Valorization of Sargassum muticum Biomass According to the Biorefinery Concept
Abstract
:1. Introduction
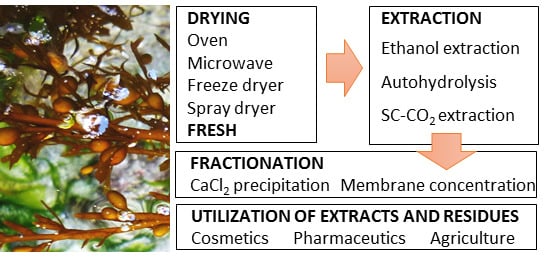
2. Results and Discussion
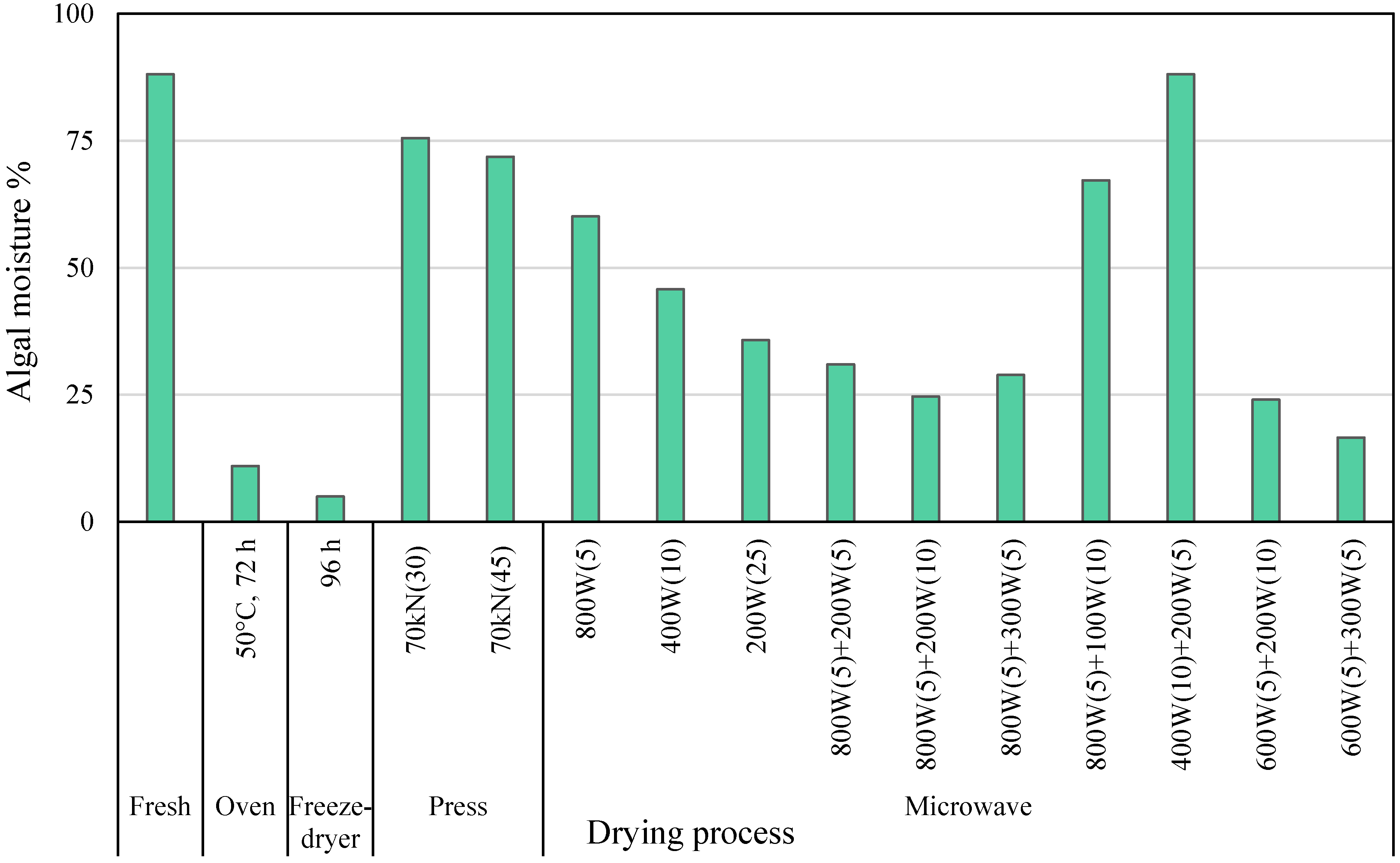
2.1. Drying
2.1.1. Sargassum muticum Biomass
2.1.2. Concentrated Extracts
2.2. Extracts Production by Green Technologies from Sm
2.2.1. Conventional Solvent Extraction.
| Fresh Sm | ovSm | fzSm | ||||||
|---|---|---|---|---|---|---|---|---|
| Soxhlet | Shaker | Soxhlet | Shaker | Shaker | ||||
| Et | Et | W | Et | Et:W (3:1) | Et:W (1:1) | |||
| Extraction yield (mg extract g−1 Sm d.b.) | 359 | 10 | 59 | 263 | 11 | 14 | 11 | 12 |
| SD | 5 | 6 | 8 | 4 | 2 | 1 | 6 | 7 |
| TPC (mg GAE g−1 extract) | 27 | 12 | 6 | 80 | 72 | 79 | 73 | 64 |
| SD | 3 | 0 | 0 | 0 | 0 | 2 | 8 | 8 |
| TEAC (mg trolox g−1 extract) | - | 46 | 3 | - | 309 | 72 | 88 | 99 |
| SD | - | 3 | 0 | - | 11 | 5 | 8 | 11 |
2.2.2. Extracts Uses and Applications
2.2.3. SC-CO2 Extraction
Direct Extraction
| A (17% Moisture mwSm) | B (24% Moisture mwSm) | |||
|---|---|---|---|---|
| SC-CO2 (45 °C, 1 h, 25 g CO2 min−1) | 10 MPa | 35 MPa | 10 MPa | 35 MPa |
| Extract yield (mg extract g−1 Sm) | 54 | 160 | 35 | 84 |
| SD | 10 | 10 | 20 | 10 |
| Fucoxanthin concentration (mg fucoxanthin g−1 Sm d.b.) | 5.13 | 0.11 | 2.77 | 0.07 |
| SD | 0 | 10 | 0 | 1 |
SC-CO2 Fractionation of Ethanol Extracts
| SC-CO2 Extraction Temperature (°C) | Fraction Yield (mg Fraction g−1 Sm Extract) Vessel 1 | Fucoxanthin Content (mg Fucoxanthin g−1 Extract) |
|---|---|---|
| 40 | 3 | 7 |
| 50 | 4 | 1 |
| 60 | 4 | 1 |
2.2.4. Hydrothermal Treatments: Autohydrolysis or Subcritical Water Extraction
Membrane Processing of Alginate Free Liquors
Purification of AH Liquors and Arsenic Removal by Membranes
| (i) Stream | Calculated Salt Concentration (g CaCl2E L−1) | (ii) Stream | Calculated Salt Concentration (g CaCl2E L−1) | Phenolic Content (g GAE g−1 Extract) |
|---|---|---|---|---|
| Liquor + 1% CaCl2 | 12.49 | Concentrate | 32.95 | 0.05 |
| Ret 1 | 5.58 | Perm | 30.36 | 0.03 |
| Perm 1 | 5.38 | Ret 1 ׀ Perm 1 | 19.27 ׀ 19.98 | - ׀ 0.03 |
| Ret 2 | 1.01 | Ret 2 ׀ Perm 2 | 11.87 ׀ 11.42 | 0.14 ׀ 0.02 |
| Perm 2 | 0.71 | Ret 3 ׀ Perm 3 | - ׀ 6.60 | 0.08 ׀ 0.03 |
| Ret 3 | 0.24 | Ret 4 ׀ Perm 4 | 5.53 ׀ 4.46 | 0.08 ׀ 0.03 |
| Perm 3 | 0.20 | Ret 5 ׀ Perm 5 | 4.28 ׀ 2.85 | 0.08 ׀ 0.03 |
| Pooled Perm | 1.81 | Ret 6 ׀ Perm 6 | 3.12 ׀ 1.56 | 0.09 ׀ 0.03 |
| Ret 7 ׀ Perm 7 | 2.23 ׀ 0.98 | 0.09 ׀ 0.02 |
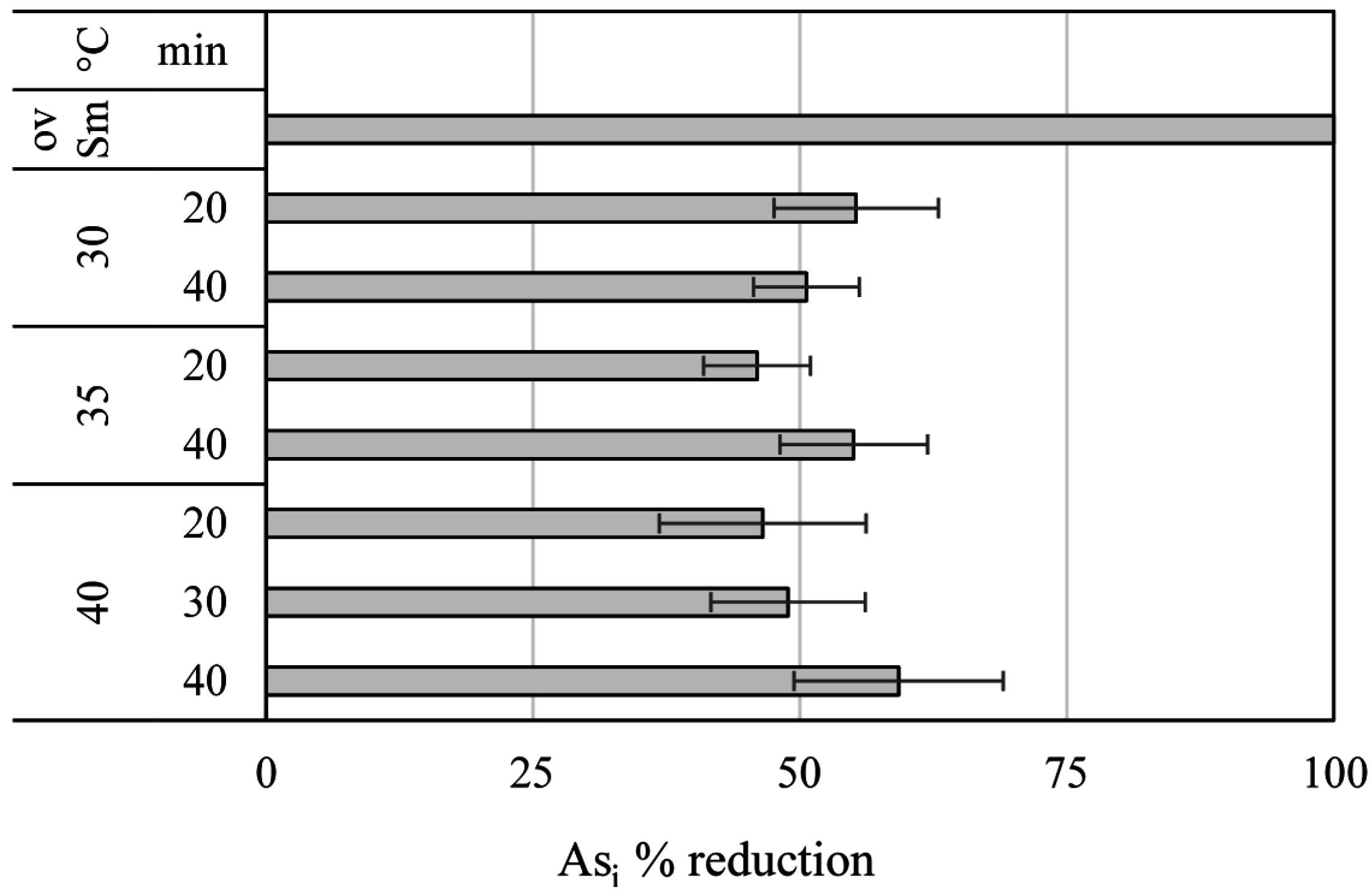
| Asi μg g−1 ± SD | |
|---|---|
| Autohydrolysis liquor | 29.4 ± 2.9 |
| 1 kDa retentate | 2.8 ± 0.6 |
| 1 kDa permeate | 24.1 ± 2.0 |
| Product 4 | 1.1 ± 0.3 |
| Product 5 | 0.4 ± 0.1 |
Extracts Uses and Applications
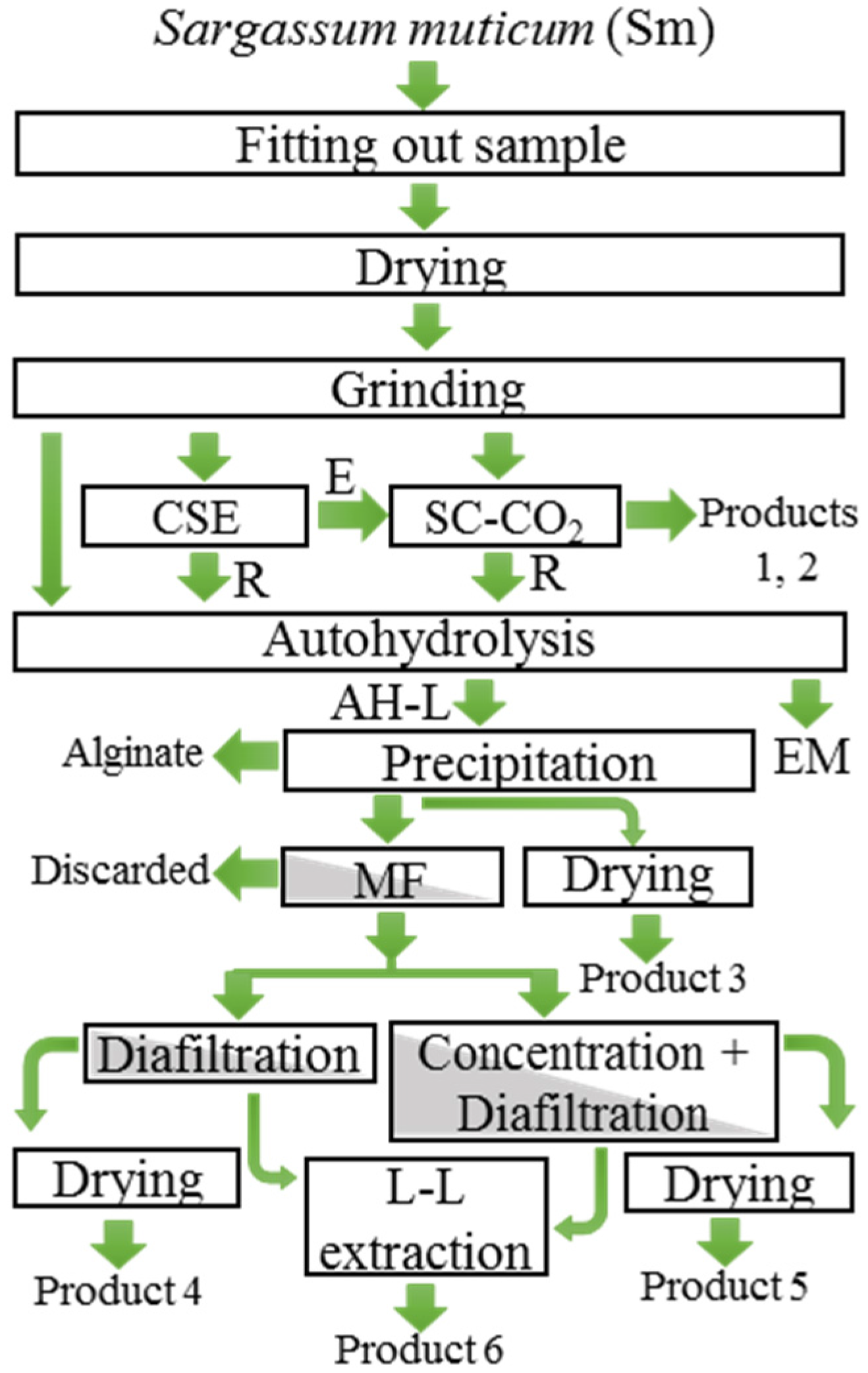
3. Materials and Methods
3.1. Fitting Out Sample
3.2. Drying of Sm and Extracts
3.3. Grinding
3.4. Proposal of Global Valorization of Sm for the Recovery of Alginate, Fucoxanthin and Antioxidant Compounds
3.4.1. Conventional Solvent Extraction
3.4.2. SC-CO2 Extraction
3.4.3. Fucoxanthin Concentration Enhancement by SC-CO2 Fractionation
3.4.4. Autohydrolysis or Subcritical Water Extraction
3.4.5. Alginate Precipitation
3.4.6. Fractionation by Means of Membrane Technology
3.4.7. Fractionation by Means of Immiscible Solvents (Partition)
3.5. Analytical Methods
3.5.1. Non-Volatile Compounds Yield
3.5.2. Inorganic Arsenic Determination
3.5.3. Total Phenolic Content (TPC) and Antioxidant Activity
3.5.4. HPLC Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Soto, M.L.; Nogueira, D.R.; González-López, N.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential of antioxidant extracts produced by aqueous processing of renewable resources for the formulation of cosmetics. Ind. Crop. Prod. 2014, 58, 104–110. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Kolanjinathan, K.; Ganesh, P.; Saranraj, P. Pharmacological Importance of Seaweeds: A Review. World J. Fish Mar. Sci. 2014, 6, 1–15. [Google Scholar]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). Biomed Res. Int. 2013, 13, 604787. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- González-López, N.; Moure, A.; Domínguez, H. Hydrothermal fractionation of Sargassum muticum biomass. J. Appl. Phycol. 2012, 24, 1569–1578. [Google Scholar] [CrossRef]
- Garrote, G.H.; Domínguez, H.; Parajó, J.C. Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood. J. Chem. Technol. Biotechnol. 1999, 74, 1101–1109. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibáñez, E.; Reglero, G.; Señoráns, J. Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himanthalia elongata. J. Appl. Phycol. 2011, 23, 909–917. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.; silva Filho, E.A.; Melo, D.F.; Feitosa, P.; de Paula, R.C.; Lima, M.G. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar]
- Pérez, L.; Conde, E.; Domínguez, H. Microwave hydrodiffusion and gravity processing of Sargassum muticum. Process Biochem. 2014, 49, 981–988. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Mujumdar, A.S.; Wang, S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Ibáñez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 4, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Moure, A.; Domínguez, H.; (Department of Chemical Engineering, University of Vigo, Ourense, Spain); Gallego-Fabrega, C.; (Stroke Genetics and Pharmacogenetics, Fundació Docència i Recerca Mútua Terrassa, Terrassa, Spain). Unpublished work. 2015.
- Balboa, E.M.; Domínguez, H.; Department of Chemical Engineering, University of Vigo, Ourense, Spain. Unpublished work. 2015.
- Kim, S.K.; Pangestuti, R. Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: Salt Lake City, UT, USA, 2011; Volume 64, pp. 111–128. [Google Scholar]
- Lee, B.M.; Kim, C.J.; Kim, C.T.; Seo, J.J.; Kim, I.H. Concentration of fucoxanthin from Ecklonia cava using supercritical carbon dioxide. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1452–1456. [Google Scholar] [CrossRef]
- Sugawa-Katayama, Y.; Katayama, M. Release of minerals from dried Hijiki, Sargassum fusiforme (Harvey) Setchell, during water-soaking. Trace Nutr. Res. 2007, 24, 106–109. [Google Scholar]
- Katayama, M.; Sugawa-Katayama, Y.; Yamaguchi, Y.; Murakami, K.; Hirata, S. Effect of temperature on the extraction of various arsenic compounds from dried Hijiki, Sargassum fusiforme by water-soaking as a pre-cooking process. Trace Nutr. Res. 2008, 25, 134–138. [Google Scholar]
- Gallagher, P.A.; Shoemaker, J.A.; Wei, X.; Brockhoff-Schwegel, C.A.; Creed, J.T. Extraction and detection of arsenicals in seaweed via accelerated solvent extraction with ion chromatographic separation and ICP-MS detection. Fresenius J. Anal. Chem. 2001, 369, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kamsonlain, S.; Balomajumder, C.; Chand, S. Studies on surface characterisation and isotherm modelling: Biosorption of arsenic(III) onto low cost biosorbent derived from orange peel. J. Sci. Ind. Res. Indian 2012, 71, 810–816. [Google Scholar]
- Muñoz, O.; Devesa, V.; Suñer, M.A.; Vélez, D.; Montoro, R.; Urieta, I.; Macho, M.L.; Jalón, M. Total and inorganic arsenic in fresh and processed fish products. J. Agric. Food Chem. 2000, 48, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Balboa, E.M.; Domínguez, H.; (Department of Chemical Engineering, University of Vigo, Ourense, Spain); Taboada, C.; (Department of Physiology, University of Santiago Compostela, Santiago de Compostela, Spain). Unpublished work. 2015.
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Sultana, V.; Ehteshamul-Haque, S.; Ara, J.; Athar, M. Effect of brown seaweeds and pesticides on root rotting fungi and root-knot nematode infecting tomato roots. J. Appl. Bot. Food Qual. 2012, 83, 50–53. [Google Scholar]
- Pérez-López, P.; Balboa, E.M.; González-García, S.; Domínguez, H.; Feijoo, G.; Moreira, M.T. Comparative environmental assessment of valorization strategies of the invasive macroalgae Sargassum muticum. Bioresour. Technol. 2014, 161, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Romaní, A.; Garrote, G.; Alonso, J.L.; Parajó, J.C. Bioethanol production from hydrothermally pretreated Eucalyptus globulus wood. Bioresour. Technol. 2010, 101, 8706–8712. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- De Quirós, A.R.B.; Frecha-Ferreiro, S.; Vidal-Perez, A.M.; López-Hernández, J. Antioxidant compounds in edible brown seaweeds. Eur. Food Res. Technol. 2010, 231, 495–498. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT and BHA. J. Agric. Food Chem. 1997, 45, 632–637. [Google Scholar] [CrossRef]
- Balboa, E.M.; Rivas, S.; Moure, A.; Domínguez, H.; Parajó, J.C. Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar. Drugs 2013, 11, 4612–4627. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balboa, E.M.; Moure, A.; Domínguez, H. Valorization of Sargassum muticum Biomass According to the Biorefinery Concept. Mar. Drugs 2015, 13, 3745-3760. https://doi.org/10.3390/md13063745
Balboa EM, Moure A, Domínguez H. Valorization of Sargassum muticum Biomass According to the Biorefinery Concept. Marine Drugs. 2015; 13(6):3745-3760. https://doi.org/10.3390/md13063745
Chicago/Turabian StyleBalboa, Elena M., Andrés Moure, and Herminia Domínguez. 2015. "Valorization of Sargassum muticum Biomass According to the Biorefinery Concept" Marine Drugs 13, no. 6: 3745-3760. https://doi.org/10.3390/md13063745
APA StyleBalboa, E. M., Moure, A., & Domínguez, H. (2015). Valorization of Sargassum muticum Biomass According to the Biorefinery Concept. Marine Drugs, 13(6), 3745-3760. https://doi.org/10.3390/md13063745