Myrothecols G and H, Two New Analogues of the Marine-Derived Quinone Sesquiterpene Penicilliumin A
Abstract
:1. Introduction
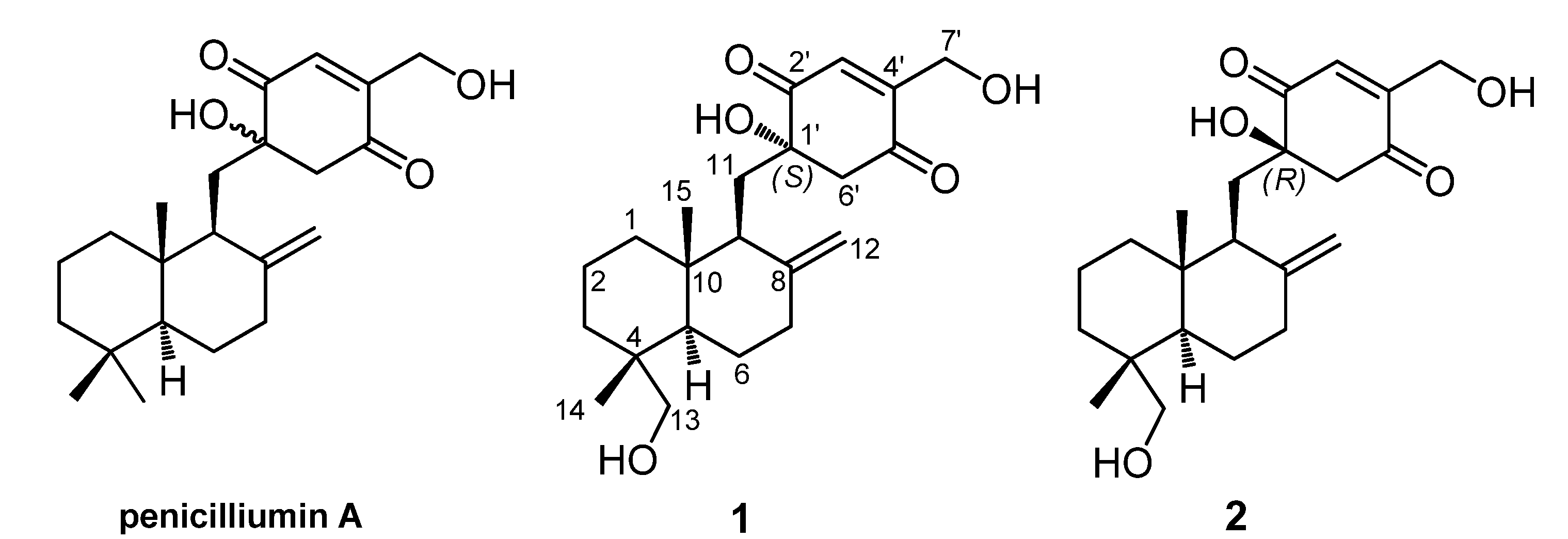
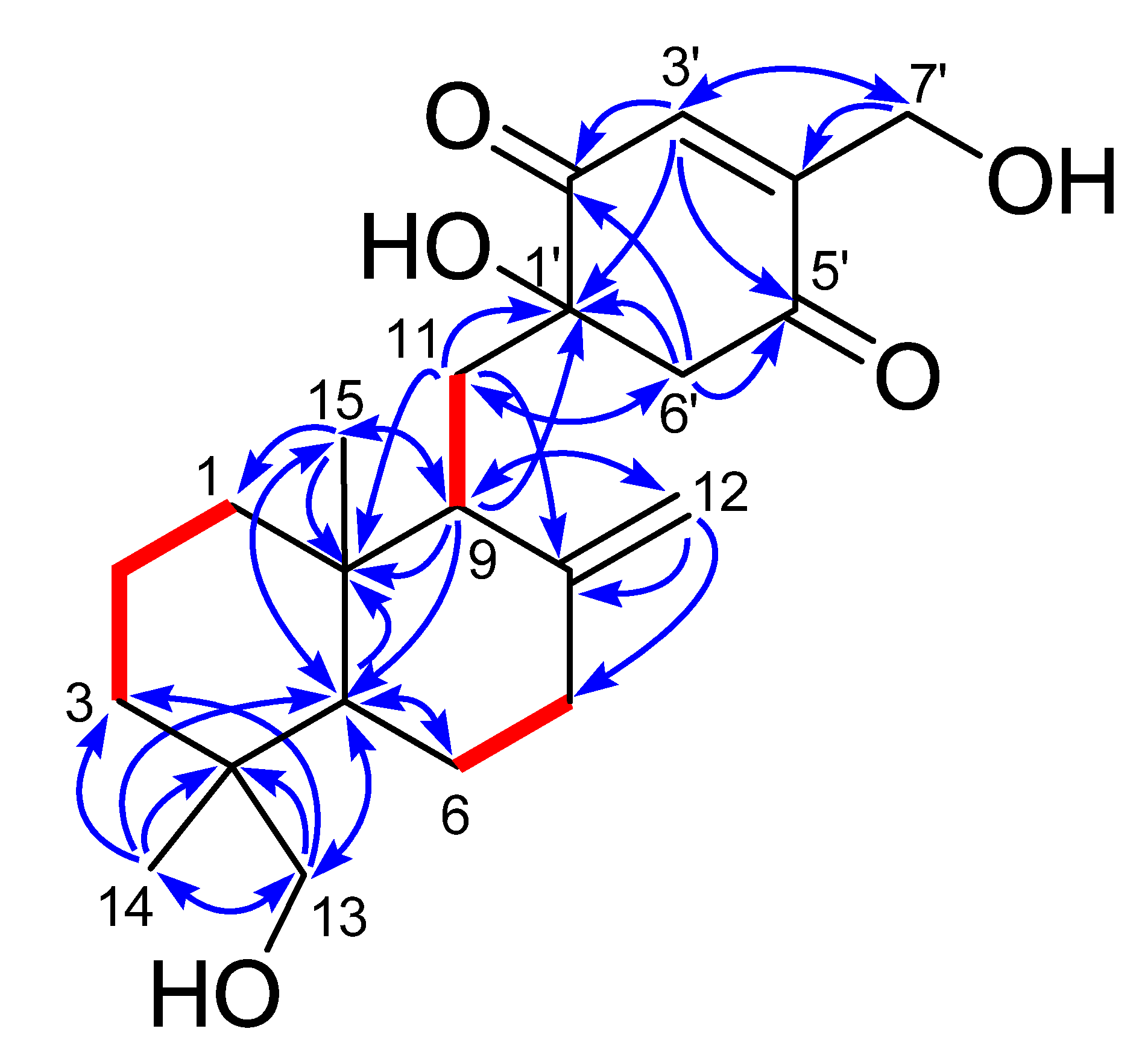
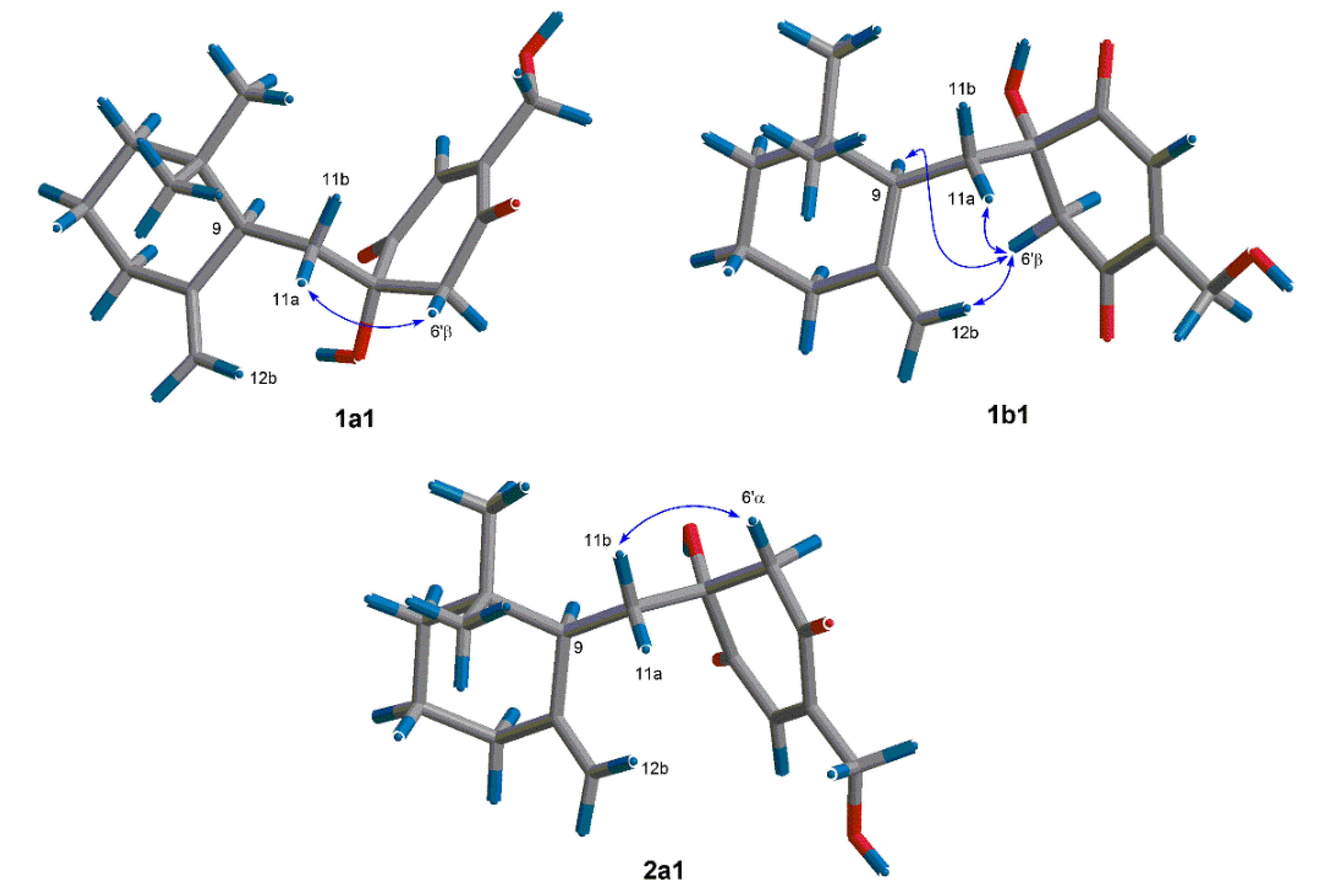
2. Results and Discussion
| 1 | 2 | |||
|---|---|---|---|---|
| Position | δC, type | δH, mult. (J in Hz) | δC, type | δH, mult. (J in Hz) |
| 1α | 38.9, CH2 | 1.31–1.36 m | 38.9, CH2 | 1.47 dt (13.0, 3.0) |
| 1β | 1.76 br d (12.9) | 1.76–1.83 overlapped | ||
| 2α | 19.5, CH2 | 1.40–1.44 m | 19.6, CH2 | 1.52–1.57 m |
| 2β | 1.57 qt (13.4, 3.0) | 1.61 qt (13.3. 3.0) | ||
| 3α | 36.3, CH2 | 1.83 m | 36.5, CH2 | 1.79 overlapped |
| 3β | 1.38 br d (12.3) | 1.40 dt (12.8, 3.1) | ||
| 4 | 39.3, C | 38.9, C | ||
| 5 | 49.2, CH | 1.91 dd (12.9, 2.6) | 49.2, CH2 | 1.76–1.83 overlapped |
| 6α | 24.9, CH2 | 1.81–1.86 overlapped | 25.1, CH2 | 1.76–1.83 overlapped |
| 6β | 1.27–1.31 overlapped | 1.26 qd (12.3, 4.1) | ||
| 7α | 38.8, CH2 | 2.04 td (12.9, 4.5) | 38.8, CH2 | 2.02 td (12.5 5.0) |
| 7β | 2.34 dt (12.6, 3.0) | 2.23–2.28 overlapped | ||
| 8 | 150.5, C | 149.7, C | ||
| 9 | 51.5, CH | 2.43 br d (7.0) | 51.2, CH | 2.48 br d (8.5) |
| 10 | 40.9, C | 40.6, C | ||
| 11a | 32.5, CH2 | 2.25 dd (15.1, 7.3) | 35.5, CH2 | 2.27 dd (14.7, 8.7) |
| 11b | 2.24 br d (15.1) | 2.21 br d (14.7) | ||
| 12a | 108.3, CH2 | 4.94 br s | 107.5, CH2 | 4.81 br s |
| 12b | 4.71 br s | 4.64 br s | ||
| 13a | 71.6, CH2 | 3.62 d (10.7) | 71.7, CH2 | 3.54 d (10.6) |
| 13b | 3.29 d (10.7) | 3.27 d (10.6) | ||
| 14 | 18.4, CH3 | 0.79 s | 18.2, CH3 | 0.78 s |
| 15 | 15.9, CH3 | 0.72 s | 16.0, CH3 | 0.69 s |
| 1ʹ | 78.1, C | 78.8, C | ||
| 2ʹ | 202.2, C | 201.8, C | ||
| 3ʹ | 134.5, CH | 7.49 t (2.0) | 135.4, CH | 7.49 t (2.0) |
| 4ʹ | 154.9, C | 153.7, C | ||
| 5ʹ | 197.7, C | 197.9, C | ||
| 6ʹα | 51.8, CH2 | 3.24 d (16.3) | 55.0, CH2 | 3.41 d (15.7) |
| 6ʹβ | 3.32 d (16.3) | 3.47 d (15.7) | ||
| 7ʹa | 58.7, CH2 | 4.94 dd (18.6, 2.1) | 58.8, CH2 | 5.01 dd (18.4, 2.1) |
| 7′b | 4.86 dd (18.6, 2.1) | 4.85 dd (18.4, 2.1) | ||
| Cell Lines | |||
|---|---|---|---|
| A549 | HeLa | HepG2 | |
| 1 | 46.7 ± 0.8 | 15.9 ± 0.8 | 31.9 ± 0.9 |
| 2 | 40.2 ± 1.5 | 28.7 ± 0.8 | 25.7 ± 1.6 |
| Adriamycin b | 0.69 ± 0.06 | 0.47 ± 0.05 | 1.22 ± 0.02 |
3. Experimental Section
3.1. General
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
3.4. Theoretical Conformational Analysis Method
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, G.P.; Yuan, J.; Sun, L.; She, Z.G.; Wu, J.H.; Lan, X.J.; Zhu, X.Z.; Liu, Y.C.; Chen, S.P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.Q.; Zhang, J.G.; Cheng, J.F. Overview of chirality and chiral drugs. In Chiral Drugs: Chemistry and Biological Action, 1st ed.; Lin, G.Q., You, Q.D., Cheng, J.F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 14–18. [Google Scholar]
- Lee, M.R. Plants against malaria. Part 1: Cinchona or the Peruvian bark. J. R. Coll. Physicians Edinb. 2002, 32, 189–196. [Google Scholar] [PubMed]
- Williams, E.M.V. The mode of action of quinidine on isolated rabbit atria interpreted from intracellular potential records. Br. J. Pharmacol. 1958, 13, 276–287. [Google Scholar]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2012, 30, 1229–1230. [Google Scholar]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Diez, D.; Urones, J.G. Quinone/hydroquinone sesquiterpenes. Mini Rev. Org. Chem. 2010, 7, 230–254. [Google Scholar] [CrossRef]
- Lin, X.P.; Zhou, X.F.; Wang, F.Z.; Liu, K.S.; Yang, B.; Yang, X.W.; Peng, Y.; Liu, J.; Ren, Z.; Liu, Y.H. A new cytotoxic sesquiterpene quinone produced by Penicillium sp. F00120 isolated from a deep sea sediment sample. Mar. Drugs 2012, 10, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wu, P.; Xue, J.H.; Wei, X.Y. Cytotoxic and antibacterial quinone sesquiterpenes from a Myrothecium Fungus. J. Nat. Prod. 2014, 77, 1791–1799. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, revision C.01; Gaussian: Wallingford, CT, USA, 2010.
- Shi, J.F.; Wu, P.; Jiang, Z.H.; Wei, X.Y. Synthesis and tumor cell growth inhibitory activity of biotinylated annonaceous acetogenins. Eur. J. Med. Chem. 2014, 71, 219–228. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wu, P.; Xue, J.; Li, H.; Wei, X. Myrothecols G and H, Two New Analogues of the Marine-Derived Quinone Sesquiterpene Penicilliumin A. Mar. Drugs 2015, 13, 3360-3367. https://doi.org/10.3390/md13063360
Fu Y, Wu P, Xue J, Li H, Wei X. Myrothecols G and H, Two New Analogues of the Marine-Derived Quinone Sesquiterpene Penicilliumin A. Marine Drugs. 2015; 13(6):3360-3367. https://doi.org/10.3390/md13063360
Chicago/Turabian StyleFu, Ying, Ping Wu, Jinghua Xue, Hanxiang Li, and Xiaoyi Wei. 2015. "Myrothecols G and H, Two New Analogues of the Marine-Derived Quinone Sesquiterpene Penicilliumin A" Marine Drugs 13, no. 6: 3360-3367. https://doi.org/10.3390/md13063360
APA StyleFu, Y., Wu, P., Xue, J., Li, H., & Wei, X. (2015). Myrothecols G and H, Two New Analogues of the Marine-Derived Quinone Sesquiterpene Penicilliumin A. Marine Drugs, 13(6), 3360-3367. https://doi.org/10.3390/md13063360






