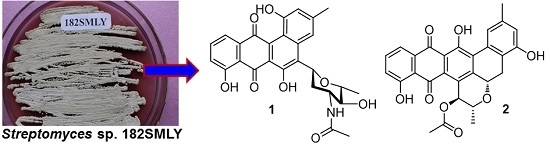
Bioactive Polycyclic Quinones from Marine Streptomyces sp. 182SMLY
Abstract
:1. Introduction
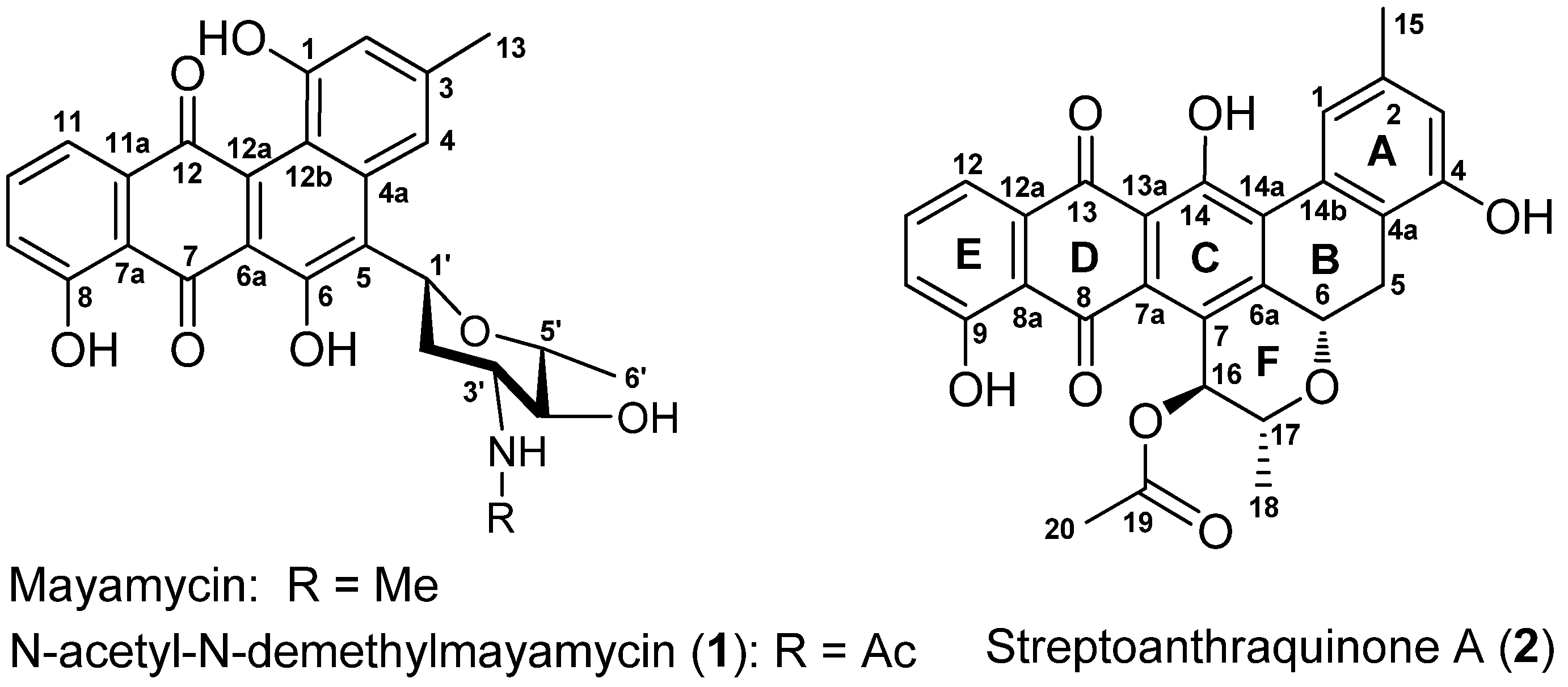
2. Results and Discussion
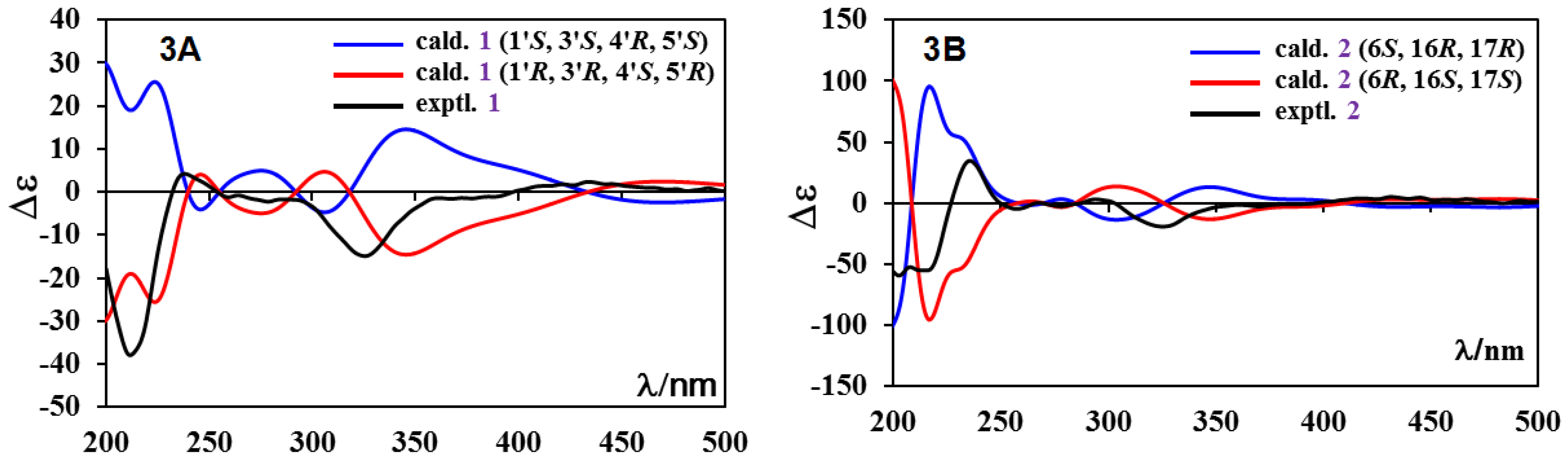
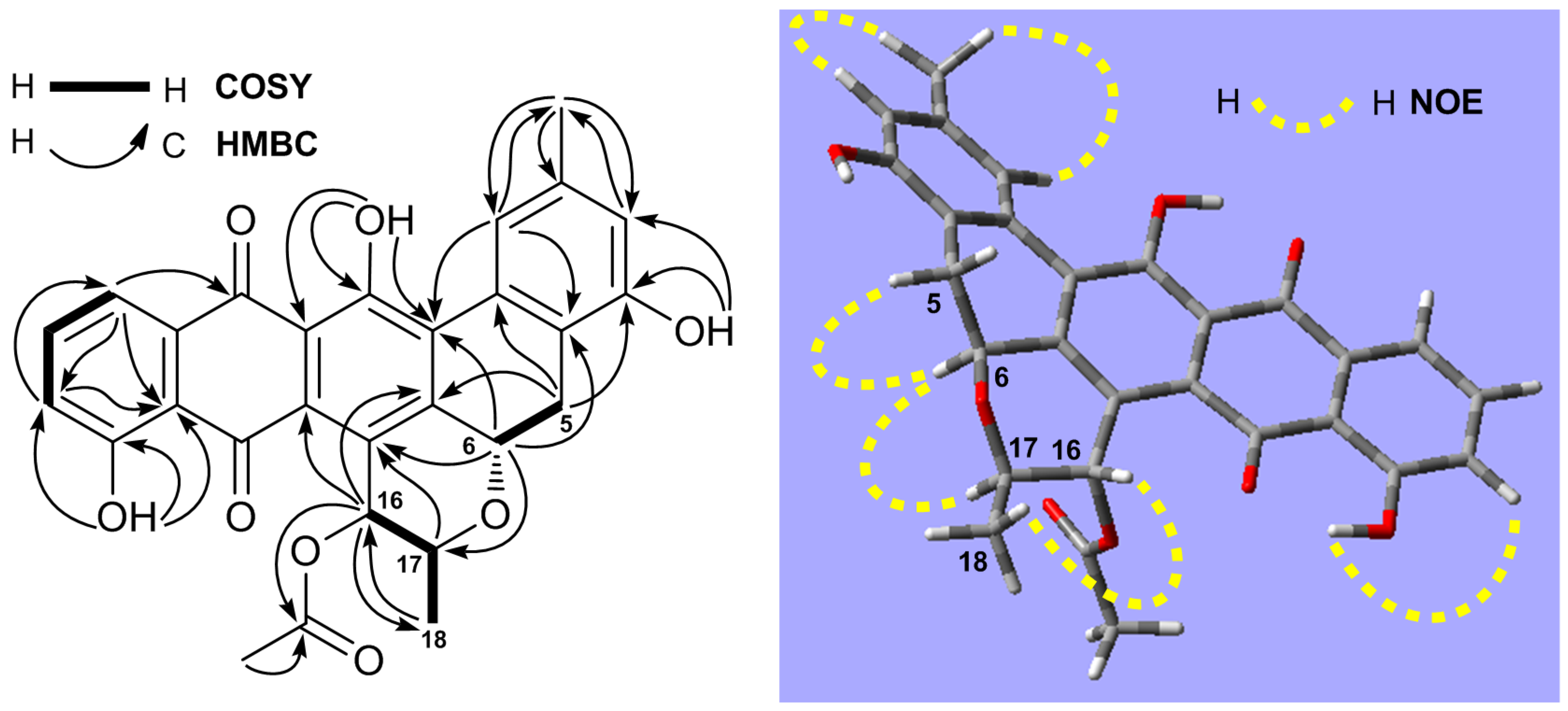
2.1. Structure Elucidation
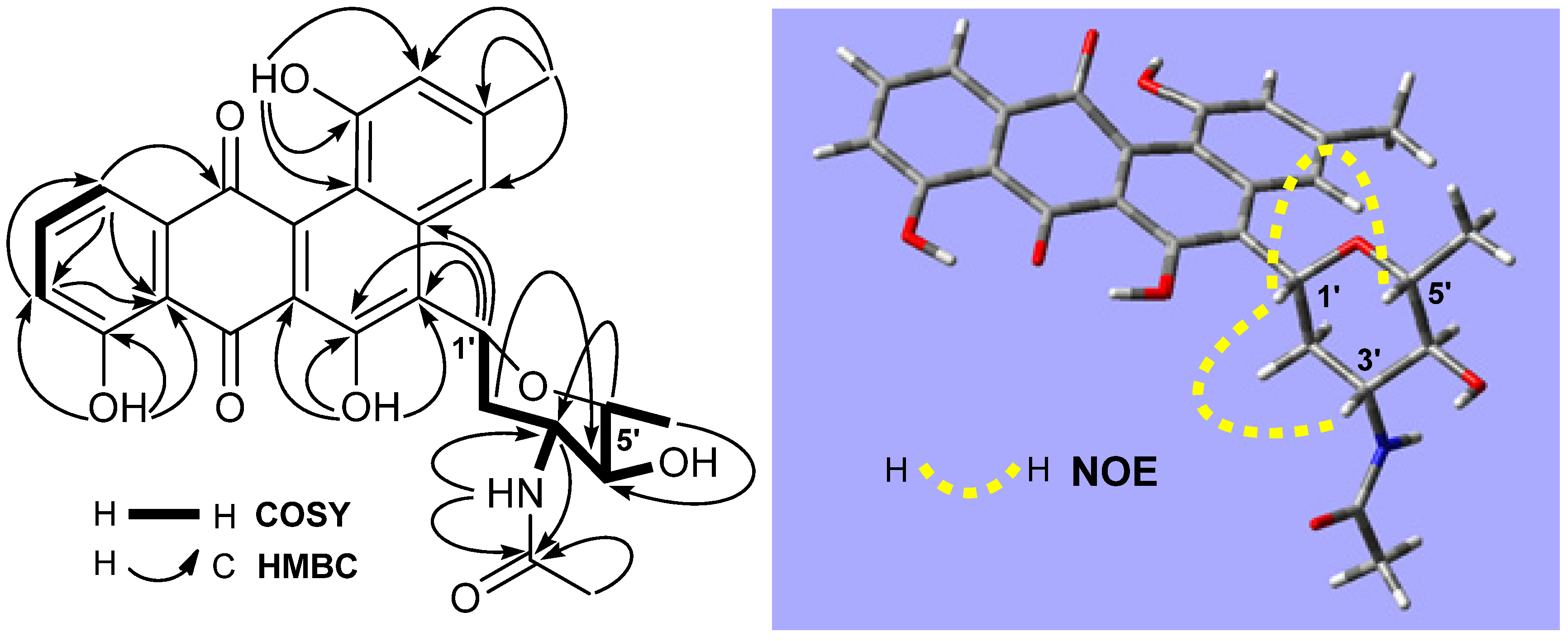
| No. | δC, Type | δH (J = Hz) | HMBC | No. | δC, Type | δH (J = Hz) | HMBC |
|---|---|---|---|---|---|---|---|
| 1 | 155.2, C | 12a | 137.7 a, C | ||||
| 2 | 111.5, CH | 6.64, brs | 1, 4, 12b, 13 | 12b | 115.4, C | ||
| 3 | 141.0, C | 13 | 22.3, CH3 | 2.40, s | 2, 3, 4 | ||
| 4 | 115.4, CH | 7.89, brs | 2, 5, 12b, 13 | 1′ | 71.0, CH | 5.44, dd (12.1, 2.3) | 4a, 5, 6 |
| 4a | 137.6 a, C | 2′ | 36.0, CH2 | 1.86, m; 2.10, 1H, m | 1′, 3′, 4′ | ||
| 5 | 124.2, C | 3′ | 52.5, CH | 3.85, m | 7′ | ||
| 6 | 152.1, C | 4′ | 74.2, CH | 3.20, m | 3′ | ||
| 6a | 117.6, C | 5′ | 77.7, CH | 3.40, dd (9.1, 6.1) | 3′ | ||
| 7 | 191.6, C | 6′ | 18.6, CH3 | 1.27, d (6.1) | 4′, 5′ | ||
| 7a | 115.6, C | 7′ | 169.2, C | ||||
| 8 | 160.2, C | 8′ | 22.3, CH3 | 1.80, s | 7′ | ||
| 9 | 122.9, CH | 7.30, dd (7.5, 1.1) | 7a, 8, 11 | 1-OH | 10.26, s | 1, 2, 12b | |
| 10 | 137.5, CH | 7.76, t (7.5) | 8, 11, 11a | 6-OH | 12.26, s | 5, 6, 6a | |
| 11 | 117.9, CH | 7.43, dd (7.5, 1.1) | 7a, 9, 12 | 8-OH | 11.36, s | 7a, 8, 9 | |
| 11a | 136.6, C | 3′-NH | 7.87, d (8.2) | 3′, 7′ | |||
| 12 | 184.6, C | 4′-OH | 5.02, d (5.1) |
| No. | δC, Type | δH (J = Hz) | HMBC | No. | Δc, Type | δH (J = Hz) | HMBC |
|---|---|---|---|---|---|---|---|
| 1 | 118.1, CH | 8.14, d (1.6) | 3, 4a, 14a, 14b, 15 | 12a | 135.3, C | ||
| 2 | 143.0, C | 13 | 189.6, C | ||||
| 3 | 119.1, CH | 7.01, d (1.6) | 1, 4, 4a, 15 | 13a | 118.9 a, C | ||
| 4 | 154.7, C | 14 | 154.3, C | ||||
| 4a | 139.1, C | 14a | 129.7, C | ||||
| 5 | 29.8, CH2 | 2.88, dd (15.6, 12.1); 3.57, dd (15.6, 3.3) | 4, 6, 6a 6, 6a, 14b | 14b | 118.8 a, C | ||
| 6 | 72.3, CH | 5.77, dd (12.1, 3.3) | 4a, 7, 14a, 17 | 15 | 22.6, CH3 | 2.50, s | 1, 2, 3 |
| 6a | 152.9, C | 16 | 73.1, CH | 5.33, d (9.5) | 6a, 7a, 17, 18, 19 | ||
| 7 | 127.7, C | 17 | 77.1, CH | 3.93, dd (9.5, 6.1) | 7 | ||
| 7a | 133.4, C | 18 | 19.0, CH3 | 1.43, d (6.1) | 16, 17 | ||
| 8 | 193.5, C | 19 | 170.3, C | ||||
| 8a | 114.9, C | 20 | 21.0, CH3 | 2.23, s | 19 | ||
| 9 | 162.2, C | 4-OH | 9.25, s | 3, 4 | |||
| 10 | 125.5, CH | 7.34, dd (7.5, 1.2) | 8a, 9, 12 | 9-OH | 11.69, s | 8a, 9, 10 | |
| 11 | 138.1, CH | 7.71, t (7.5) | 9, 12a | 14-OH | 12.84, s | 13a, 14, 14a | |
| 12 | 121.7, CH | 7.81, dd (7.5, 1.2) | 8a, 10, 13 |
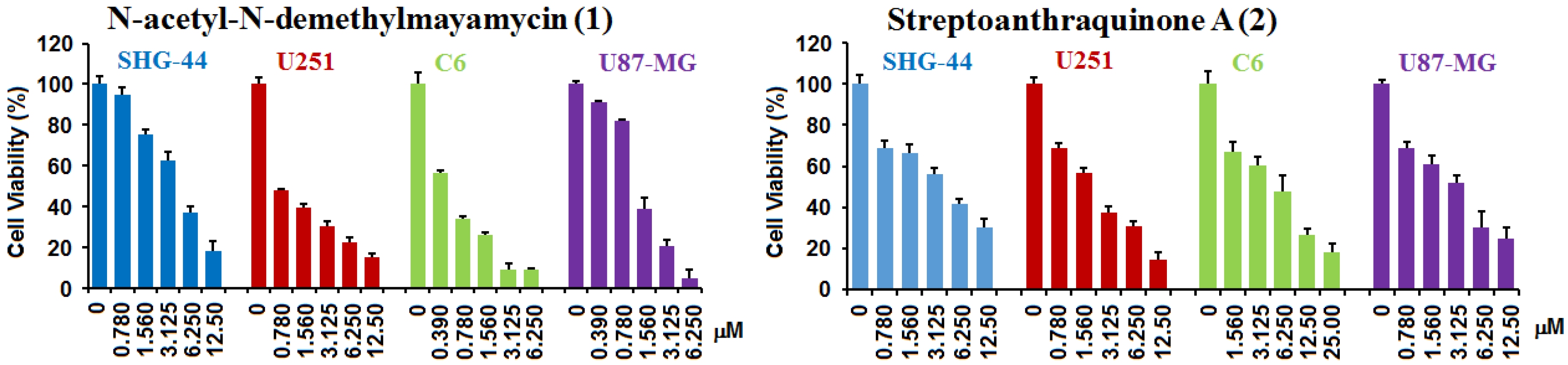
2.2. Biological Activities
| Compounds | U251 | U87-MG | SHG-44 | C6 | HA |
|---|---|---|---|---|---|
| N-acetyl-N-demethylmayamycin (1) | 0.7 ± 0.2 | 1.4 ± 0.1 | 3.9 ± 0.4 | 0.5 ± 0.1 | 25 ± 1.3 |
| Ratios of IC50HA/IC50gc | 35 | 18 | 6.4 | 53 | |
| Streptoanthraquinone A (2) | 3.3 ± 0.3 | 4.6 ± 0.3 | 6.5 ± 1.1 | 7.3 ± 1.4 | >100 |
| Ratios of IC50HA/IC50gc | >31 | >22 | >16 | >14 | |
| Doxorubicin (DOX) | 6.7 ± 1.1 | 0.9 ± 0.1 | 9.0 ± 0.8 | 1.0 ± 0.1 | NT |

3. Experimental Section
3.1. General Experimental Procedures
3.2. Isolation and Taxonomic Identity of Marine Streptomyces sp. 182SMLY
3.3. Culture of Strain Streptomyces sp. 182SMLY
3.4. Extraction and Isolation of Compounds
3.5. Computational Methods
3.6. Cells Culture
3.7. Sulforhodamine B (SRB) Assay
3.8. Antimicrobial Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DFT | Density functional theory |
| DOX | Doxorubicin |
| ECD | Electronic circular dichroism |
| ODS | Octadecyl-functionalized silica gel |
| OPLS | Optimized potentials for liquid simulations |
| SRB | Sulforhodamine B |
| TDDFT | Time-dependent density functional theory |
| TMZ | Temozolomide |
References
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013, 15 (Suppl. 2), ii1–ii56. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Hosni-Ahmed, A.; Jones, T.S.; Patil, R.; Pfeffer, L.M.; Miller, D.D. Novel approaches to glioma drug design and drug screening. Expert Opin. Drug Discov. 2013, 8, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Temozolomide: Therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev. Neurother. 2010, 10, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. Gold from the sea: Marine compounds as inhibitors of the hallmarks of cancer. Biotechnol. Adv. 2011, 29, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Petit, K.; Biard, J.F. Marine natural products and related compounds as anticancer agents: An overview of their clinical status. Anticancer Agents Med. Chem. 2013, 13, 603–631. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.X.; Ye, X.W.; Yu, S.R.; Lian, X.Y.; Zhang, Z.Z. New capoamycin-type antibiotics and polyene acids from marine Streptomyces fradiae PTZ0025. Mar. Drugs 2012, 10, 2388–2402. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.R.; Ye, X.W.; Chen, L.; Lian, X.Y.; Zhang, Z.Z. Polyoxygenated 24,28-epoxyergosterols inhibiting the proliferation of glioma cells from sea anemone Anthopleura midori. Steroids 2014, 88, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.R.; Ye, X.W.; Huang, H.C.; Peng, R.; Su, Z.H.; Lian, X.Y.; Zhang, Z.Z. Bioactive sulfated saponins from sea cucumber Holothuria moebii. Planta Med. 2015, 81, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liang, L.; Song, T.F.; Anjum, K.; Wang, W.L.; Yu, S.R.; Huang, H.C.; Lian, X.Y.; Zhang, Z.Z. Synthesis and bioactivity of tripolinolate A from Tripolium vulgare and its analogs. Bioorg. Med. Chem. Lett. 2015, 25, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.R.; Ye, X.W.; Chen, L.; Xie, X.; Zhou, Q.; Lian, X.Y.; Zhang, Z.Z. Cytotoxic and anti-colorectal tumor effects of sulfated saponins from sea cucumber Holothuria moebii. Phytomedicine 2015, 22, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.W.; Anjum, K.; Song, T.F.; Wang, W.L.; Yu, S.R.; Huang, H.C.; Lian, X.Y.; Zhang, Z.Z. A new curvularin glycoside and its cytotoxic and antibacterial analogues from marine actinomycete Pseudonocardia sp. HS7. Nat. Prod. Res. 2015. [Google Scholar] [CrossRef]
- Werner, G.; Hagenmaier, H.; Drautz, H.; Baumgartner, A.; Zähner, H. Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity. J. Antibiot. 1984, 37, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.G.; Putnam, A.R.; Mishra, S.K.; Mulks, M.H.; Taft, W.H.; Keller, J.E.; Miller, J.R.; Zhu, P.P.; Meinhart, J.D.; Lynn, D.G. Faeriefungin: A new broad-spectrum antibiotic from Streptomyces griseus var. autotrophicus. J. Nat. Prod. 1989, 52, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Shinagawa, S.; Nozaki, Y.; Asai, M.; Kishi, T. C-19393 S2 and H2, new carbapenem antibiotics. II. isolation and structures. J. Antibiot. 1980, 33, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Nozaki, Y.; Shinagawa, S.; Kitano, K. C-19393 E5, a new carbapenem antibiotic. Fermentation, isolation and structure. J. Antibiot. 1982, 35, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Yamawaki, I.; Matsumoto, H.; Minami, Y.; Yamada, Y.; Marunaka, T.; Qi, C.; Tian, T.; Zhang, R. New antibiotics 4181-A and B from Streptomyces griseus; taxonomy, fermentation, isolation and characterization. J. Antibiot. 1988, 41, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Tohma, S.; Kondo, H.; Yokotsuka, J.; Iwamoto, J.; Matsuhashi, G.; Ito, T.; Seto, H. Ashimycins A and B, new streptomycin analogues. J. Antibiot. 1989, 42, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Ma, M.; Rateb, M.E.; Shaaban, K.A.; Yu, Z.; Huang, S.X.; Zhao, L.X.; Zhu, X.; Yan, Y.; Peterson, R.M.; et al. Biosynthetic potential-based strain prioritization for natural product discovery: A showcase for diterpenoid-producing actinomycetes. J. Nat. Prod. 2014, 77, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, Y.; Adachi, H.; Nakamura, H.; Nishimura, Y.; Naganawa, H.; Okami, Y.; Takeuchi, T. The structure of diphenazithionin, a novel antioxidant from Streptomyces griseus ISP 5236. Tetrahedron Lett. 1996, 37, 9227–9228. [Google Scholar] [CrossRef]
- Byrne, K.M.; Gonda, S.K.; Hilton, B.D. Largomycin FII chromophore component 4, a new pluramycin antibiotic. J. Antibiot. 1985, 38, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, I.; Kajahn, I.; Ohlendorf, B.; Zinecker, H.; Erhard, A.; Nagel, K.; Wiese, J.; Imhoff, J.F. Mayamycin, a cytotoxic polyketide from a Streptomyces strain isolated from the marine sponge Halichondria panicea. J. Nat. Prod. 2010, 73, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Cheng, X.C.; Halley, K. Biosynthesis of dehydrorabelomycin and PD 116740: Prearomatic deoxygenation as evidence for different polyketide synthases in the formation of benz[a]anthraquinones. J. Am. Chem. Soc. 1992, 114, 10066–10068. [Google Scholar] [CrossRef]
- Mitra, P.; Behera, B.; Maiti, T.K.; Mal, D. Angucycline C5 glycosides: Regio- and stereocontrolled synthesis and cytotoxicity. J. Org. Chem. 2013, 78, 9748–9757. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wang, M.; Yao, Q.; Zhang, A. Synthesis study toward mayamycin. Chin. J. Chem. 2013, 31, 93–99. [Google Scholar] [CrossRef]
- Fernekorn, U.; Heinze, T.; Schlegel, B.; Dahse, H.M.; Gräfe, U. Synthesis of a new polycyclic quinone by reduction of a dihydrobenzo. J. Antibiot. 2001, 54, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.P.; Shen, Y.H.; Zhang, S.D.; Tian, J.M.; Zeng, H.W.; Ye, J.; Li, H.L.; Shan, L.; Zhang, W.D. Incarvilleatone, a new cyclohexylethanoid dimer from Incarvillea younghusbandii and its inhibition against nitric oxide (NO) release. Org. Lett. 2012, 14, 1954–1957. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Su, Y.F.; Chen, L.; Que, M.; Gao, X.M.; Chang, J.B. Polygonumosides A–D, stilbene derivatives from processed roots of Polygonum multiflorum. J. Nat. Prod. 2014, 77, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Ritzau, M.; Vettermann, R.; Fleck, W.F.; Gutsche, W.; Dornberger, K. Benaphthamycin, a new dihydrobenzo[a]naphthacenequinone antibiotic from Streptomyces sp. HKI-0057. J. Antibiot. 1997, 50, 791–793. [Google Scholar] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Xie, X.; Chen, L.; Yan, S.; Ye, X.; Anjum, K.; Huang, H.; Lian, X.; Zhang, Z. Bioactive Polycyclic Quinones from Marine Streptomyces sp. 182SMLY. Mar. Drugs 2016, 14, 10. https://doi.org/10.3390/md14010010
Liang Y, Xie X, Chen L, Yan S, Ye X, Anjum K, Huang H, Lian X, Zhang Z. Bioactive Polycyclic Quinones from Marine Streptomyces sp. 182SMLY. Marine Drugs. 2016; 14(1):10. https://doi.org/10.3390/md14010010
Chicago/Turabian StyleLiang, Ying, Xin Xie, Lu Chen, Shilun Yan, Xuewei Ye, Komal Anjum, Haocai Huang, Xiaoyuan Lian, and Zhizhen Zhang. 2016. "Bioactive Polycyclic Quinones from Marine Streptomyces sp. 182SMLY" Marine Drugs 14, no. 1: 10. https://doi.org/10.3390/md14010010
APA StyleLiang, Y., Xie, X., Chen, L., Yan, S., Ye, X., Anjum, K., Huang, H., Lian, X., & Zhang, Z. (2016). Bioactive Polycyclic Quinones from Marine Streptomyces sp. 182SMLY. Marine Drugs, 14(1), 10. https://doi.org/10.3390/md14010010