Pinnisterols A–C, New 9,11-Secosterols from a Gorgonian Pinnigorgia sp.
Abstract
:1. Introduction
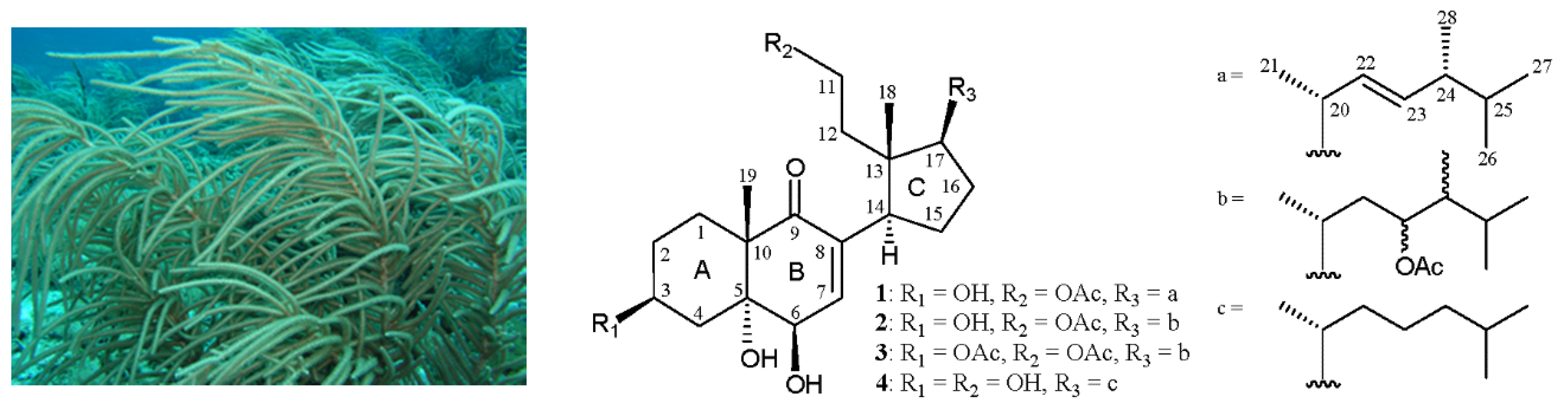
2. Results and Discussion
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 1.94 m; 1.60 m | 27.6, CH2 | H2-2 | C-5 |
| 2 | 1.91 m; 1.48 m | 30.2, CH2 | H2-1, H-3 | n. o. b |
| 3 | 3.99 br s a | 67.1, CH | H2-2, H2-4 | C-5 |
| 4 | 2.07 dd (12.0, 12.0); 1.74 br d (12.0) | 38.7, CH2 | H-3 | C-2, -3, -5 |
| 5 | 76.7, C | |||
| 6 | 4.00 br s a | 71.7, CH | H-7 | C-5, -7, -8, -10 |
| 7 | 6.40 d (4.4) | 139.5, CH | H-6 | C-5, -8, -9, -14 |
| 8 | 136.6, C | |||
| 9 | 204.9, C | |||
| 10 | 48.0, C | |||
| 11 | 4.14 t (7.2) | 61.6, CH2 | H2-12 | C-12, -13, acetate carbonyl |
| 12 | 1.65 m; 1.25 m | 36.5, CH2 | H2-11 | C-11, -13, -14, -17, -18 |
| 13 | 45.9, C | |||
| 14 | 3.19 dd (9.6, 9.2) | 42.6, CH | H2-15 | C-7, -8, -9, -12, -13, -15, -18 |
| 15 | 1.60 m | 26.9, CH2 | H-14, H2-16 | n. o. |
| 16 | 1.69 m; 1.47 m | 25.5, CH2 | H2-15, H-17 | C-15 |
| 17 | 1.69 m | 50.5, CH | H2-16, H-20 | C-15, -16 |
| 18 | 0.74 s | 17.2, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.31 s | 21.7, CH3 | C-1, -5, -9, -10 | |
| 20 | 2.19 m | 38.7, CH | H-17, H3-21, H-22 | C-16, -17, -22, -23 |
| 21 | 1.04 d (6.8) | 21.6, CH3 | H-20 | C-17, -20, -22 |
| 22 | 5.25 dd (15.2, 6.4) | 134.3, CH | H-20, H-23 | C-20, -23, -24 |
| 23 | 5.21 dd (15.2, 6.8) | 133.1, CH | H-22, H-24 | C-20, -22, -24, 28 |
| 24 | 1.85 q (6.4) | 43.1, CH | H-23, H-25, H3-28 | C-22, -23, -25, -26, -27, -28 |
| 25 | 1.45 m | 33.1, CH | H-24, H3-26, H3-27 | C-23, -24, -26, -27, -28 |
| 26 | 0.83 d (7.2) | 20.0, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.81 d (6.8) | 19.7, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.91 d (6.8) | 17.6, CH3 | H-24 | C-23, -24, -25 |
| 11-OAc | 171.7, C | |||
| 2.00 s | 21.2, CH3 | Acetate carbonyl |

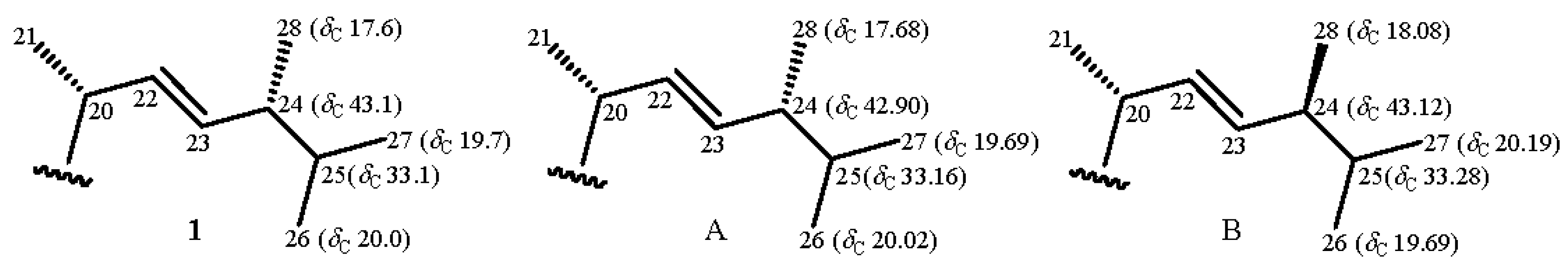
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 1.99 m; 1.70 m | 27.6, CH2 | H2-2 | C-19 |
| 2 | 1.99 m; 1.55 m | 30.5, CH2 | H2-1, H-3 | n. o. a |
| 3 | 4.07 m | 67.1, CH | H2-2, H2-4 | C-5 |
| 4 | 2.14 dd (12.4, 12.0); 1.78 dd (12.4, 3.2) | 39.2, CH2 | H-3 | C-2, -3, -5, -10 |
| 5 | 76.8, C | |||
| 6 | 4.05 m | 72.2, CH | H-7 | C-5, -7, -8, -10 |
| 7 | 6.43 d (5.2) | 138.2, CH | H-6 | C-5, -9, -14 |
| 8 | 137.5, C | |||
| 9 | 203.2, C | |||
| 10 | 48.2, C | |||
| 11 | 4.15 m | 61.2, CH2 | H2-12 | C-12, acetate carbonyl |
| 12 | 1.67 m; 1.28 m | 36.8, CH2 | H2-11 | C-11, -13, -14, -17 |
| 13 | 46.1, C | |||
| 14 | 3.27 dd (10.8, 9.2) | 42.6, CH | H2-15 | C-7, -8, -13, -15, -18 |
| 15 | 1.65 m | 27.0, CH2 | H-14, H2-16 | C-14 |
| 16 | 1.87 m; 1.47 m | 26.1, CH2 | H2-15, H-17 | C-13 |
| 17 | 1,66 m | 51.1, CH | H2-16, H-20 | C-13, -14, -20, -21, -22 |
| 18 | 0.76 s | 17.1, CH3 | C-12, -13, -14, -17, -23 | |
| 19 | 1.36 s | 21.8, CH3 | C-1, -5, -9, -10 | |
| 20 | 1.53 m | 33.4, CH | H-17, H3-21, H2-22 | C-22 |
| 21 | 1.02 d (6.8) | 20.4, CH3 | H-20 | C-17, -20 |
| 22 | 1.70 m; 1.22 m | 35.6, CH2 | H-20, H-23 | C-17, -20, -21, -23, -24 |
| 23 | 5.02 m | 76.9, CH | H2-22, H-24 | n. o. |
| 24 | 1.49 m | 42.7, CH | H-23, H-25, H3-28 | C-22, -23, -25, -26, -27, -28 |
| 25 | 1.58 m | 28.5, CH | H-24, H3-26, H3-27 | C-23, -24, 26, -27, -28 |
| 26 | 0.94 d (6.8) | 21.6, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.84 d (6.8) | 21.5, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.81 d (6.8) | 11.0, CH3 | H-24 | C-23, -24, -25 |
| 11-OAc | 171.2, C | |||
| 2.00 s | 21.1, CH3 | Acetate carbonyl | ||
| 23-OAc | 170.8, C | |||
| 2.03 s | 21.5, CH3 | Acetate carbonyl |
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.06 m; 1.71 m | 27.4, CH2 | H2-2 | C-5, -10 |
| 2 | 1.98 m; 1.61 m | 26.4, CH2 | H2-1, H-3 | C-3 |
| 3 | 5.10 m | 70.3, CH | H2-2, H2-4 | n. o. a |
| 4 | 2.21 dd (13.2, 11.6); 1.87 m | 35.5, CH2 | H-3 | C-3, -5, -10, -12 |
| 5 | 76.5, C | |||
| 6 | 4.03 d (4.8) | 72.3, CH | H-7 | C-5, -7, -8, -10 |
| 7 | 6.41 d (4.8) | 138.1, CH | H-6 | C-5, -9, -14 |
| 8 | 137.4, C | |||
| 9 | 202.8, C | |||
| 10 | 48.0, C | |||
| 11 | 4.16 m | 61.2, CH2 | H2-12 | C-12, -13, acetate carbonyl |
| 12 | 1.61 m; 1.28 m | 36.8, CH2 | H2-11 | C-11, -13, -14, -17 |
| 13 | 46.1, C | |||
| 14 | 3.27 dd (11.6, 8.0) | 42.6, CH | H2-15 | C-7, -8, -13 |
| 15 | 1.61 m | 27.0, CH2 | H-14, H2-16 | C-4, -13 |
| 16 | 1.87 m; 1.47 m | 26.1, CH2 | H2-15, H-17 | n. o. |
| 17 | 1.68 m | 51.2, CH | H2-16, H-20 | n. o. |
| 18 | 0.75 d (6.4) | 17.1, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.31 s | 21.6, CH3 | C-1, -5, -10 | |
| 20 | 1.53 m | 33.4, CH | H-17, H3-21, H2-22 | n. o. |
| 21 | 1.03 d (6.4) | 20.4, CH3 | H-20 | C-17, -20, -22 |
| 22 | 1.71 m; 1.26 m | 35.5, CH2 | H-20, H-23 | C-21, -23 |
| 23 | 5.02 m | 76.9, CH | H2-22, H-24 | n. o. |
| 24 | 1.49 m | 42.8, CH | H-23, H-25, H3-28 | C-22, -23, -27, -28 |
| 25 | 1.57 m | 28.5, CH | H-24, H3-26, H3-27 | C-24, -27, -28 |
| 26 | 0.94 d (6.4) | 21.6, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.84 d (6.4) | 18.6, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.81 d (6.4) | 11.0, CH3 | H-24 | C-23, -24, -25 |
| 3-OAc | 170.8, C | |||
| 2.01 s | 21.4, CH3 | Acetate carbonyl | ||
| 11-OAc | 171.1, C | |||
| 2.00 s | 21.1, CH3 | Acetate carbonyl | ||
| 23-OAc | 170.8, C | |||
| 2.05 s | 21.5, CH3 | Acetate carbonyl |
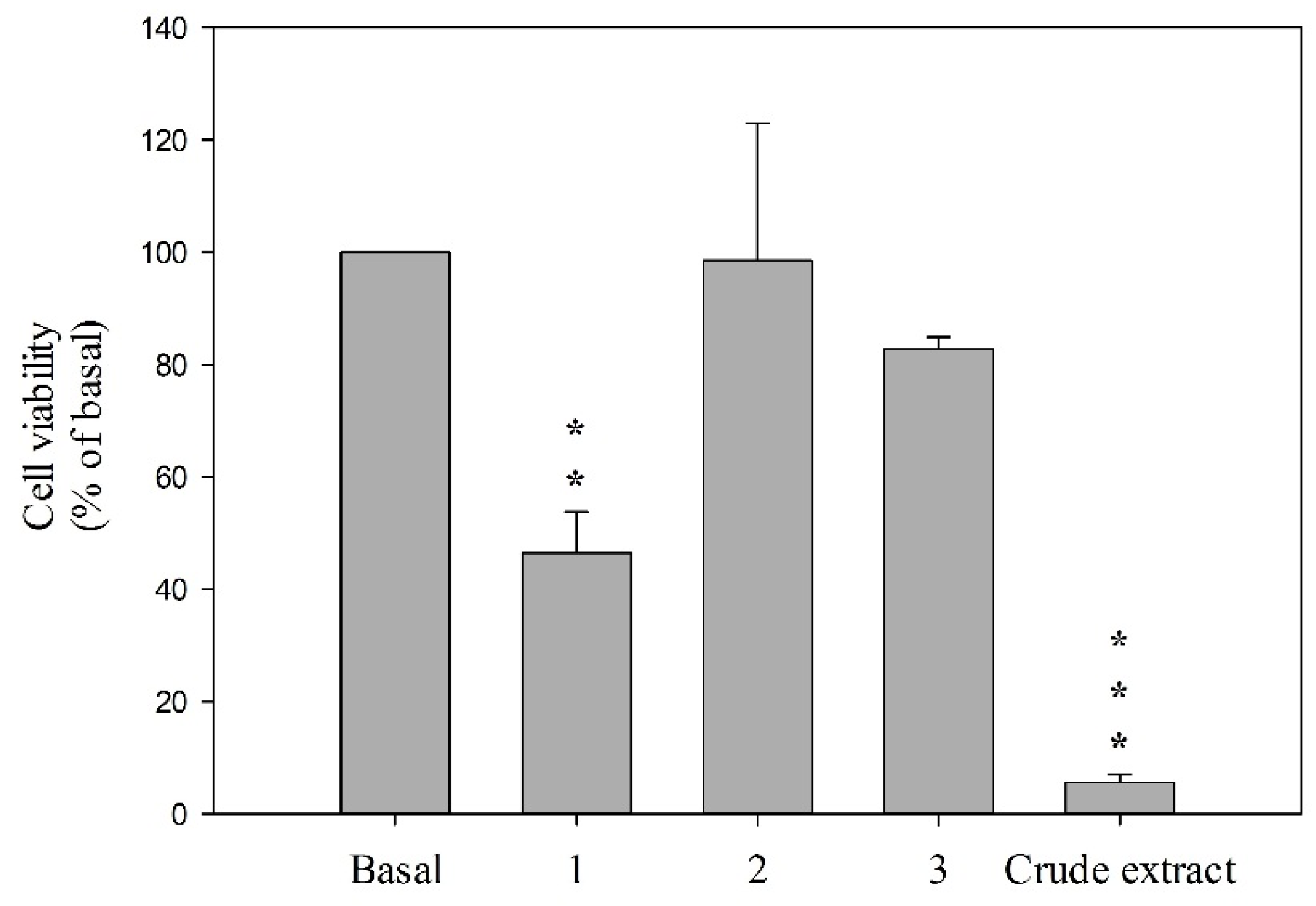
| Elastase Release | Superoxide Anion | |
|---|---|---|
| Compound | IC50 (μM) a | IC50 (μM) a |
| 1 | 3.32 | 2.33 |
| 2 | >10 | >10 |
| 3 | 2.81 | 2.50 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.4. Anti-Hepatofibric Assay
3.5. Generation of Superoxide Anions and Release of Elastase by Human Neutrophils
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duh, C.-Y.; Li, C.-H.; Wang, S.-K.; Dai, C.-F. Diterpenoids, norditerpenoids, and secosteroids from the Formosan soft coral Cespitularia hypotentaculata. J. Nat. Prod. 2006, 69, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-W.; Chang, S.-M.; Huang, C.-Y.; Su, J.-H.; Wen, Z.-H.; Wu, Y.-C.; Sheu, J.-H. Hirsutosterols A–G, polyoxygenated steroids from a Formosan soft coral Cladiella hirsuta. Org. Biomol. Chem. 2011, 9, 3272–3278. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Su, J.-H.; Duh, C.-Y.; Chen, B.-W.; Wen, Z.-H.; Kuo, Y.-H.; Sheu, J.-H. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg. Med. Chem. Lett. 2012, 22, 4373–4376. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Tseng, Y.-J.; Huang, H.-H.; Ahmed, A.F.; Lu, C.-K.; Wu, Y.-C.; Sheu, J.-H. 9,11-Secosterols from the soft corals Sinularia lochmodes and Sinularia leptoclados. J. Nat. Prod. 2006, 69, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Chen, H.-P.; Wang, S.-K.; Duh, C.-Y. Three new 9,11-secosterols from the Formosan soft coral Sinularia leptoclados. Bull. Chem. Soc. Jpn. 2011, 84, 648–652. [Google Scholar] [CrossRef]
- Tseng, Y.-J.; Wang, S.-K.; Duh, C.-Y. Secosteroids and norcembranoids from the soft coral Sinularia nanolobata. Mar. Drugs 2013, 11, 3288–3296. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Esposito, G.; Fattorusso, E.; Iuvone, T.; Luciano, P.; Menna, M. Aplidiasterols A and B, two new cytotoxic 9,11-secosterols from the mediterranean ascidian Aplidium conicum. Steroids 2003, 68, 719–723. [Google Scholar] [CrossRef]
- Wright, J.L.C.; McInnes, A.G.; Shimizu, S.; Smith, D.G.; Walter, J.A.; Idler, D.; Khalil, W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonances spectroscopy. Can. J. Chem. 1978, 56, 1898–1903. [Google Scholar]
- Yu, H.-P.; Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; et al. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed.; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 218–219. [Google Scholar]
- Kuo, L.-M.; Kuo, C.-Y.; Lin, C.-Y.; Hung, M.-F.; Shen, J.-J.; Hwang, T.-L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 2014, 19, 3327–3344. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-C.; Sung, P.-J.; Duh, C.-Y.; Chen, B.-W.; Sheu, J.-H.; Yang, N.-S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083–4126. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Kim, S.-K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Kuo, L.-M.; Hwang, T.-L.; Yeh, J.; Wen, Z.-H.; Fang, L.-S.; Wu, Y.-C.; Lin, C.-S.; Sheu, J.-H.; Sung, P.-J. Pinnisterols A–C, New 9,11-Secosterols from a Gorgonian Pinnigorgia sp. Mar. Drugs 2016, 14, 12. https://doi.org/10.3390/md14010012
Chang Y-C, Kuo L-M, Hwang T-L, Yeh J, Wen Z-H, Fang L-S, Wu Y-C, Lin C-S, Sheu J-H, Sung P-J. Pinnisterols A–C, New 9,11-Secosterols from a Gorgonian Pinnigorgia sp. Marine Drugs. 2016; 14(1):12. https://doi.org/10.3390/md14010012
Chicago/Turabian StyleChang, Yu-Chia, Liang-Mou Kuo, Tsong-Long Hwang, Jessica Yeh, Zhi-Hong Wen, Lee-Shing Fang, Yang-Chang Wu, Chan-Shing Lin, Jyh-Horng Sheu, and Ping-Jyun Sung. 2016. "Pinnisterols A–C, New 9,11-Secosterols from a Gorgonian Pinnigorgia sp." Marine Drugs 14, no. 1: 12. https://doi.org/10.3390/md14010012
APA StyleChang, Y.-C., Kuo, L.-M., Hwang, T.-L., Yeh, J., Wen, Z.-H., Fang, L.-S., Wu, Y.-C., Lin, C.-S., Sheu, J.-H., & Sung, P.-J. (2016). Pinnisterols A–C, New 9,11-Secosterols from a Gorgonian Pinnigorgia sp. Marine Drugs, 14(1), 12. https://doi.org/10.3390/md14010012








