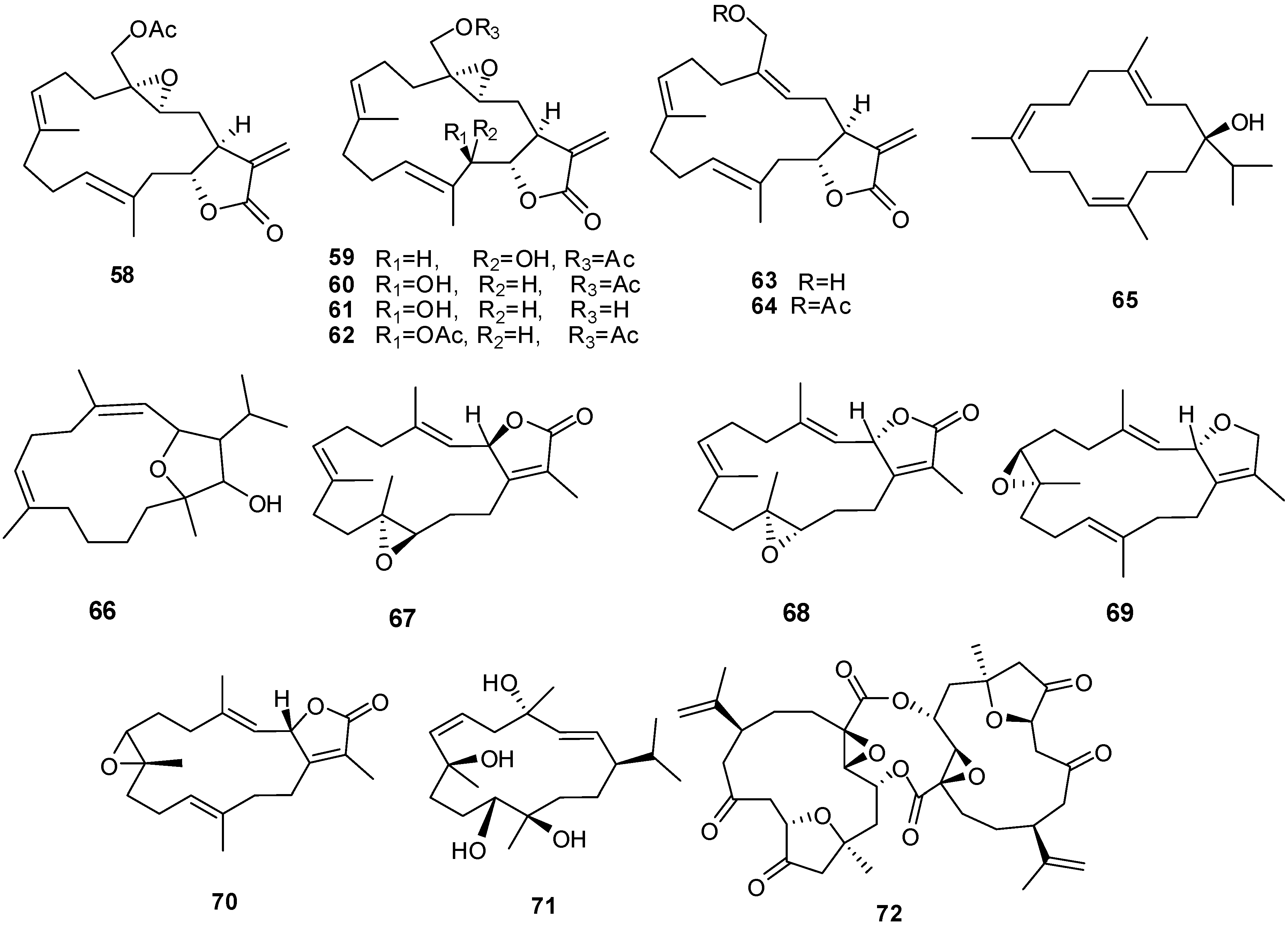
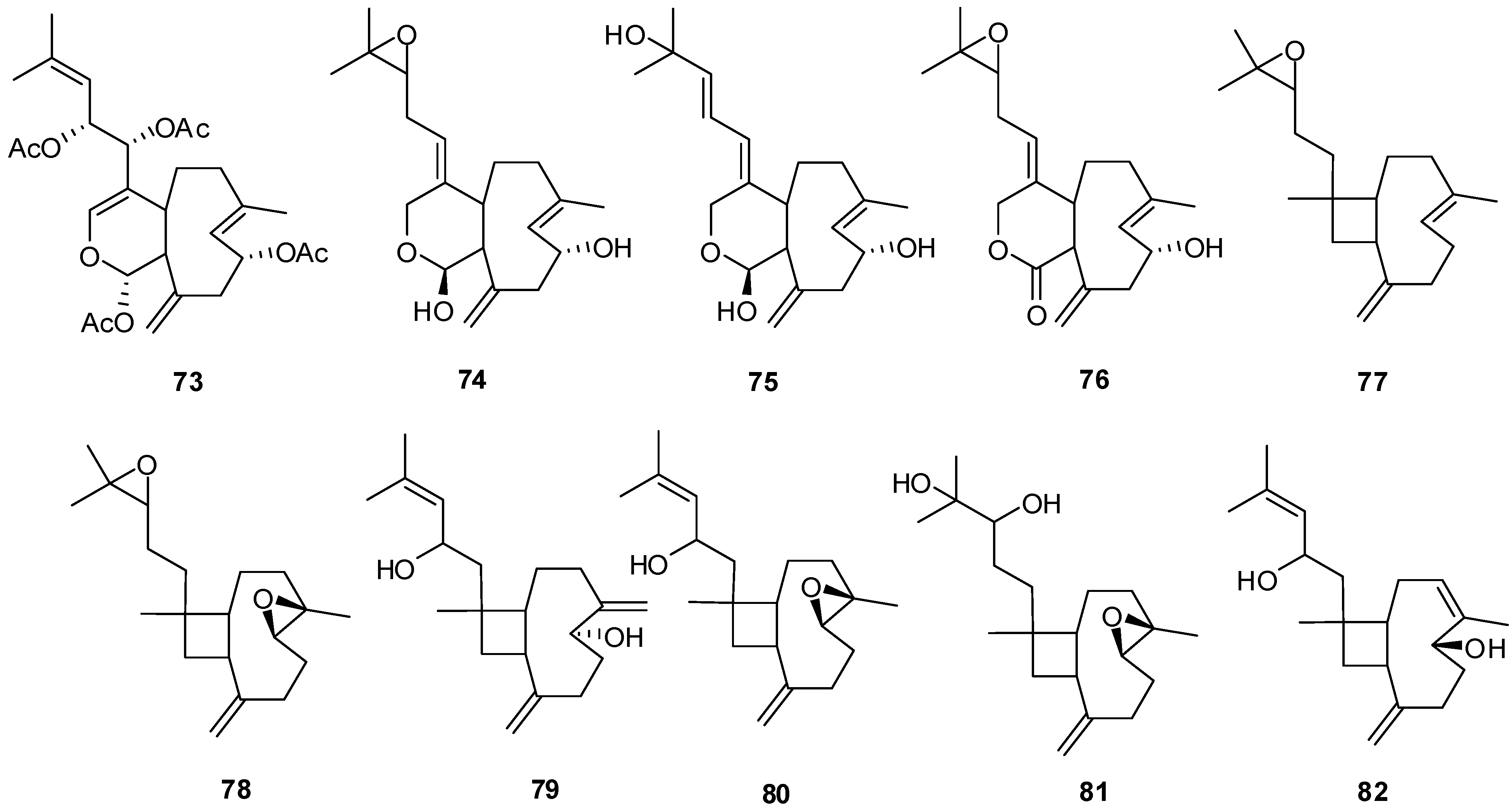
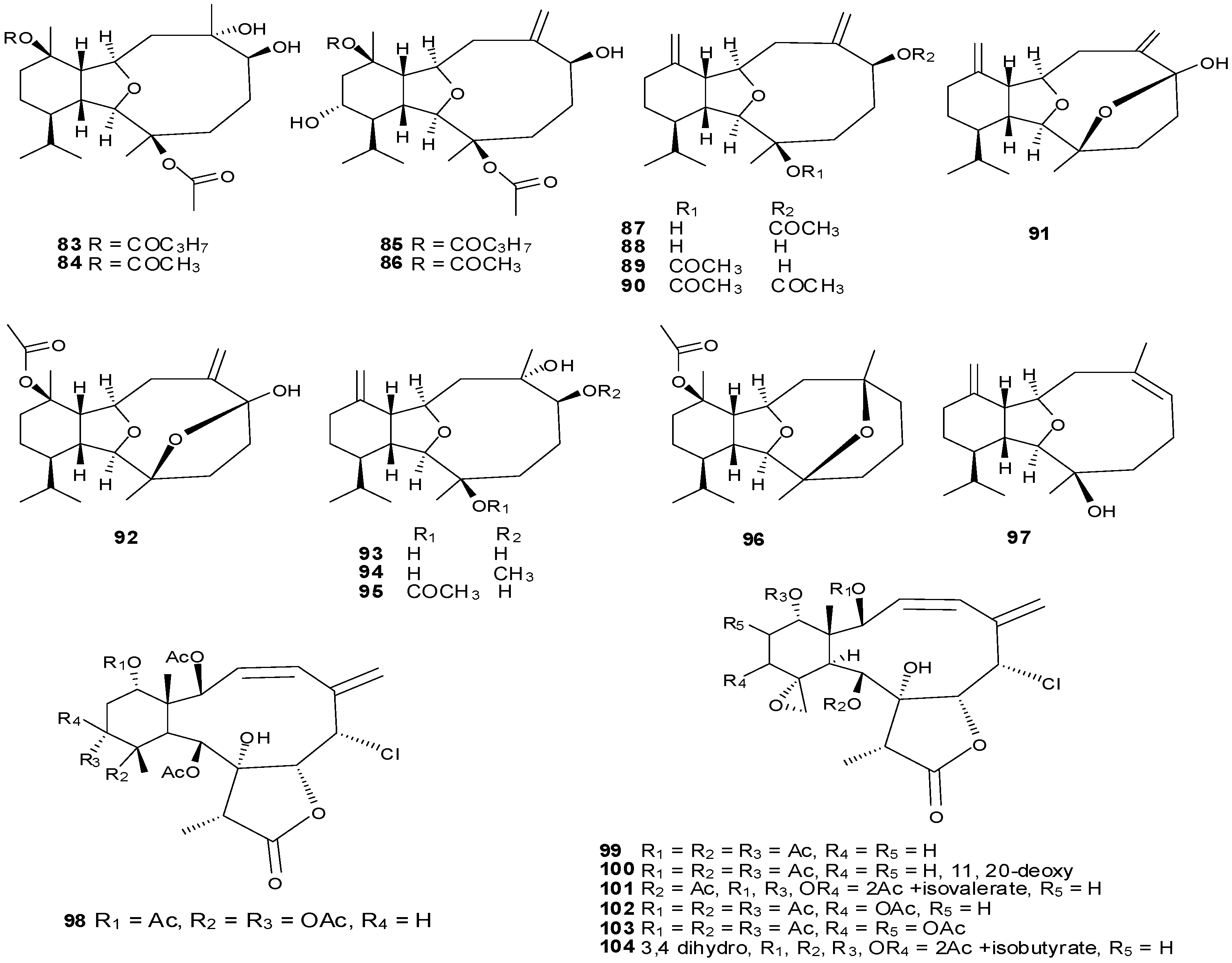
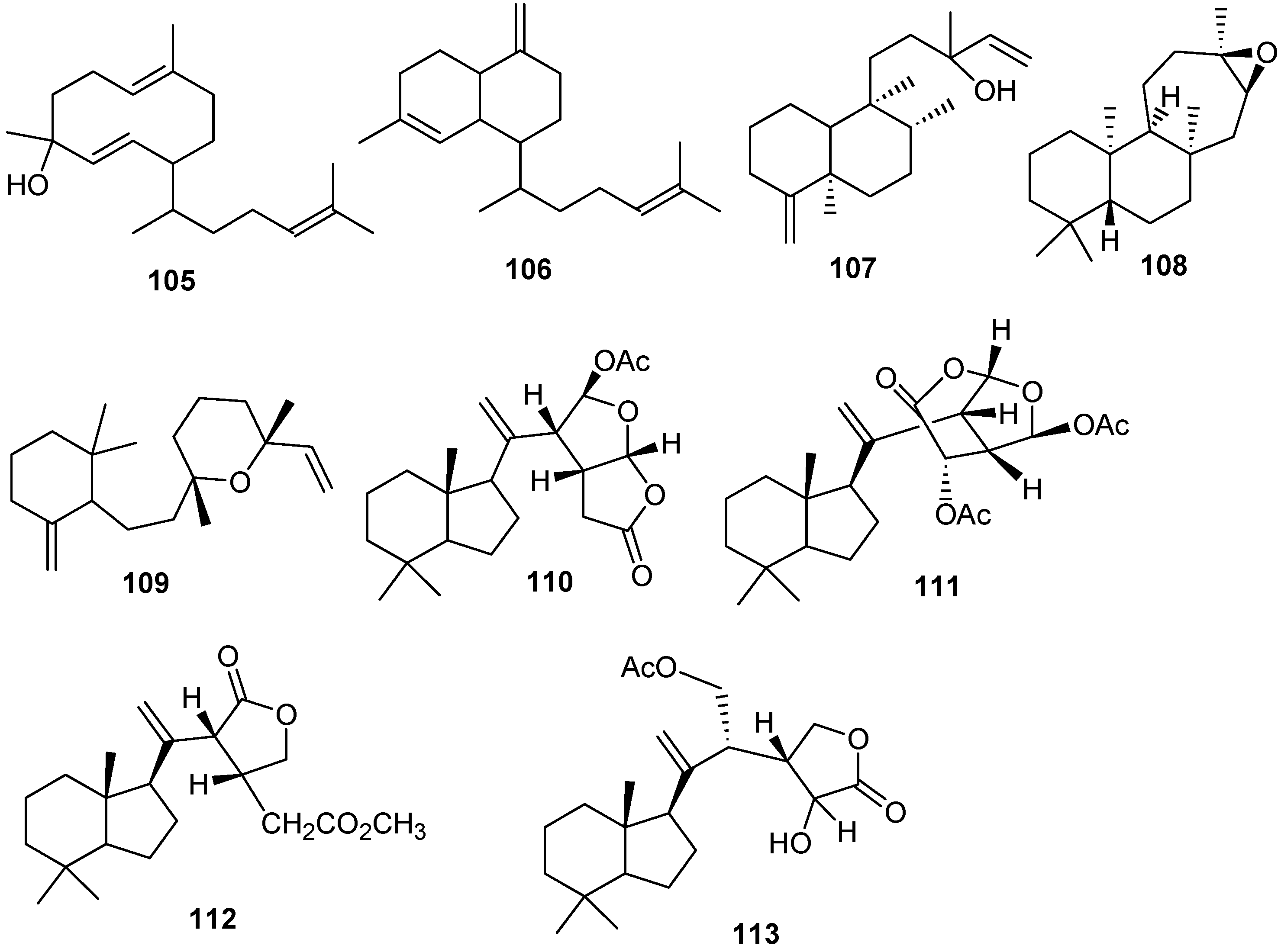
1. Red Sea Ecosystem
Marine ecosystems cover nearly 70% of the earth’s surface, averaging almost 4 km in depth and are proposed to contain over 80% of the world’s plant and animal species [
1]. Exact marine biodiversity is less certain since between one-third and two-thirds of marine organisms have yet to be described [
2]. Worldwide there are approximately 226,000 marine eukaryotes currently reported, while close to a million total species are estimated, based on calculations by marine biologists using statistical predictions [
2]. Considering that constituents from higher plants along with metabolites from terrestrial microorganisms have provided a substantial fraction of the natural-product-derived drugs currently used in Western medicine [
3], the potential to vastly expand the number and diversity of natural products by mining marine eukaryotes as well as associated prokaryotes from the richly diverse Red Sea ecosystem seems attainable. In fact, just within the past quarter century, the search for new marine metabolites has resulted in the isolation of upward of 10,000 compounds [
4], many of which exhibit biological activity. Despite the fact that marine biodiversity far exceeds that of terrestrial ecosystems, research of marine natural products as pharmaceutical agents, is still in its infancy. Factors that contribute to the gap between terrestrial and marine derived natural products include a paucity of ethno-medical history from marine sources as well as impediments associated with collecting, identifying and chemical analysis of marine materials [
5].
Notwithstanding, a combination of new diving techniques and implementation of remotely operated pods over the last decade has facilitated the characterization of marine-derived metabolites. This review encompasses secondary metabolites derived from marine invertebrates, a largely diverse group of fixed or sessile organisms, many in a stationary form although some are capable of slow primitive movement. While invertebrates lack physical defences such as a protective shell or spines, they are often rich in defence metabolites that can be utilized to attack prey or defend a habitat.
This review focused on a class of secondary defence metabolites abundant in marine invertebrates, five-carbon isoprenoid-derived terpenes. Extensive speciation from microorganisms to mammals can be attributed, at least in part to a wide range of temperatures (0 to 35 °C in arctic waters versus hydrothermal vents, respectively), pressures (1–1000 atm.), nutrient availabilities (oligotrophic to eutrophic) and lighting conditions that exist in this marine biome [
6]. The analysis will be limited to the Red Sea which is considered an epicenter for marine biodiversity with an extremely high endemic biota including over 50 genera of hermatypic coral. Indeed soft coral (Cnidaria: Anthozoa: Octocorallia), which are an important structural component of coral reef communities [
7,
8], are approximately 40% native to the Red Sea [
6]. The Red Sea, in which extensive reef formation occurs, is arguably the world’s warmest (up to 35 °C in summer) and most saline habitat (
ca. 40 psu in the northern Red Sea) [
6]. Despite the Red Sea’s size and diversity of reef-associated inhabitants (for examples, see
Figure 1), marine invertebrates in this ecosystem remain poorly studied compared to other large coral reef systems around the world such as the Great Barrier Reef or the Caribbean. This review will cover terpenes isolated from marine invertebrates of the Red Sea (
Figure 2) as well as identified biological activities for compounds reported during the time period from 1980 to 2014.
Figure 1.
Samples of marine invertebrate diversity from the Red Sea including (from left to right starting at the top left corner) Sarcophyton glaucum, S. regulare, S. ehrenbergi, Nephthea molle, Acropora humilis, Porites solida, Pocillopora verrucosa, Clothraria rubrinoidis and Cystoseira trinode. Marine species exhibit greater phyta diversity than land species.
Figure 1.
Samples of marine invertebrate diversity from the Red Sea including (from left to right starting at the top left corner) Sarcophyton glaucum, S. regulare, S. ehrenbergi, Nephthea molle, Acropora humilis, Porites solida, Pocillopora verrucosa, Clothraria rubrinoidis and Cystoseira trinode. Marine species exhibit greater phyta diversity than land species.
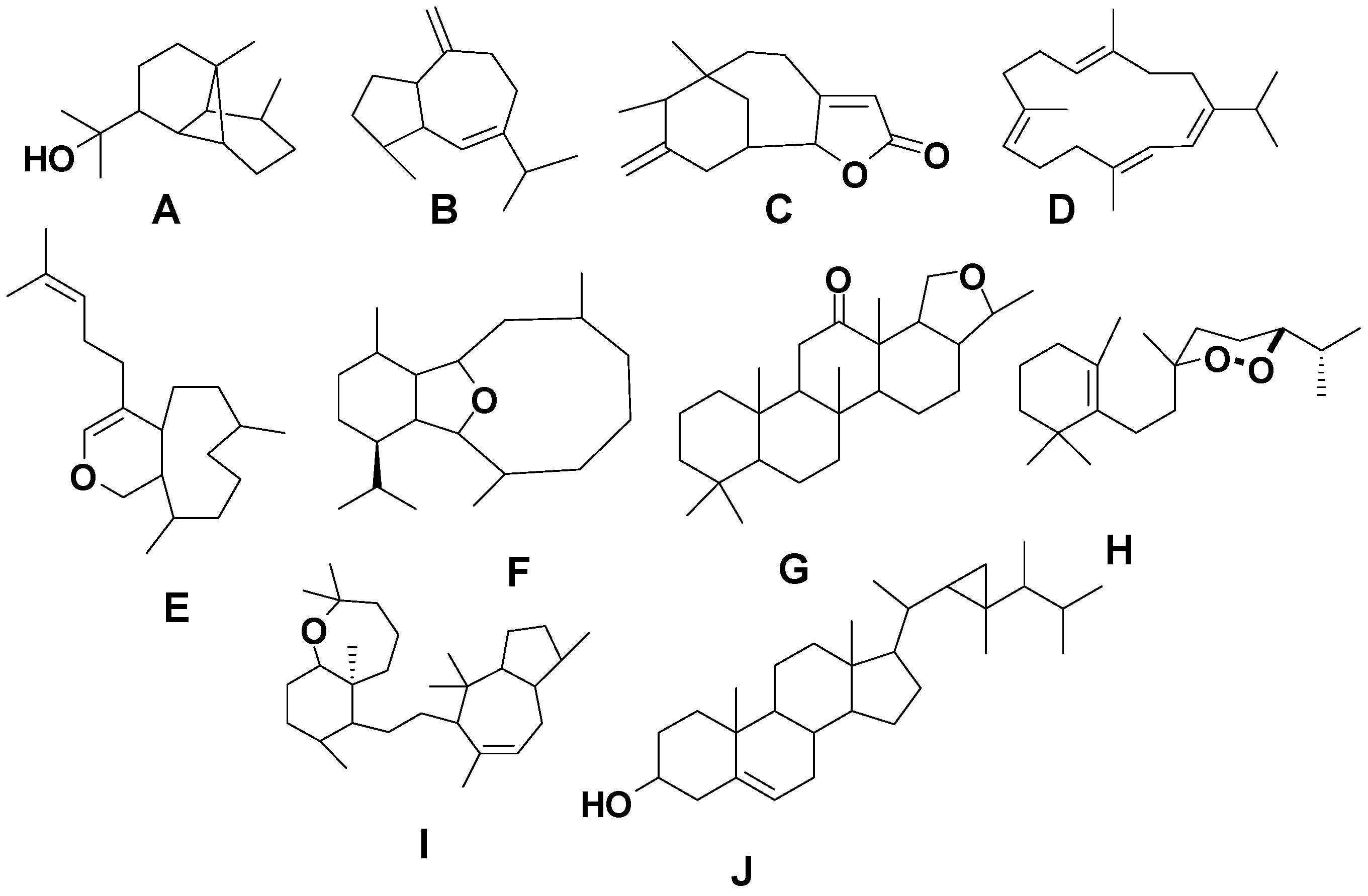
Figure 2.
Terpene skeletal types including ylangene (A), aromadendrane (B), tricycle-[6,7,5]-sesquiterpene (C), cembrane (D), xeniolide and xeniaphyllane (E), eunicellin diterpene (F), sesterterpene (G), norsesterterpene (H), triterpene (I) and steroid (J) types.
Figure 2.
Terpene skeletal types including ylangene (A), aromadendrane (B), tricycle-[6,7,5]-sesquiterpene (C), cembrane (D), xeniolide and xeniaphyllane (E), eunicellin diterpene (F), sesterterpene (G), norsesterterpene (H), triterpene (I) and steroid (J) types.
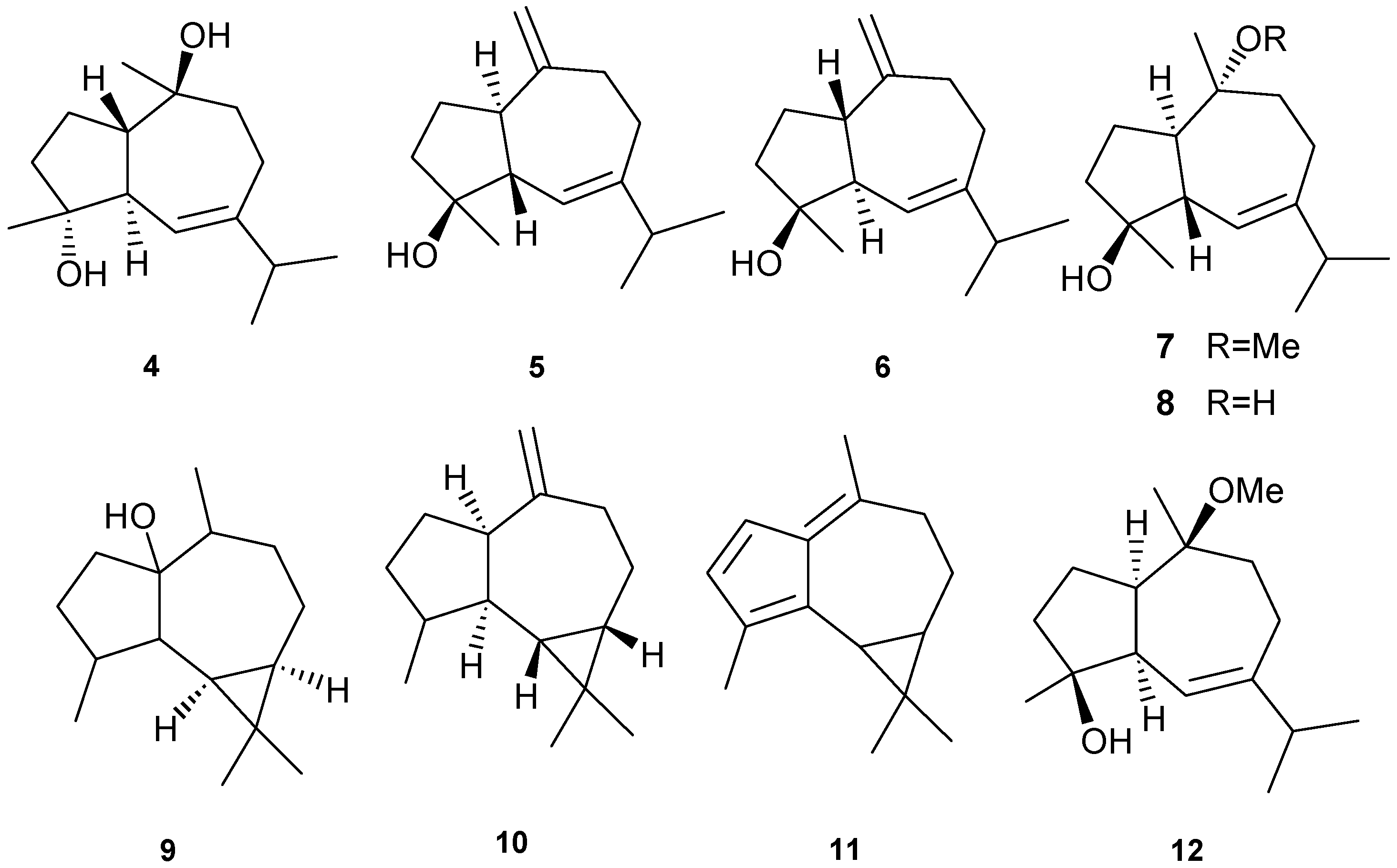
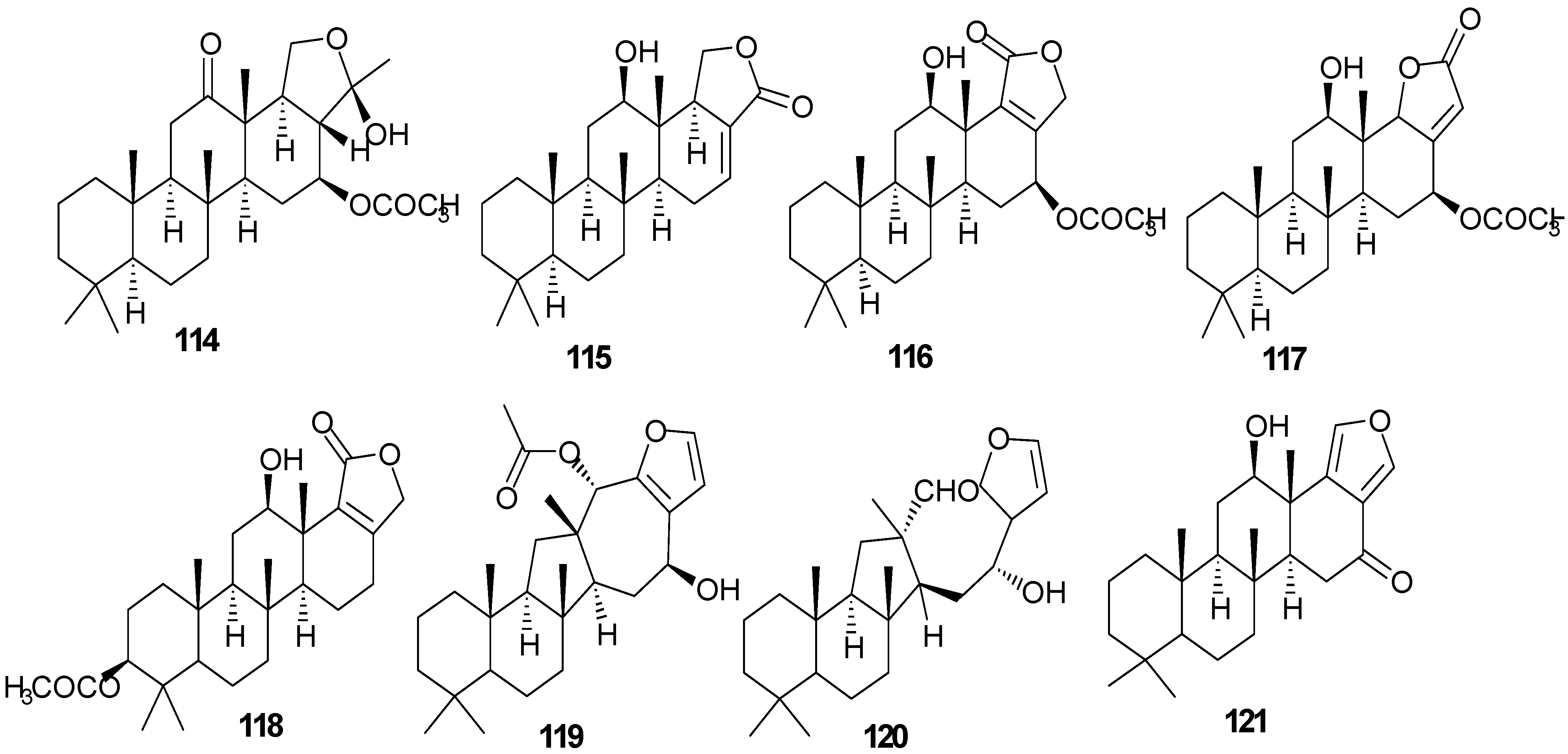
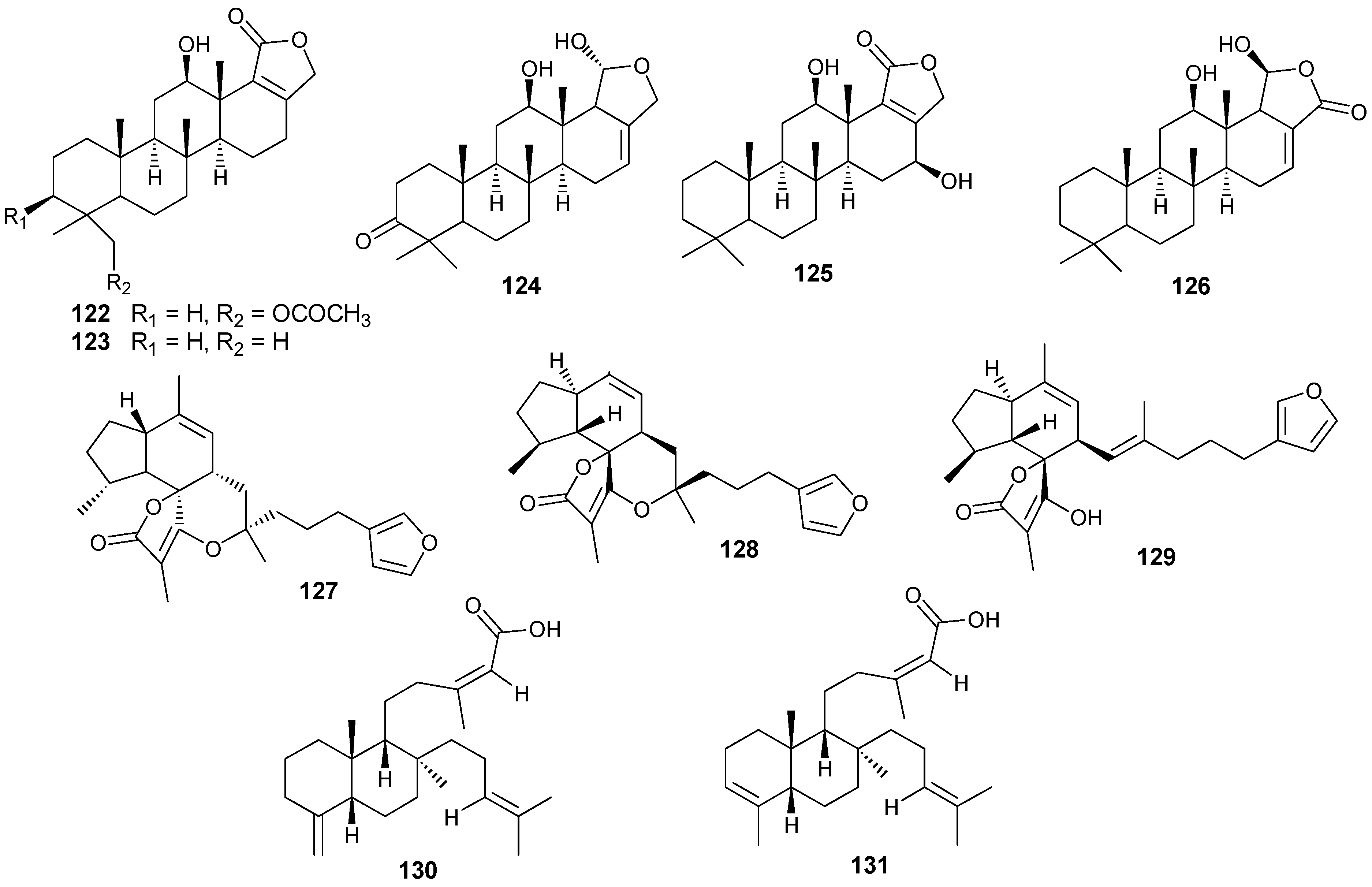
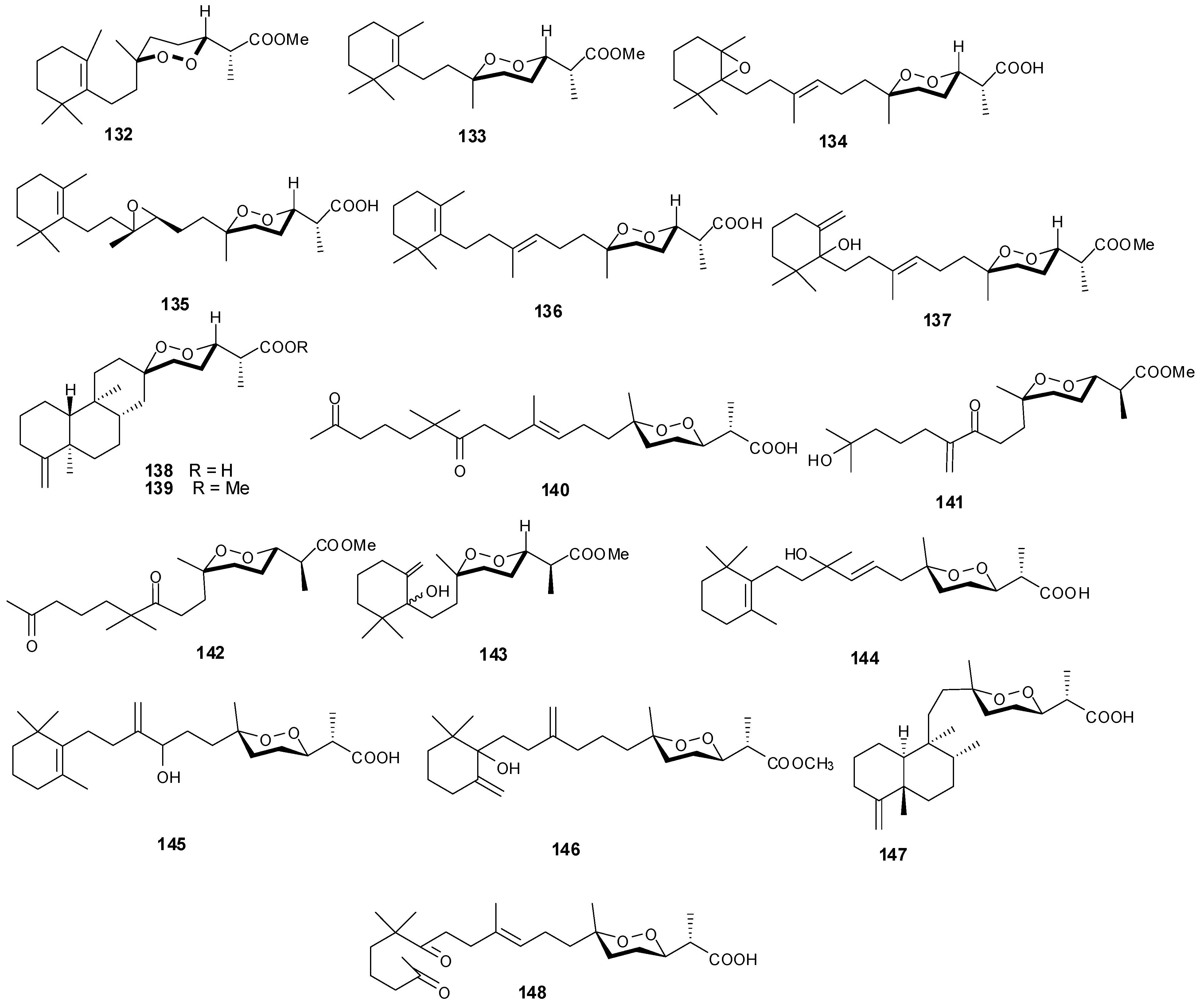
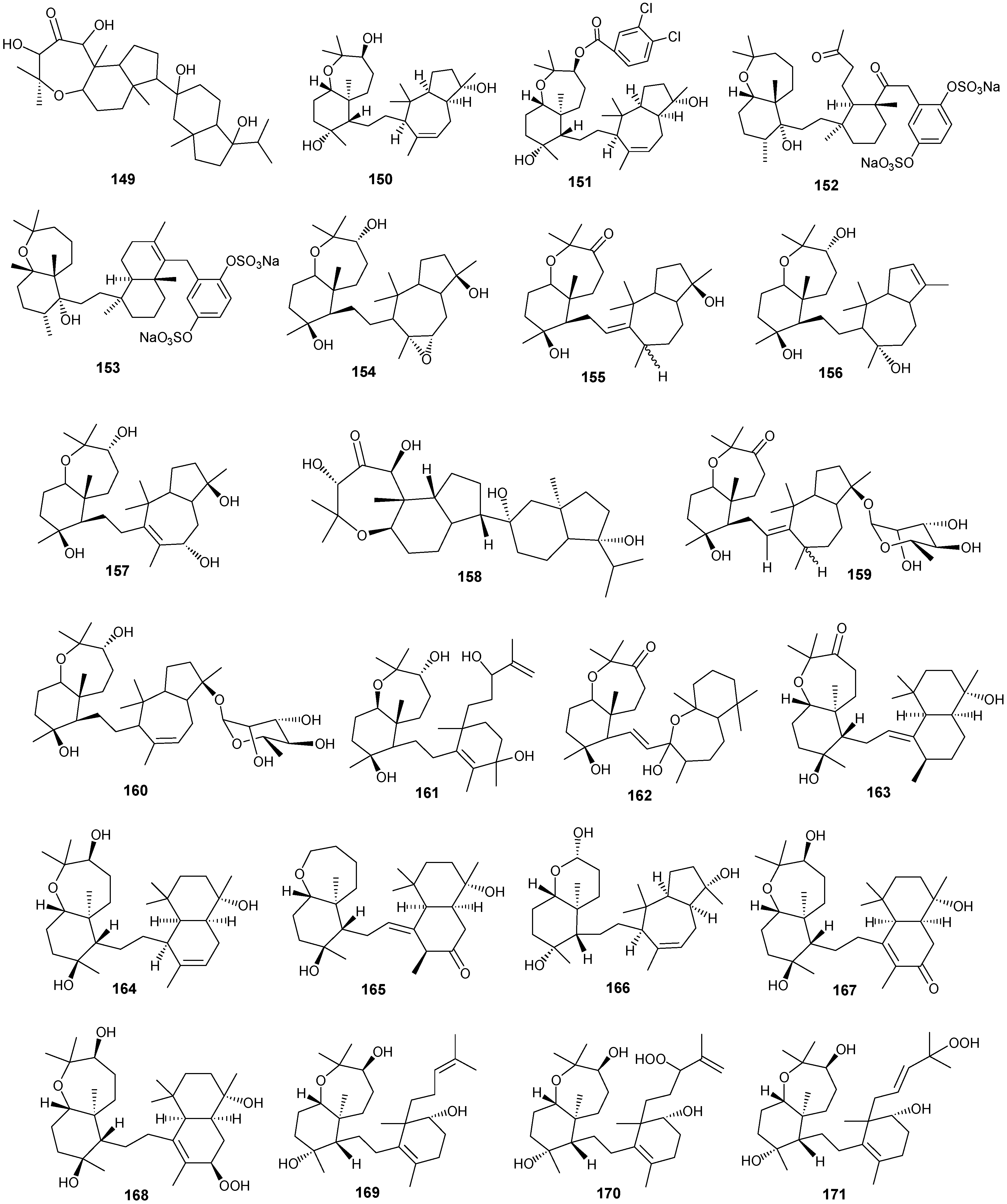
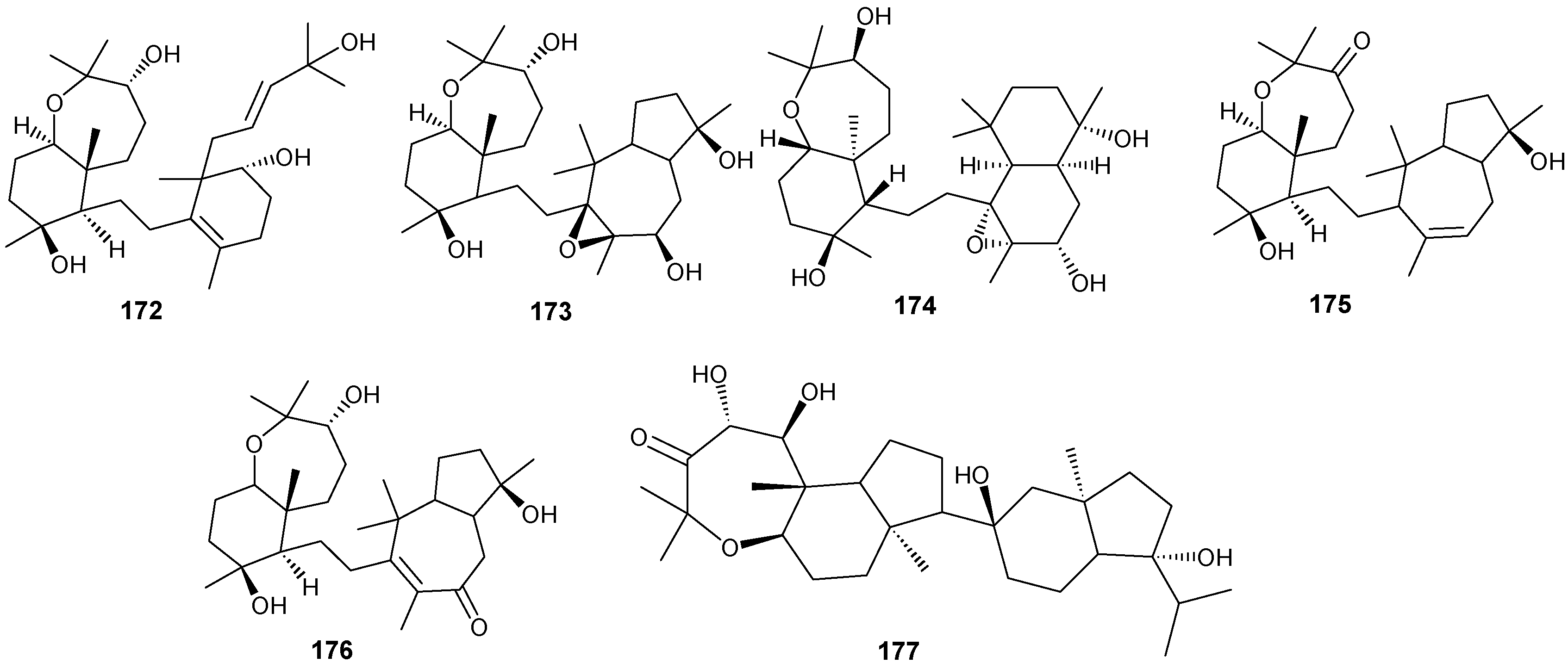
5. Triterpenes
Structurally diverse triterpenes are widespread in Red Sea sponges with examples shown in
Table 9 and
Figure 12. Compound
149 inhibits growth of human breast cancer cells, MDA-MB-231, MCF-7, BT-474 and T-47D, in a dose-dependent manner [
46,
47]. Triterpenes have also been studied for their efficacy in reducing the appearance of drug resistance. In the presence of many cytotoxic drugs, resistant cell variants appear, a process referred to as multidrug resistance (MDR). Overexpression of the ATP-binding cassette (ABC) transporter ABCB1/P-glycoprotein (P-gp) is one of the most common causes of MDR in cancer cells. P-gp a 170-kD transmembrane glycoprotein functions as a drug efflux pump that extrudes a wide spectrum of compounds including amphipathic and hydrophobic drugs. Sipholane triterpenoids can serve as P-gp inhibitors and are being developed to enhance the effect of chemotherapeutic drugs with MDR cancer cells
in vitro and
in vivo [
33,
36]. Compounds
162–
163 enhanced cytotoxicity of several P-gp substrate-anticancer drugs, including colchicine, vinblastine and paclitaxel. These sipholane triterpenes significantly reversed the MDR-phenotype in P-gp-over expressing MDR cancer cells, KB-C2, in a dose-dependent manner. Moreover, these sipholanes have no effect on the response to cytotoxic agents in cells lacking P-gp expression or expressing MRP1 (ABCC1) or MRP7 (ABCC10) or with the breast cancer resistance protein (BCRP/ABCG2). Perhaps most importantly, these sipholanes with a low IC
50 of
ca. 50 μM are not toxic to the assayed cell lines [
48].
Table 9.
Triterpenes, sources and activities.
Table 9.
Triterpenes, sources and activities.
| No. | Name | Source | Activity |
|---|
| 149 | Neviotine-A [46,47] | Siphonochalina siphonella | |
| 150 | Sipholenol A [47,49,50,51,52,53] | S. siphonella | anti-tumor |
| 151 | SipholenolA-4-O-3′,4′-dichlorobenzoate [49] | S. siphonella | |
| 152 | Shaagrockol B [54] | Toxiclona toxius | |
| 153 | Shaagrockol C [54] | T. toxius | |
| 154 | Sipholenol G [55] | S. siphonella | |
| 155 | Sipholenone D [55] | S. siphonella | |
| 156 | Sipholenol F [55] | S. siphonella | |
| 157 | Sipholenol H [55] | S. siphonella | |
| 158 | Neviotine B [55] | S. siphonella | |
| 159 | Sipholenoside A [55] | S. siphonella | |
| 160 | Sipholenoside B [55] | S. siphonella | |
| 161 | Siphonellinol B [55] | S. siphonella | |
| 162 | Dahabinone A [55] | S. siphonella | |
| 163 | Sipholenone E [51] | S. siphonella | anti-tumor |
| 164 | Sipholenol L [47,51] | S. siphonella | anti-tumor |
| 165 | Sipholenol J [51] | S. siphonella | |
| 166 | (2S,4aS,5S,6R,8aS)-5-(2-((1S,3aS,5R,8aS,Z)-1-hydroxy-1,4,4,6-tetramethyl-1,2,3,3a,4,5,8,8a-octahydroazulen-5-yl)-ethyl)-4a,6-dimethyloctahydro-2H-chromene-2,6-diol [51] | S. siphonella | |
| 167 | Sipholenol K [51] | S. siphonella | |
| 168 | Sipholenol M [51] | S. siphonella | |
| 169 | Siphonellinol D [51] | S. siphonella | |
| 170 | Siphonellinol E [51] | S. siphonella | |
| 171 | Siphonellinol-C-23-hydroperoxide [51] | S. siphonella | |
| 172 | Siphonellinol C [56] | S. siphonella | |
| 173 | epi-Sipholenol I [56] | S. siphonella | |
| 174 | Sipholenol I [51] | S. siphonella | |
| 175 | Sipholenone A [56,47] | S. siphonella | |
| 176 | Sipholenol D [52] | S. siphonella | |
| 177 | Neviotine-C [47] | Siphonochalina siphonella | cytotoxic |
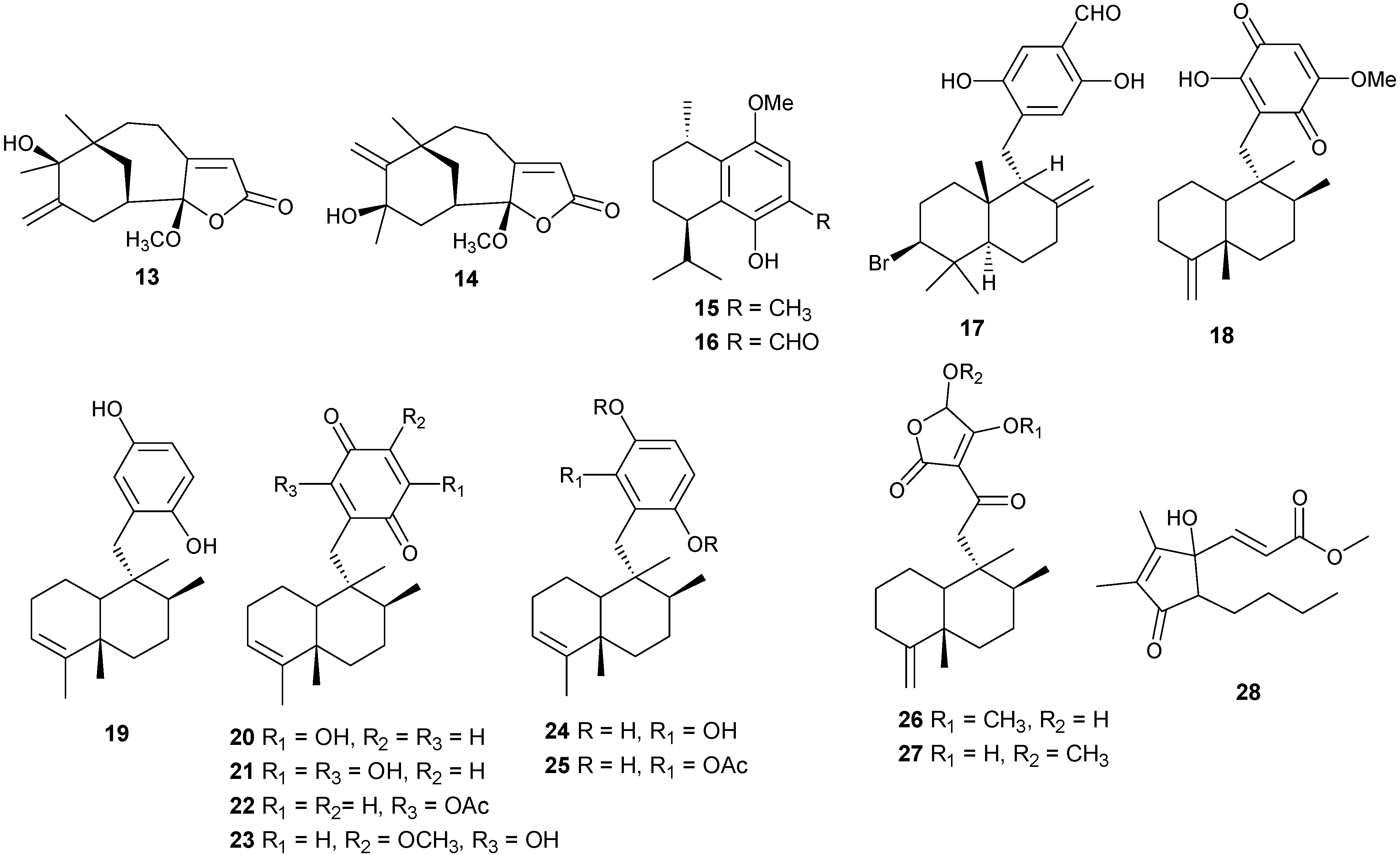
Figure 12.
Structures of triterpenes (149–177).
Figure 12.
Structures of triterpenes (149–177).
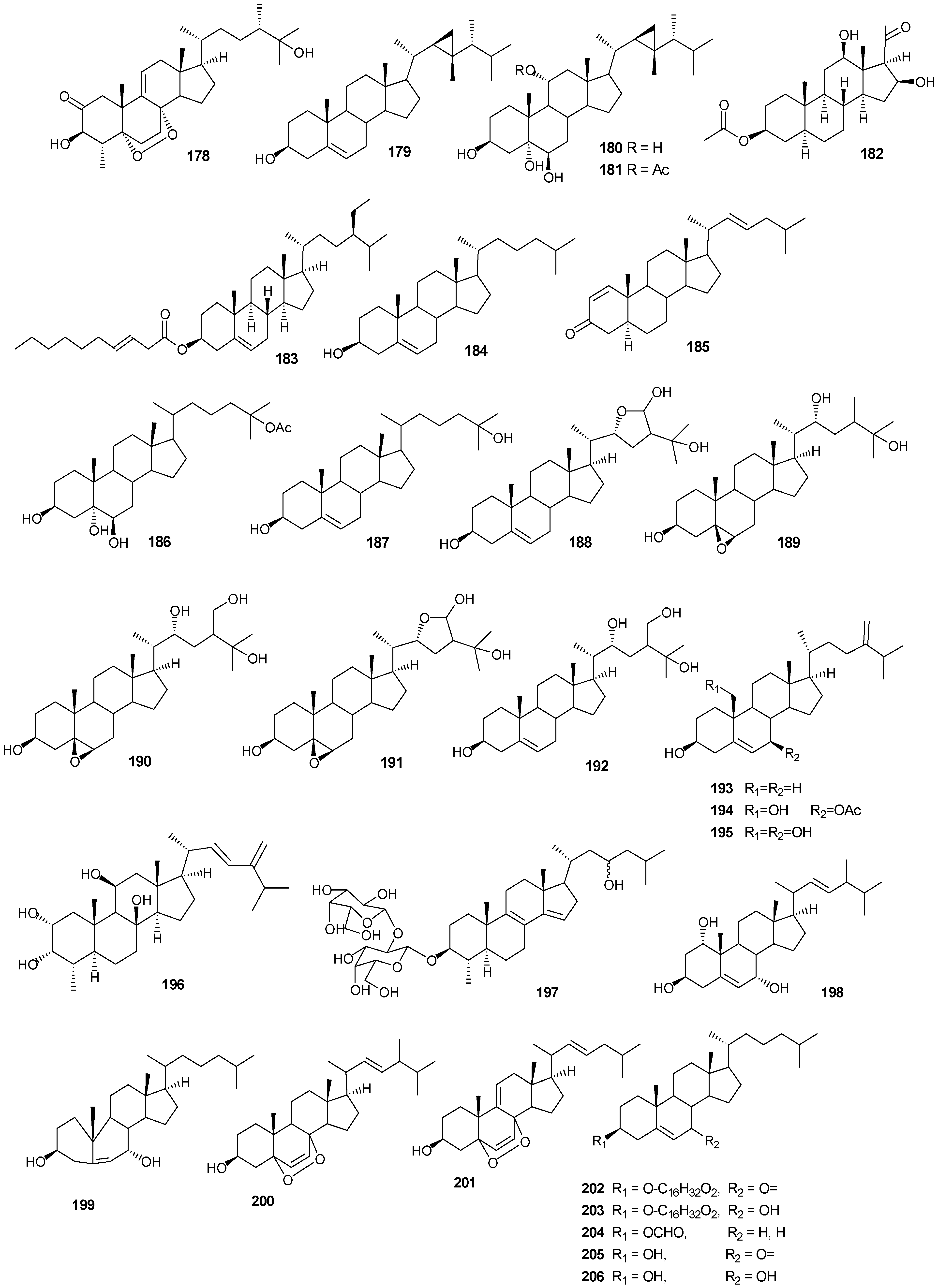
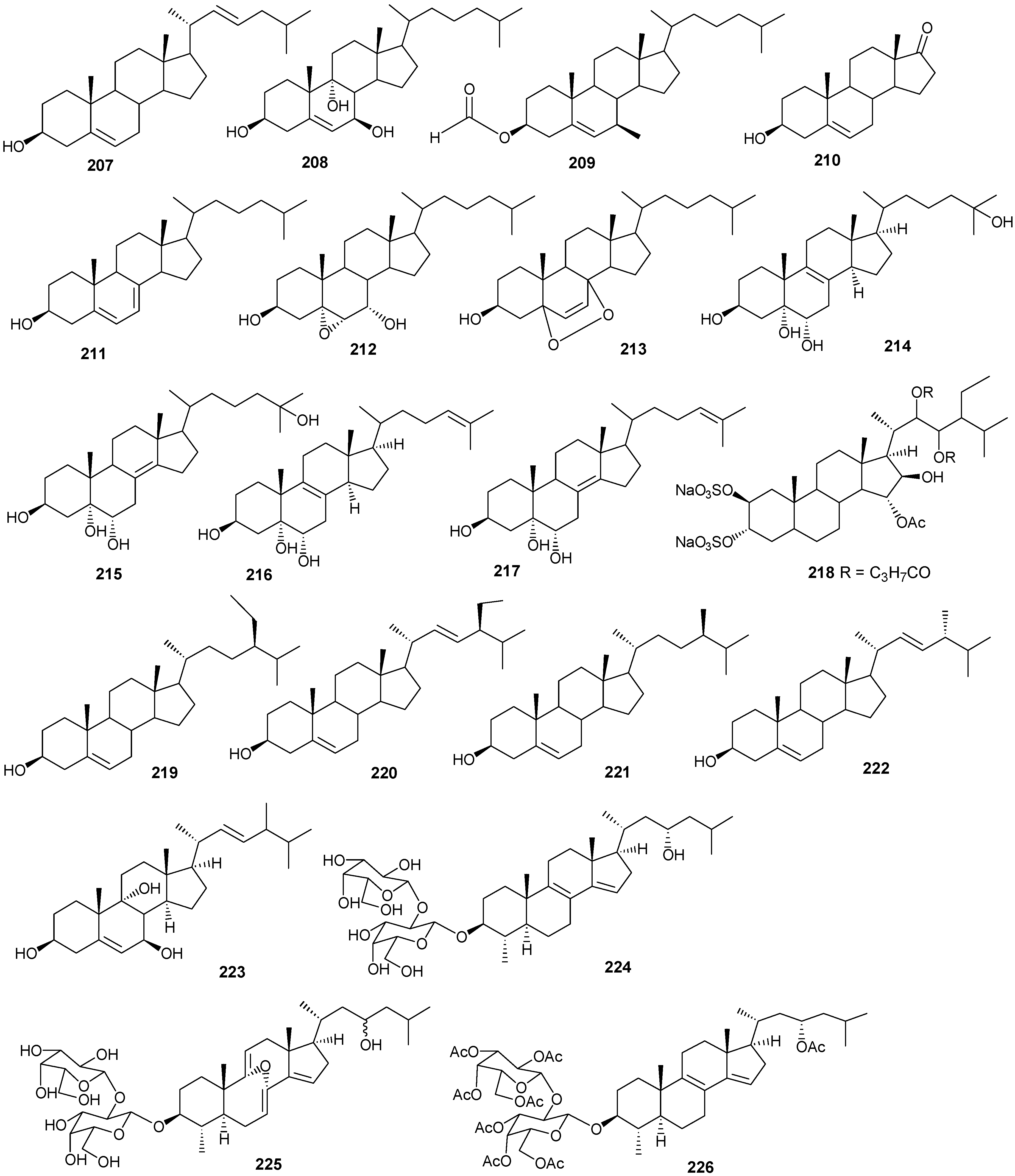
6. Steroids
Steriods are widespread throughout the marine biome with recent chemical reports including soft coral (
Sinularia candidula,
S. polydactyla,
Heteroxenia ghardaqensis,
Dendronephthya sp
.,
Lobophytom depressum and
Litophyton arboreum) [
9,
11,
24,
57,
58,
59,
60], black coral (
Antipathes dichotoma) [
38,
40], and sponges (
Echinoclathria gibbosa,
Hyrtios sp.,
Erylus sp., and
Petrosia sp.) [
18,
64,
65,
66]. Steroid examples are shown in
Table 10 and
Figure 13.
Table 10.
Steroids, sources and activities.
Table 10.
Steroids, sources and activities.
| No. | Name | Source | Activity |
|---|
| 178 | 3β-25-Dihydroxy-4-methyl-5α,8α-epidioxy-2-ketoergost-9-ene [57] | Sinularia candidula | anti-viral |
| 179 | Gorgosten-5(E)-3β-ol [58] | Heteroxenia ghardaqensis | anti-tumor |
| 180 | Gorgostan-3β,5α,6β,11α-tetraol (sarcoaldosterol A) [58] | H. ghardaqensis | |
| 181 | Gorgostan-3β,5α,6β-triol-11α-acetate [58] | H. ghardaqensis | |
| 182 | 5α-Pregna-3β-acetoxy-12β,16β-diol-20-one [59] | Echinoclathria gibbosa | anti-tumor |
| 183 | β-Sitosterol-3-O-(3Z)-pentacosenoate [59] | E. gibbosa | anti-tumor |
| 184 | Cholesterol [9] | Dendronephthya | |
| 185 | Dendronesterone A [9] | Dendronephthya | |
| 186 | 24-Methylcholestane-3β,5α,6β,25-tetrol-25-monoacetate [24] | Sinularia polydactyla | anti-tumor |
| 187 | 24-Methylcholestane-5-en-3β,25-diol [24] | S. polydactyla | antimicrobial |
| 188 | Lobophytosterol [60] | L. depressum | |
| 189 | 5β,6β-Epoxy-24E-methylchloestan-3β,22(R),25-triol [60] | L. depressum | |
| 190 | Depresosterol [60] | L. depressum | |
| 191 | (22R,24E,28E)-5β,6β-Epoxy-22,28-oxido-24-methyl-5α-cholestan-3β,25,28-triol [60] | L. depressum | |
| 192 | (22R,24E)-24-Methylcholest-5-en-3β,22,25,28-tetraol [60] | L. depressum | |
| 193 | 24-Methylcholesta-5,24(28)-diene-3β-ol [11] | Litophyton arboreum | |
| 194 | 7β-Acetoxy-24-methylcholesta-5-24(28)-diene-3,19-diol [11] | L. arboreum | cytotoxic |
| 195 | 24-Methylcholesta-5,24(28)-diene-3β,7β,19-triol [11] | L. arboreum | |
| 196 | Hyrtiosterol [16] | Hyrtios Species | |
| 197 | Eryloside A [61,62,63] | Genus Erylus | cytotoxic |
| 198 | (22E)-Methylcholesta-5,22-diene-1α,3β,7α-triol [64] | Antipathes dichotoma | anti-bacterial |
| 199 | 3β,7α-Dihydroxy-cholest-5-ene [64] | A. dichotoma | anti-bacterial |
| 200 | (22E,24S)-5α,8α-Epidioxy-24 methylcholesta -6,22-dien-3β-ol [64] | A. dichotoma | anti-bacterial |
| 201 | (22E,24S)-5α,8α-Epidioxy-24-methylcholesta-6,9(11),22-trien-3β-ol [64] | A. dichotoma | anti-bacterial |
| 202 | 3β-Hexadecanoylcholest-5-en-7-one [65] | A. dichotoma | anti-tumor |
| 203 | 3β-Hexadecanoylcholest-5-en-7β-ol [65] | A. dichotoma | anti-tumor |
| 204 | Cholest-5-en-3β-yl-formate [65] | A. dichotoma | anti-tumor |
| 205 | 3β-Hydroxycholest-5-en-7-one [65] | A. dichotoma | |
| 206 | Cholest-5-en-3β,7β-diol [65] | A. dichotoma | |
| 207 | 22-Dehydrocholestrol [65] | A. dichotoma | |
| 208 | 3β,7β,9α-Trihydroxycholest-5-en [66] | Petrosia | cytotoxic (anti-tumor) |
| 209 | Cholest-5-en-7β-methyl-3β-yl formate [66] | Petrosia sp. | cytotoxic (anti-tumor) |
| 210 | Dehydroepiandrosterone [66] | Petrosia sp. | cytotoxic (anti-tumor) |
| 211 | 7-Dehydrocholesterol [66] | Petrosia sp. | cytotoxic (anti-tumor) |
| 212 | 5α,6α-Epoxycholest-8(14)-ene-3β,7α-diol [66] | Petrosia sp. | cytotoxic (anti-tumor) |
| 213 | 5α,8α-Epidioxycholesta-6-en-3β-ol [66] | Petrosia sp. | cytotoxic (anti-tumor) |
| 214 | Cholesta-8-en-3β,5α,6α,25-tetrol [67] | Lamellodysidea herbacea | |
| 215 | Cholesta-8(14)-en-3β,5α,6α,25-tetrol [67] | L. herbacea | |
| 216 | Cholesta-8,24-dien-3β,5α,6α-triol [67] | L. herbacea | anti-fungal |
| 217 | Cholesta-8(14),24-dien-3β,5α,6α-triol [67] | L. herbacea | anti-fungal |
| 218 | Clathsterol [68] | Clathria sp. | |
| 219 | Clionasterol [69] | Dragmacidon coccinea | |
| 220 | Stigmasterol [69] | D. coccinea | |
| 221 | Campesterol [69] | D. coccinea | |
| 222 | Brassicasterol [69] | D. coccinea | |
| 223 | Dendrotriol [70] | Dendronephthya hemprichi | |
| 224 | Erylosides K [62] | Erylus lendenfeldi | |
| 225 | Erylosides L [62] | E. lendenfeldi | |
| 226 | Erylosides B [63] | E. lendenfeldi | |
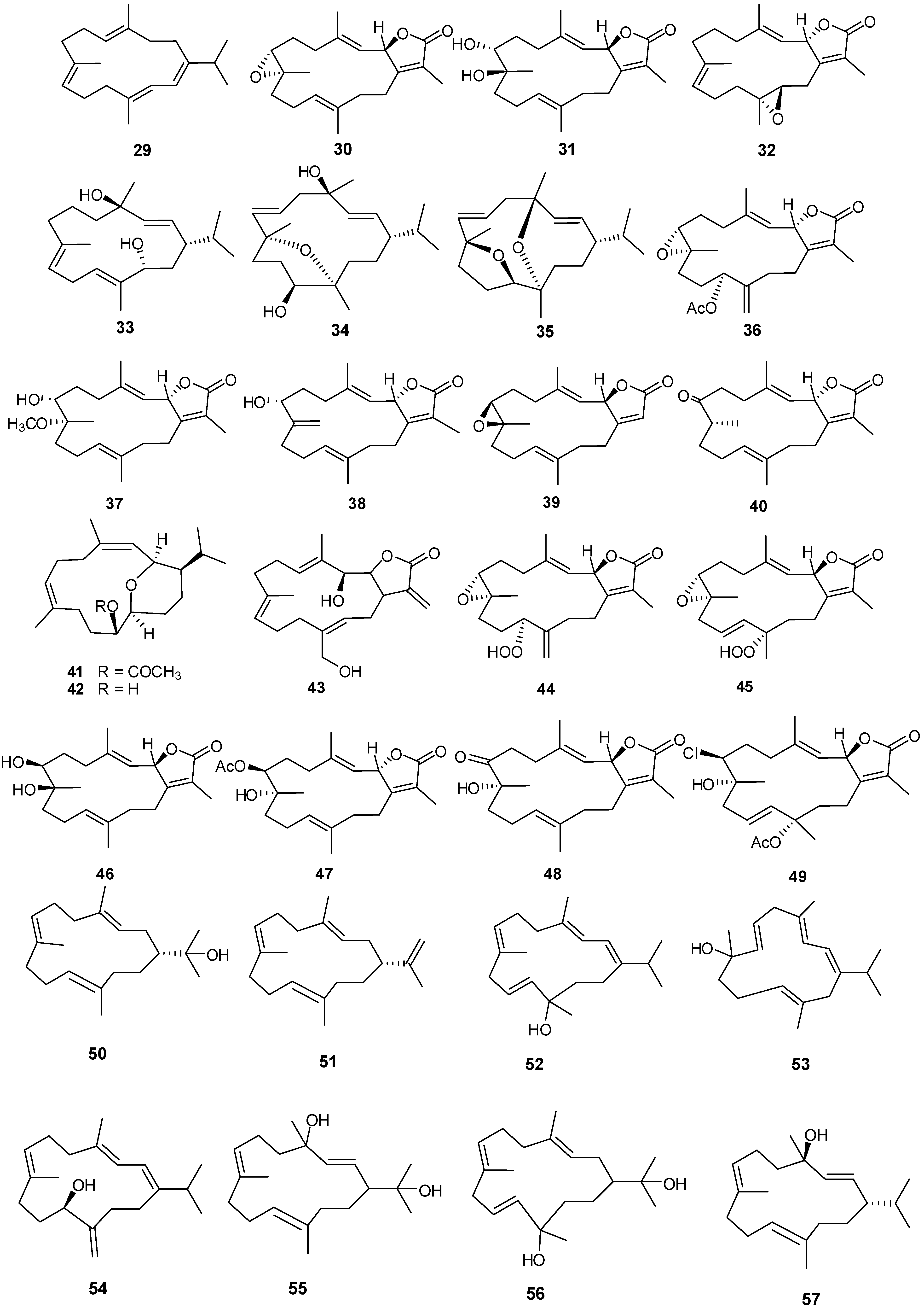
Figure 13.
Steriod structures (178–226).
Figure 13.
Steriod structures (178–226).
Moderate growth inhibition for a human colon tumor cell line was observed with
180 [
58]. Compounds
184 and
186 exhibited activity against three human tumor cell lines including the lung non-small cell line A549, the glioblastoma line U373 and the prostate line PC-3 [
59]. Compound
187 showed an IC
50 of 6.1 and 8.2 μg/mL against the human cancer cell lines HepG2 and HCT, respectively [
24].
Compounds
204–
207 show antibacterial activity against Gram-positive (
Bacillus subtilis) and Gram-negative bacteria (
Pseudomonas aeruginosa), at a 1 μg/ml concentration [
64]. Compounds
208–
210 exhibited antitumor activity based on four cancer panels: HepG2, WI 38, VERO, and MCF-7 [
65]. Compounds
215–
220 exhibited cytotoxic effects in the tumor cell lines, HepG2 and MCF-7 with IC
50 in the range of 20-500 μM. Interestingly,
217 showed the highest affinity to DNA with IC
50 30 μg/mL [
66]. Compounds
223 and
224 showed antifungal activity against
Candida tropicalis, with petri dish inhibition at 10 μg/disc [
67].
7. Drug Leads
Even though terpenes are the largest group of natural products with over 25,000 structures thus far reported, a small subset of these metabolites have been investigated for biological function and/or activity. Basic biological constituents such as membrane components, hormones, antioxidants and chemical defenses require the isoprenoid building module. Future chemical studies of marine organisms are expected to generate an ensemble of novel terpenes based on progressive knowledge on enzymatic machinery and selective pressures under which such organisms have evolved. The expanding chemo-diversity of marine terpenes is being assisted in part by advanced analytical chemistry methods for structure determination and sophisticated diving techniques for sample collection.
Methods for assaying for
in vitro biological activity can be more variable in terms of stardardized protocols. The same positive or native chemical controls are not always utilized making direct comparisons of biological activity between different testing laboratories unreliable or at least not reproducible. Moreover, with the paucity of ethnomedical knowledge from marine sources, the basis for selecting the most promising bioassay can be more of an art than a science. The screening for anti-cancer activity in facilities such as the National Cancer Institute (NCI) Chemotherapeutic Agents Repository operated by Fisher BioServices [
71] can provide invaluable, cost-free, sensitive screening of hits against multiple-target 60 cancer cell line panels, broadening the opportunity to conduct more comprehensive and mechanistic studies. In the case where a set of metabolites has already been identified possessing a given biological activity, computational,
in silico, and pharmacophore modeling can guide future design of druggable analogues with better biological activity, without expected toxicity, even if the structural characterization of the biological target(s) is/are not feasible. Such virtual models utilizes steric and electronic descriptors to identify pharmacophoric features such as hydrophobic centroids, aromatic rings, hydrogen bonding acceptors/donors and cation/anion interactions to match optimal supramolecular interactions with a specific biological target that triggers or blocks a response. Functional group properties can also be identified for the rational semi-synthetic design of biologically active marine natural scaffolds. Strategies such as the Topliss scheme designate a series of substituents based on lipophilic, electronic and steric properties to generate multiple analogues with slight controlled chemical property differences that can be used for comprehensive structure-activity studies to obtain superior biological activity relative to the parent natural product. While these techniques and tools are not distinct or exclusive for exploring marine sources and marine-derived natural products, such methods can be effective for enhancing biological activity. For example, sipholenol A is a noteworthy example of developing a marine metabolite using medicinal chemistry approaches to generate biologically active analogue libraries [
49]. These natural product examples with exceptional biological potency outcomes (IC
50 in the low μM range for invasive breast cancer) demonstrate the potential of marine natural products for the discovery of future novel druggable entities useful for the control and management of human diseases.