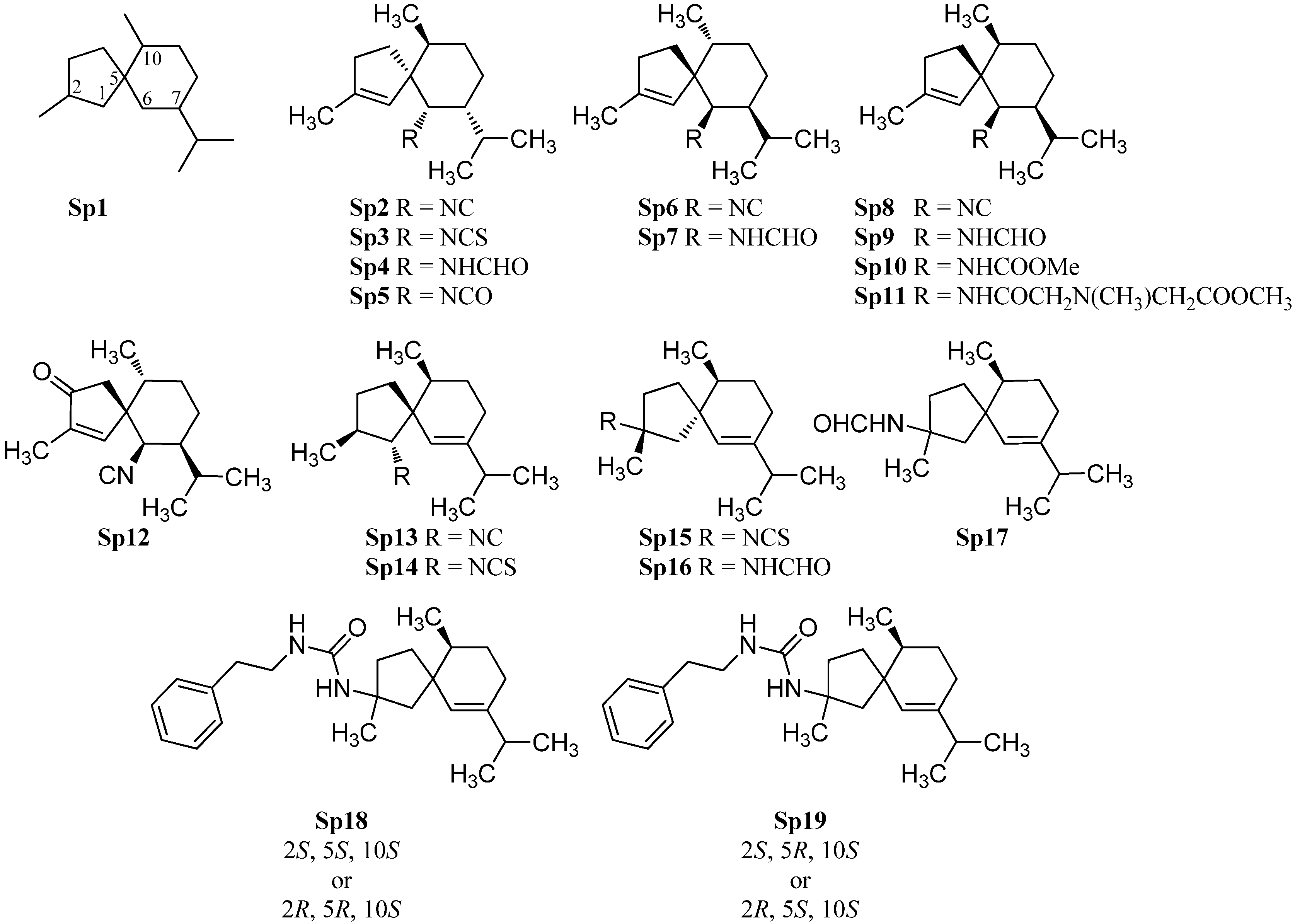
2.1.5. Aromadendranes
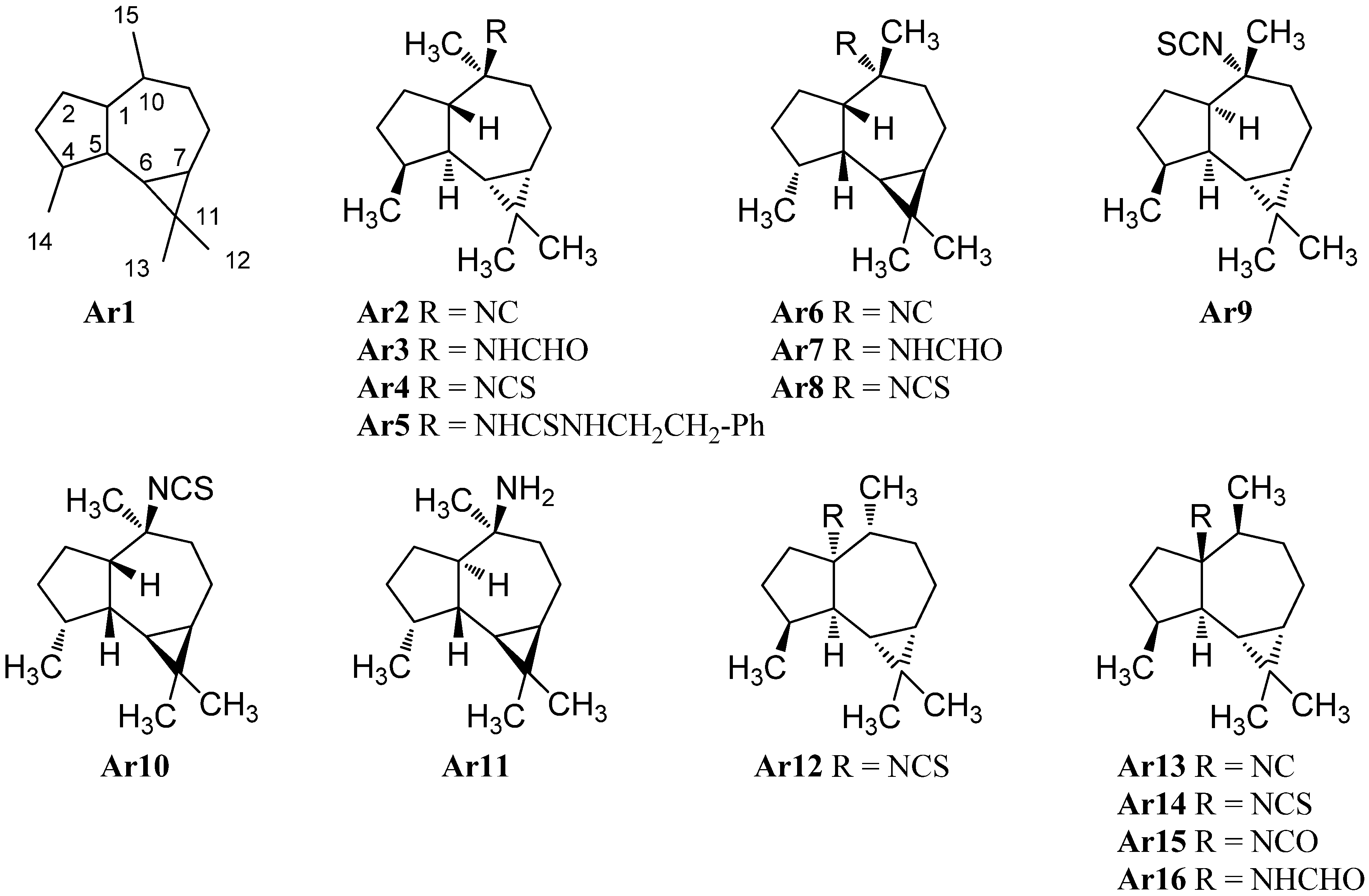
2.1.6. Epimaalianes
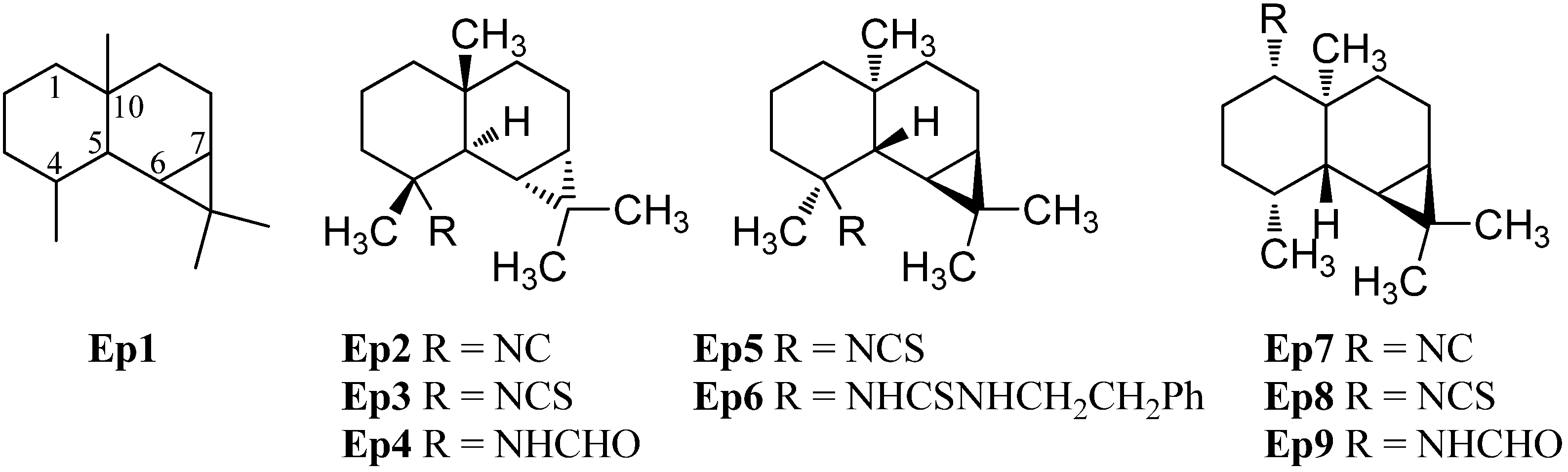
2.1.7. Pupukeananes
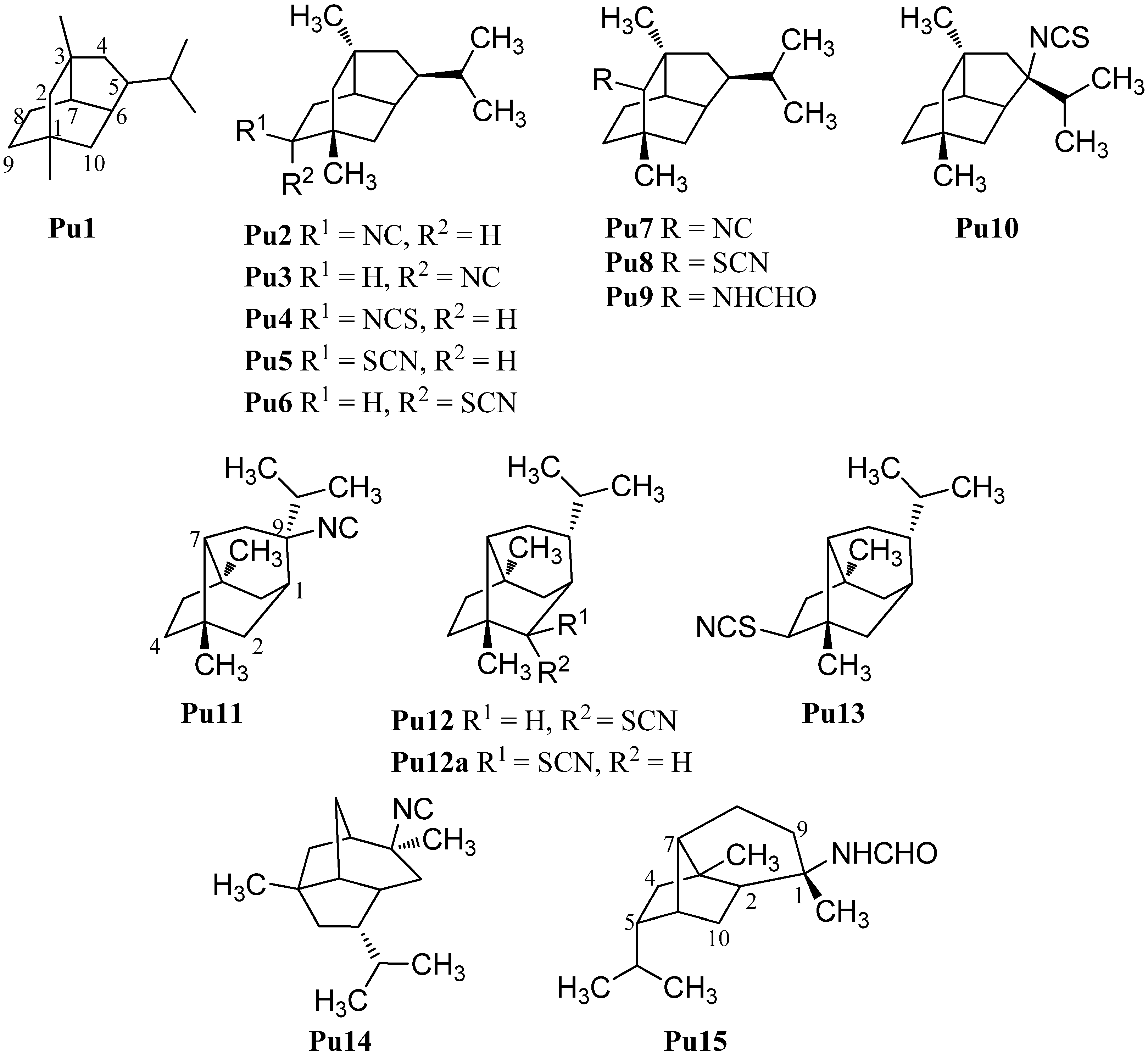
2.1.8. Bisabolanes
2.1.9. Guaianes
2.1.10. Other Sesquiterpenoids
2.2. Diterpenoids
2.2.1. Acyclic
2.2.2. Kalihinanes
Kalihinols
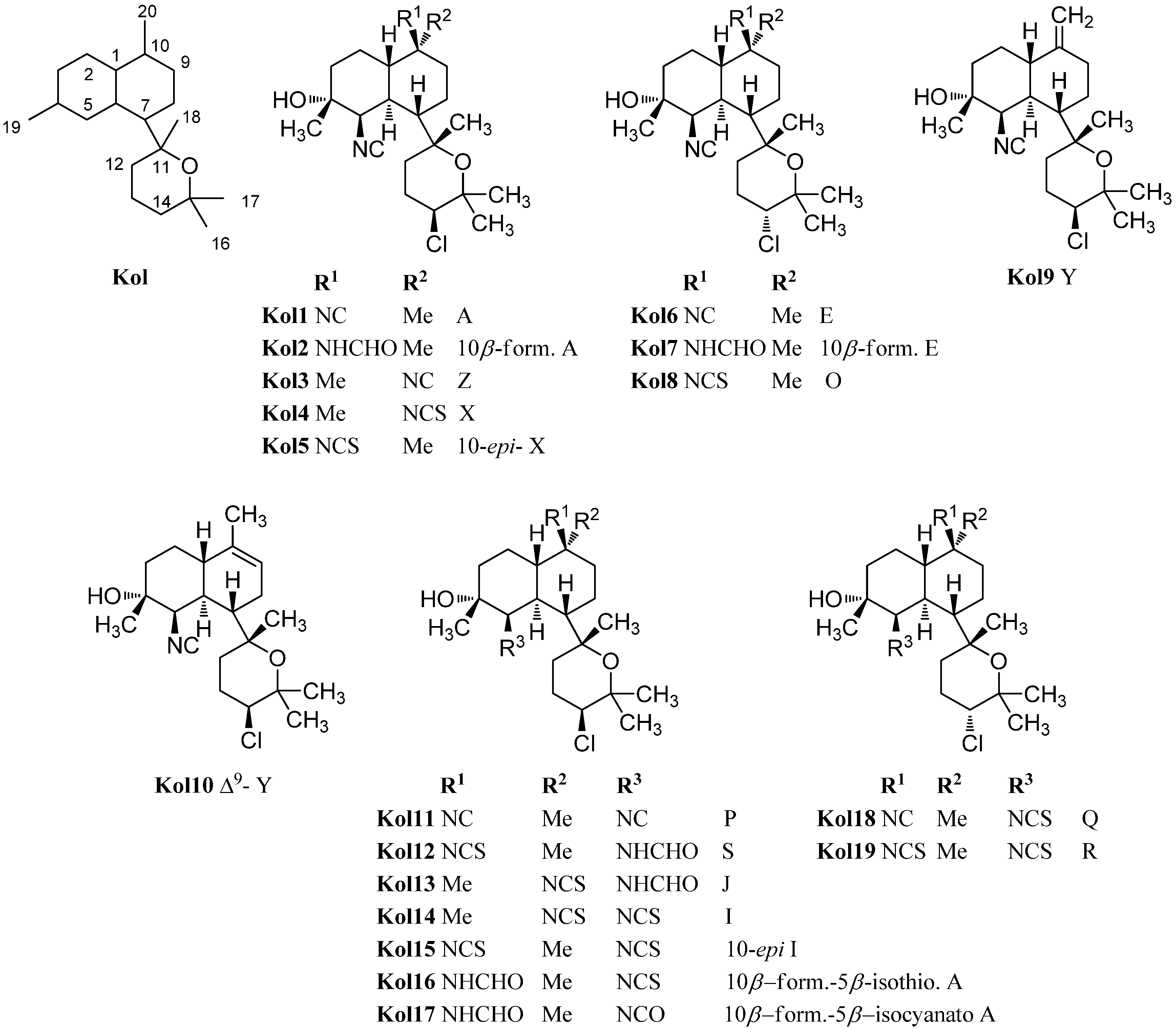
Kalihinenes
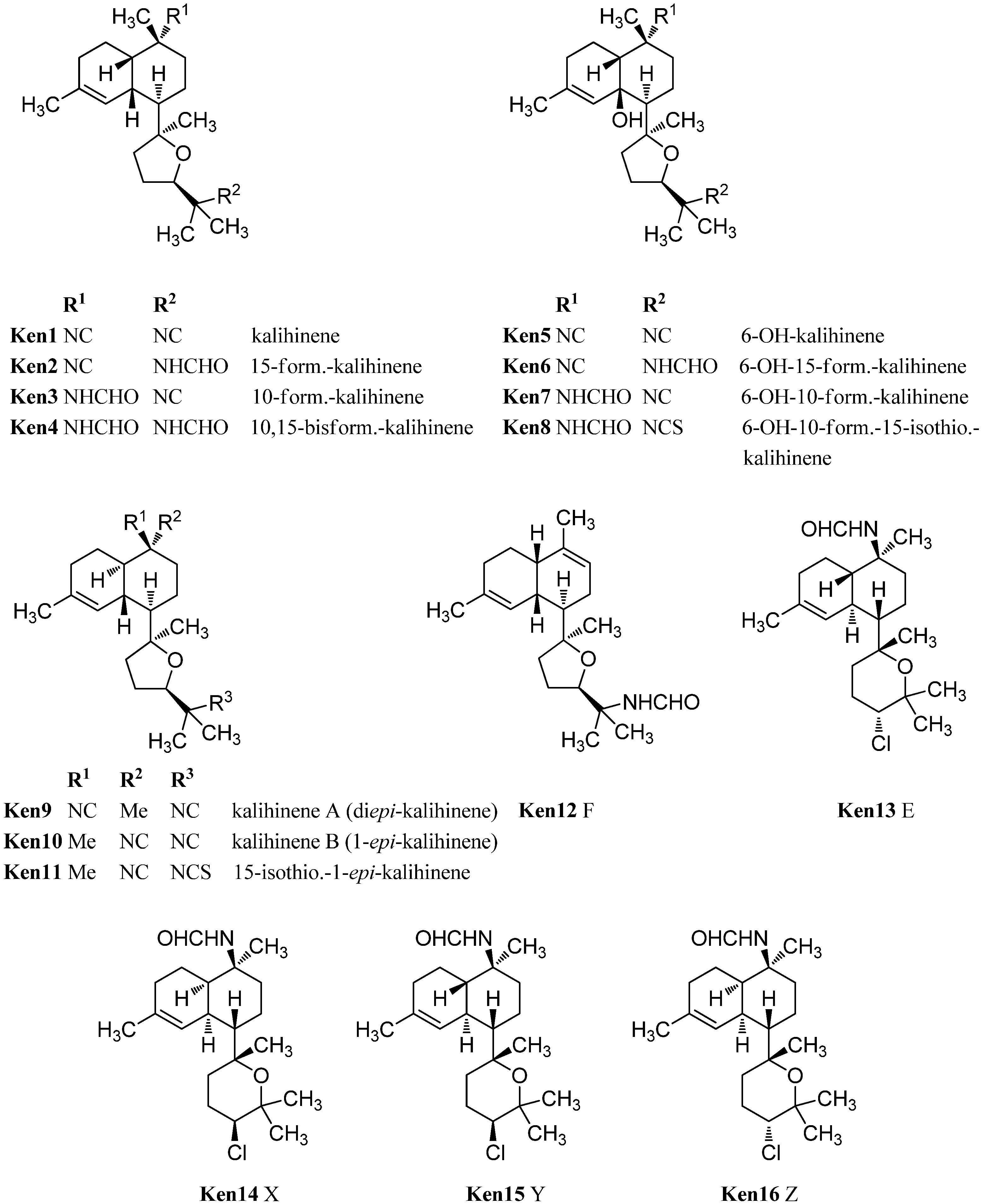
Cavernenes and Other Intermediates
2.2.3. Amphilectanes
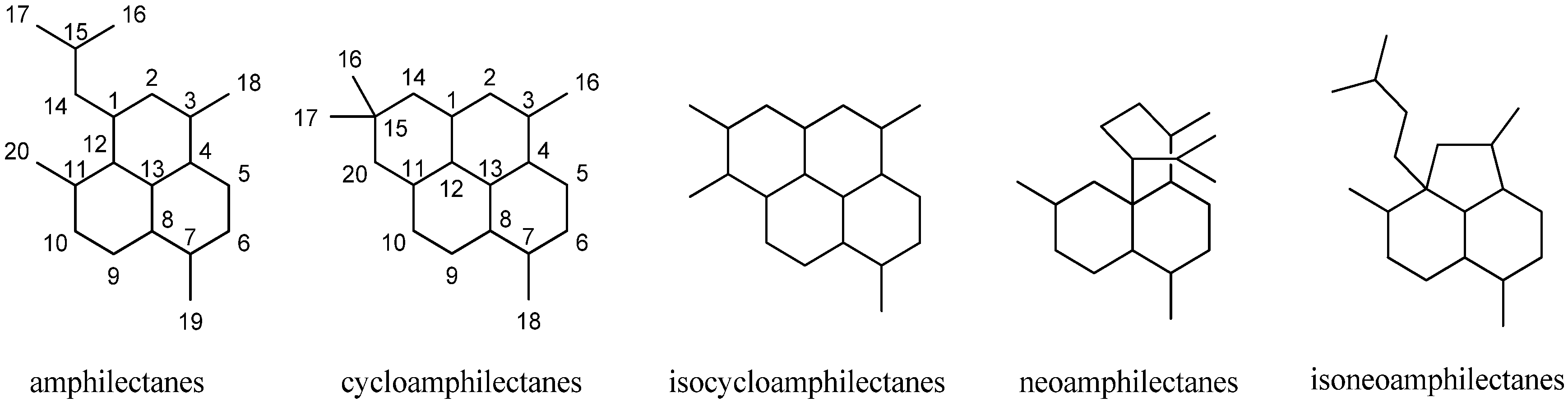
Amphilectenes

Cycloamphilectenes
Isocycloamphilectanes
Neoamphilectene
Isoneoamphilectenes

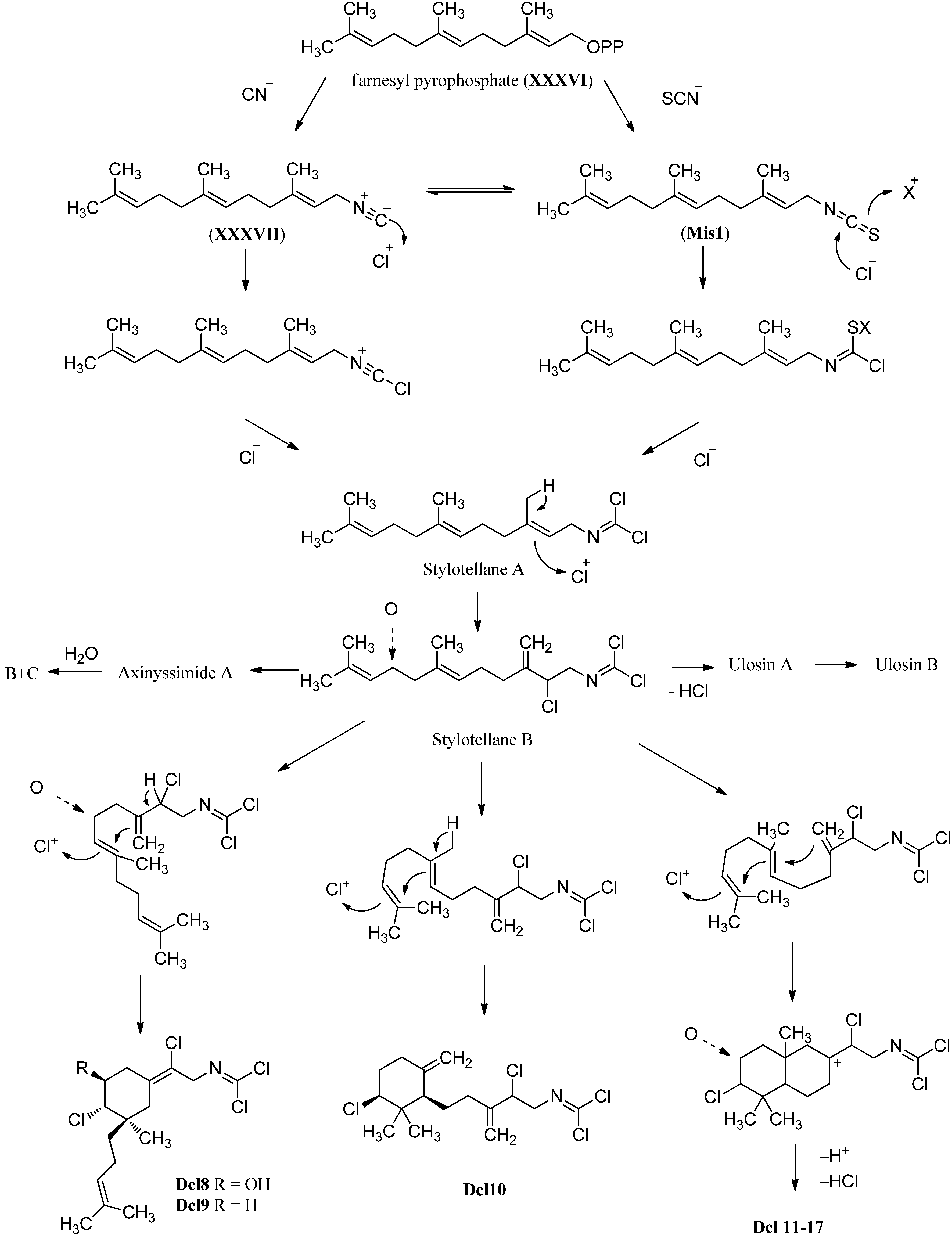
2.3. Carbonimidic Dichlorides
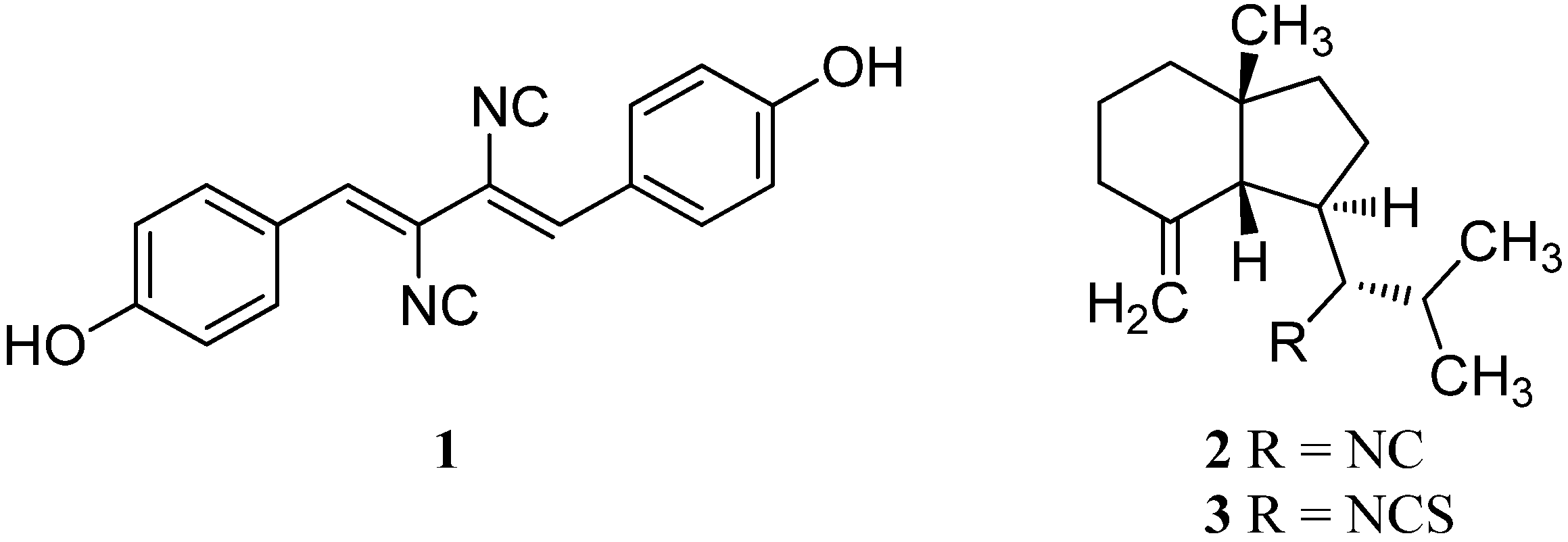
2.4. Other Marine Isonitriles and Related Compounds (Miscellaneous Structures)
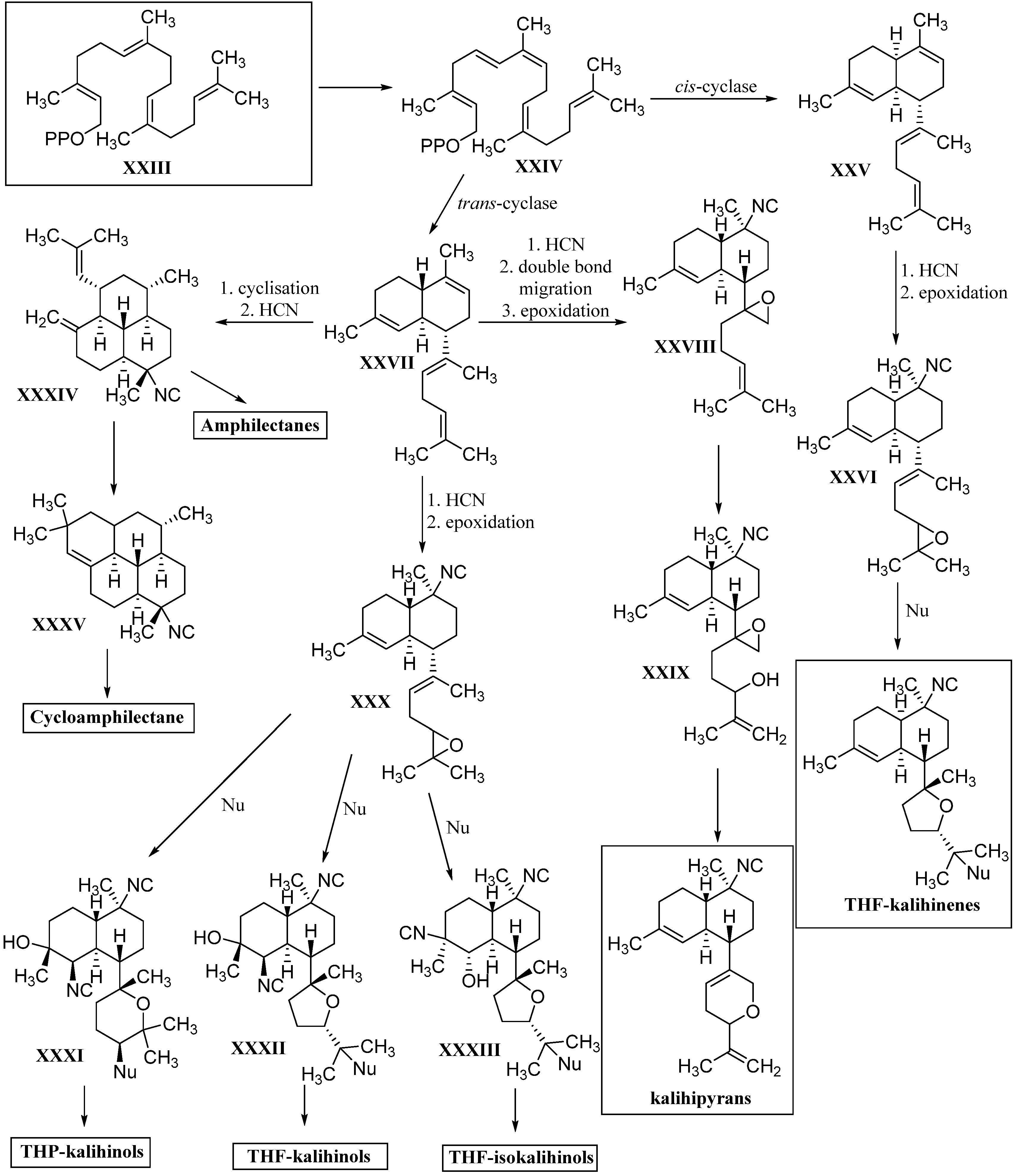
3. Biosynthesis
3.1. Sesquiterpenoids
3.2. Diterpenoids
3.3. Carbonimidic Dichlorides
4. Biological Activity
5. Conclusions
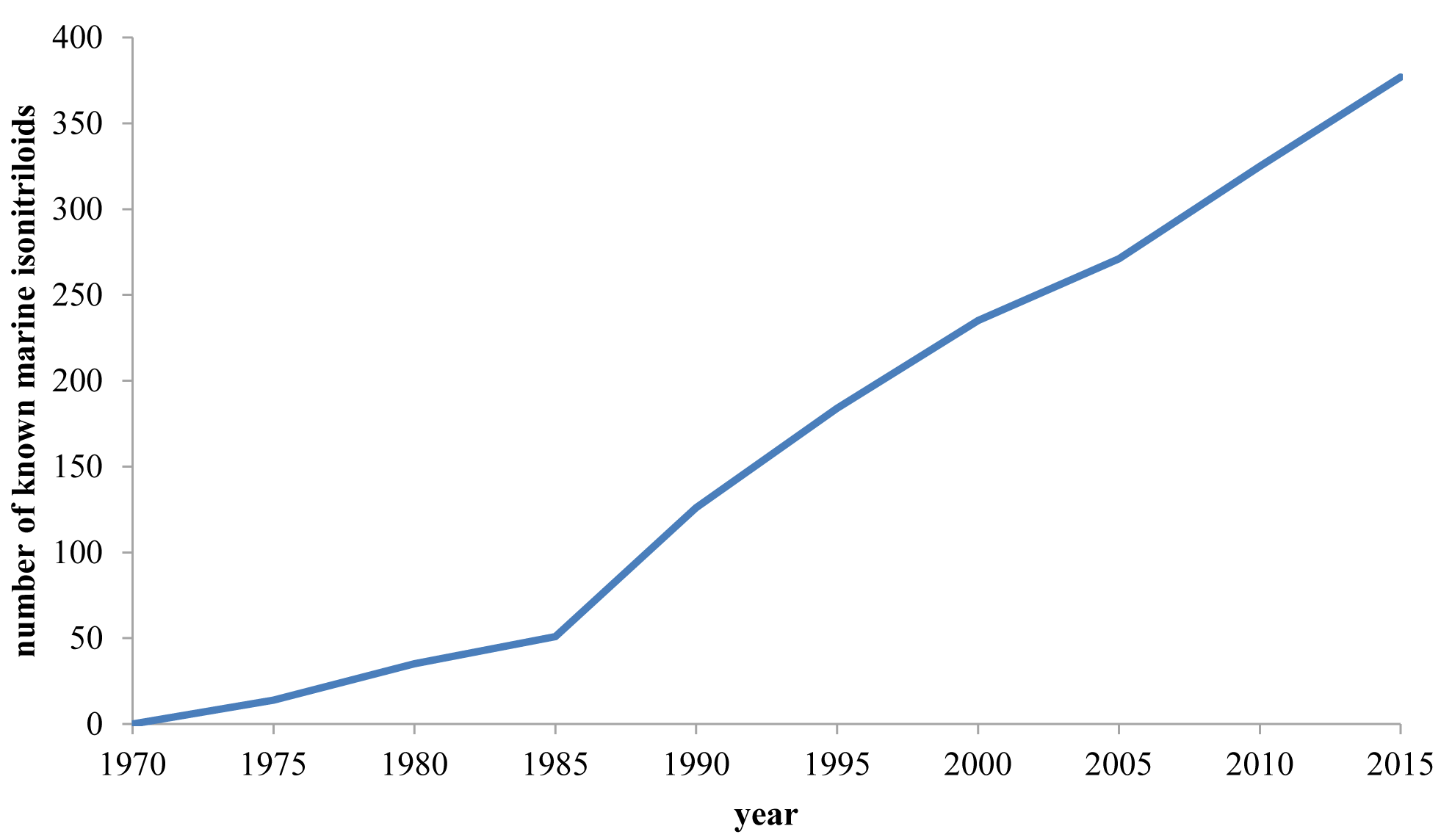
6. Overview about All Marine Isonitriles and Their Related Compounds
| Trivial Name (Structure) | Organism | Origin | Year | References |
|---|---|---|---|---|
| Sesquiterpenoids | ||||
| Axanes | ||||
| axisonitrile-1 (Ax2) | Axinella cannabina | Bay of Taranto, Italy | 1973 | [2] |
| axisothiocyanate-1 (Ax3) | Axinella cannabina | Bay of Taranto, Italy | 1973 | [2] |
| axamide-1 (Ax4) | Axinella cannabina | Bay of Taranto, Italy | 1974 | [4] |
| axisonitrile-4 (Ax5) | Axinella cannabina | Bay of Taranto, Italy | 1977 | [16] |
| axisothiocyanate-4 (Ax6) | Axinella cannabina | Bay of Taranto, Italy | 1977 | [16] |
| axamide-4 (Ax7) | Axinella cannabina | Bay of Taranto, Italy | 1977 | [16] |
| cavernoisonitrile (Ax8) | Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] |
| Acanthella cavernosa | Hachijo-jima Island, Japan | 1996 | [20] | |
| (−)-cavernothiocyanate (Ax9) | Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] |
| Acanthella cavernosa | Hachijo-jima Island, Japan | 1996 | [20] | |
| 10-isothiocyanato-11-axene (Ax10) | Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] |
| Phyllidia ocellata | Hachijo-jima Island, Japan | 1992 | [19] | |
| Acanthella cavernosa | Hachijo-jima Island, Japan | 1996 | [20] | |
| Eudesmanes | ||||
| acanthellin-1 (Eu2) | Acanthella acuta | Bay of Naples, Italy | 1974 | [3] |
| Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1984 | [21] | |
| R=NCS (Eu3) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1984 | [21] |
| R=NHCHO (Eu4) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1984 | [21] |
| R=NC (Eu5) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1984 | [21] |
| R=NCS (Eu6) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1984 | [21] |
| R=NHCHO (Eu7) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1984 | [21] |
| 6α-isocyano-5α-H,7α-H, 10α-eudesm-4(14)-ene (Eu8) | Axinella cannabina and Acanthella acuta | Bay of Taranto, Porto Cesareo, Italy | 1987 | [22] |
| 6α-isothiocyano-5α-H,7α-H, 10α-eudesm-4(14)-ene (Eu9) | Axinella cannabina and Acanthella acuta | Bay of Taranto, Porto Cesareo, Italy | 1987 | [22] |
| 6α-formamido-5α-H,7α-H, 10α-eudesm-4(14)-ene (Eu10) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1987 | [22] |
| halichonadin C (Eu11) | Halichondria sp. | Unten Port, Okinawa, Japan | 2005 | [24] |
| Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] | |
| acanthene B (Eu12) | Acanthella sp. | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| acanthene C (Eu13) | Cadlina luteomarginata | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] |
| halichonadin B (Eu14) | Halichondria sp. | Unten Port, Okinawa, Japan | 2005 | [24] |
| halichonadin A (Eu15) | Halichondria sp. | Unten Port, Okinawa, Japan | 2005 | [24] |
| 11-isocyano-7β-H-eudesm-5-ene (Eu16) | Axinella cannabina | Taranto, near Porto Cesareo, Italy | 1987 | [25] |
| Phyllidia pustulosa | Negros Island, Cebu Island, San Sebastian, Cebu, Philippines | 1991 | [86] | |
| Acanthella sp. and Cadlina luteomarginata | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] | |
| Axinyssa ambrosia | Santa Marta Bay, Caribbean Coast, Colombia | 2002 | [26] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| Phyllidiella pustulosa | Vietnam | 2010 | [27] | |
| 11-isothiocyano-7β-H-eudesm-5-ene (Eu17) | Axinella cannabina | Taranto, near Porto Cesareo, Italy | 1987 | [25] |
| Acanthella pulcherrima | Weed Reef, Darwin, Australia | 1988 | [28] | |
| Phyllidia pustulosa | Negros Island, Cebu Island, San Sebastian, Cebu, Philippines | 1991 | [86] | |
| Acanthella klethra | Pelorus Islands, Queensland, Australia | 1992 | [30] | |
| Acanthella sp. and Cadlina luteomarginata | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] | |
| Axinyssa ambrosia | Santa Marta Bay, Caribbean Coast, Colombia | 2002 | [26] | |
| Acanthella cavernosa | Coral Gardens, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| 11-formamido-7β-H-eudesm-5-ene (Eu18) | Axinella cannabina | Taranto, near Porto Cesareo, Italy | 1987 | [25] |
| Axinyssa ambrosia | Santa Marta Bay, Caribbean Coast, Colombia | 2002 | [26] | |
| 4-Isothiocyanatoeudesm-11-ene (Eu19) | Axinyssa ambrosia | Santa Marta Bay, Caribbean Coast, Colombia | 2002 | [26] |
| 4-isothiocyanatoeudesm-11-ene (Eu20) | Acanthella klethra | Pelorus Islands, Queensland, Australia | 1992 | [30] |
| Acanthella cavernosa | Heron Island, Great Barrier Reef, Australia | 2000 | [31] | |
| Acanthella cavernosa | Mudjimba Island, Mooloolaba, Australia | 2007 | [32,58] | |
| Axinyssa isabela | Isabel Island, Nayarit, Mexico | 2008 | [33] | |
| 4-formamidoeudesm-11-ene (Eu21) | Axinyssa ambrosia | Santa Marta Bay, Caribbean Coast, Colombia | 2002 | [26] |
| stylostelline (Eu22) | Stylotella sp. | South East of New Caledonia | 1987 | [34] |
| ent-stylotelline (Eu23) | Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] |
| 4-isothiocyanatoeudesm-11-ene (Eu24) | Acanthella klethra | Pelorus Islands, Queensland, Australia | 1992 | [30] |
| Acanthella sp. and Cadlina luteomarginata | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] | |
| axinisothiocyanate M (Eu25) | Axinyssa isabela | Isabel Island, Nayarit, Mexico | 2008 | [33] |
| axinisothiocyanate N (Eu26) | Axinyssa isabela | Isabel Island, Nayarit, Mexico | 2008 | [33] |
| 4-formamidoeudesm-7-ene (Eu27) | Axinyssa sp. | South China Sea | 2008 | [36] |
| 4-formamidoeudesman-11-ol (Eu28) | Axinyssa sp. | South China Sea | 2008 | [36] |
| halichonadin E (Eu29) | Halichondria sp. | Unten Port, Okinawa, Japan | 2008 | [37] |
| halichonadin G (Eu30) | Halichondria sp. | Unten Port, Okinawa, Japan | 2011 | [38] |
| halichonadin H (Eu31) | Halichondria sp. | Unten Port, Okinawa, Japan | 2011 | [38] |
| halichonadin I (Eu32) | Halichondria sp. | Unten Port, Okinawa, Japan | 2011 | [38] |
| halichonadin J (Eu33) | Halichondria sp. | Unten Port, Okinawa, Japan | 2011 | [38] |
| Cadinanes | ||||
| (–)-10-isocyano-4-amorphene (Ca2) | Halichondria sp. | North coast of O’ahu, Hawaii | 1975 | [6,39,40] |
| Axinyssa | Gun Beach, Guam | 1989 | [43] | |
| Phyllidia ocelata | Kamikoshiki-jima Island, Japan | 1996 | [42] | |
| (–)-10-isothiocyanato-4-amorphene (Ca3) | Halichondria sp. | North coast of O’ahu, Hawaii | 1975 | [6,39,40] |
| Phyllidia pustulosa | Yakushima Island, Japan | 1996 | [42] | |
| Phyllidiella pustulosa | Vietnam | 2010 | [27] | |
| (–)-10-isoformamido-4-amorphene (Ca4) | Halichondria sp. | North coast of O’ahu, Hawaii | 1975 | [6,39,40] |
| R=NC (Ca5) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1986 | [44] |
| R=NCS (Ca6) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1986 | [44] |
| R=NHCHO (Ca7) | Axinella cannabina | Bay of Taranto, Porto Cesareo, Italy | 1986 | [44] |
| (3S*,5R*,6R*,9R*)-3-isocyano-1(10)-cadinene (Ca8) | Axinyssa aplysinoides | West of Malakal Harbor, Palau | 1995 | [46] |
| halipanicine (Ca9) | Halochondria panacea | Okinawa, Japan | 1991 | [45] |
| (3S*,5R*,6R*,9R*)-3-formamido-1(10)-cadinene (Ca10) | Axinyssa aplysinoides | West of Malakal Harbor , Palau | 1995 | [46] |
| Halichondria sp. | PP Island, Andaman Sea, Southern Thailand | 2011 | [59] | |
| 4α-isocyano-9-amorphene (Ca11) | Phyllidia pustulosa | Hachijo-jima Island, Japan | 1991 | [47] |
| 10α-isocyano-4-amorphene (Ca12) | Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] |
| Phyllidia ocellata | Hachijo-jima Island, Japan | 1992 | [19] | |
| 10-isocyano-4-cadinene (Ca13) | Phyllidia pustulosa + varicosa | Kamikoshiki-jima/Shimokoshiki Island, Japan | 1996 | [42] |
| 10-isothiocyanate-4-cadinene (Ca14) | Acanthella cavernosa | Heron Island, Great Barrier Reef, Australia | 2000 | [31] |
| Stylissa sp. | Iriomote Island, Okinawa, Japan | 2004 | [50] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| R=NCS (Ca15) | Acanthella pulcherrima | Weed Reef, Darwin, Australia | 1988 | [28] |
| Acanthella cavernosa | Coral Gardens, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| (1R,6S,7S,10S)-10-isothiocyanato-4-amorphene (Ca16) | Axinella fenestratus | Fiji | 1991 | [49] |
| Topsentia sp., Acanthella cavernosa | Thailand | 1991 | [49] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| (1R*,6R*,7S*,10S*)-10-isothiocyanatocadin-4-ene (Ca17) | Stylissa sp. | Coral reef, Iriomote Island, Okinawa, Japan | 2004 | [50] |
| axinisothiocyanate K (Ca18) | Axinyssa | Gulf of California | 2008 | [51] |
| (1R*,4S*,6R*,7S*)-4-isothiocyanato-9-amorphene (Ca19) | Axinella fenestratus | Fiji | 1991 | [49] |
| Topsentia sp., Acanthella cavernosa | Thailand | 1991 | [49] | |
| (1S*,4S*,6S*,7R*)-4-thiocyanato-9-cadinene (Ca20) | Trachyopsis aplysinoides | Palau | 1989 | [52] |
| Phyllidia pustulosa | Katsuura, Kii Penisula, Japan | 1998 | [53] | |
| (1S*,4S*,7R*,10S*)-10-isocyano-5-cadinen-4-ol (Ca21) | Phyllidia pustulosa | Katsuura, Kii Penisula, Japan | 1998 | [53] |
| 10-isothiocyanatoamorph-5-en-4-ol (Ca22) | Axinella fenestratus | Fiji | 1991 | [49] |
| Topsentia sp., Acanthella cavernosa | Thailand | 1991 | [49] | |
| axinisothiocyanate J (Ca23) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate A (Ca24) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate B (Ca25) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate C (Ca26) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate D (Ca27) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate E (Ca28) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate F (Ca29) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate G (Ca30) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate H (Ca31) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate I (Ca32) | Axinyssa | Gulf of California | 2008 | [51] |
| axinisothiocyanate L (Ca33) | Axinyssa | Gulf of California | 2008 | [51] |
| Axiplyn C (Ca34) | Axinyssa aplysinoides | Misali Island, Tanzania | 2008 | [54] |
| Spiroaxanes | ||||
| (+)-axisonitril-3 (Sp2) | Axinella cannabina | Bay of Taranto, Italy | 1976 | [55] |
| Acanthella acuta | Mediterranean sea | 1987 | [57] | |
| Topsentia sp. | Thailand | 1991 | [49] | |
| Acanthella klethra | Pelorus Islands, Queensland, Australia | 1992 | [30] | |
| Axinyssa aplysinoides | Mutok Harbor, Pohnpei | 1992 | [69] | |
| Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] | |
| Phyllidia ocellata | Hachijo-jima Island, Japan | 1992 | [19] | |
| Phyllidia pustulosa | Yakushima/Kuchinoerabu-jima/Tenegashima Islands, Japan | 1996 | [42] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| Acanthella sp. | Yalong Bay, Hainan Province, China | 2009 | [103] | |
| Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] | |
| (+)-axisothiocyanate-3 (Sp3) | Axinella cannabina | Bay of Taranto, Italy | 1976 | [55] |
| Acanthella klethra | Pelorus Islands, Queensland, Australia | 1992 | [30] | |
| Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] | |
| Phyllidia ocellata | Hachijo-jima Island, Japan | 1992 | [19] | |
| Acanthella cavernosa | Hachijo-jima Island, Japan | 1996 | [20] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| (−)-axamide-3 (Sp4) | Axinella cannabina | Bay of Taranto, Italy | 1976 | [55] |
| Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] | |
| Phyllidia ocellata | Hachijo-jima Island, Japan | 1992 | [19] | |
| axisocyanate-3 (Sp5) | Acanthella cavernosa | Mudjimba Island, Mooloolaba, Australia | 2007 | [32,58] |
| (−)-axisonitrile-3 (Sp6) | Halichondria sp. | PP Island, Andaman Sea, Southern Thailand | 2011 | [59] |
| (+)-axamide (Sp7) | Axinella cannabina | Bay of Taranto, Italy | 1976 | [55] |
| Acanthella cavernosa | Hachijo-jima Island, Japan | 1996 | [20] | |
| Halichondria sp. | PP Island, Andaman Sea, Southern Thailand | 2011 | [59] | |
| 10-epi-axisonitrile-3 (Sp8) | Phyllidia pustulosa | Yakushima/Kuchinoerabu-jima Islands, Japan | 1996 | [42] |
| Geodia exigua | Oshima, Kagoshima Prefecture, Japan | 2003 | [60] | |
| exiguamide (Sp9) | Geodia exigua | Oshima, Kagoshima Prefecture, Japan | 2003 | [60] |
| exicarbamate (Sp10) | Geodia exigua | Oshima, Kagoshima Prefecture, Japan | 2003 | [60] |
| exigurin (Sp11) | Geodia exigua | Oshima, Kagoshima Prefecture, Japan | 2003 | [60] |
| 3-Oxoaxisonitrile-3 (Sp12) | Acanthella sp. | South China Sea | 2006 | [61] |
| R=NC (Sp13) | Acanthella acuta | Bay of Naples, Italy | 1987 | [62] |
| R=NCS (Sp14) | Acanthella acuta | Bay of Naples, Italy | 1987 | [62] |
| (2R,5R,10S)-2-isothiocyanato-6-axene (Sp15) | Trachyopsis aplysinoides | Palau | 1989 | [52] |
| Axinyssa aplysinoides | Palau | 1992 | [69] | |
| Amorphinopsis foetida | Madang region, Papua New Guinea | 2006 | [63] | |
| (2R,5R,10S)-2-formamido-6-axene (Sp16) | Trachyopsis aplysinoides | Palau | 1989 | [52] |
| Axinyssa aplysinoides | Palau | 1992 | [69] | |
| Axinyssa aplysinoides | Mele Bay, Vanuatu | 2006 | [63] | |
| R=NHCHO (Sp17) | Amorphinopsis foetida | Madang region, Papua New Guinea | 2006 | [63] |
| N-Phenethyl-2-formamido-6-axene (Sp18) | Amorphinopsis foetida and Axinyssa aplysinoides | Madang region, Papua New guinea and Mele Bay, Vanuatu | 2006 | [63] |
| N-Phenethyl-2-formamido-6-axene (Sp19) | Amorphinopsis foetida and Axinyssa aplysinoides | Madang region, Papua New guinea and Mele Bay, Vanuatu | 2006 | [63] |
| Aromadendranes | ||||
| axisonitrile-2 (Ar2) | Axinella cannabina | Bay of Taranto, Italy | 1974 | [4] |
| Acanthella cannabina | Taranto, near Porto Cesareo, Italy | 1986 | [44] | |
| Phyllidia pustulosa | Hachijo-jima Island, Japan | 1991 | [47] | |
| Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] | |
| Acanthella cavernosa | Hachiji-jima Island, Japan | 1996 | [20] | |
| Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] | |
| axamide-2 (Ar3) | Axinella cannabina | Bay of Taranto, Italy | 1974 | [4] |
| Hexabrandies sanguinens | South China Sea | 2007 | [68] | |
| Halichondria sp. | PP Island, Andaman Sea, Southern Thailand | 2011 | [59] | |
| axisothiocyanate-2/epipolasin B (Ar4) | Axinella cannabina | Bay of Taranto, Italy | 1974 | [4] |
| Epipolasis kushimotoensis | 1985 | [64] | ||
| Axinyssa aplysinoides | Ant Atoll, Pohnpei | 1992 | [69] | |
| Acanthella cavernosa | Hachijo-jima Island, Japan | 1996 | [20] | |
| Axinyssa sp. | Tsutsumi Island, Fukuoka prefecture, Japan | 2002 | [165] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| epipolasinthiourea-B (Ar5) | Epipolasis kushimotoensis | 1985 | [64] | |
| 10α-isocyanoalloaromadendrane (Ar6) | Acanthella cannabina | Taranto, near Porto Cesareo, Italy | 1987 | [25] |
| 10α-formamidoalloaromadendrane (Ar7) | Acanthella cannabina | Taranto, near Porto Cesareo, Italy | 1987 | [25] |
| 10α-isothiocyanatoalloaromadendrane (Ar8) | Acanthella cannabina | Taranto, near Porto Cesareo, Italy | 1987 | [25] |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| (1R,4S,5S,6R,7S,10R)-(+)-isothiocyanatoalloaromadendrane (Ar9) | Acanthella cavernosa | Hachijo-jima Island, Japan | 1996 | [20] |
| Acanthella sp. | Yalong Bay, Hainan Province, China | 2009 | [103] | |
| Phyllidiella pustulosa | Vietnam | 2010 | [27] | |
| Halochonadin F (Ar11) | Halichondria sp. | Unten Port, Okinawa, Japan | 2008 | [66] |
| R=NCS (Ar12) | Axinyssa aplysinoides | Ant Atoll, Pohnpei | 1992 | [69] |
| R=NC (Ar13) | Acanthella acuta | Bay of Naples, Italy | 1987 | [62] |
| Acanthella acuta | Banyuls, France | 1988 | [67] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| Phyllidiella pustulosa | Vietnam | 2010 | [27] | |
| Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] | |
| R=NCS (Ar14) | Acanthella acuta | Bay of Naples, Italy | 1987 | [62] |
| Acanthella acuta | Banyuls, France | 1988 | [67] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| Phyllidiella pustulosa | Vietnam | 2010 | [27] | |
| R=NCO (Ar15) | Acanthella cavernosa | Coral gardens, Gneerings reef, Mooloolba, Australia | 2007 | [32,58] |
| Acanthella acuta | Banyuls, France | 1988 | [67] | |
| R=NHCHO (Ar16) | Hexabrandies sanguinens | South China Sea | 2007 | [68] |
| Epimaalianes | ||||
| R=NC (Ep2) | Cadlina luteomarginata | San Diego, California | 1982 | [70] |
| Acanthella sp. and Cadlina luteomarginata | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] | |
| (−)-epipolasin A (Ep3) | Cadlina luteomarginata | San Diego, California | 1982 | [70] |
| Acanthella pulcherrima | Weed Reef, Darwin, Australia | 1988 | [28] | |
| Acanthella sp. | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] | |
| Axinyssa sp. nov. | Great Barrier Reef | 1997 | [71] | |
| Axinyssa sp. | Tsutsumi Island, Fukuoka prefecture, Japan | 2003 | [165] | |
| R=NHCHO (Ep4) | Acanthella sp. and Cadlina luteomarginata | Conehead Point, Rennell Sound, Graham Island, British Columbia | 1993 | [23] |
| (+)-epipolasin A (Ep5) | Epipolasis kushimotoensis | 1985 | [64] | |
| Axinyssa aplysinoides | Ant Atoll, Pohnpei | 1992 | [69] | |
| R=NHCSNHCH2CH2Ph (Ep6) | Epipolasis kushimotoensis | 1985 | [64] | |
| R=NC (Ep7) | Axinella cannabina | Bay of Taranto, Italy | 1985 | [72] |
| R=NCS (Ep8) | Axinella cannabina | Bay of Taranto, Italy | 1985 | [72] |
| R=NHCHO (Ep9) | Axinella cannabina | Bay of Taranto, Italy | 1985 | [72] |
| Pupukeananes | ||||
| 9-isocyanopupukeanane (Pu2) | Phyllidia varicosa and Cyocalypta sp. | Pupukea, north shore of O’ahu, Hawaii | 1975 | [73] |
| Phyllidia varicosa Lamarck 1801 | Pupukea, north shore of O’ahu, Hawaii | 1979 | [77] | |
| Phyllidia bourguini | Hachijo-jima Island, Japan | 1990 | [75] | |
| Phyllidia pustulosa | Hachijo-jima Island, Japan | 1991 | [47] | |
| 9-epi-isocyanopupukeanane (Pu3) | Phyllidia bourguini | Hachijo-jima Island, Japan | 1990 | [75] |
| Phyllidia pustulosa | Hachijo-jima Island, Japan | 1991 | [47] | |
| 9-isothiocyanatopupukeanane (Pu4) | Axinyssa sp. nov. | Great Barrier Reef | 1997 | [71] |
| 9-thiocyanatopupukeanane (Pu5) | Phyllidia varicosa Axinyssa aculeata | Pramuka Island, Indonesia | 2003 | [76] |
| Phyllidiella pustulosa | Vietnam | 2010 | [27] | |
| 9-epi-thiocyanatopupukeanane (Pu6) | Phyllidia varicosa Axinyssa aculeata | Pramuka Island, Indonesia | 2003 | [76] |
| Phyllidiella pustulosa | Vietnam | 2010 | [27] | |
| 2-isocyanopupukeanane (Pu7) | Phyllidia varicosa Lamarck 1801 | Pupukea, north shore of O’ahu, Hawaii | 1979 | [77] |
| 2-thiocyanatopupukeanane (Pu8) | Axinyssa aplysinoides | Palau | 1992 | [69] |
| 2-formamidopupukeanane (Pu9) | Phyllidia coelestis Bergh | Koh-Ha Islets, Thailand | 2013 | [78] |
| 5-isothiocyanatopupukeanane (Pu10) | Axinyssa | Gun Beach, Guam | 1989 | [43] |
| 9-isocyanoneopupukeanane (Pu11) | Ciocalypta sp. | O’ahu, Hawaii | 1989 | [155] |
| 2-thiocyanatoneopupukeanane (Pu12) | Phycopsis terpnis | Okinawa, Japan and Pohnpei | 1991 | [80] |
| Axinyssa aplysinoides | Mutok Harbor, Pohnpei | 1992 | [69] | |
| Phyllidia pustulosa | Kuchinoerabu/Tanegoshima Island, Japan | 1996 | [42] | |
| 4-thiocyanatoneopupukeanane (Pu13) | Phycopsis terpnis | Okinawa, Japan and Pohnpei | 1991 | [80] |
| Phyllidia pustulosa | Tanegoshima Island, Japan | 1996 | [42] | |
| 2-isocyanoallopupukeanane (Pu14) | Phyllidia pustulosa | Hachijo-jima Island, Japan | 1991 | [47] |
| 1-formamido-10(1 → 2)-abeopupukeanane (Pu15) | Phyllidia coelestis Bergh | Koh-Ha Islets, Thailand | 2013 | [78] |
| Bisabolanes | ||||
| 3-isocyanotheonellin (Bi2) | Phyllidia sp. | Colombo, Sri Lanka | 1986 | [74] |
| Phyllidia pustulosa | Hachijo-jima Island, Japan | 1991 | [47] | |
| Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] | |
| Lipastrotethya ana | Lingshui Bay, Hainan | 2007 | [166] | |
| Raphoxya sp. | Blue Hole, Guam | 2012 | [82] | |
| 3-isothiocyanatotheonellin (Bi3) | Theonella cf. swinhoei | Okinawa | 1984 | [81] |
| Phyllidia pustulosa | Tanegoshima Island, Japan | 1996 | [42] | |
| Axinyssa | Micronesia | 1999 | [90] | |
| Lipastrotethya ana | Lingshui Bay, Hainan | 2007 | [91] | |
| Raphoxya sp. | Blue Hole, Guam | 2012 | [82] | |
| 3-formamidotheonellin (Bi4) | Theonella cf. swinhoei | Okinawa | 1984 | [81] |
| Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] | |
| 3-isocyanatotheonellin (Bi5) | Raphoxya sp. | Blue Hole, Guam | 2012 | [82] |
| 7-isocyano-7,8-dihydro-α-bisabolene (Bi6) | Ciocalypta sp. | Pupukea, O’ahu, Hawaii | 1986 | [74] |
| Phyllidia pustulosa | Hachijo-jima Island, Japan | 1991 | [47] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| 7-isothiocyanato-7,8-dihydro-α-bisabolene (Bi7) | Halichondria sp. | Ponape, Marshall Islands | 1986 | [83] |
| Phyllidia pustulosa | Negros Island, Cebu Island, San Sebastian, Cebu, Philippines | 1991 | [86] | |
| Acanthella cf. cavernosa | Hachijo-jima Island, Japan | 1992 | [19] | |
| Phyllidia pustulosa | Hachijo-jima Island, Japan | 1992 | [19] | |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| N,N′-Bis(6R,7S)-7,8-dihydro-α-bisabolane (Bi8) | Halichondria sp. | Ponape, Marshall Islands | 1986 | [83] |
| 7-formamido-7,8-dihydro-α-bisabolene (Bi9) | Axinyssa sp. | Sanya, Hainan Province, China | 2008 | [84] |
| 7-isocyano-7,8-dihydro-α-bisabolene (Bi10) | Ciocalypta sp. | Pupukea, O’ahu, Hawaii | 1986 | [74] |
| 7-isocyanato-7,8-dihydro-α-bisabolene (Bi11) | Ciocalypta sp. | Pupukea, O’ahu, Hawaii | 1986 | [74] |
| R=NC (Bi12) | Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] |
| (E)-4-isocyanobisabolane-7,10-diene (Bi13) | Axinyssa | Okinawa | 2002 | [85] |
| 3-isocyanobisabolane-8,10-diene (Bi14) | Phyllidia pustulosa | Negros Island, Cebu Island, San Sebastian, Cebu, Philippines | 1991 | [86] |
| 3-formamidobisabolene-8,10-diene (Bi15) | Halichondria cf. lengenfeldi | Palau | 1991 | [86] |
| axinythiocyanate A (Bi16) | Axinyssa isabela | Isabel Island, Nayarit, Mexico | 2008 | [33] |
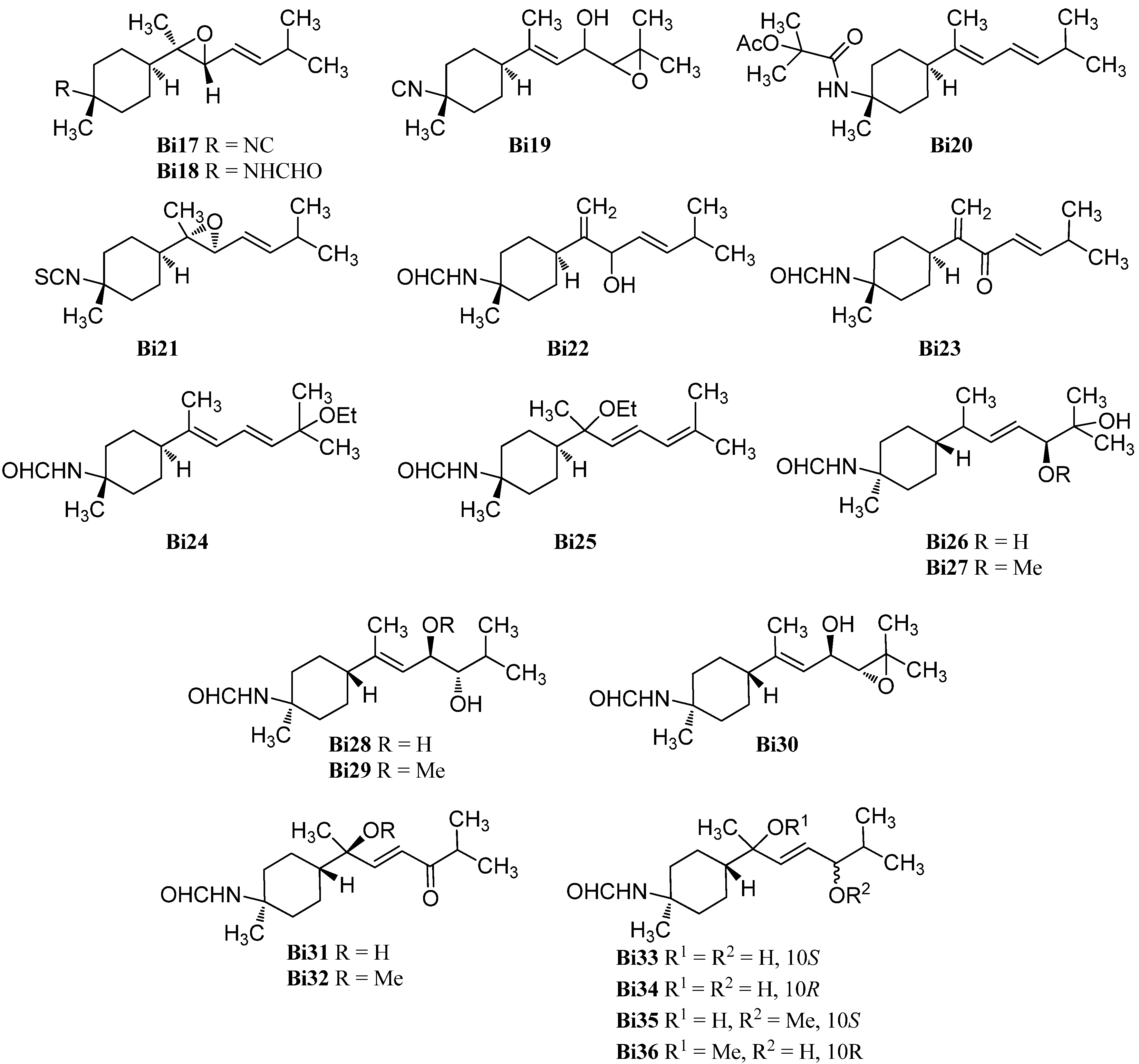
| 3-isocyano-7,8-epoxy-α-bisabolane (Bi17) | Axinyssa sp. | Hainan | 2010 | [87] |
| 3-formamido-7,8-epoxy-α-bisabolane (Bi18) | Axinyssa sp. | Hainan | 2010 | [87] |
| Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] | |
| axinysaline B (Bi19) | Axinyssa sp. | Formosa | 2014 | [88] |
| axinysaline A (Bi20) | Axinyssa sp. | Formosa | 2014 | [88] |
| 7α,8α-epoxy theonellin isothiocyanate (Bi21) | Phycopsis sp. | Mandapam Coast, Gulf of Mannar, Tamilnadu, India | 2009 | [89] |
| 3-formamidobisabolane-14(7),9-dien-8-ol (Bi22) | Axinyssa | Micronesia | 1999 | [90] |
| 3-formamidobisabolane-14(7),9-dien-8-one (Bi23) | Axinyssa | Micronesia | 1999 | [90] |
| 11-ethoxy-3-formamidotheonellin (Bi24) | Axinyssa aff. variabilis | Lingshui Bay, Hainan | 2007 | [91] |
| 7-ethoxy-3-formamidobisabolane-8,10-diene (Bi25) | Axinyssa aff. variabilis | Lingshui Bay, Hainan | 2007 | [91] |
| axinyssine C (Bi26) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine D (Bi27) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine E (Bi28) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine F (Bi29) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine G (Bi30) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine H (Bi31) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine I (Bi32) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine J (Bi33) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine K (Bi34) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| axinyssine L (Bi35) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| 3-formamido-8-methoxybisabolan-9-en-10-ol (Bi36) | Axinyssa | Micronesia | 1999 | [90] |
| Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] | |
| Guaianes | ||||
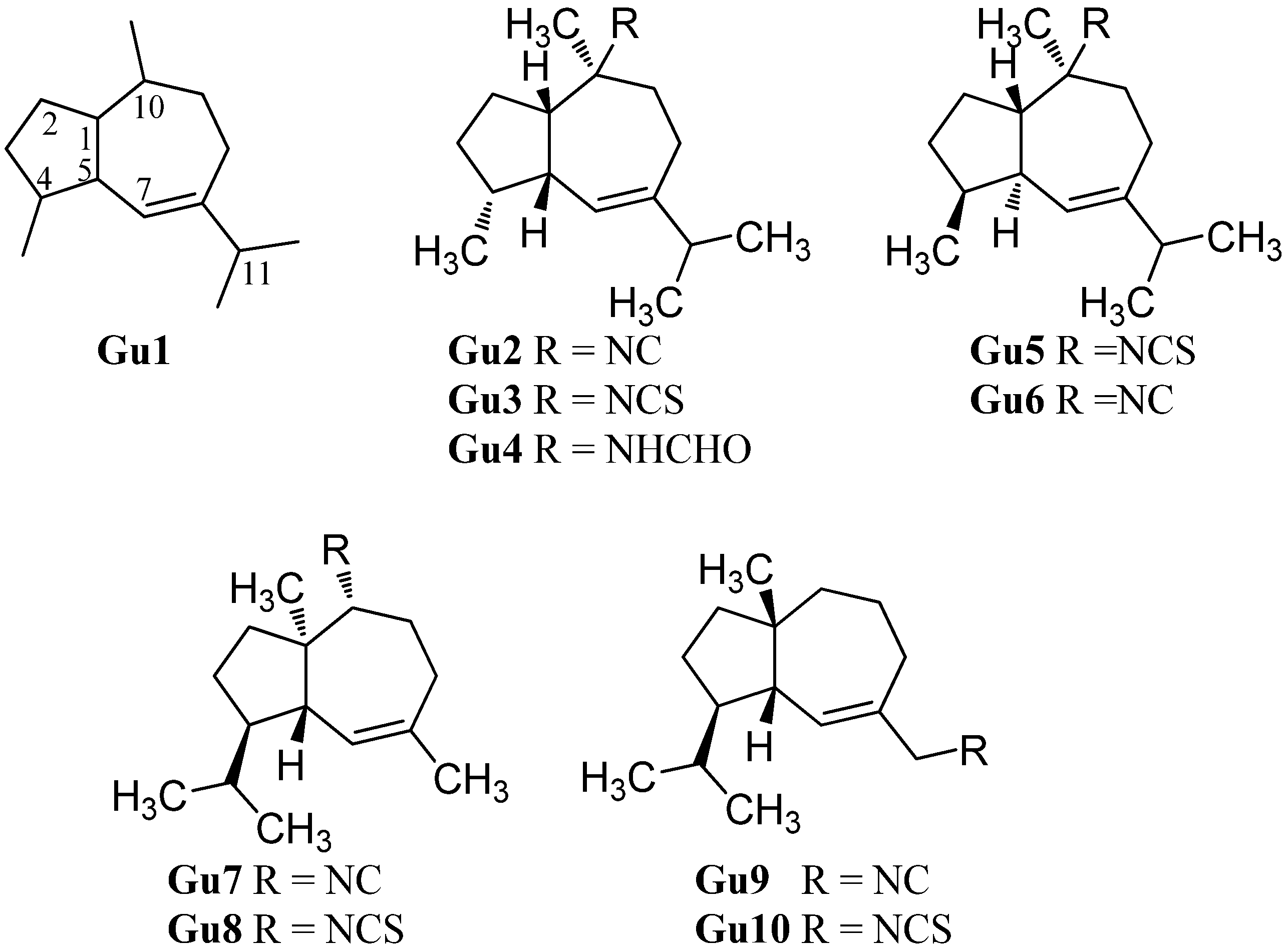
| Guai-6-ene isocyanide (Gu2) | Not identified | Wakayama Prefecture, Japan | 1988 | [93] |
| Guai-6-ene isothiocyanide (Gu3) | Not identified | Wakayama Prefecture, Japan | 1988 | [93] |
| Acanthella cavernosa | Tani’s Reef, Gneerings Reef, Mooloolaba, Australia | 2007 | [32,58] | |
| Guai-6-ene formamide (Gu4) | Not identified | Wakayama Prefecture, Japan | 1988 | [93] |
| (1S*,4S*,5R*,10S*)-10-Isothiocyanatoguaia-6-ene (Gu5) | Trachyopsis aplysinoides | Palau | 1989 | [52] |
| Axinyssa aplysinoides | Palau | 1992 | [69] | |
| (1S*,4S*,5R*,10S*)-10-Isocyanoguaia-6-ene (Gu6) | Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] |
| R=NC (Gu7) | Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] |
| 0R=NCS (Gu8) | Axinyssa sp. | Sanya Island, Hainan, China | 2008 | [84] |
| R=NC (Gu9) | Acanthella acuta | Bay of Naples, Italy | 1987 | [62] |
| R=NCS (Gu10) | Acanthella acuta | Bay of Naples, Italy | 1987 | [62] |
| Further sesquiterpenoids | ||||
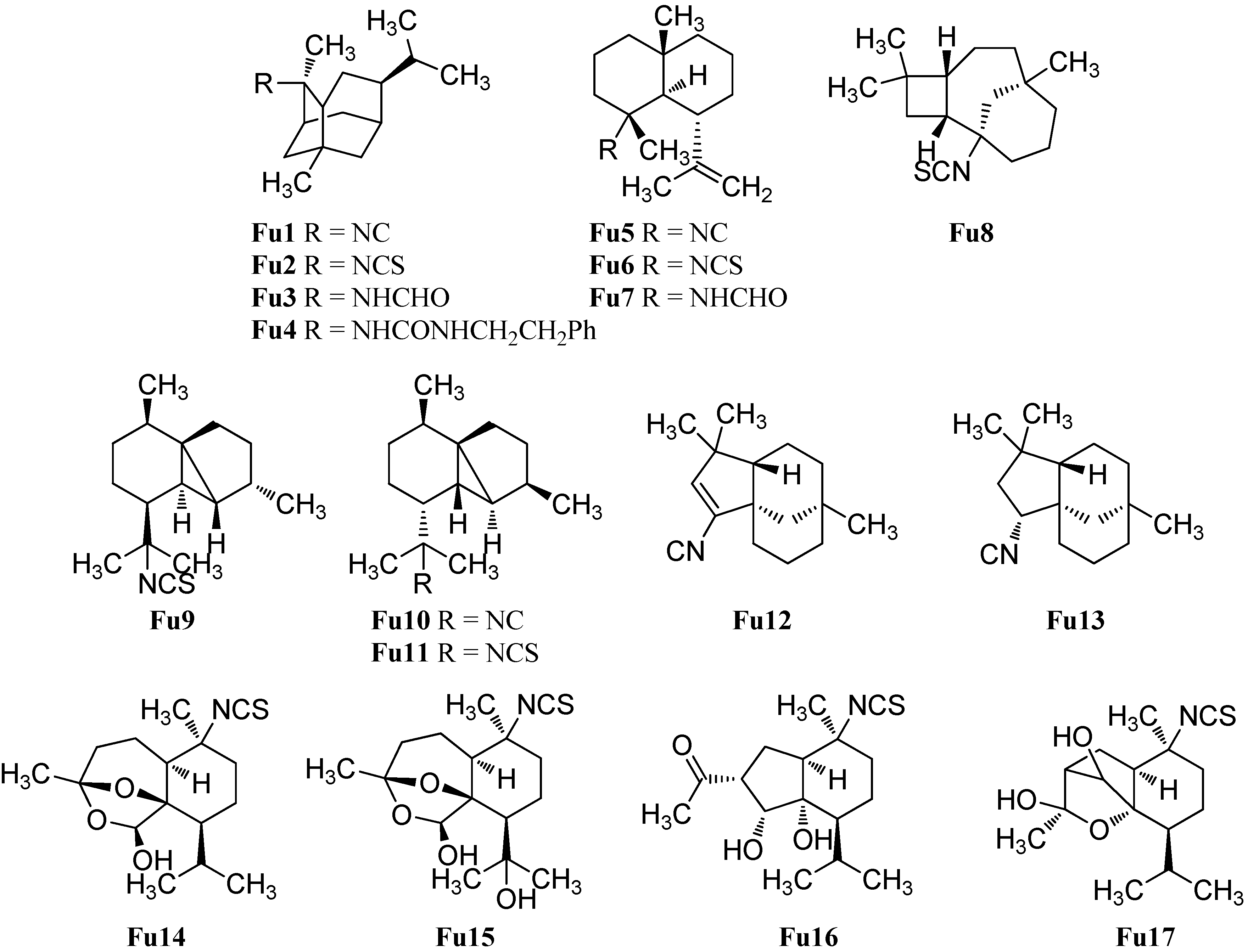
| 2-isocyanotrachyopsane (Fu1) | Phyllidia varicosa | Shimokoshiki Island, Japan | 1996 | [42] |
| 2-isothiocyanatotrachyopsane (Fu2) | Trachyopsis aplysinoides | Palau | 1989 | [52] |
| Axinyssa aplysinoides | Palau | 1992 | [69] | |
| 2-(formylamino)trachyopsane (Fu3) | Axinyssa aplysinoides | Malakal Harbor, Palau | 1997 | [95] |
| N-phenethyl-N′-2-trachyopsane (Fu4) | Axinyssa aplysinoides | Malakal Harbor, Palau | 1997 | [95] |
| 4α-isocyanogorgon-11-ene (Fu5) | Phyllidia varicosa and Phyllidia pustulosa | Negros Island, Cebu Island, San Sebastian, Cebu, Philippines | 1991 | [86] |
| 4α-isothiocyanatogorgon-11-ene (Fu6) | Phyllidia pustulosa | Negros Island, Cebu Island, San Sebastian, Cebu, Philippines | 1991 | [86] |
| 4α-formamidogorgon-11-ene (Fu7) | Phyllidia varicosa and Phyllidia pustulosa | Negros Island, Cebu Island, San Sebastian, Cebu, Philippines | 1991 | [86] |
| (−)-(1S,2R,5R,8R)-1-isothiocyanatoepicaryolane (Fu8) | Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] |
| (1S*,2R*,5S*,6S*,7R*,8S*)-13-isothiocyanatocubebane (Fu9) | Axinyssa aplysinoides | Ant Atoll, Pohnpei | 1992 | [69] |
| (1S*,2S*,5S*,6S*,7R*,8S*)-13-isocyanocubebane (Fu10) | Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] |
| (1S*,2S*,5S*,6S*,7R*,8S*)-13-isothiocyanatocubebane (Fu11) | Stylissa sp. | Iriomote Island, Okinawa, Japan | 2004 | [50] |
| (−)-(1S,5S,8R)-2-isocyanoclovene (Fu12) | Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] |
| (−)-(1S,2R,5S,8R)-2-isocyanoclovane (Fu13) | Phyllidia ocellata | Mudjimba Island, Mooloolaba, Australia | 2015 | [94] |
| axiplyn A (Fu14) | Axinyssa aplysinoides | Misali Island, Tanzania | 2008 | [54] |
| axiplyn B (Fu15) | Axinyssa aplysinoides | Misali Island, Tanzania | 2008 | [54] |
| axiplyn D (Fu16) | Axinyssa aplysinoides | Misali Island, Tanzania | 2008 | [54] |
| axiplyn E (Fu17) | Axinyssa aplysinoides | Misali Island, Tanzania | 2008 | [54] |
| Diterpenoids | ||||
| Acyclic | ||||
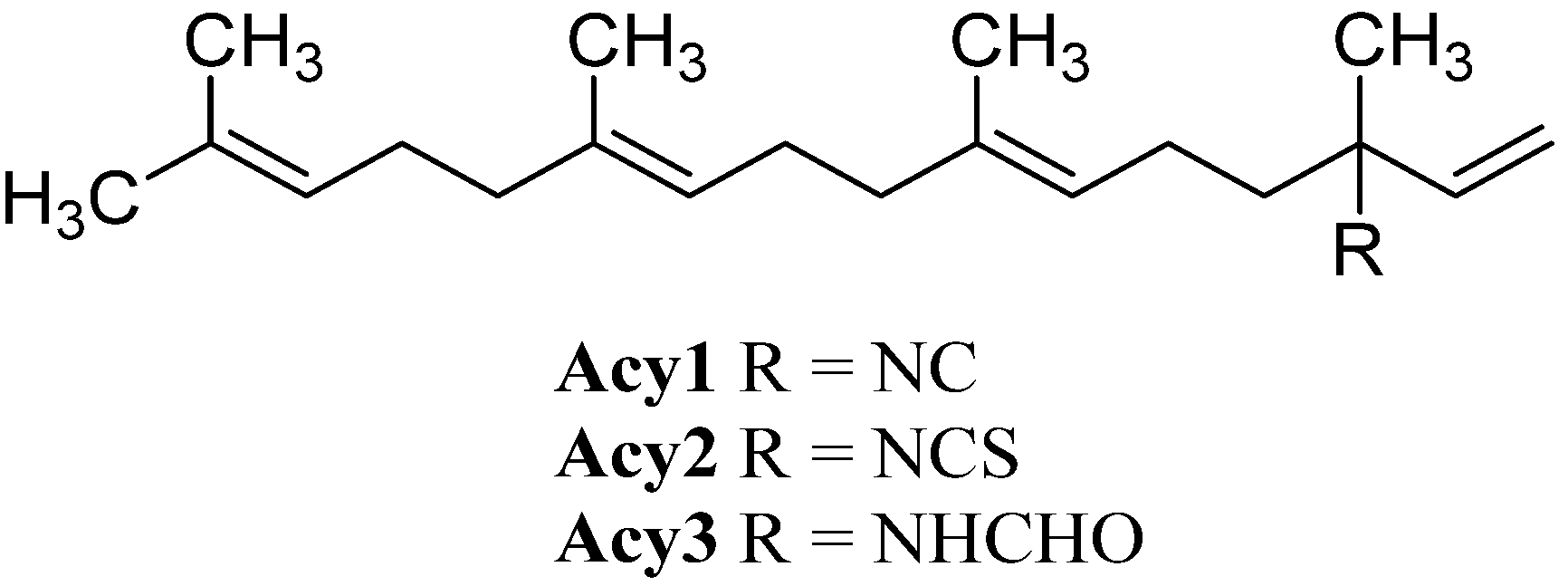
| R=NC (Acy1) | Halichondria sp. | North coast of O’ahu, Hawaii | 1974 | [39,40] |
| R=NCS (Acy2) | Halichondria sp. | North coast of O’ahu, Hawaii | 1974 | [39,40] |
| R=NHCHO (Acy3) | Halichondria sp. | North coast of O’ahu, Hawaii | 1974 | [39,40] |
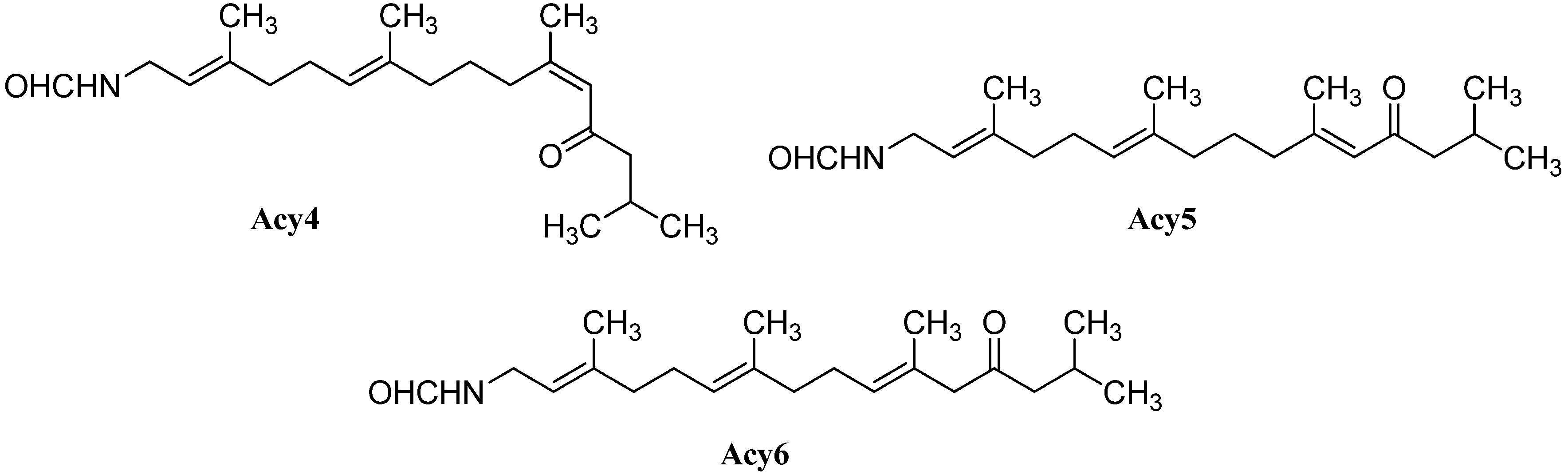
| malonganenone C (Acy4) | Leptogorgia gilchristi | Ponto Malongane, Mozambique | 2006 | [96] |
| Euplexaura nuttingi | Uvinage, Pemba Island, Tanzania | 2007 | [97] | |
| Δ11,12-(E) (Acy5) | Euplexaura nuttingi | Uvinage, Pemba Island, Tanzania | 2007 | [97] |
| malonganenone K (Acy6) | Euplexaura robusta | Wei Zhou Island (Guanxi, China) | 2012 | [98] |
| Kalihinoles | ||||
| kalihinol A (Kol1) | Acanthella sp. | Apra Harbor, Guam | 1984 | [100,101,102] |
| Acanthella cavernosa | Fiji | 1988 | [106] | |
| Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] | |
| Acanthella cavernosa | Yakushima Island, Japan | 1995 | [111] | |
| Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] | |
| Phakellia pulcherrima | Davao, Philippines | 1998 | [104] | |
| Acanthella cavernosa | Coral reef, Ishigaki Island, Okinawa, Japan | 1998 | [105] | |
| Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] | |
| Acanthella sp. | Yalong Bay, Hainan Province, China | 2009 | [103] | |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] | |
| 10β-formamido kalihinol A (Kol2) | Acanthella cavernosa | Hachiji-jima Island, Japan | 1996 | [20] |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] | |
| kalihinol Z (Kol3) | Acanthella sp. | Fish Patch, Vita Levu, Fiji | 1987 | [102] |
| Acanthella cavernosa | Fiji | 1988 | [106] | |
| Phakellia pulcherrima | Davao, Philippines | 1998 | [104] | |
| kalihinol X (Kol4) | Acanthella sp. | Fish Patch, Vita Levu, Fiji | 1987 | [102] |
| Acanthella cavernosa | Fiji | 1988 | [106] | |
| Acanthella cavernosa | Thailand | 1991 | [49] | |
| Phakellia pulcherrima | Philippines | 1998 | [104] | |
| Acanthella cavernosa | Dibud, Philippines | 2004 | [109] | |
| 10-epi-kalihinol X (Kol5) | Acanthella sp. | Yalong Bay, Hainan Province, China | 2009 | [103] |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] | |
| kalihinol E (Kol6) | Acanthella sp. | Apra Harbor, Guam | 1987 | [102] |
| Acanthella cavernosa | Hachiji-jima Island, Japan | 1996 | [20] | |
| Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] | |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] | |
| 10β-formamido kalihinol E (Kol7) | Acanthella cavernosa | Hachiji-jima Island, Japan | 1996 | [20] |
| kalihinol O (Kol8) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
| kalihinol Y(Kol9) | Acanthella sp. | Fish Patch, Vita Levu, Fiji | 1987 | [102] |
| Acanthella cavernosa | Fiji | 1988 | [106] | |
| Acanthella cavernosa | Thailand | 1991 | [49] | |
| Phakellia pulcherrima | Philippines | 1998 | [104] | |
| Acanthella cavernosa | Dibud, Philippines | 2004 | [109] | |
| Δ9-kalihinol Y(Kol10) | Phakellia pulcherrima | Davao, Philippines | 1998 | [104] |
| Acanthella cavernosa | Coral reef, Ishigaki Island, Okinawa, Japan | 1998 | [105] | |
| kalihinol P (Kol11) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
| kalihinol S (Kol12) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
| kalihinol J (Kol13) | Acanthella cavernosa | Thailand | 1991 | [49] |
| Acanthella cavernosa | Dibud, Philippines | 2004 | [109] | |
| kalihinol I (Kol14) | Acanthella cavernosa | Thailand | 1991 | [49] |
| 10-epi-kalihinol I (Kol15) | Acanthella cavernosa | Coral reef, Ishigaki Island, Okinawa, Japan | 1998 | [105] |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] | |
| 10β-formamido-5β-isothiocyanato kalihinol A (Kol16) | Acanthella cavernosa | Hachiji-jima Island, Japan | 1996 | [20] |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] | |
| 10β-formamido-5β-isocyanato kalihinol A (Kol17) | Acanthella cavernosa | Hachiji-jima Island, Japan | 1996 | [20] |
| kalihinol Q (Kol18) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
| kalihinol R (Kol19) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
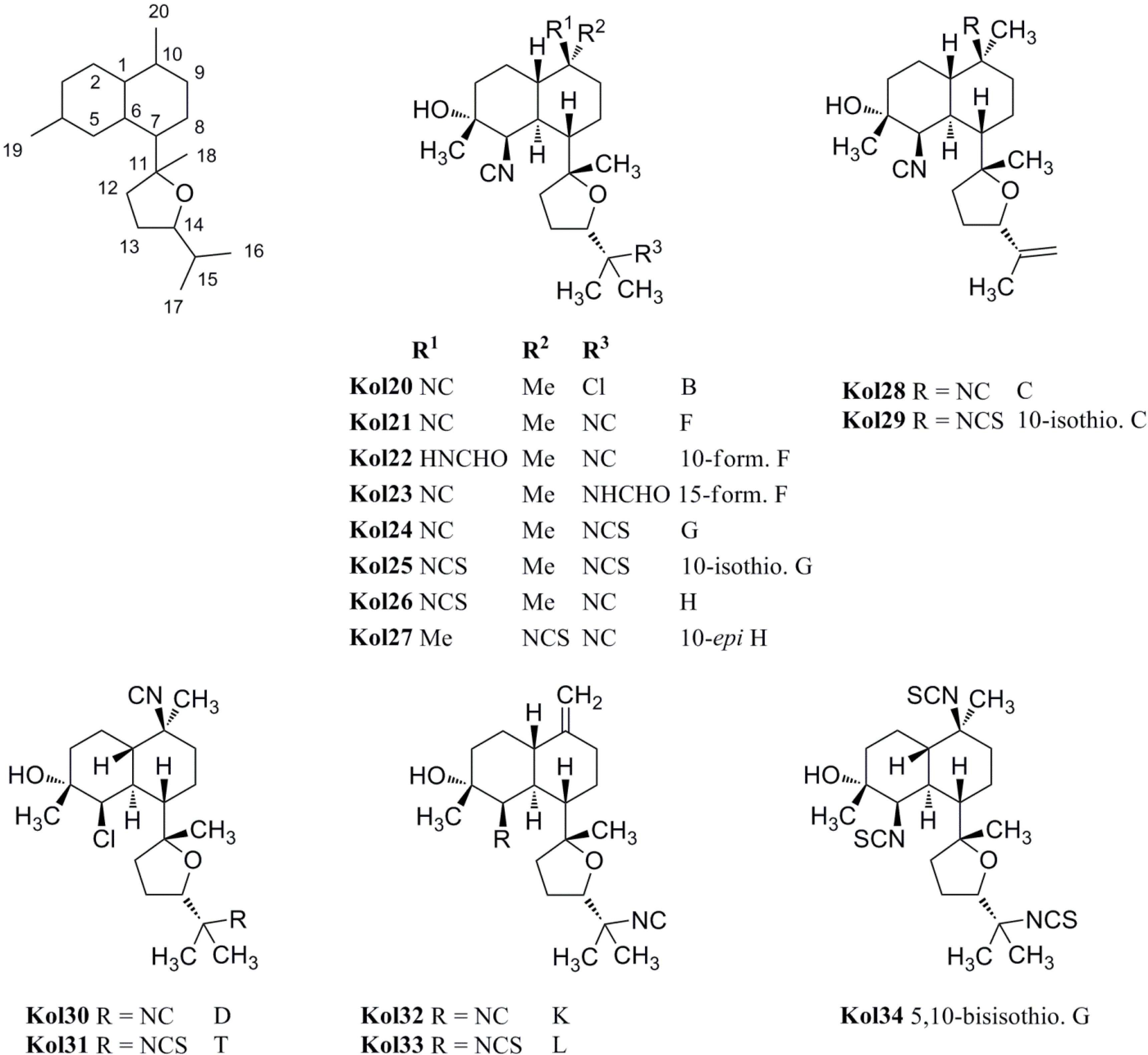
| kalihinol B (Kol20) | Acanthella sp. | Apra Harbor, Guam | 1987 | [102] |
| Phakellia pulcherrima | Davao, Philippines | 1998 | [104] | |
| kalhinol F (Kol21) | Acanthella sp. | Apra Harbor, Guam | 1987 | [100,101,102] |
| Acanthella cavernosa | Fiji | 1988 | [106] | |
| Acanthella sp. | Cape Sada, Ehime Prefecture, Japan | 2003 | [108] | |
| Acanthella cavernosa | Davao Gulf, Mindanao, Philippines | 2004 | [109] | |
| 10-formamido kalihinol F (Kol22) | Acanthella cavernosa | Davao Gulf, Mindanao, Philippines | 2004 | [109] |
| 15-formamido kalihinol F(Kol23) | Acanthella cavernosa | Davao Gulf, Mindanao, Philippines | 2004 | [109] |
| kalihinol G (Kol24) | Acanthella sp. | Apra Harbor, Guam | 1987 | [102] |
| Acanthella cavernosa | Davao Gulf, Mindanao, Philippines | 2004 | [109] | |
| 10-isothiocyanato kalihinol G (Kol25) | Phakellia pulcherrima | Philippines | 1998 | [104] |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] | |
| kalihinol H (Kol26) | Acanthella sp. | Apra Harbor, Guam | 1987 | [102] |
| 10-epi-kalihinol H (Kol27) | Phakellia pulcherrima | Davao, Philippines | 1998 | [104] |
| kalihinol C (Kol28) | Acanthella sp. | Apra Harbor, Guam | 1987 | [102] |
| Phakellia pulcherrima | Philippines | 1998 | [104] | |
| 10-isothiocyanato kalihinol C (Kol29) | Phakellia pulcherrima | Philippines | 1998 | [104] |
| kalihinol D (Kol30) | Acanthella sp. | Apra Harbor, Guam | 1987 | [102] |
| Acanthella sp. | Yalong Bay, Hainan Province, China | 2009 | [103] | |
| kalihinol T (Kol31) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
| kalihinol K (Kol32) | Phakellia pulcherrima | Davao, Philippines | 1998 | [104] |
| kalihinol L (Kol33) | Phakellia pulcherrima | Davao, Philippines | 1998 | [104] |
| 5,10-bisisothiocyanato kalihinol G (Kol34) | Acanthella cavernosa | Coral reef, Ishigaki Island, Okinawa, Japan | 1998 | [105] |
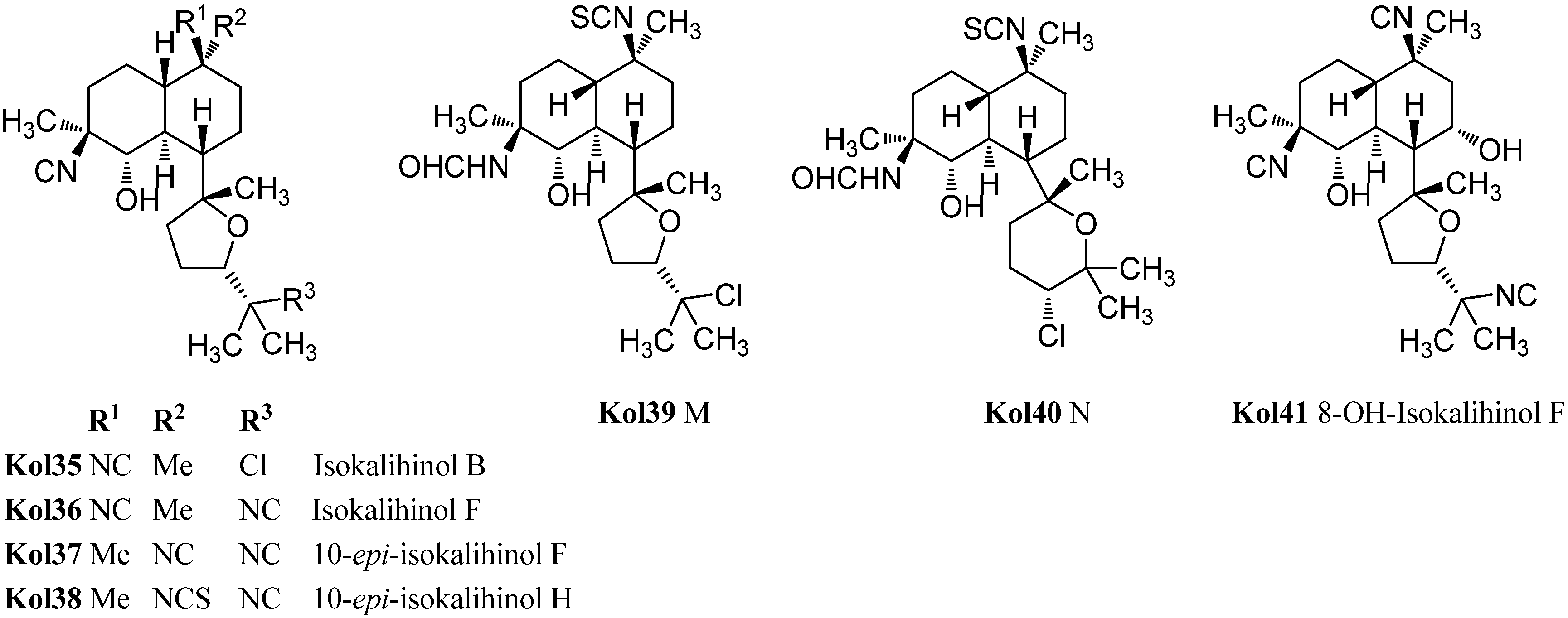
| isokalihinol B (Kol35) | Acanthella cavernosa | Kuchihoerabu Island, Satsunan Archipel, Japan | 1990 | [110] |
| Acanthella cavernosa | Beau Vallon Beach, Mahé , Seychelles | 1994 | [112] | |
| isokalihinol F (Kol36) | Acanthella cavernosa | Fiji | 1988 | [106] |
| Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] | |
| 10-epi-isokalihinol F (Kol37) | Acanthella cavernosa | Beau Vallon Beach, Mahé , Seychelles | 1994 | [112] |
| 10-epi-isokalihinol H (Kol38) | Acanthella cavernosa | Beau Vallon Beach, Mahé , Seychelles | 1994 | [112] |
| kalihinol M (Kol39) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
| kalihinol N (Kol40) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [107] |
| 8-OH-isokalihinol F (Kol41) | Acanthella cavernosa | Heron Island, Great Barrier Reef, Australia | 2000 | [31] |
| kalihinenes | ||||
| kalihinene (Ken1) | Acanthella cavernosa | Kuchihoerabu Island, Satsunan Archipel, Japan | 1990 | [110] |
| Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] | |
| Phakellia pulcherrima | Davao, Philippines | 1998 | [104] | |
| Acanthella cavernosa | Coral reef, Ishigaki Island, Okinawa, Japan | 1998 | [105] | |
| Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] | |
| Acanthella cavernosa | Davao Gulf, Mindanao, Philippines | 2004 | [109] | |
| 15-formamido kalihinene (Ken2) | Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] |
| Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] | |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] | |
| 10-formamido-kalihinene (Ken3) | Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] |
| Acanthella cavernosa | Yakushima Island, Japan | 1995 | [111] | |
| Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] | |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] | |
| 10,15-bisformamido kalihinene (Ken4) | Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] |
| 6-hydroxy-kalihinene (Ken5) | Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] |
| Acanthella cavernosa | Coral reef, Ishigaki Island, Okinawa, Japan | 1998 | [105] | |
| 6-hydroxy-15-formamido-kalihinene (Ken6) | Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] |
| 6-hydroxy-10-formamido-kalihinene (Ken7) | Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] |
| 6-hydroxy-10-formamido-15-isothiocyanato-kalihinene (Ken8) | Acanthella cavernosa | Pacific Harbor/Benga (=Beqa) Lagoon, Fiji | 1994 | [99] |
| Kalihinene A/1,10-diepi-kalihinene (Ken9) | Acanthella cavernosa | Beau Vallon Beach, Mahé , Seychelles | 1994 | [112] |
| Kalihinene B/1-epi-kalihinene (Ken10) | Acanthella cavernosa | Beau Vallon Beach, Mahé , Seychelles | 1994 | [112] |
| Phakellia pulcherrima | Davao, Philippines | 1998 | [104] | |
| Acanthella cavernosa | Heron Island, Great Barrier Reef, Australia | 2000 | [31] | |
| 15-isothiocyanato-1-epi-kalihinene (Ken11) | Acanthella cavernosa | Beau Vallon Beach, Mahé , Seychelles | 1994 | [112] |
| Kalihinene F (Ken12) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] |
| Kalihinene E (Ken13) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] |
| Kalihinene X (Ken14) | Acanthella cavernosa | Yakushima Island, Japan | 1995 | [111] |
| Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] | |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] | |
| Kalihinene Y (Ken15) | Acanthella cavernosa | Yakushima Island, Japan | 1995 | [111] |
| Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] | |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] | |
| Kalihinene Z (Ken16) | Acanthella cavernosa | Yakushima Island, Japan | 1995 | [111] |
| Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] | |
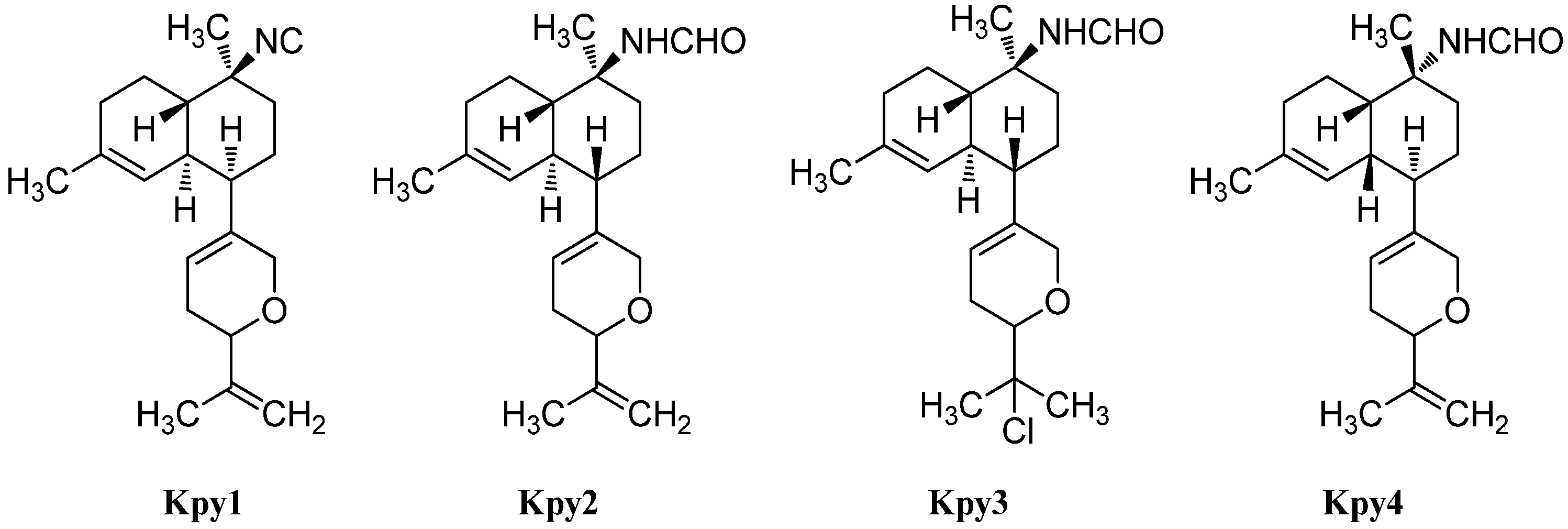
| Kalihipyranes | ||||
| Kalihipyran (Kpy1) | Acanthella cavernosa | Beau Vallon Beach, Mahé , Seychelles | 1994 | [112] |
| Acanthella cavernosa | Heron Island, Great Barrier Reef, Australia | 2000 | [31] | |
| Kalihipyran A (Kpy2) | Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] |
| Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] | |
| Kalihipyran B (Kpy3) | Acanthella cavernosa | Yakushima Island, Japan | 1996 | [114] |
| Kalihipyran C (Kpy4) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] |
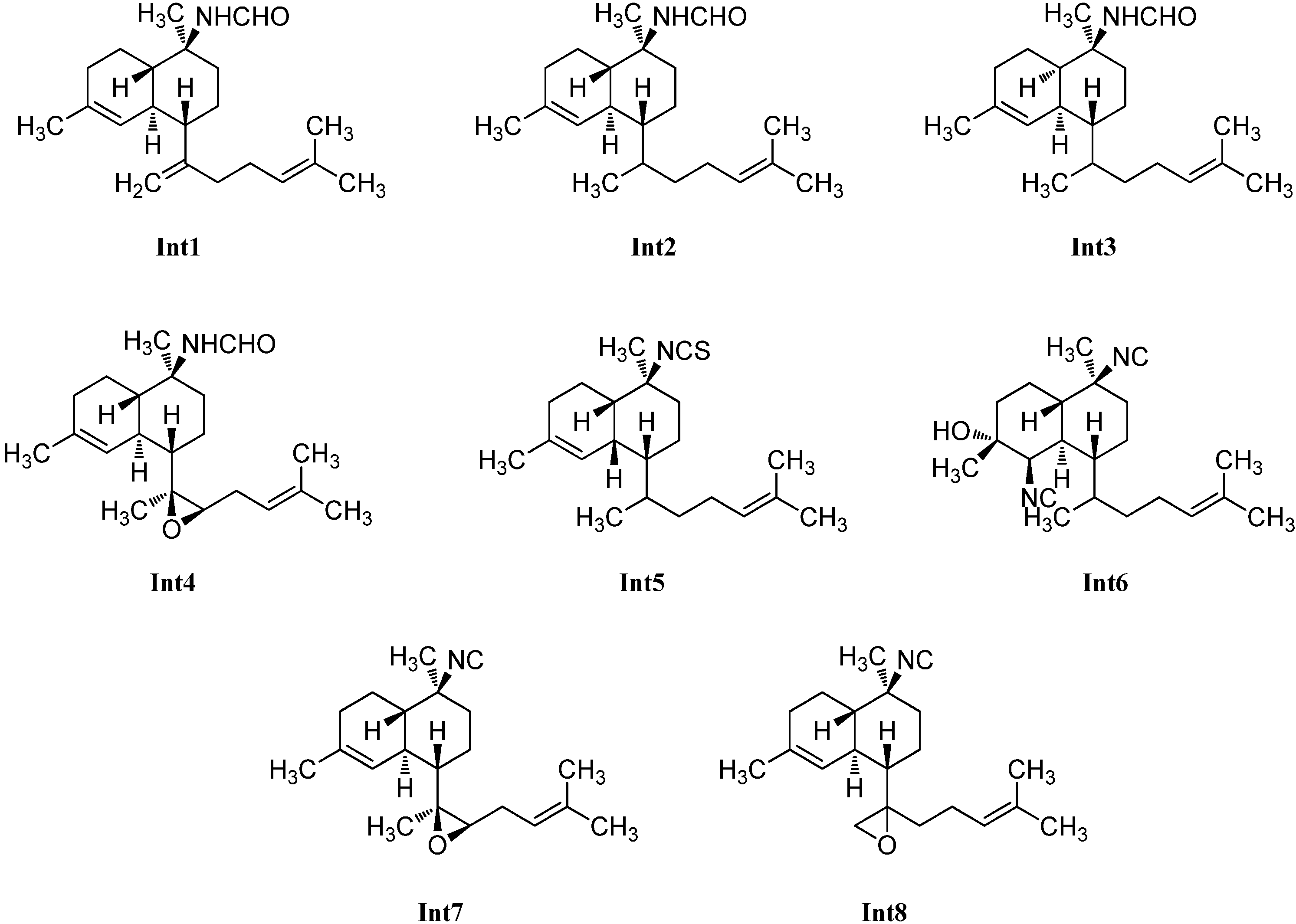
| Intermediates | ||||
| Cavernene A (Int1) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] |
| Cavernene B (Int2) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] |
| Cavernene C (Int3) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] |
| Cavernene D (Int4) | Acanthella cavernosa | Xisha Islets, South China Sea | 2012 | [113] |
| (Int5) planar | Adocia | Miyako Island, Japan | 1992 | [116] |
| (1S*,6R*,7R*,10S*,11R*)-10-isothiocyanatobiflora-1,14-diene (Int5) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| Pulcherrimol (Int6) | Phyllidiella pulcherrima | Davao, Philippines | 1998 | [104] |
| 11,12-epoxy-10-isocyano-4,14-bifloradiene (Int7) | Acanthella cavernosa | Heron Island, Great Barrier Reef, Australia | 2000 | [31] |
| 11,18-epoxy-10-isocyano-4,14-bifloradiene (Int8) | Acanthella cavernosa | Heron Island, Great Barrier Reef, Australia | 2000 | [31] |
| Amphilectenes | ||||
| 8,15-diisocyano-11(20)-amphilectene/(−)-DINCA (Amp1) | Pseudoaxinella amphilecta | Glover Reef, Belize | 1978 | [119] |
| Phyllidiella pustulosa | Hainan Island, South China Sea | 2004 | [35] | |
| Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] | |
| Ciocalapata sp. (Halichondriidae) | Koh-Tao, Surat-Thani province, Thailand | 2009 | [122] | |
| Pseudoaxinella flava | Sweeting Cay, Grand Bahamas | 2011 | [128] | |
| Svenzea flava | Great Inagua Island, Bahamas | 2013 | [133] | |
| Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2012/2015 | [125,160] | |
| 8-isocyano-15-formamido-11(20)-amphilectene (Amp2) | Pseudoaxinella amphilecta | Glover Reef, Belize | 1978 | [119] |
| Svenzea flava | Great Inagua Island, Bahamas | 2013 | [133] | |
| Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2012/2015 | [125,160] | |
| 8-isocyano-15-isothiocyanato-11(20)-amphilectene (Amp3) | Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] |
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*)-8-isocyanato-amphilecta-11(20),14-diene (Amp4) | Stylissa sp. | Coral reef, Iriomote Island, Okinawa, Japan | 2004 | [50] |
| Ciocalapata sp. (Halichondriidae) | Koh-Tao, Surat-Thani province, Thailand | 2009 | [122] | |
| 8-isocyano-10,14-amphilectadiene (Amp5) | Halichondria sp. | Palau | 1987 | [121] |
| (3S*,4R*,7S*,8S*,11S*,13S*)-8-isocyanoamphilecta-1(12),14-diene (Amp6) | Stylissa sp. | Coral reef, Iriomote Island, Okinawa, Japan | 2004 | [50] |
| 8-isocyanoamphilecta-11(20),15-diene (Amp7) | Ciocalapata sp. (Halichondriidae) | Koh-Tao, Surat-Thani province, Thailand | 2009 | [123] |
| 8-isocyanato-15-formamidoamphilect-11(20)-ene (Amp8) | Stylissa cf. massa | Koh-Tao, Surat-Thani province, Thailand | 2012 | [123] |
| 8-isothiocyanato-15-formamidoamphilect-11(20)-ene (Amp9) | Stylissa cf. massa | Koh-Tao, Surat-Thani province, Thailand | 2012 | [123] |
| Monoamphilectine A (Amp10) | Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 15 December 2010 | [124,125,160] |
| Monoamphilectine B (Amp11) | Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2015 | [125] |
| Monoamphilectine C (Amp12) | Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2015 | [125] |
| 7-isocyano-11(20),14-epiamphilectadien/(1R*,3S*,4R*,7S*,8S*,12S*,13S*)-7-isocyanoamphilecta-11(20),14-diene (Amp13) | Adocia sp. | 1980 | [126] | |
| Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] | |
| Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] | |
| (1R*,3S*,4R*,7S*,8S*,12S*,13S*)-7-formamidoamphilecta-11(20),14-diene (Amp14) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 2009 | [127] |
| (1R*,3S*,4R*,7S*,8S*,13R*)-7-isocyanoamphilecta-11,14-diene (Amp15) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*)-7-isocyanoamphilecta-11(20),15-diene (Amp16) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| Adocia sp. | 1980 | [126] | ||
| Ciocalapata sp. (Halichondriidae) | Koh-Tao, Surat-Thani province, Thailand | 2009 | [122] | |
| Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2015 | [125] | |
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*)-7-Formamidoamphilecta-11(20),15-diene (Amp17) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 2009 | [127] |
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*-7-isocyano-amphilecta-10,14-diene (Amp18) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| (1R*,3S*,4R*,7S*,8S*,12S*,13S*)-7-isocyanoamphilecta-10,14-diene (Amp19) | Stylissa sp. | Iriomote Island, Okinawa, Japan | 2004 | [50] |
| (1R*,3S*,4R*,7S*,8S*,12R*,13R*)-12-hydroxy-7-isothiocyanato-amphilecta-11(20),14-diene (Amp20) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| 7,15-diisocyano-11(20)-epiamphilectene (Amp21) | Adocia sp. | 1980 | [126] | |
| Svenzea flava | Great Inagua Island, Bahamas | 2013 | [133] | |
| Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2015 | [125] | |
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*)-7-isocyano-15-isothiocyanatoamphilecta-11(20)-ene (Amp22) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| 7,15-diisocyano-11(20)-amphilectene (Amp23) | Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] |
| Pseudoaxinella flava | Sweeting Cay, Grand Bahamas | 2011 | [128] | |
| Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2012 | [160] | |
| 7-isocyano-15-isothiocyanato-11(20)-amphilectene (Amp24) | Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] |
| (1S,3S,4R,7S,8R,12S,13S)-7-isocyanoamphilecta-11(20),15-diene (Amp25) | Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] |
| Pseudoaxinella flava | Sweeting Cay, Grand Bahamas | 2011 | [128] | |
| Svenzea flava | Cabo Norte, Mona Island, Puerto Rico | 2012 | [160] | |
| (Amp26) | Pseudoaxinella flava | Sweeting Cay, Grand Bahamas | 2011 | [128] |
| Biflora-4,9,15-triene (Amp27) | Acanthella cavernosa | Hachiji-jima Island, Japan | 1996 | [20] |
| Acanthella cavernosa | Yakusha Island, Japan | 1996 | [114] | |
| Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] | |
| Cycloamphilectenes | ||||
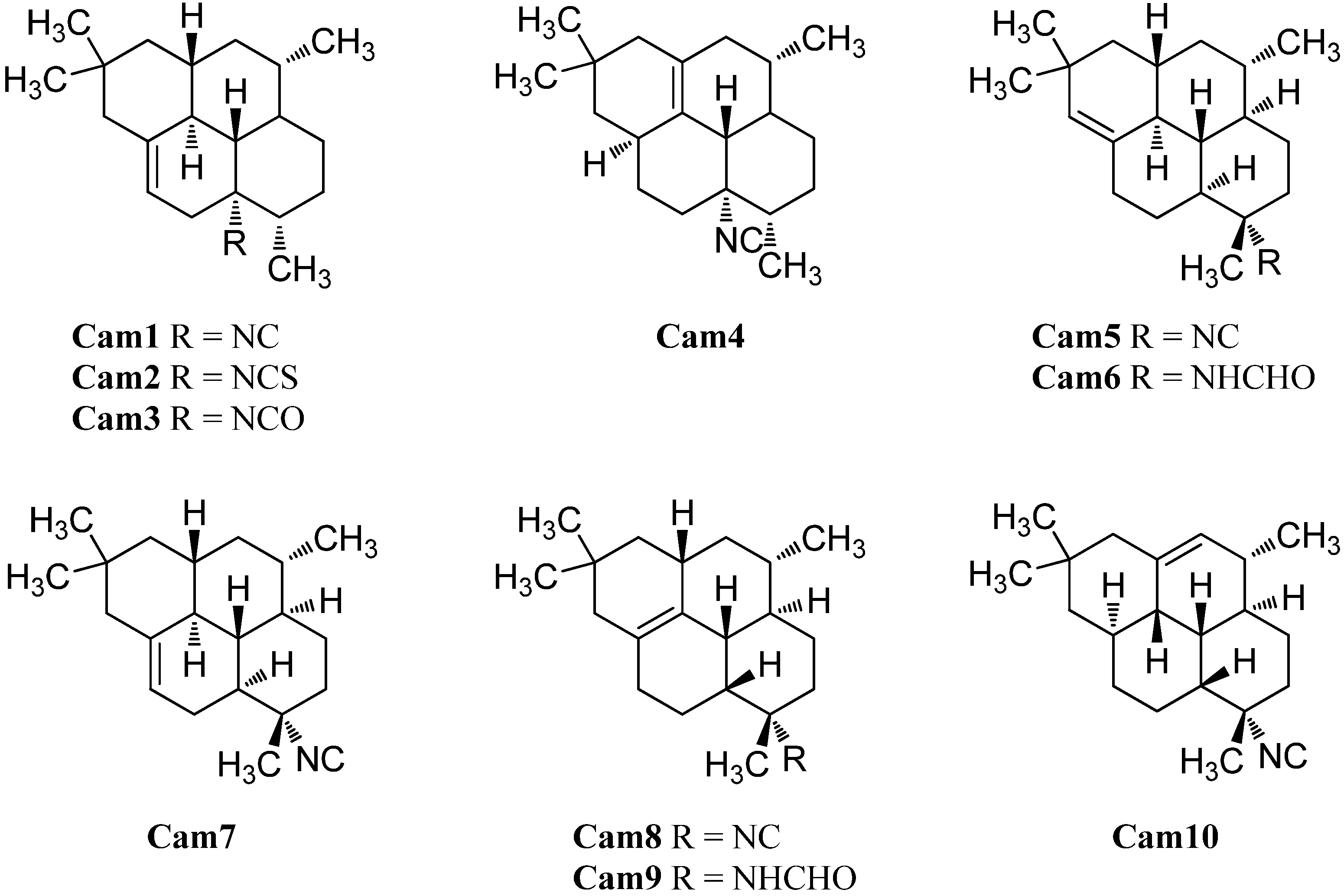
| 8-isocyano-10-cycloamphilectene (Cam1) | Adocia sp. | 1980 | [126] | |
| Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] | |
| 8-isothiocyanato-amphilect-10-ene (Cam2) | Stylissa sp. | Coral reef, Iriomote Island, Okinawa, Japan | 2004 | [50] |
| 8-isocyanatocycloamphilect-10-ene (Cam3) | Stylissa sp. | Coral reef, Iriomote Island, Okinawa, Japan | 2004 | [50] |
| (3S*,4R*,7S*,8S*,11S*,13R*)-8-isocyano-1(12)-cycloamphilectene (Cam4) | Halichondria sp. | Palau | 1987 | [121] |
| Adocia sp. | 1980 | [126] | ||
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*)-7-isocyanocycloamphilect-11(20)-ene (Cam5) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*)-7-Formamidocycloamphilect-11(20)-ene (Cam6) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 2009 | [127] |
| (1S*,3S*,4R*,7S*,8S*,12S*,13S*)-7-isocyanocylcoamphilect-10-ene (Cam7) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| (1S*,3S*,4R*,7S*,8R*,13R*)-7-isocyano-11-cycloamphilectene (Cam8) | Halichondria sp. | Palau | 1987 | [121] |
| (1S*,3S*,4R*,7S*,8R*,13R*)-N-formyl-7-amino-11-cycloamphilectene (Cam9) | Axinella sp. | Vanuatu | 2002 | [129] |
| (3S*,4R*,7S*,8R*,11S*,12R*,13S*)-7-isocyano-1-cycloamphilectene (Cam10) | Halichondria sp. | Palau | 1987 | [121] |
| Isocycloamphilectanes | ||||
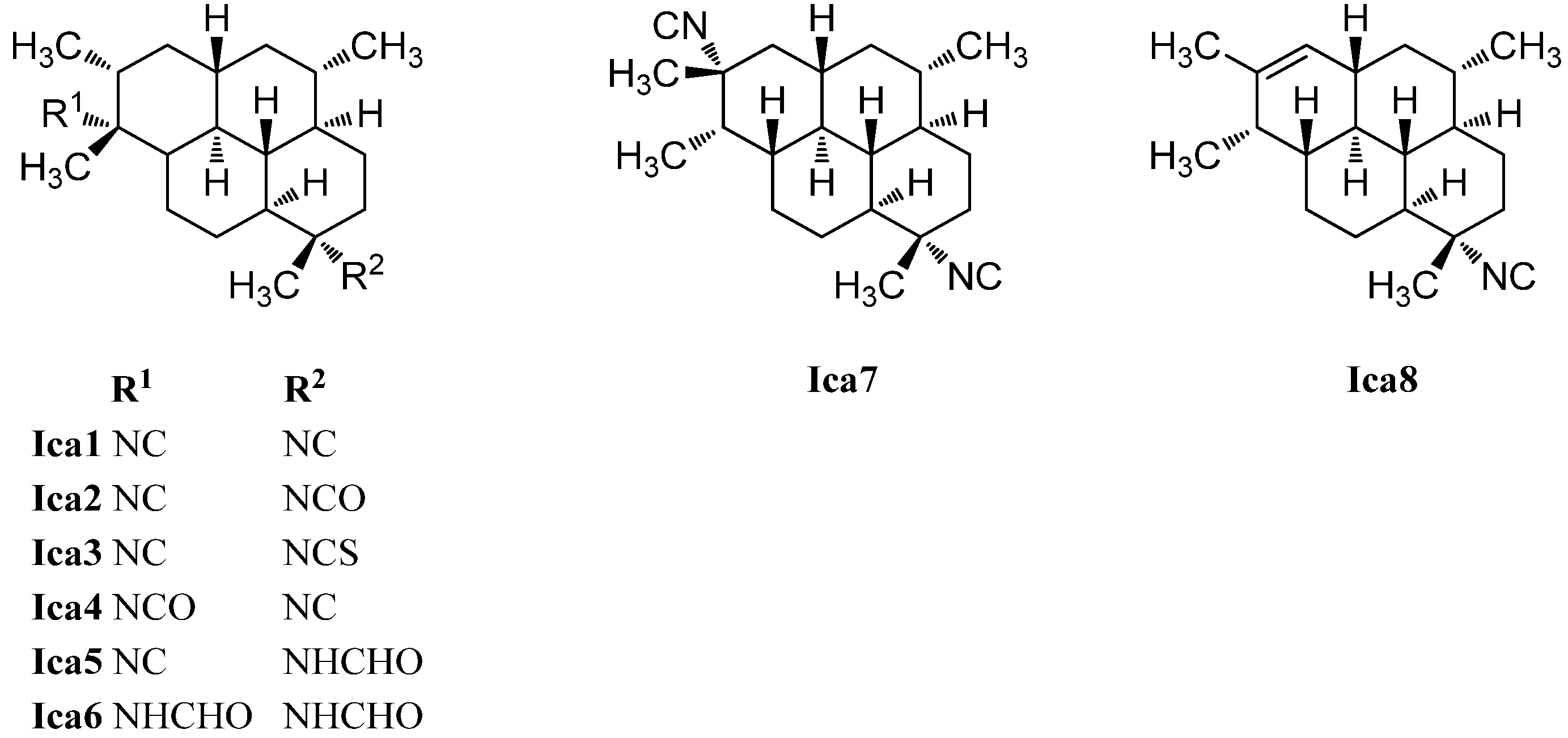
| diisocyanoadociane/(1S,3S,4R,7S,8S,11S,12S,13S,15R,20R)-7,20-diisocyanoisocycloamphilectane (Ica1) | Adocia | Great Barrier Reef, Townsville, Australia | 1976 | [130] |
| Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] | |
| Cribochalina sp. | Caribbean coast of Mexico | 2005 | [120] | |
| (1S,3S,4R,7S,8S,11S,12S,13S,15R,20R)-20-isocyano-7-isocyanatoisocycloamphilectane (Ica2) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| (1S,3S,4R,7S,8S,11S,12S,13S,15R,20R)-20-isocyano-7-isothiocyanatoisocycloamphilectane (Ica3) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| (1S,3S,4R,7S,8S,11S,12S,13S,15R,20R)-20-isocyanato-7-isocyanoisocycloamphilectane (Ica4) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| (1S,3S,4R,7S,8S,11S,12S,13S,15R,20R)-7-Formamido-10-isocyanoisocycloamphilectane (Ica5) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 2009 | [127] |
| (1S,3S,4R,7S,8S,11S,12S,13S,15R,20R)-7,20-Diformamidoisocycloamphilectane (Ica6) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 2009 | [127] |
| 7,15-diisocyanoadociane (Ica7) | Adocia sp. | 1980 | [126] | |
| (1S*,3S*,4R*,7S*,8S*,11R*,12R*,13S*,20S*)-7-isocyanoisocycloamphilect-14-ene (Ica8) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| Neoamphilectenes | ||||
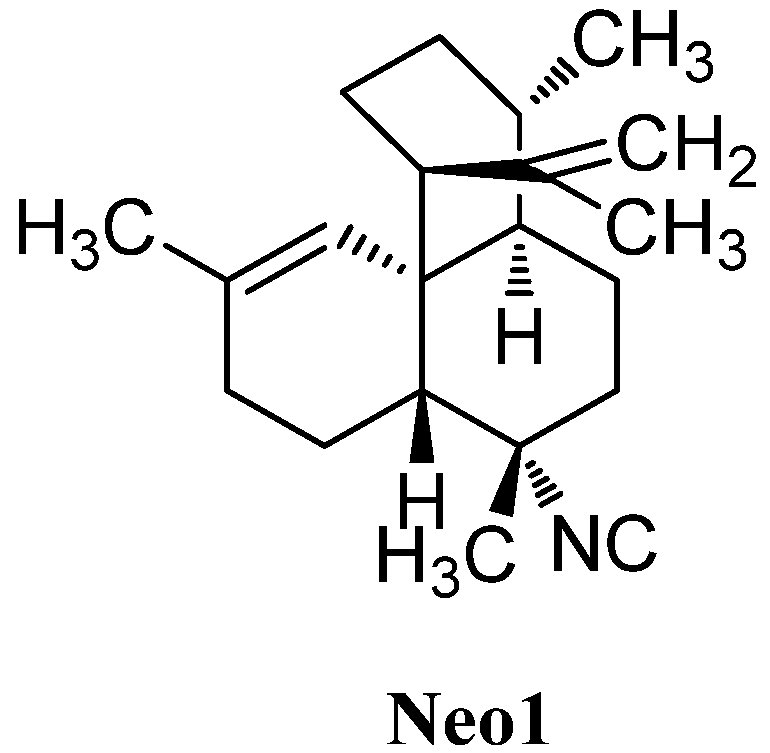
| 7-isocyanoneoamphilecta-11,15-diene (Neo1) | Adocia | Miyako Island, Japan | 1992 | [116] |
| Isoneoamphilectenes | ||||
| 7-isocyanoneoamphilecta-1(14),15-diene (Ina1) | Cymbastela hooperi | Kelso Reef, Queensland, Australia | 1996 | [115] |
| Svenzea flava | Great Inagua Island, Bahamas | 2013 | [133] | |
| 7-formamidoisoneoamphilecta-1(14),15-diene (Ina2) | Svenzea flava | Great Inagua Island, Bahamas | 2013 | [133] |
| 7-methylaminoisoneoamphilecta-1(14),15-diene (Ina3) | Svenzea flava | Great Inagua Island, Bahamas | 2013 | [133] |
| Carbonimidic dichlorides | ||||
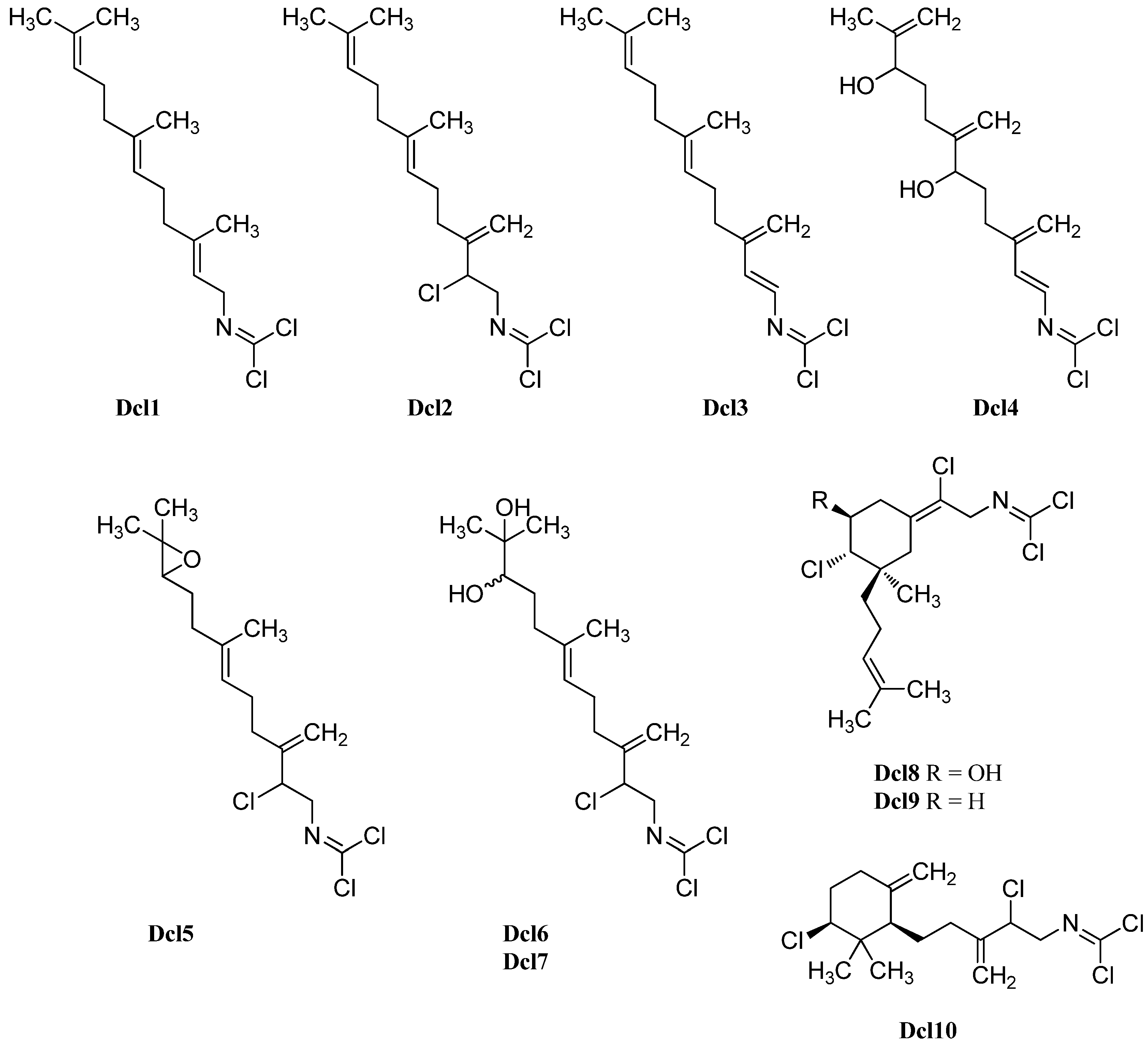
| Stylotellane A (Dcl1) | Stylotella aurantium | Coral Garden, Heron Island, Great Barrier Reef, Australia | 1997 | [71] |
| Stylotellane B (Dcl2) | Pseudoaxinella pitys | Indo-pacific | 1977 | [134] |
| Stylotella aurantium | Coral Gardens, Heron Island, Great Barrier Reef, Australia | 1997 | [71] | |
| Ulosa spongia | Wistari Reef, Great Barrier Reef, Australia | 2001 | [136] | |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| Ulosin A (Dcl3) | Ulosa spongia | Wistari Reef, Great Barrier Reef, Australia | 2001 | [136] |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| Ulosin B (Dcl4) | Ulosa spongia | Wistari Reef, Great Barrier Reef, Australia | 2001 | [136] |
| Axinyssimide A (Dcl5) | Axinyssa | Hachijo-jima Island, Japan | 1998 | [53] |
| Axinyssimide B (Dcl6) | Axinyssa | Hachijo-jima Island, Japan | 1998 | [53] |
| Axinyssimide C (Dcl7) | Axinyssa | Hachijo-jima Island, Japan | 1998 | [53] |
| (1R*,5S*,6S*)-6,14-dichloro-5-hydroxy-9,3(14)-(Z)-axinyssadien-15-yl-carbonimidic dichloride (Dcl8) | Pseudoaxinella pitys | Indo-pacific | 1977 | [134] |
| Reticulidia fungia | Irabu Island, Okinawa, Japan | 1999 | [141] | |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| R=H (Dcl9) | Stylotella aurantium | 2004 | [138] | |
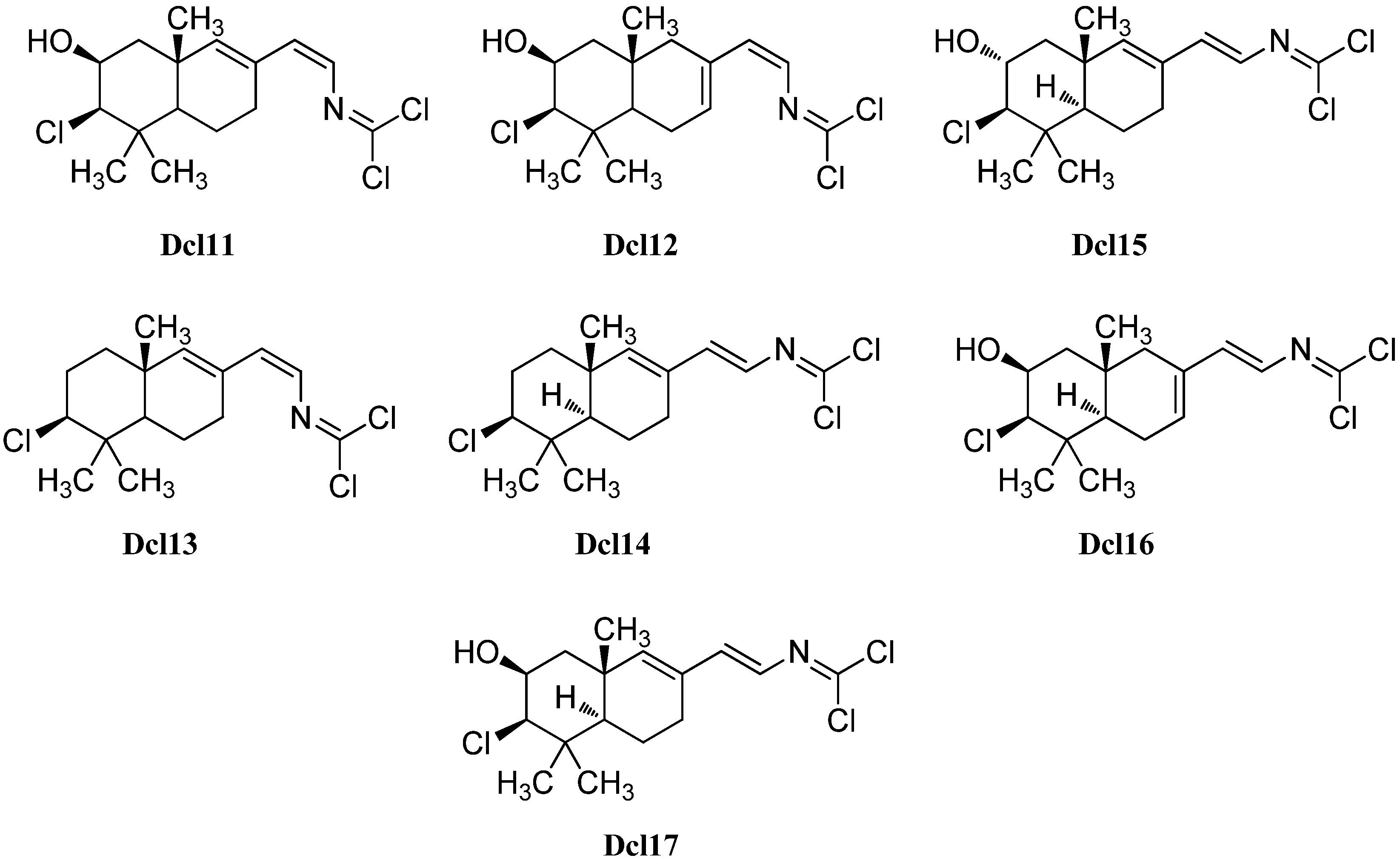
| (Dcl10) | Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] |
| (Dcl11) | Pseudoaxinella pitys | Indo-pacific | 1978 | [140] |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| (Dcl12) | Pseudoaxinella pitys | Indo-pacific | 1978 | [140] |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| (Dcl13) | Pseudoaxinella pitys | Indo-pacific | 1978 | [140] |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| (Dcl14) | Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] |
| Reticulidin A (Dcl15) | Reticulidia fungia | Irabu Island, Okinawa, Japan | 1999 | [141] |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| Reticulidin B (Dcl16) | Reticulidia fungia | Irabu Island, Okinawa, Japan | 1999 | [141] |
| Ulosa spongia | Wistari Reef, Great Barrier Reef, Australia | 2001 | [136] | |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| Isoreticulidin B (Dcl17) | Pseudoaxinella pitys | Indo-pacific | 1977 | [134] |
| Reticulidia fungia | Irabu Island, Okinawa, Japan | 1999 | [141] | |
| Ulosa spongia | Wistari Reef, Great Barrier Reef, Australia | 2001 | [136] | |
| Stylotella aurantium | Iriomate Island, Okinawa, Japan | 2001 | [137] | |
| Other marine isonitriles | ||||
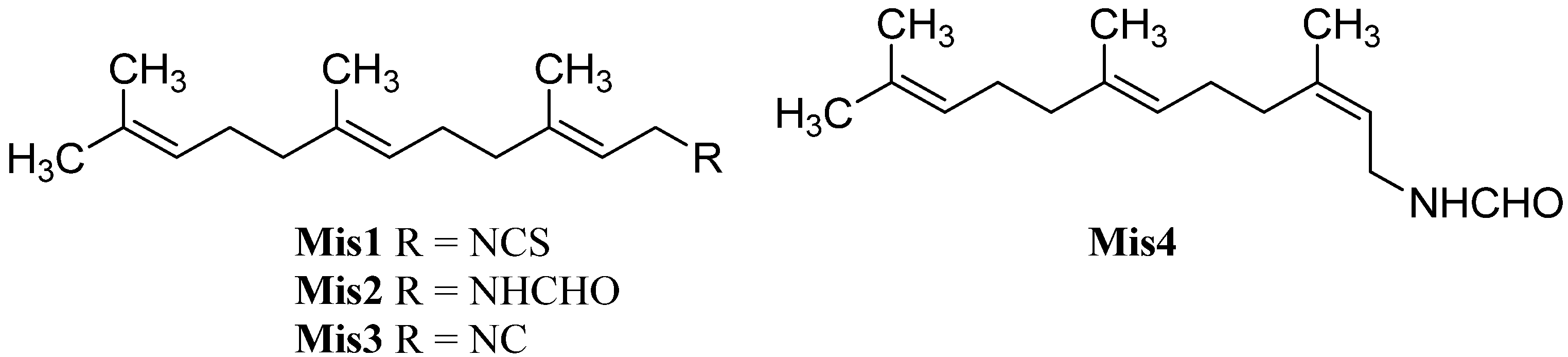
| Farnesyl isothiocyanate (Mis1) | Stylotella aurantium | Heron Island, Great Barrier Reef, Australia | 1997 | [71] |
| Farnesyl formamide (Mis2) | Axinyssa sp. | Sanya, Hainan Province, China | 2008 | [84] |
| Isofarnesyl formamide (Mis4) | Axinyssa sp. | Sanya, Hainan Province, China | 2008 | [84] |
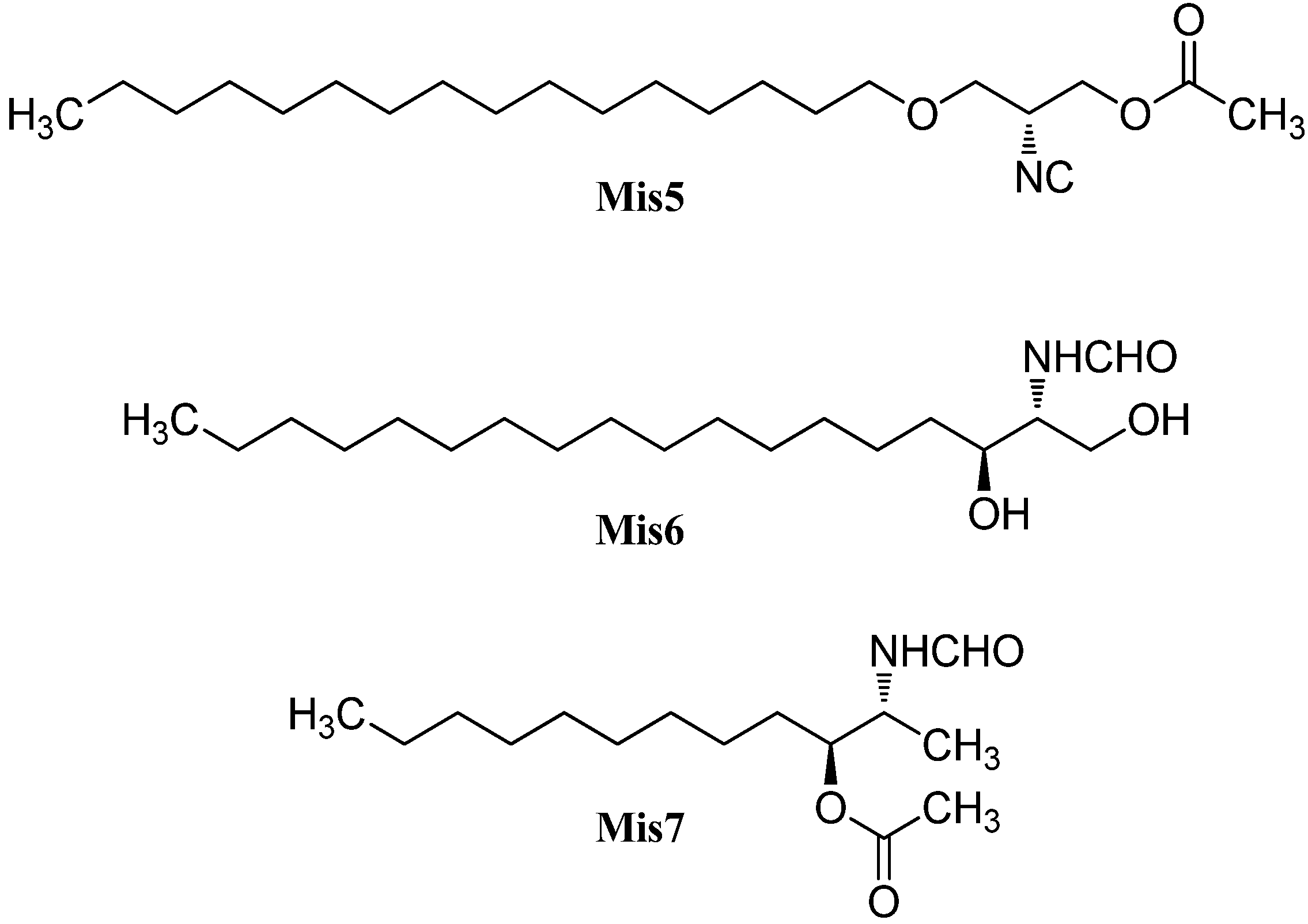
| (R)-(−)-actisonitrile (Mis5) | Actinocyclis papillatus | Wei Zhou Island, South China Sea, China | 2011 | [142] |
| (2R,3S)-2-formamide-1,3dihydroxy-octadecane (Mis6) | Gracilaria verrucosa | Jeju Island, South Korea | 2008 | [143] |
| Clavaminol L (Mis7) | Clavelina plegraea | Bay of Naples, Itlay | 2009 | [144] |
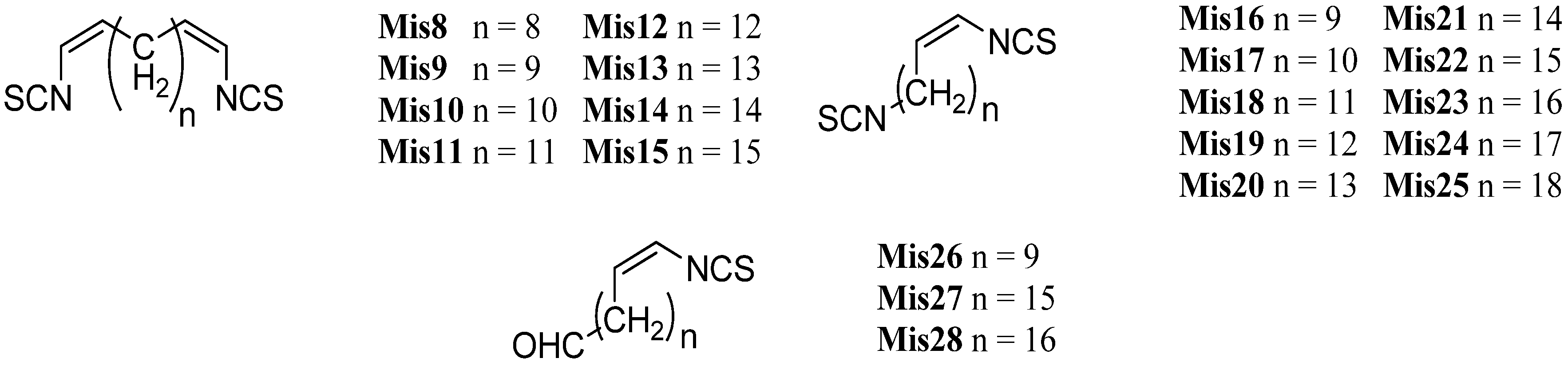
| (Mis8–Mis28) | Pseudoaxinyssa sp. | Fiji | 1987 | [145] |
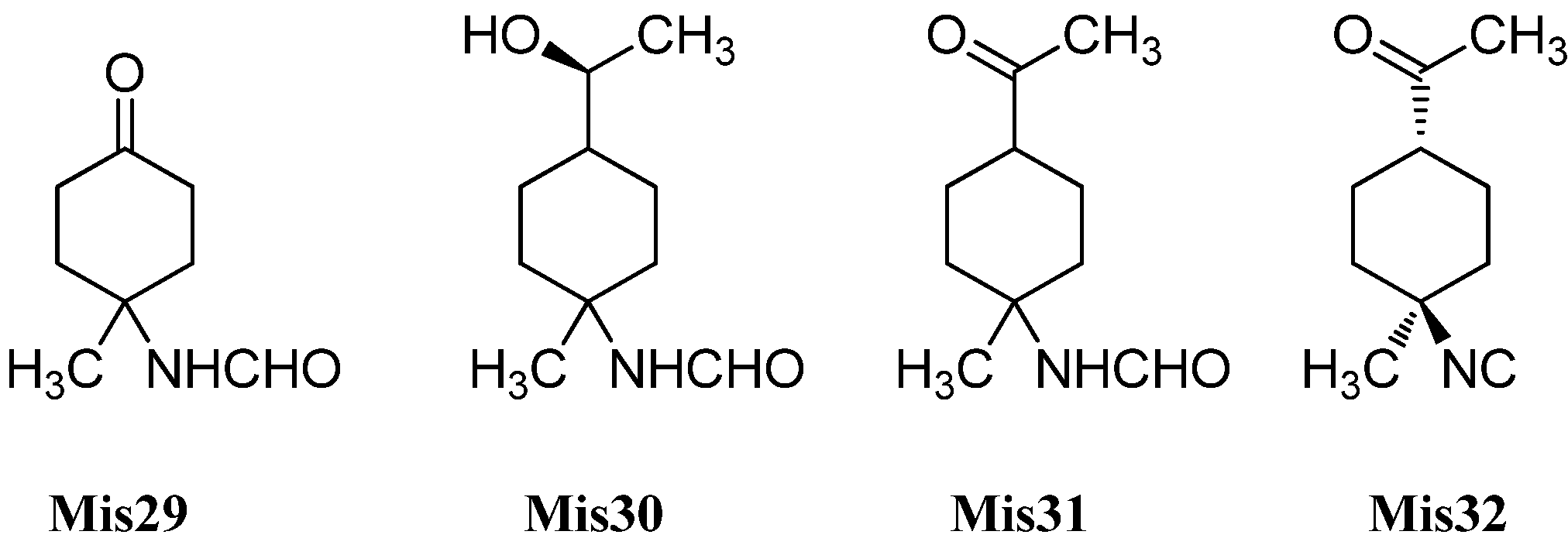
| Axinyssina A (Mis29) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| Axinyssine B (Mis30) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| 1-acetyl-4-formamido-4-methylcyclohexane (Mis31) | Axinyssa sp. | Inner coral reef, Andaman Sea, Thailand | 2014 | [92] |
| 1-acetyl-4-isocyano-4-methylcyclohexane (Mis32) | Phyllidia sp. | Colombo, Sri Lanka | 1986 | [74] |
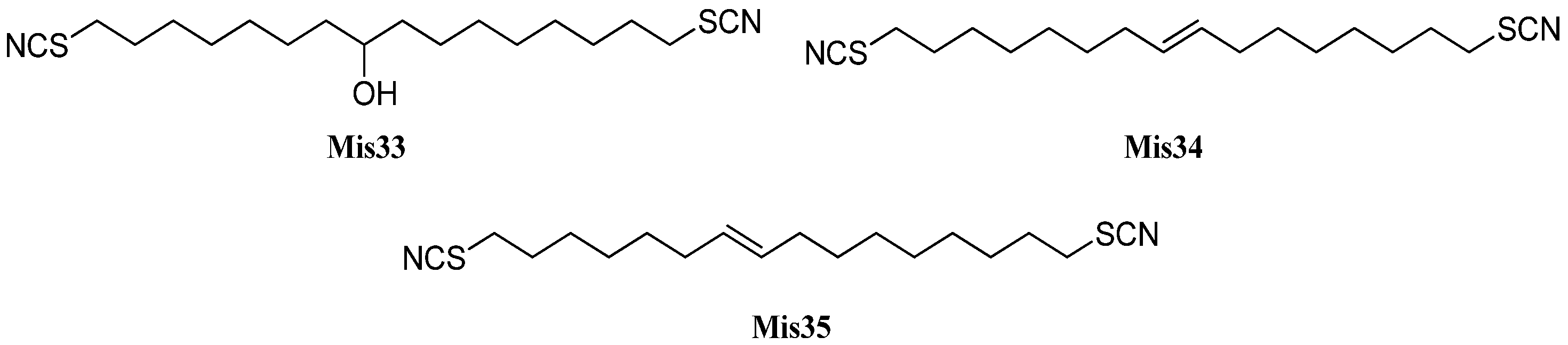
| Thiocyanatin A (Mis33) | Oceanapia sp. | Northern Rottnest shelf, Australia | 2001 | [146] |
| Thiocyanatin B (Mis34) | Oceanapia sp. | Northern Rottnest shelf, Australia | 2001 | [146] |
| Thiocyanatin C (Mis35) | Oceanapia sp. | Northern Rottnest shelf, Australia | 2001 | [146] |
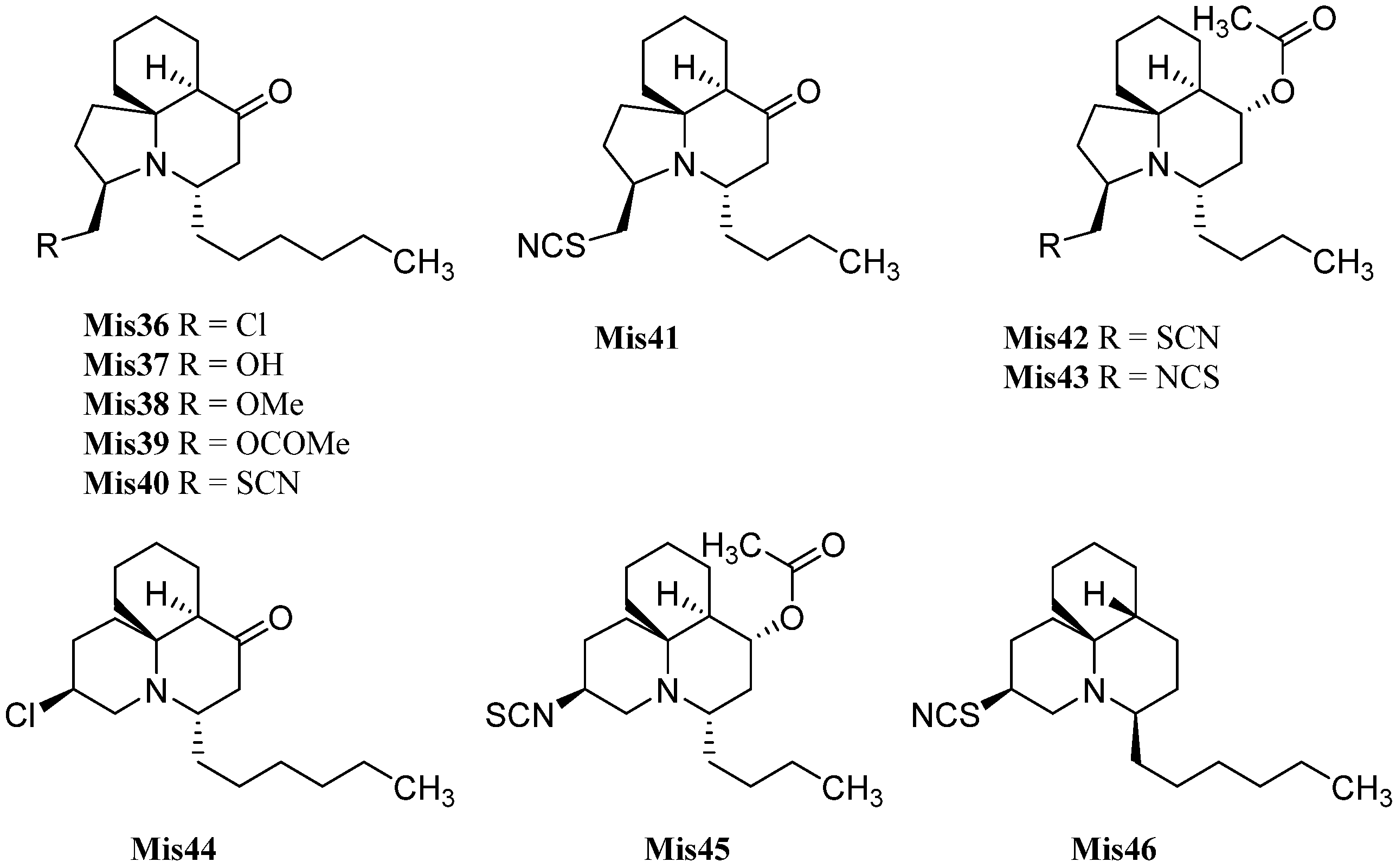
| Cylindricine A (Mis36) | Clavelina cylindrica | Deep Glen Bay, East Coast, Tasmania | 1994 | [147] |
| Cylindricine C (Mis37) | Clavelina cylindrica | Deep Glen Bay, East Coast, Tasmania | 1994 | [147] |
| Cylindricine D (Mis38) | Clavelina cylindrica | Deep Glen Bay, East Coast, Tasmania | 1994 | [147] |
| Cylindricine E (Mis39) | Clavelina cylindrica | Deep Glen Bay, East Coast, Tasmania | 1994 | [147] |
| Cylindricine F (Mis40) | Clavelina cylindrica | Bay of Islands, South Bruny Island, Tasmania | 1994 | [147] |
| Cylindricine G (Mis41) | Clavelina cylindrica | Bay of Islands, South Bruny Island, Tasmania | 1994 | [147] |
| Cylindricine H (Mis42) | Clavelina cylindrica | Tasmania | 1995 | [148] |
| Cylindricine I (Mis43) | Clavelina cylindrica | Tasmania | 1995 | [148] |
| Cylindricine B (Mis44) | Clavelina cylindrica | Deep Glen Bay, East Coast, Tasmania | 1994 | [147] |
| Cylindricine J (Mis45) | Clavelina cylindrica | Tasmania | 1995 | [148] |
| Fasicularin (Mis46) | Nephtheis fasicularis | Micronesia | 1997 | [149] |
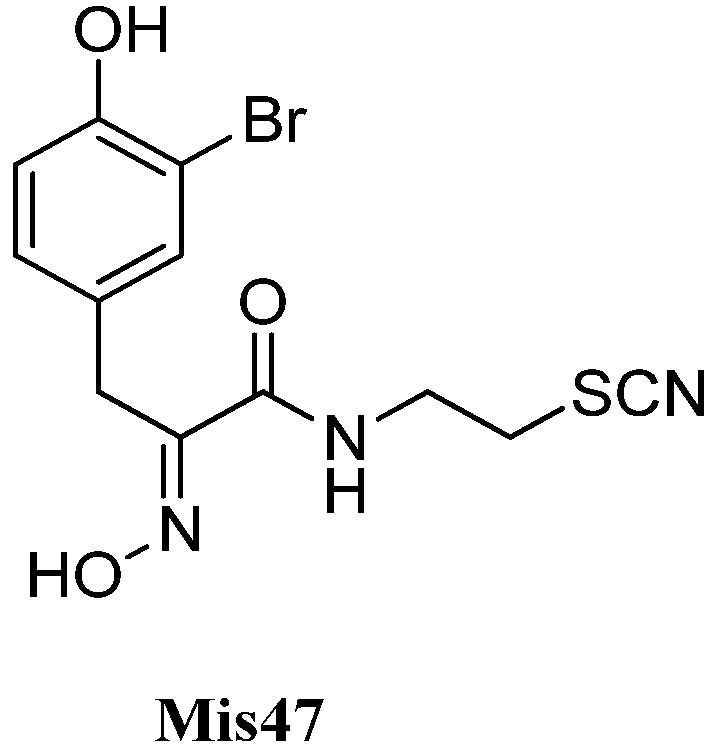
| Psammaplin B (Mis47) | Psammaplysilla purpurea | 1991 | [150] | |
Acknowledgments
Conflicts of Interest
References
- Rothe, W. Vorläufige Mitteilung über ein neues Antibiotikum. Pharmazie 1950, 5, 190. [Google Scholar]
- Cafieri, F.; Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Isolation and structure of axisonitrile-1 and axisothiocyanate-1 two unusual sesquiterpenoids from the marine sponge Axinella cannabina. Tetrahedron 1973, 29, 4259–4262. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Sodano, G. Acanthellin-1, an unique isonitrile sesquiterpene from the sponge Acanthella acuta. Tetrahedron 1974, 30, 1341–1343. [Google Scholar] [CrossRef]
- Fattorusso, E.; Magno, S.; Mayol, L.; Santacroce, C.; Sica, D. Isolation and structure of axisonitrile-2. Tetrahedron 1974, 30, 3911–3913. [Google Scholar] [CrossRef]
- Fattorusso, E.; Magno, S.; Mayol, L.; Santacroce, C.; Sica, D. New sesquiterpenoids from the sponge Axinella cannabina. Tetrahedron 1975, 31, 269–270. [Google Scholar] [CrossRef]
- Burreson, B.J.; Christophersen, C.; Scheuer, P.J. Cooccurrence of a terpenoid isocyanide-formamide pair in the marine sponge Halichondria species. J. Am. Chem. Soc. 1975, 97, 201–202. [Google Scholar] [CrossRef]
- Edenborough, M.S.; Herbert, R.B. Naturally occurring isocyanides. Nat. Prod. Rep. 1988, 5, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.J.; Scheuer, P.J. Marine isocyano compounds. In Studies in Natural Products Chemistry; Scheuer, P.J., Ed.; Springer-Verlag: Berlin, Germany, 1993; Volume 167, pp. 33–75. [Google Scholar]
- Chang, C.W.J. Naturally occurring isocyano/isothiocyanato and related compounds. In Fortschritte der Chemie Organischer Naturstoffe; Springer: New York, NY, USA, 2000; Volume 80. [Google Scholar]
- Garson, M.J.; Simpson, J.S.; Flowers, A.E.; Dumdei, E.J. Cyanide and thiocyanate-derived functionality in marine organisms—Structures, biosynthesis and ecology. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 21, Part B; pp. 329–372. [Google Scholar]
- Garson, M.J. Biosynthetic studies on marine natural products. Nat. Prod. Rep. 1989, 6, 143–170. [Google Scholar] [CrossRef]
- Chang, C.W.J.; Scheuer, P.J. Biosynthesis of marine isocyanoterpenoids in sponges. Comparative Biochem. Physiol. Part B: Comp. Biochem. 1990, 97, 227–233. [Google Scholar] [CrossRef]
- Garson, M.J. The biosynthesis of marine natural products. Chem. Rev. 1993, 93, 1699–1733. [Google Scholar] [CrossRef]
- Garson, M.J.; Simpson, J.S. Marine isocyanides and related natural products—Structure, biosynthesis and ecology. Nat. Prod. Rep. 2004, 21, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Schnermann, M.J.; Shenvi, R.A. Syntheses and biological studies of marine terpenoids derived from inorganic cyanide. Nat. Prod. Rep. 2015, 32, 543–577. [Google Scholar] [CrossRef] [PubMed]
- Iengo, A.; Mayol, L.; Santacroce, C. Minor sesquiterpenoids from the sponge Axinella cannabina. Experientia 1977, 33, 11–12. [Google Scholar] [CrossRef]
- Adinolfi, M.; de Napoli, L.; Di Blasio, B.; Iengo, A.; Pedone, C.; Santacroce, C. The absolute configuration of the axane sesquiterpenes from the sponge Axinella cannabina. Tetrahedron Lett. 1977, 18, 2815–2816. [Google Scholar] [CrossRef]
- Piers, E.; Yeung, B.W.A. Total syntheses of the sesquiterpenoids (±)-axamide-1, (±)-axisonitrile-1, and the corresponding C-10 epimers. Can. J. Chem. 1986, 64, 2475–2476. [Google Scholar] [CrossRef]
- Fusetani, N.; Wolstenholme, H.J.; Shinoda, K.; Asai, N.; Matsunaga, S.; Onuki, H.; Hirota, H. Two sesquiterpene isocyanides and a sesquiterpene thiocyanate from the marine sponge Acanthella cf. cavernosa and the Nudibranch Phyllidia ocellata. Tetrahedron Lett. 1992, 33, 6823–6826. [Google Scholar] [CrossRef]
- Hirota, H.; Tomono, Y.; Fusetani, N. Terpenoids with antifouling activity against barnacle larvae from the marine sponge Acanthella cavernosa. Tetrahedron 1996, 52, 2359–2368. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mayol, L. New nitrogenous sesquiterpenes from the marine sponge Axinella cannabina. J. Org. Chem. 1984, 49, 3949–3951. [Google Scholar] [CrossRef]
- Ciminiello, P.; Magno, S.; Mayol, L.; Piccialli, V. Cis-Eudesmane nitrogenous metabolites from the marine sponges Axinella cannabina and Acanthella acuta. J. Nat. Prod. 1987, 50, 217–220. [Google Scholar] [CrossRef]
- Burgoyne, D.L.; Dumdei, E.J.; Andersen, R.J. Acanthenes A to C: A chloro, isothiocyanate, formamide sesquiterpene triad isolated from the Northeastern Pacific marine sponge Acanthella sp. and the dorid nudibranch Cadlina luteomarginata. Tetrahedron 1993, 49, 4503–4510. [Google Scholar] [CrossRef]
- Ishiyama, H.; Hashimoto, A.; Fromont, J.; Hoshino, Y.; Mikami, Y.; Kobayashi, J.I. Halichonadins A–D, new sesquiterpenoids from a sponge Halichondria sp. Tetrahedron 2005, 61, 1101–1105. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mayol, L. New nitrogenous sesquiterpenes based on alloaromadendrane and epi-eudesmane skeletons from the marine sponge Axinella cannabina. Can. J. Chem. 1987, 65, 518–522. [Google Scholar] [CrossRef]
- Petrichtcheva, N.V.; Duque, C.; Dueñas, A.; Zea, S.; Hara, N.; Fujimoto, Y. New nitrogenous eudesmane-type compounds isolated from the Caribbean sponge Axinyssa ambrosia. J. Nat. Prod. 2002, 65, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Lyakhova, E.G.; Kolesnikova, S.A.; Kalinovskii, A.I.; Stonik, V.A. Secondary metabolites of the Vietnamese nudibranch mollusk Phyllidiella pustulosa. Chem. Nat. Compd. 2010, 46, 534–538. [Google Scholar] [CrossRef]
- Capon, R.; Macleod, J. New Isothiocyanate Sesquiterpenes From the Australian Marine Sponge Acanthella pulcherrima. Aust. J. Chem. 1988, 41, 979–983. [Google Scholar] [CrossRef]
- Angerhofer, C.K.; Pezzuto, J.M.; König, G.M.; Wright, A.D.; Sticher, O. Antimalarial activity of sesquiterpenes from the marine sponge Acanthella klethra. J. Nat. Prod. 1992, 55, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D.; Sticher, O.; Fronczek, F.R. Two New Sesquiterpene Isothiocyanates from the Marine Sponge Acanthella klethra. J. Nat. Prod. 1992, 55, 633–638. [Google Scholar] [CrossRef]
- Clark, R.J.; Stapleton, B.L.; Garson, M.J. New Isocyano and Isothiocyanato Terpene Metabolites from the Tropical Marine Sponge Acanthella cavernosa. Tetrahedron 2000, 56, 3071–3076. [Google Scholar] [CrossRef]
- Jumaryatno, P.; Stapleton, B.L.; Hooper, J.N.A.; Brecknell, D.J.; Blanchfield, J.T.; Garson, M.J. A Comparison of Sesquiterpene Scaffolds across Different Populations of the Tropical Marine Sponge Acanthella cavernosa. J. Nat. Prod. 2007, 70, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Zubía, E.; Ortega, M.J.; Carballo, J.L. Sesquiterpenes from the Sponge Axinyssa isabela. J. Nat. Prod. 2008, 71, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.; Fontaine, C.; Lauren, D.; La Barre, S.; Guittet, E. Stylotelline, a new sesquiterpene isocyanide from the sponge Stylotella sp., application of 2D-NMR in structure determination. Tetrahedron Lett. 1987, 28, 1409–1412. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Gavagnin, M.; Mollo, E.; Guo, Y.-W.; Cimino, G. Isocyanide Terpene Metabolites of Phyllidiella pustulosa, a Nudibranch from the South China Sea. J. Nat. Prod. 2004, 67, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.-J.; Wan, H.-P.; Li, G.-X.; Li, H.-J.; Chen, Y.-Y.; Liao, C.-Z.; Cai, J.-W. New Sesquiterpene Formamides from the Marine Sponge Axinyssa sp. Helv. Chim. Acta 2008, 91, 426–434. [Google Scholar] [CrossRef]
- Kozawa, S.; Ishiyama, H.; Fromont, J.; Kobayashi, J. Halichonadin E. a Dimeric Sesquiterpenoid from the Sponge Halichondria sp. J. Nat. Prod. 2008, 71, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Suto, S.; Tanaka, N.; Fromont, J.; Kobayashi, J.I. Halichonadins G–J, new sesquiterpenoids from a sponge Halichondria sp. Tetrahedron Lett. 2011, 52, 3470–3473. [Google Scholar] [CrossRef]
- Burreson, B.J.; Christophersen, C.; Scheuer, P.J. Co-occurrence of two terpenoid isocyanide-formamide pairs in a marine sponge (Halichondria sp.). Tetrahedron 1975, 31, 2015–2018. [Google Scholar] [CrossRef]
- Burreson, B.J.; Scheuer, P.J. Isolation of a diterpenoid isonitrile from a marine sponge. J. Chem. Soc., Chem. Commun. 1974, 1035–1036. [Google Scholar] [CrossRef]
- Young, R.M.; Adendorff, M.R.; Wright, A.D.; Davies-Coleman, M.T. Antiplasmodial activity: The first proof of inhibition of heme crystallization by marine isonitriles. Eur. J. Med. Chem. 2015, 93, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New antifouling sesquiterpenes from four nudibranchs of the family Phyllidiidae. Tetrahedron 1996, 52, 9447–9454. [Google Scholar] [CrossRef]
- Marcus, A.H.; Molinski, T.F.; Fahy, E.; Faulkner, D.J.; Xu, C.; Clardy, J. 5-Isothiocyanatopupukeanane from a sponge of the genus Axinyssa. J. Org. Chem. 1989, 54, 5184–5186. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mayol, L. Minor nitrogenous sesquiterpenes from the marine sponge Axinella cannabina. A hypothesis for the biogenesis of the spiro-axane skeleton. Experientia 1986, 42, 625–627. [Google Scholar] [CrossRef]
- Nakamura, H.; Deng, S.; Takamatsu, M.; Kobayashi, J.I.; Ohizumi, Y.; Hirata, Y. Structure of Halipanicine, a New Sesquiterpene Isothiocyanate from the Okinawan Marine Sponge Halichondria panicea (Pallas). Agricultural and Biological Chemistry 1991, 55, 581–583. [Google Scholar] [CrossRef]
- Compagnone, R.S.; Faulkner, D.J. Metabolites of the Palauan Sponge Axinyssa aplysinoides. J. Nat. Prod. 1995, 58, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N.; Wolstenholme, H.J.; Matsunaga, S.; Hirota, H. Two new sesquiterpene isonitriles from the nudibranch, phyllida pustulosa. Tetrahedron Lett. 1991, 32, 7291–7294. [Google Scholar] [CrossRef]
- Nishikawa, K.; Umezawa, T.; Garson, M.J.; Matsuda, F. Confirmation of the Configuration of 10-Isothiocyanato-4-cadinene Diastereomers through Synthesis. J. Nat. Prod. 2012, 75, 2232–2235. [Google Scholar] [CrossRef] [PubMed]
- Alvi, K.A.; Tenenbaum, L.; Crews, P. Anthelmintic Polyfunctional Nitrogen-Containing Terpenoids from Marine Sponges. J. Nat. Prod. 1991, 54, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mitome, H.; Shirato, N.; Miyaoka, H.; Yamada, Y.; van Soest, R.W.M. Terpene Isocyanides, Isocyanates, and Isothiocyanates from the Okinawan Marine Sponge Stylissa sp. J. Nat. Prod. 2004, 67, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Zubía, E.; Ortega, M.J.; Hernández-Guerrero, C.J.; Carballo, J.L. Isothiocyanate Sesquiterpenes from a Sponge of the Genus Axinyssa. J. Nat. Prod. 2008, 71, 608–614. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; Faulkner, D.J.; Shumsky, J.S.; Hong, K.; Clardy, J. A sesquiterpene thiocyanate and three sesquiterpene isothiocyanates from the sponge Trachyopsis aplysinoides. J. Org. Chem. 1989, 54, 2511–2514. [Google Scholar] [CrossRef]
- Hirota, H.; Okino, T.; Yoshimura, E.; Fusetani, N. Five new antifouling sesquiterpenes from two marine sponges of the genus Axinyssa and the nudibranch Phyllidia pustulosa. Tetrahedron 1998, 54, 13971–13980. [Google Scholar] [CrossRef]
- Sorek, H.; Zelikoff, A.L.; Benayahu, Y.; Kashman, Y. Axiplyns A–E, new sesquiterpene isothiocyanates from the marine sponge Axinyssa aplysinoides. Tetrahedron Lett. 2008, 49, 2200–2203. [Google Scholar] [CrossRef]
- Di Blasio, B.; Fattorusso, E.; Magno, S.; Mayol, L.; Pedone, C.; Santacroce, C.; Sica, D. Axisonitrile-3, axisothiocyanate-3 and axamide-3. Sesquiterpenes with a novel spiro[4,5]decane skeleton from the sponge Axinella cannabina. Tetrahedron 1976, 32, 473–478. [Google Scholar] [CrossRef]
- Caine, D.; Deutsch, H. Total synthesis of (–)-axisonitrile-3. An application of the reductive ring opening of vinylcyclopropanes. J. Am. Chem. Soc. 1978, 100, 8030–8031. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Deneubourg, F.; Huysecom, J.; Vandevyver, G. I-Isocyanoaromadendrane, A New Isonitrile Sesquiterpene from the Sponge Acanthella Acuta. Bull. Soc. Chim. Belg. 1987, 96, 539–543. [Google Scholar] [CrossRef]
- Jumaryatno, P.; Rands-Trevor, K.; Blanchfield, J.T.; Garson, M.J. Isocyanates in marine sponges: Axisocyanate-3, a new sesquiterpene from Acanthella cavernosa. Arkivoc 2007, 157–166. [Google Scholar]
- Prawat, H.; Mahidol, C.; Wittayalai, S.; Intachote, P.; Kanchanapoom, T.; Ruchirawat, S. Nitrogenous sesquiterpenes from the Thai marine sponge Halichondria sp. Tetrahedron 2011, 67, 5651–5655. [Google Scholar] [CrossRef]
- Uy, M.M.; Ohta, S.; Yanai, M.; Ohta, E.; Hirata, T.; Ikegami, S. New spirocyclic sesquiterpenes from the marine sponge Geodia exigua. Tetrahedron 2003, 59, 731–736. [Google Scholar] [CrossRef]
- Yan, X.H.; Zhu, X.Z.; Yu, J.L.; Jin, D.Z.; Guo, Y.W.; Mollo, E.; Cimino, G. 3-Oxo-axisonitrile-3, a new sesquiterpene isocyanide from the Chinese marine sponge Acanthella sp. J. Asian Nat. Prod. Res. 2006, 8, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Piccialli, V.; Sica, D. Nitrogenous sesquiterpenes from the marine sponge acanthella acuta: Three new isocyanide-isothiocyanate pairs. Tetrahedron 1987, 43, 5381–5388. [Google Scholar] [CrossRef]
- Wegerski, C.J.; Sonnenschein, R.N.; Cabriales, F.; Valeriote, F.A.; Matainaho, T.; Crews, P. Stereochemical challenges in characterizing nitrogenous spiro-axane sesquiterpenes from the Indo-Pacific sponges Amorphinopsis and Axinyssa. Tetrahedron 2006, 62, 10393–10399. [Google Scholar] [CrossRef]
- Tada, H.; Yasuda, F. Metabolites from the Marine Sponge Epipolasis kushimotoensis. Chem. Pharm. Bull. 1985, 33, 1941–1945. [Google Scholar] [CrossRef]
- Da Silva, C.C.; Almagro, V.; Zukerman-Schpector, J.; Castellano, E.E.; Marsaioli, A.J. An Easy Route to (–)-10(R)-Isothiocyanoaromadendrane and (–)-10(S)-Isothiocyanoalloaromadendrane. J. Org. Chem. 1994, 59, 2880–2881. [Google Scholar] [CrossRef]
- Ishiyama, H.; Kozawa, S.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J.I. Halichonadin F and the Cu(I) Complex of Halichonadin C from the Sponge Halichondria sp. J. Nat. Prod. 2008, 71, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Braekman, J.-C.; Daloze, D.; Stoller, C.; Declercq, J.-P. The Configuration of Palustrol and Related Compounds. Bull. Soc. Chim. Belg. 1989, 98, 869–875. [Google Scholar] [CrossRef]
- Zhang, W.; Gavagnin, M.; Guo, Y.-W.; Mollo, E.; Ghiselin, M.T.; Cimino, G. Terpenoid metabolites of the nudibranch Hexabranchus sanguineus from the South China Sea. Tetrahedron 2007, 63, 4725–4729. [Google Scholar] [CrossRef]
- He, H.Y.; Salva, J.; Catalos, R.F.; Faulkner, D.J. Sesquiterpene thiocyanates and isothiocyanates from Axinyssa aplysinoides. J. Org. Chem. 1992, 57, 3191–3194. [Google Scholar] [CrossRef]
- Thompson, J.E.; Walker, R.P.; Wratten, S.J.; Faulkner, D.J. A chemical defense mechanism for the nudibranch cadlina luteomarginata. Tetrahedron 1982, 38, 1865–1873. [Google Scholar] [CrossRef]
- Simpson, J.S.; Garson, M.J.; Hooper, J.N.A.; Cline, E.I.; Angerhofer, C.K. Terpene Metabolites from the Tropical Marine Sponge Axinyssa sp. nov. Aust. J. Chem. 1997, 50, 1123–1128. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Magno, S.; Mayol, L. Sesquiterpenoids Based on the Epi-Maaliane Skeleton from the Marine Sponge Axinella cannabina. J. Nat. Prod. 1985, 48, 64–68. [Google Scholar] [CrossRef]
- Burreson, B.J.; Scheuer, P.J.; Finer, J.; Clardy, J. 9-Isocyanopupukeanane, a marine invertebrate allomone with a new sesquiterpene skeleton. J. Am. Chem. Soc. 1975, 97, 4763–4764. [Google Scholar] [CrossRef]
- Gulavita, N.K.; De Silva, E.D.; Hagadone, M.R.; Karuso, P.; Scheuer, P.J.; Van Duyne, G.D.; Clardy, J. Nitrogenous bisabolene sesquiterpenes from marine invertebrates. J. Org. Chem. 1986, 51, 5136–5139. [Google Scholar] [CrossRef]
- Fusetani, N.; Wolstenholme, H.J.; Matsunaga, S. Co-occurrence of 9-isocyanopupukeanane and its C-9 epimer in the nudibranch phyllidia bourguini. Tetrahedron Lett. 1990, 31, 5623–5624. [Google Scholar] [CrossRef]
- Yasman, Y.; Edrada, R.A.; Wray, V.; Proksch, P. New 9-Thiocyanatopupukeanane Sesquiterpenes from the Nudibranch Phyllidia varicosa and Its Sponge-Prey Axinyssa aculeata. J. Nat. Prod. 2003, 66, 1512–1514. [Google Scholar] [CrossRef] [PubMed]
- Hagadone, M.R.; Burreson, B.J.; Scheuer, P.J.; Finer, J.S.; Clardy, J. Defense Allomones of the Nudibranch Phyllidia varicosa Lamarck 1801. Helv. Chim. Acta 1979, 62, 2484–2494. [Google Scholar] [CrossRef]
- Jaisamut, S.; Prabpai, S.; Tancharoen, C.; Yuenyongsawad, S.; Hannongbua, S.; Kongsaeree, P.; Plubrukarn, A. Bridged Tricyclic Sesquiterpenes from the Tubercle Nudibranch Phyllidia coelestis Bergh. J. Nat. Prod. 2013, 76, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- Karuso, P.; Poiner, A.; Scheuer, P.J. Isocyanoneopupukeanane, a new tricyclic sesquiterpene from a sponge. J. Org. Chem. 1989, 54, 2095–2097. [Google Scholar] [CrossRef]
- Pham, A.T.; Ichiba, T.; Yoshida, W.Y.; Scheuer, P.J.; Uchida, T.; Tanaka, J.-I.; Higa, T. Two marine sesquiterpene thiocyanates. Tetrahedron Lett. 1991, 32, 4843–4846. [Google Scholar] [CrossRef]
- Nakamura, H.; Kobayashi, J.I.; Ohizumi, Y.; Mitsubishi, K.; Hirata, Y. Novel bisabolene-type sesquiterpenoids with a conjugated diene isolated from the okinawan sea sponge theonella cf. swinhoei. Tetrahedron Lett. 1984, 25, 5401–5404. [Google Scholar] [CrossRef]
- Wright, A.D.; Schupp, P.J.; Schrör, J.-P.; Engemann, A.; Rohde, S.; Kelman, D.; de Voogd, N.; Carroll, A.; Motti, C.A. Twilight Zone Sponges from Guam Yield Theonellin Isocyanate and Psammaplysins I and J. J. Nat. Prod. 2012, 75, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.W.; Faulkner, D.J.; Okamoto, K.T.; Chen, M.H.M.; Clardy, J. (6R,7S)-7-amino-7,8-dihydro-alpha-bisabolene, an antimicrobial metabolite from the marine sponge Halichondria sp. J. Org. Chem. 1986, 51, 5134–5136. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yu, Z.-G.; Guo, Y.-W. New N-Containing Sesquiterpenes from Hainan Marine Sponge Axinyssa sp. Helv. Chim. Acta 2008, 91, 1553–1558. [Google Scholar] [CrossRef]
- Iwashima, M.; Terada, I.; Iguchi, K.; Yamori, T. New Biologically Active Marine Sesquiterpenoid and Steroid from the Okinawan Sponge of the Genus Axinyssa. Chem. Pharm. Bull. 2002, 50, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Kassuhlke, K.E.; Potts, B.C.M.; Faulkner, D.J. New nitrogenous sesquiterpenes from two Philippine nudibranchs, Phyllidia pustulosa and P. varicosa, and from a Palauan sponge, Halichondria cf. lendenfeldi. J. Org. Chem. 1991, 56, 3747–3750. [Google Scholar] [CrossRef]
- Sun, J.-Z.; Chen, K.-S.; Liu, H.-L.; van Soest, R.; Guo, Y.-W. New Epoxy-Substituted Nitrogenous Bisabolene-Type Sesquiterpenes from a Hainan Sponge Axinyssa sp. Helv. Chim. Acta 2010, 93, 517–521. [Google Scholar] [CrossRef]
- Liu, W.; Liang, K.-J.; Chiang, C.-Y.; Lu, M.-C.; Su, J.-H. New Nitrogenous Bisabolene-Type Sesquiterpenes from a Formosan Sponge Axinyssa sp. Chem. Pharm. Bull. 2014, 62, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Kondempudi, C.M.; Singanaboina, R.; Manchala, N.; Gunda, V.G.; Janapala, V.R.; Yenamandra, V. Chemical Examination of the Sponge Phycopsis sp. Chem. Pharm. Bull. 2009, 57, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Schmitz, F.J.; Kelly, M. New Nitrogenous Bisabolene-Type Sesquiterpenes from a Micronesian Marine Sponge, Axinyssa Species. J. Nat. Prod. 1999, 62, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.-C.; Guo, Y.-W.; van Soest, R.; Cimino, G. New Nitrogenous Bisabolene-Type Sesquiterpenes from a Hainan Sponge Axinyssa aff. variabilis. Helv. Chim. Acta 2007, 90, 588–593. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, D.; Zhang, F.; Zhang, Q.; Pedpradab, P.; Proksch, P.; Liang, H.; Lin, W. Formamidobisabolene-based derivatives from a sponge Axinyssa sp. Tetrahedron 2014, 70, 3576–3583. [Google Scholar] [CrossRef]
- Tada, H.; Tozyo, T.; Shiro, M. A new isocyanide from a sponge. Is the formamide a natural product? J. Org. Chem. 1988, 53, 3366–3368. [Google Scholar] [CrossRef]
- White, A.M.; Pierens, G.K.; Skinner-Adams, T.; Andrews, K.T.; Bernhardt, P.V.; Krenske, E.H.; Mollo, E.; Garson, M.J. Antimalarial Isocyano and Isothiocyanato Sesquiterpenes with Tri- and Bicyclic Skeletons from the Nudibranch Phyllidia ocellata. J. Nat. Prod. 2015, 78, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Reichwein, R.; Bean, M.F.; Faucette, L.; Johnson, R.K.; Haltiwanger, R.C.; Eggleston, D.S. Two New Nitrogenous Sesquiterpenes from the Sponge Axinyssa aplysinoides. J. Nat. Prod. 1997, 60, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Keyzers, R.A.; Gray, C.A.; Schleyer, M.H.; Whibley, C.E.; Hendricks, D.T.; Davies-Coleman, M.T. Malonganenones A–C, novel tetraprenylated alkaloids from the Mozambique gorgonian Leptogorgia gilchristi. Tetrahedron 2006, 62, 2200–2206. [Google Scholar] [CrossRef]
- Sorek, H.; Rudi, A.; Benayahu, Y.; Ben-Califa, N.; Neumann, D.; Kashman, Y. Nuttingins A−F and Malonganenones D−H, Tetraprenylated Alkaloids from the Tanzanian Gorgonian Euplexaura nuttingi. J. Nat. Prod. 2007, 70, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-R.; Li, P.-L.; Tang, X.-L.; Qi, X.; Li, G.-Q. Cytotoxic Tetraprenylated Alkaloids from the South China Sea Gorgonian Euplexaura robusta. Chem. Biodivers. 2012, 9, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Nieto, R.M.; Hunter, L.M.; Diaz, M.C.; Crews, P.; Lobkovsky, E.; Clardy, J. Variation among known kalihinol and new kalihinene diterpenes from the sponge Acanthella cavernosa. Tetrahedron 1994, 50, 11079–11090. [Google Scholar] [CrossRef]
- Chang, C.W.J.; Patra, A.; Roll, D.M.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Kalihinol-A, a highly functionalized diisocyano diterpenoid antibiotic from a sponge. J. Am. Chem. Soc. 1984, 106, 4644–4646. [Google Scholar] [CrossRef]
- Patra, A.; Chang, C.W.J.; Scheuer, P.J.; Van Duyne, G.D.; Matsumoto, G.K.; Clardy, J. An unprecedented triisocyano diterpenoid antibiotic from a sponge. J. Am. Chem. Soc. 1984, 106, 7981–7983. [Google Scholar] [CrossRef]
- Chang, C.W.J.; Patra, A.; Baker, J.A.; Scheuer, P.J. Kalihinols, multifunctional diterpenoid antibiotics from marine sponges Acanthella sp. J. Am. Chem. Soc. 1987, 109, 6119–6123. [Google Scholar] [CrossRef]
- Sun, J.-Z.; Chen, K.-S.; Yao, L.-G.; Liu, H.-L.; Guo, Y.-W. A new kalihinol diterpene from the Hainan sponge Acanthella sp. Arch. Pharmacal Res. 2009, 32, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Schmitz, F.J. New Diterpene Isonitriles from the Sponge Phakellia pulcherrima. J. Nat. Prod. 1998, 61, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, H.; Shimomura, M.; Kimura, H.; Yamada, Y.; Kim, H.-S.; Yusuke, W. Antimalarial activity of kalihinol A and new relative diterpenoids from the Okinawan sponge, Acanthella sp. Tetrahedron 1998, 54, 13467–13474. [Google Scholar] [CrossRef]
- Omar, S.; Albert, C.; Fanni, T.; Crews, P. Polyfunctional diterpene isonitriles from marine sponge Acanthella carvenosa. J. Org. Chem. 1988, 53, 5971–5972. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Jiao, W.-H.; Wang, R.-P.; Peng, Y.; Qi, S.-H.; Song, S.-J.; Chen, W.-S.; Lin, H.-W. Antifouling and cytotoxic constituents from the South China Sea sponge Acanthella cavernosa. Tetrahedron 2012, 68, 2876–2883. [Google Scholar] [CrossRef]
- Ohta, E.; Ohta, S.; Hongo, T.; Hamaguchi, Y.; Andoh, T.; Shioda, M.; Ikegami, S. Inhibition of Chromosome Separation in Fertilized Starfish Eggs by Kalihinol F, a Topoisomerase I Inhibitor Obtained from a Marine Sponge. Biosci. Biotechnol. Biochem. 2003, 67, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Singh, M.P.; Chen, L.; Arias, D.A.; Harper, M.K.; Greenstein, M.; Maiese, W.M.; Concepción, G.P.; Mangalindan, G.C.; Ireland, C.M. Kalihinols from two Acanthella cavernosa sponges: Inhibitors of bacterial folate biosynthesis. Tetrahedron 2004, 60, 6981–6988. [Google Scholar] [CrossRef]
- Fusetani, N.; Yasumuro, K.; Kawai, H.; Natori, T.; Brinen, L.; Clardy, J. Kalihinene and isokalihinol B, cytotoxic diterpene isonitriles from the marine sponge Acanthella klethra. Tetrahedron Lett. 1990, 31, 3599–3602. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. Antifouling kalihinenes from the marine sponge Acanthella cavernosa. Tetrahedron Lett. 1995, 36, 8637–8640. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Faulkner, D.J. Six New Diterpene Isonitriles from the Sponge Acanthella cavernosa. J. Nat. Prod. 1994, 57, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lang, J.-H.; Jiao, W.-H.; Wang, R.-P.; Peng, Y.; Song, S.-J.; Zhang, B.-H.; Lin, H.-W. Formamido-Diterpenes from the South China Sea Sponge Acanthella cavernosa. Marine Drugs 2012, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New Antifouling Kalihipyrans from the Marine Sponge Acanthella cavernosa. J. Nat. Prod. 1996, 59, 1081–1083. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Angerhofer, C.K. Novel Potent Antimalarial Diterpene Isocyanates, Isothiocyanates, and Isonitriles from the Tropical Marine Sponge Cymbastela hooperi. J. Org. Chem. 1996, 61, 3259–3267. [Google Scholar] [CrossRef]
- Sharma, H.A.; Tanaka, J.-I.; Higa, T.; Lithgow, A.; Bernardinelli, G.; Jefford, C.W. Two New Diterpene Isocyanides from a Sponge of the Family Adocidae. Tetrahedron Lett. 1992, 33, 1593–1596. [Google Scholar] [CrossRef]
- Braekman, J.-C.; Daloze, D.; Stoller, C.; Van Soest, R.W.M. Chemotaxonomy of Agelas (Porifera: Demospongiae). Biochem. Syst. Ecol. 1992, 20, 417–431. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Fontana, A.; Puliti, R.; Scognamiglio, G.; Cimino, G. Structures and absolute stereochemistry of isocyanide and isothiocyanate amphilectenes from the Caribbean sponge Cribochalina sp. Tetrahedron 1999, 55, 12629–12636. [Google Scholar] [CrossRef]
- Wratten, S.J.; Faulkner, D.J.; Hirotsu, K.; Clardy, J. Diterpenoid isocyanides from the marine sponge hymeniacidon amphilecta. Tetrahedron Lett. 1978, 19, 4345–4348. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Gavagnin, M.; Manzo, E.; Puliti, R.; Mattia, C.A.; Mazzarella, L.; Cimino, G.; Simpson, J.S.; Garson, M.J. Structural and stereochemical revision of isocyanide and isothiocyanate amphilectenes from the Caribbean marine sponge Cribochalina sp. Tetrahedron 2005, 61, 8049–8053. [Google Scholar] [CrossRef]
- Molinski, T.F.; Faulkner, D.J.; Van Duyne, G.D.; Clardy, J. Three new diterpene isonitriles from a Palauan sponge of the genus Halichondria. J. Org. Chem. 1987, 52, 3334–3337. [Google Scholar] [CrossRef]
- Wattanapiromsakul, C.; Chanthathamrongsiri, N.; Bussarawit, S.; Yuenyongsawad, S.; Plubrukarn, A.; Suwanborirux, K. 8-Isocyanoamphilecta-11(20),15-diene, a new antimalarial isonitrile diterpene from the sponge Ciocalapata sp. Can. J. Chem. 2009, 87, 612–618. [Google Scholar] [CrossRef]
- Chanthathamrongsiri, N.; Yuenyongsawad, S.; Wattanapiromsakul, C.; Plubrukarn, A. Bifunctionalized Amphilectane Diterpenes from the Sponge Stylissa cf. massa. J. Nat. Prod. 2012, 75, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Avilés, E.; Rodríguez, A.D. Monamphilectine A, a Potent Antimalarial β-Lactam from Marine Sponge Hymeniacidon sp: Isolation, Structure, Semisynthesis, and Bioactivity. Org. Lett. 2010, 12, 5290–5293. [Google Scholar] [CrossRef] [PubMed]
- Avilés, E.; Prudhomme, J.; Le Roch, K.G.; Rodríguez, A.D. Structures, semisyntheses, and absolute configurations of the antiplasmodial α-substituted β-lactam monamphilectines B and C from the sponge Svenzea flava. Tetrahedron 2015, 71, 487–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kazlauskas, R.; Murphy, P.T.; Wells, R.J.; Blount, J.F. New diterpene isocyanides from a sponge. Tetrahedron Lett. 1980, 21, 315–318. [Google Scholar] [CrossRef]
- Wright, A.D.; Lang-Unnasch, N. Diterpene Formamides from the Tropical Marine Sponge Cymbastela hooperi and Their Antimalarial Activity in Vitro. J. Nat. Prod. 2009, 72, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Lamoral-Theys, D.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Kiss, R.; Costantino, V. Evaluation of the Antiproliferative Activity of Diterpene Isonitriles from the Sponge Pseudoaxinella flava in Apoptosis-Sensitive and Apoptosis-Resistant Cancer Cell Lines. J. Nat. Prod. 2011, 74, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- Ciasullo, L.; Cutignano, A.; Casapullo, A.; Puliti, R.; Mattia, C.A.; Debitus, C.; Riccio, R.; Gomez-Paloma, L. A New Cycloamphilectene Metabolite from the Vanuatu Sponge Axinella sp. J. Nat. Prod. 2002, 65, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Wells, R.J.; Oberhaensli, W.E.; Hawes, G.B. A new diisocyanide of novel ring structure from a sponge. J. Am. Chem. Soc. 1976, 98, 4010–4012. [Google Scholar] [CrossRef]
- Fookes, C.J.R.; Garson, M.J.; MacLeod, J.K.; Skelton, B.W.; White, A.H. Biosynthesis of diisocyanoadociane, a novel diterpene from the marine sponge Amphimedon sp. Crystal structure of a monoamide derivative. J. Chem. Soc. Perkin Trans. 1 1988, 1003–1011. [Google Scholar] [CrossRef]
- Corey, E.J.; Magriotis, P.A. Total synthesis and absolute configuration of 7,20-diisocyanoadociane. J. Am. Chem. Soc. 1987, 109, 287–289. [Google Scholar] [CrossRef]
- Avilés, E.; Rodríguez, A.D.; Vicente, J. Two Rare-Class Tricyclic Diterpenes with Antitubercular Activity from the Caribbean Sponge Svenzea flava. Application of Vibrational Circular Dichroism Spectroscopy for Determining Absolute Configuration. J. Org. Chem. 2013, 78, 11294–11301. [Google Scholar] [CrossRef] [PubMed]
- Wratten, S.J.; Faulkner, D.J. Carbonimidic dichlorides from the marine sponge Pseudaxinyssa pitys. J. Am. Chem. Soc. 1977, 99, 7367–7368. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.S.; Raniga, P.; Garson, M.J. Biosynthesis of dichloroimines in the tropical marine sponge Stylotella aurantium. Tetrahedron Lett. 1997, 38, 7947–7950. [Google Scholar] [CrossRef]
- Kehraus, S.; König, G.M.; Wright, A.D. New Carbonimidic Dichlorides from the Australian Sponge Ulosa spongia and Their Possible Taxonomic Significance. J. Nat. Prod. 2001, 64, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Musman, M.; Tanaka, J.; Higa, T. New Sesquiterpene Carbonimidic Dichlorides and Related Compounds from the Sponge Stylotella aurantium. J. Nat. Prod. 2001, 64, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Brust, A.; Garson, M.J. Dereplication of complex natural product mixtures by 2D NMR: Isolation of a new carbonimidic dichloride of biosynthetic interest from the tropical marine sponge Stylotella aurantium. ACGC Chem. Res. Commun. 2004, 17, 33–37. [Google Scholar]
- Simpson, J.S.; Brust, A.; Garson, M.J. Biosynthetic pathways to dichloroimines; precursor incorporation studies on terpene metabolites in the tropical marine sponge Stylotella aurantium. Org. Biomol. Chem. 2004, 2, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Wratten; John, D.; Faulkner, S.J.; Van Engen, D.; Clardy, J. A vinyl carbonimidic dichloride from the marine sponge pseudaxinyssa pitys. Tetrahedron Lett. 1978, 19, 1391–1394. [Google Scholar] [CrossRef]
- Tanaka, J.; Higa, T. Two New Cytotoxic Carbonimidic Dichlorides from the Nudibranch Reticulidia fungia. J. Nat. Prod. 1999, 62, 1339–1340. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Carbone, M.; Mollo, E.; Irace, C.; Di Pascale, A.; Li, Y.; Ciavatta, M.L.; Cimino, G.; Guo, Y.-W.; Gavagnin, M. Structure and Synthesis of a Unique Isonitrile Lipid Isolated from the Marine Mollusk Actinocyclus papillatus. Org. Lett. 2011, 13, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Shinde, P.B.; Lee, Y.M.; Hong, J.; Kim, D.K.; Jung, J.H. Anti-inflammatory Constituents of the Red Alga Gracilaria verrucosa and Their Synthetic Analogues. J. Nat. Prod. 2008, 71, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Fattorusso, E.; Giordano, A.; Menna, M.; Navarrete, C.; Muñoz, E. Clavaminols G–N, six new marine sphingoids from the Mediterranean ascidian Clavelina phlegraea. Tetrahedron 2009, 65, 4384–4388. [Google Scholar] [CrossRef]
- Karuso, P.; Scheuer, P.J. Long-chain α,ω-bisisothiocyanates from a marine sponge. Tetrahedron Lett. 1987, 28, 4633–4636. [Google Scholar] [CrossRef]
- Capon, R.J.; Skene, C.; Liu, E.H.-T.; Lacey, E.; Gill, J.H.; Heiland, K.; Friedel, T. The Isolation and Synthesis of Novel Nematocidal Dithiocyanates from an Australian Marine Sponge, Oceanapia sp. J. Org. Chem. 2001, 66, 7765–7769. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Blackman, A. Cylindricines C-G, Perhydropyrrolo[2,1-j]quinolin-7-one Alkaloids From the Ascidian Clavelina cylindrica. Aust. J. Chem. 1994, 47, 1355–1361. [Google Scholar] [CrossRef]
- Li, C.; Blackman, A. Cylindricines H-K, Novel Alkaloids From the Ascidian Clavelina cylindrica. Aust. J. Chem. 1995, 48, 955–965. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Reichwein, R.; Carte, B.; Killmer, L.B.; Faucette, L.; Johnson, R.K.; Faulkner, D.J. Fasicularin, a novel tricyclic alkaloid from the ascidian Nephteis fasicularis with selective activity against a DNA repair-deficient organism. Tetrahedron Lett. 1997, 38, 363–364. [Google Scholar] [CrossRef]
- Jiménez, C.; Crews, P. Novel marine sponge derived amino acids 13. Additional psammaplin derivatives from Psammaplysilla purpurea. Tetrahedron 1991, 47, 2097–2102. [Google Scholar] [CrossRef]
- Iengo, A.; Santacroce, C.; Sodano, G. Metabolism in Porifera. X. On the intermediary of a formamide moiety in the biosynthesis of isonitrile terpenoids in sponges. Experientia 1979, 35, 10–11. [Google Scholar] [CrossRef]
- Hagadone, M.R.; Scheuer, P.J.; Holm, A. On the origin of the isocyano function in marine sponges. J. Am. Chem. Soc. 1984, 106, 2447–2448. [Google Scholar] [CrossRef]
- Garson, M.J. Biosynthesis of the novel diterpene isonitrile diisocyanoadociane by a marine sponge of the amphimedon genus: Incorporation studies with sodium [14C]cyanide and sodium [2-14C]acetate. J. Chem. Soc., Chem. Commun. 1986, 35–36. [Google Scholar] [CrossRef]
- McClintock, J.B.; Baker, B.J. Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–624. [Google Scholar]
- Karuso, P.; Scheuer, P.J. Biosynthesis of isocyanoterpenes in sponges. J. Org. Chem. 1989, 54, 2092–2095. [Google Scholar] [CrossRef]
- Parker, W.; Roberts, J.S.; Ramage, R. Sesquiterpene biogenesis. Q. Rev. Chem. Soc. 1967, 21, 331–363. [Google Scholar] [CrossRef]
- Rücker, G. Sesquiterpenes. Angew. Chem. Int. Ed. 1973, 12, 793–806. [Google Scholar] [CrossRef]
- Bülow, N.; König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar] [CrossRef]
- Ugi, I. Isonitrile Chemistry; Academic press: New York, NY, USA, 1971; pp. 1–278. [Google Scholar]
- Mayer, A.M.S.; Avilés, E.; Rodríguez, A.D. Marine sponge Hymeniacidon sp. amphilectane metabolites potently inhibit rat brain microglia thromboxane B2 generation. Bioorg. Med. Chem. 2012, 20, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; McCluskey, A.; Robertson, M.J.; MacGregor, K.A.; Gordon, C.P.; Guenther, J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela hooperi. Org. Biomol. Chem. 2011, 9, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; König, G.M.; Angerhofer, C.K.; Greenidge, P.; Linden, A.; Desqueyroux-Faúndez, R. Antimalarial Activity: The Search for Marine-Derived Natural Products with Selective Antimalarial Activity. J. Nat. Prod. 1996, 59, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Wang, H.; Gurrath, M.; König, G.M.; Kocak, G.; Neumann, G.; Loria, P.; Foley, M.; Tilley, L. Inhibition of Heme Detoxification Processes Underlies the Antimalarial Activity of Terpene Isonitrile Compounds from Marine Sponges. J. Med. Chem. 2001, 44, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Daub, M.E.; Prudhomme, J.; Le Roch, K.; Vanderwal, C.D. Synthesis and Potent Antimalarial Activity of Kalihinol B. J. Am. Chem. Soc. 2015, 137, 4912–4915. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Higuchi, R.; Miyamoto, T.; Van Soest, R.W.M. (−)-Axinyssene: A Novel Cytotoxic Diterpene from a Japanese Marine Sponge Axinyssa sp. Org. Lett. 2003, 5, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.-C.; Manzo, E.; Guo, Y.-W.; Gavagnin, M.; Mollo, E.; Ciavatta, M.L.; van Soest, R.; Cimino, G. New diastereomeric bis-sesquiterpenes from Hainan marine sponges Axinyssa variabilis and Lipastrotethya ana. Tetrahedron 2007, 63, 11108–11113. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emsermann, J.; Kauhl, U.; Opatz, T. Marine Isonitriles and Their Related Compounds. Mar. Drugs 2016, 14, 16. https://doi.org/10.3390/md14010016
Emsermann J, Kauhl U, Opatz T. Marine Isonitriles and Their Related Compounds. Marine Drugs. 2016; 14(1):16. https://doi.org/10.3390/md14010016
Chicago/Turabian StyleEmsermann, Jens, Ulrich Kauhl, and Till Opatz. 2016. "Marine Isonitriles and Their Related Compounds" Marine Drugs 14, no. 1: 16. https://doi.org/10.3390/md14010016
APA StyleEmsermann, J., Kauhl, U., & Opatz, T. (2016). Marine Isonitriles and Their Related Compounds. Marine Drugs, 14(1), 16. https://doi.org/10.3390/md14010016