Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Protein Hydrolysates of STDM Using Five Proteases
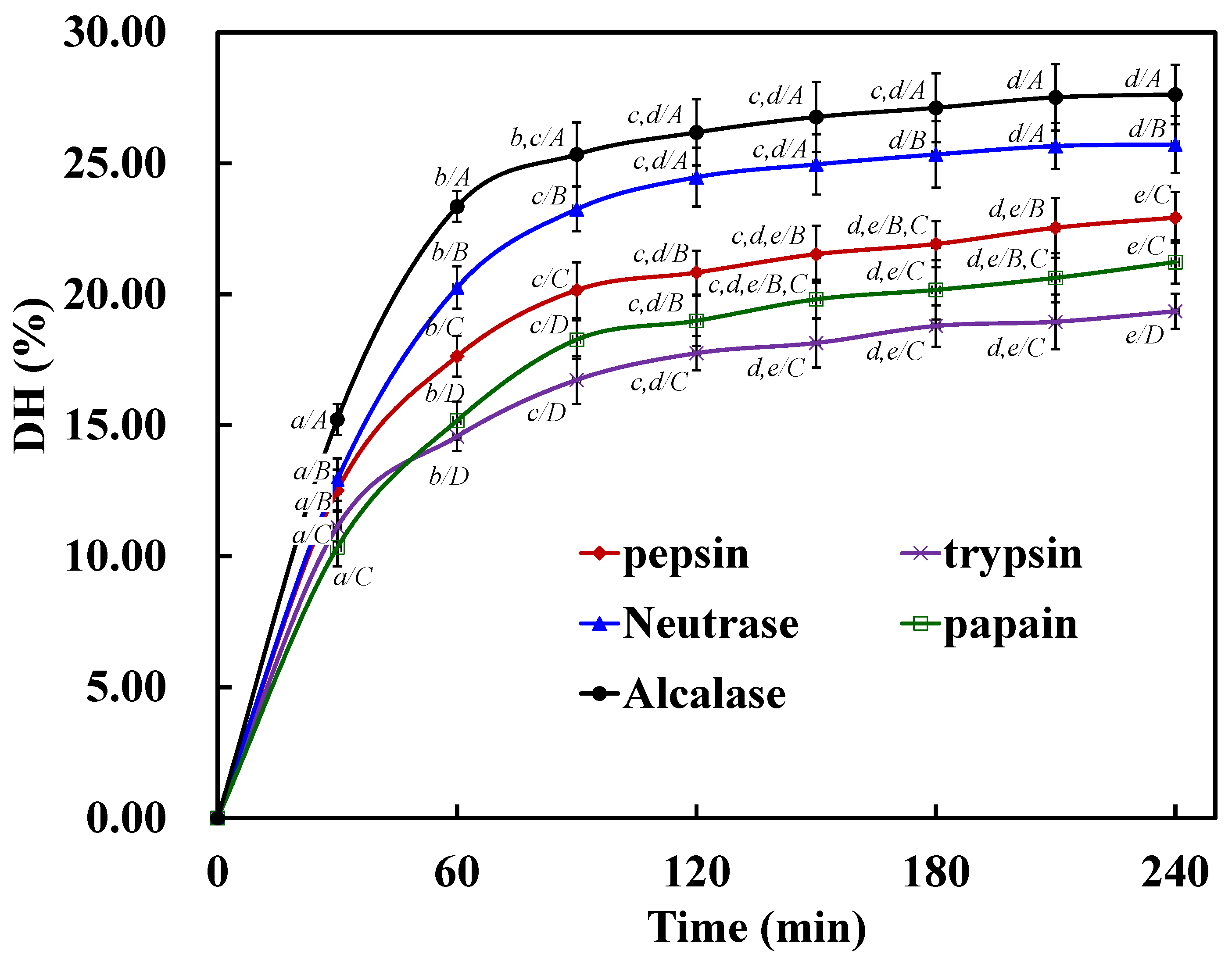
| Enzyme | DH (%) | EC50 (mg/mL) of Protein Hydrolysates | ||
|---|---|---|---|---|
| DPPH• | HO• | |||
| Pepsin | 22.93 ± 0.98 a | 7.09 ± 0.37 a | 2.37 ± 0.09 a | 7.65 ± 0.42 a |
| Trypsin | 19.35 ± 0.67 b | 9.36 ± 0.51 b | 4.09 ± 0.16 b | 9.37 ± 0.40 b |
| Neutrase | 25.72 ± 1.09 c | 5.38 ± 0.15 c | 1.58 ± 0.11 c | 6.38 ± 0.53 c |
| Papain | 21.23 ± 0.83 a | 8.65 ± 0.57 b | 3.42 ± 0.23 d | 8.66 ± 0.48 b |
| Alcalase | 27.63 ± 1.14 d | 4.54 ± 0.43 d | 1.27 ± 0.12 e | 5.67 ± 0.26 c |
2.2. Isolation of Antioxidant Peptides
2.2.1. Ultrafiltration (UF) Fractionation of ATH and NTH
| Sample | EC50 (mg/mL) | ||
|---|---|---|---|
| DPPH• | HO• | ||
| ATH and Its Fractions | |||
| ATH | 4.54 ± 0.43 a | 1.27 ± 0.12 a | 5.67 ± 0.26 a |
| ATH-I | 7.06 ± 0.63 b | 1.56 ± 0.17 b | 6.82 ± 0.47 b |
| ATH-II | 2.21 ± 0.12 c,f | 0.58 ± 0.05 c,d | 2.74 ± 0.11 c |
| Fr.A1 | 3.27 ± 0.24 d,e | 0.73 ± 0.08 d,e | 3.54 ± 0.26 d |
| Fr.A2 | 2.42 ± 0.15 f | 0.55 ± 0.06c,d,f | 2.76 ± 0.18 c |
| Fr.A3 | 1.08 ± 0.08 g | 0.22 ± 0.05 g | 1.31 ± 0.11 e |
| Fr.A4 | 1.76 ± 0.04 c | 0.37 ± 0.03 f,g | 1.69 ± 0.09 e |
| NTH and Its Fractions | |||
| NTH | 5.38 ± 0.15 h | 1.58 ± 0.11 b | 6.38 ± 0.53 b |
| NTH-I | 8.16 ± 0.42 i | 1.88 ± 0.22 h | 7.34 ± 0.58 f |
| NTH-II | 3.09 ± 0.23 d | 0.85 ± 0.06 e,i | 2.83 ± 0.18 c |
| Fr.B1 | 3.74 ± 0.24 e | 1.09 ± 0.12 j | 3.36 ± 0.32 d |
| Fr.B2 | 0.98 ± 0.07 g | 0.48 ± 0.05 c,f | 1.56 ± 0.13 e |
| Fr.B3 | 3.06 ± 0.15 d | 0.94 ± 0.06 i,j | 2.35 ± 0.22 c |
2.2.2. Gel Filtration Chromatography of ATH-II and NTH-II
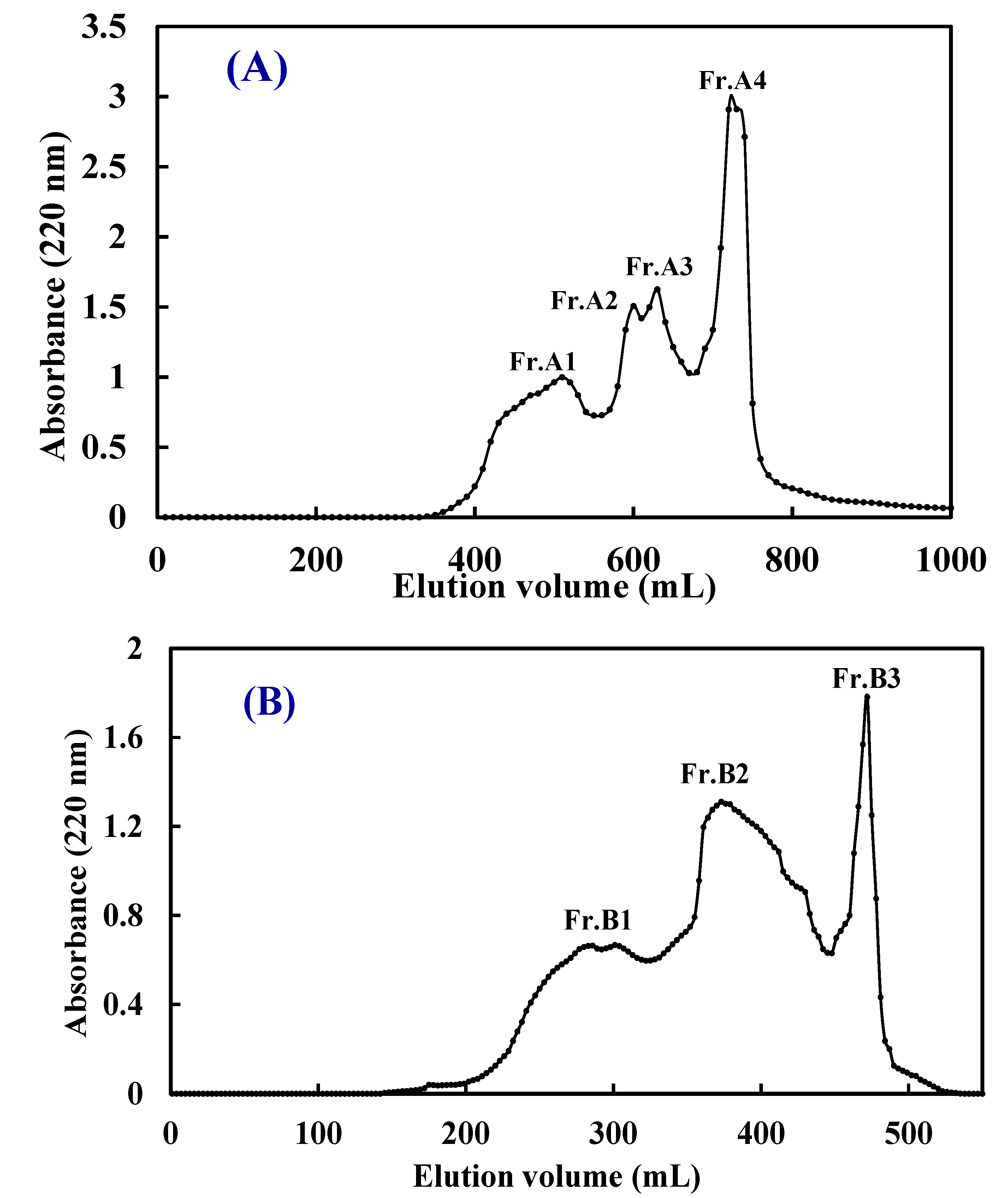
2.3. Amino Acid and Peptide Compositions of Fr.A3 and Fr.B2
2.3.1. Amino Acid Compositions of Fr.A3 and Fr.B2
| Amino Acid | Defatted STDM | ATH | NTH | Fr.A3 | Fr.B2 |
|---|---|---|---|---|---|
| Asp (D) | 98.2 | 96.9 | 97.3 | 79.9 | 91.2 |
| Glu (E) | 127.6 | 125.2 | 125.7 | 94.0 | 106.9 |
| Ser (S) | 71.4 | 70.5 | 70.8 | 65.2 | 60.8 |
| Gly (G) | 79.6 | 76.8 | 77.3 | 63.8 | 69.6 |
| His (H) | 75.4 | 74.7 | 74.6 | 81.1 | 73.9 |
| Arg (R) | 69.7 | 70.2 | 68.9 | 53.6 | 61.9 |
| Thr (T) | 48.5 | 47.7 | 48.3 | 36.5 | 41.2 |
| Cys (C) | 3.9 | 4.1 | 4.5 | 6.4 | 5.5 |
| Tyr (Y) | 11.3 | 11.7 | 12.6 | 24.0 | 22.5 |
| Lys (K) | 29.6 | 30.2 | 29.5 | 25.6 | 29.1 |
| Ala (A) | 79.4 | 80.1 | 80.5 | 89.8 | 86.1 |
| Pro (P) | 9.7 | 9.4 | 9.2 | 14.9 | 10.6 |
| Val (V) | 72.4 | 73.6 | 73.1 | 85.4 | 81.7 |
| Met (M) | 24.7 | 25.6 | 25.3 | 21.2 | 20.0 |
| Ile (I) | 53.9 | 54.7 | 53.4 | 78.7 | 70.1 |
| Leu (L) | 94.3 | 96.3 | 97.1 | 103.9 | 92.5 |
| Trp (W) | 10.8 | 12.4 | 11.6 | 26.4 | 21.9 |
| Phe (F) | 39.6 | 39.9 | 40.3 | 49.6 | 54.5 |
| Total | 1000 | 1000 | 1000 | 1000.0 | 1000.0 |
| Aromatic amino acids | 137.1 | 138.7 | 139.1 | 181.1 | 172.8 |
| Hydrophobic amino acids | 384.8 | 392 | 390.5 | 469.9 | 437.4 |
2.3.2. Peptide Profiles and Compositions of Fr.A3 and Fr.B2
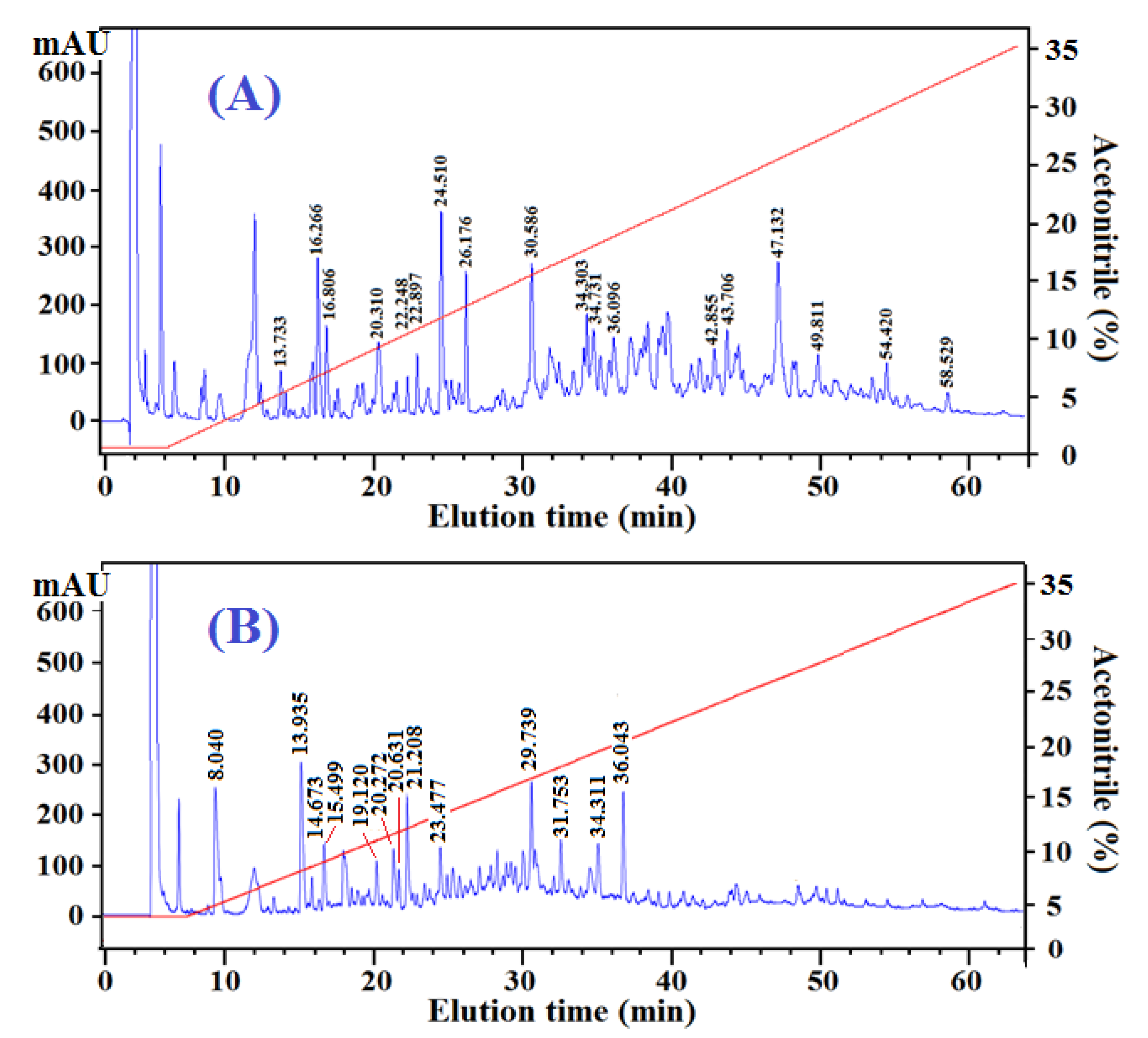
| No. | Retention Time (min) | Observed Mass (Da) | Calculated Mass (Da) | Amino Acid Sequence | Antioxidant Score |
|---|---|---|---|---|---|
| Peptides from Fr.A3 | |||||
| A01 | 13.733 | 335.33 | 335.36 | YGP | 5.5 |
| A02 | 16.266 | 482.42 | 482.44 | YEGD | 4.5 |
| A03 | 16.806 | 463.53 | 463.55 | QWM | 7.5 |
| A04 | 20.310 | 538.50 | 538.51 | EYNN | 4 |
| A05 | 22.248 | 423.41 | 423.42 | QNY | 4.5 |
| A06 | 22.897 | 331.30 | 331.33 | QAGG | 2 |
| A07 | 24.510 | 429.45 | 429.47 | QPW | 8.5 |
| A08 | 26.176 | 407.41 | 407.42 | QFN | 3 |
| A09 | 30.586 | 660.69 | 660.72 | DVIEW | 9.5 |
| A10 | 34.303 | 902.91 | 902.9 | QYDEYW | 9.5 |
| A11 | 34.731 | 574.64 | 574.67 | WVGTI | 9.5 |
| A12 | 36.096 | 563.57 | 563.60 | DLYPG | 5 |
| A13 | 42.855 | 571.61 | 571.62 | YVAGY | 6 |
| A14 | 43.706 | 489.49 | 489.52 | DVWA | 8 |
| A15 | 47.132 | 528.58 | 528.60 | QPVW | 9.5 |
| A16 | 49.811 | 681.73 | 681.74 | QELHR | 3 |
| A17 | 54.420 | 619.69 | 619.71 | EYIPV | 7.5 |
| A18 | 58.529 | 441.45 | 441.48 | QPPT | 3 |
| Peptides from Fr.B2 | |||||
| B01 | 8.040 | 446.39 | 446.41 | QGGEG | 2 |
| B02 | 13.935 | 538.48 | 538.51 | YENGG | 4.5 |
| B03 | 14.673 | 506.44 | 506.46 | QESGS | 2 |
| B04 | 15.499 | 510.46 | 510.50 | QYSGG | 4 |
| B05 | 19.120 | 521.49 | 521.52 | EGYPG | 4 |
| B06 | 20.272 | 407.40 | 407.42 | QFGG | 3 |
| B07 | 20.631 | 494.48 | 494.50 | QFGGS | 3 |
| B08 | 21.208 | 464.45 | 464.47 | QFGGG | 3 |
| B09 | 23.477 | 681.73 | 681.74 | WGYAW | 10 |
| B10 | 29.739 | 742.83 | 742.86 | YIVYW | 12 |
| B11 | 31.753 | 1214.21 | 1214.24 | WGDAGGYYYY | 10 |
| B12 | 34.311 | 544.62 | 544.64 | QILTA | 3 |
| B13 | 36.043 | 543.54 | 543.57 | QPWN | 8 |
2.4. Protective Effects of Fr.A3 and Fr.B2 on Lipid Peroxidation in a Linoleic Acid System.
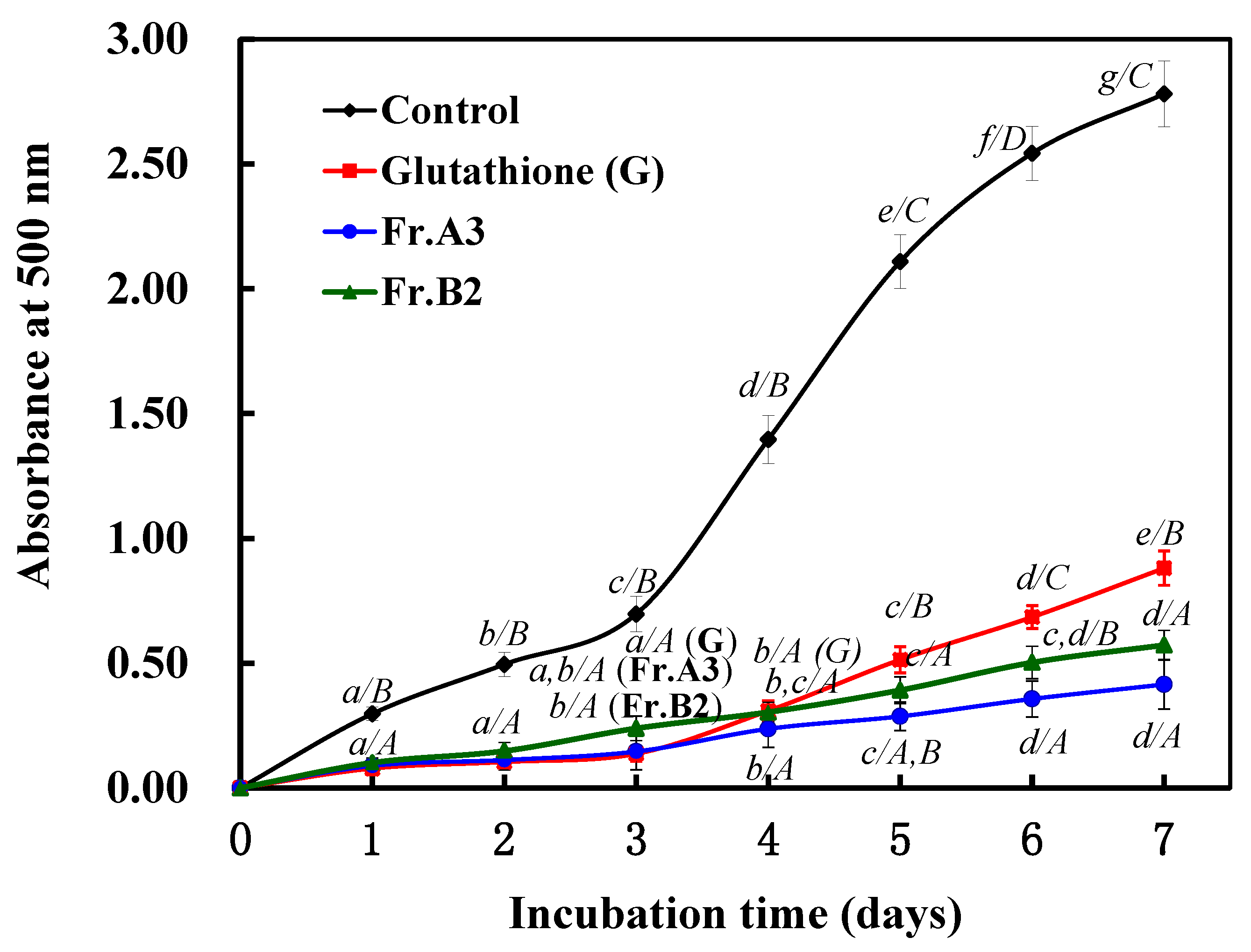
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Preparation of Protein Hydrolysates of STDM
4.3. Determination of the Degree of Hydrolysis (DH)
4.4. Determination of Amino Acid Composition
4.5. Antioxidant Activities
4.5.1. Radical (DPPH•, HO•, and •) Scavenging Assays
HO• Scavenging Activity
DPPH• Scavenging Activity
•Scavenging Activity
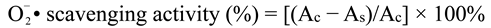
4.5.2. Lipid Peroxidation Inhibition Assay
4.5.3. Antioxidant Score
4.6. Separation of Antioxidant Peptides
4.6.1. Ultrafiltration
4.6.2. Gel Filtration Chromatography
4.6.3. Reversed-Phase High Performance Liquid Chromatography (RP-HPLC)
4.7. Identification of the Peptide Sequences and Molecular Masses
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farvin, K.H.; Lystbæk Andersen, L.; Hauch Nielsen, H.; Jacobsen, C.; Jakobsen, G.; Johansson, I.; Jessena, F. Antioxidant activity of Cod (Gadus morhua) protein hydrolysates: In vitro assays and evaluation in 5% fish oil-in-water emulsion. Food Chem. 2014, 149, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.; Ryu, B.; Ngo, D.H.; Kim, S.K. Peptide isolated from Japanese flounder skin gelatin protects against cellular oxidative damage. J. Agric. Food Chem. 2012, 60, 9112–9119. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Z.R.; Chi, C.F.; Zhang, Q.H.; Luo, H.Y. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 2012, 36, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Ren, X.J.; Deng, S.G.; Wu, C.W. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem. 2015, 168, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Mahaki, H.; Zare-Zardini, H. Antioxidant activity of protein hydrolysates and purified peptides from Zizyphus jujuba fruits. J. Funct. Foods 2014, 10, 144–153. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Hasan, F.M.; Gill, T.A.; Aluko, R.E. Antioxidant properties of Salmon (Salmo salar) protein hydrolysate and peptide fractions isolated by reverse-phase HPLC. Food Res. Int. 2013, 52, 315–322. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y.F. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Chang, O.K.; Ha, G.E.; Han, G.S.; Seol, K.H.; Kim, H.W.; Jeong, S.G.; Oh, M.H.; Park, B.Y.; Ham, J.S. Novel antioxidant peptide derived from the ultrafiltrate of ovomucin hydrolysate. J. Agric. Food Chem. 2013, 61, 7294–7300. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, T.T.; Hu, P.; Yang, J.; Wang, S.Y. Purification and characterization of an antioxidant peptide (GSQ) from Chinese leek (Allium tuberosum Rottler) seeds. J. Funct. Foods 2014, 10, 144–153. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Kishimura, H.; Shahidi, F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011, 124, 1354–1362. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.M.; Babacar, S.K.S.; Zo, R.; Claire, D.M.; Jacques, M.; Tran, L.T.; Bergé, J.P. Enzymatic hydrolysis of yellowfin tuna (Thunnus albacares) by-products using Protamex protease. Food Technol. Biotech. 2011, 49, 48–55. [Google Scholar]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (Pangasius sutchi) sarcoplasmic protein hydolysate. J. Funct. Foods 2014, 9, 280–289. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Deng, Y.Y.; Wang, Y.M.; Deng, S.G.; Ma, J.Y. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res. Int. 2014, 55, 222–228. [Google Scholar] [CrossRef]
- Luo, H.Y.; Wang, B.; Li, Z.R.; Chi, C.F.; Zhang, Q.H.; He, G.Y. Preparation and evaluation of antioxidant peptide from papain hydrolysate of Sphyrna lewini muscle protein. LWT-Food Sci. Technol. 2013, 51, 281–288. [Google Scholar] [CrossRef]
- Ko, J.Y.; Lee, J.H.; Samarakoon, K.; Kim, J.S.; Jeon, Y.J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ranathunga, S.; Rajapakse, N.; Kim, S.K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Intarasirisawat, R.; Benjakul, S.; Wu, J.; Visessanguan, W. Isolation of antioxidative and ACE inhibitory peptides from protein hydrolysate of skipjack (Katsuwana pelamis) roe. J. Funct. Foods 2013, 5, 1854–1862. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Sudhakar, S.; Nazeer, R.A. Structural characterization of an Indian squid antioxidant peptide and its protective effect against cellular reactive oxygen species. J. Funct. Foods 2015, 14, 502–512. [Google Scholar] [CrossRef]
- Kim, E.K.; Oh, H.J.; Kim, Y.S.; Hwang, J.W.; Ahn, C.B.; Lee, J.S.; Jeon, Y.J.; Moon, S.H.; Sung, S.H.; Jeon, B.T.; Park, P.J. Purification of a novel peptide derived from Mytilus coruscus and in vitro/in vivo evaluation of its bioactive properties. Fish Shellfish Immun. 2013, 34, 1078–1084. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Qian, Z.J.; Kim, S.K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Hsu, K.C.; Lu, G.H.; Jao, C.L. Antioxidative properties of peptides prepared from tuna cooking juice hydrolysates with orientase (Bacillus subtilis). Food Res. Int. 2009, 42, 647–652. [Google Scholar] [CrossRef]
- Hsu, K.C. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010, 122, 42–48. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Chotikachinda, R.; Tantikitti, C.; Benjakul, S.; Rustad, T.; Kumarnsit, E. Production of protein hydrolysates from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asian seabass (Lates calcarifer). Aquacult. Nutr. 2013, 19, 773–784. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Pednekar, M.D.; Juan, F.; Yardi, V.; Sharma, A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Ktari, N.; Jridi, M.; Bkhairia, I.; Sayari, N.; Ben Salah, R.; Nasri, M. Functionalities and antioxidant properties of protein hydrolysates from muscle of zebra blenny (Salaria basilisca) obtained with different crude protease extracts. Food Res. Int. 2012, 4, 747–756. [Google Scholar] [CrossRef]
- Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chem. 2009, 114, 976–983. [Google Scholar] [CrossRef]
- Jridi, M.; Lassoued, I.; Nasri, R.; Ayedi Mohamed, A.; Nasri, M.; Souissi, N. Characterization and potential use of cuttlefish skin gelatin hydrolysates prepared by different microbial proteases. BioMed. Res. Int. 2014, 2014, 461728. [Google Scholar] [CrossRef] [PubMed]
- Centenaro, G.S.; Salas-Mellado, M.; Pires, C.; Batista, I.; Nunes, M.L.; Prentice, C. Fractionation of protein hydrolysates of fish and chicken using membrane ultrafiltration: Investigation of antioxidant activity. Appl. Biochem. Biotechnol. 2014, 172, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014, 150, 366–373. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Inhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen species. J. Agric. Food Chem. 2011, 59, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, N.; Shahidi, F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J. Agric. Food Chem. 2011, 59, 5006–5014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mu, T.H.; Sun, M.J. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J. Funct. Foods 2014, 7, 191–200. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, B.W.; Zhou, D.Y.; Wu, H.T.; Tan, H.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT-Food Sci. Technol. 2011, 44, 1113–1118. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.C.; Barkia, A.; Nasri, M. Characterization and comparative assessment of antioxidant and ACE inhibitory activities of thornback ray gelatin hydrolysates. J. Funct. Foods 2015, 13, 225–238. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Zhang, B.; Deng, S.G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamaguchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.T. Lipid peroxidation and neurodegenerative disease. Free Radical Bio. Med. 2011, 51, 1302–1319. [Google Scholar] [CrossRef]
- Siswoyo, T.A.; Mardiana, E.; Lee, K.O.; Hoshokawa, K. Isolation and characterization of antioxidant protein fractions from melinjo (Gnetum gnemon) seeds. J. Agric. Food Chem. 2011, 59, 5648–5656. [Google Scholar] [CrossRef] [PubMed]
- Conway, V.; Gauthier, S.F.; Pouliot, Y. Antioxidant activities of buttermilk proteins, whey proteins, and their enzymatic hydrolysates. J. Agric. Food Chem. 2013, 61, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.M.; Chi, C.F.; Luo, H.Y.; Deng, S.G.; Ma, J.Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, Y.D.; Li, Z.R.; Yu, D.; Chi, C.F.; Ma, J.Y. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods 2014, 6, 176–185. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, Z.-R.; Luo, H.-Y. Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle. Mar. Drugs 2015, 13, 2580-2601. https://doi.org/10.3390/md13052580
Chi C-F, Hu F-Y, Wang B, Li Z-R, Luo H-Y. Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle. Marine Drugs. 2015; 13(5):2580-2601. https://doi.org/10.3390/md13052580
Chicago/Turabian StyleChi, Chang-Feng, Fa-Yuan Hu, Bin Wang, Zhong-Rui Li, and Hong-Yu Luo. 2015. "Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle" Marine Drugs 13, no. 5: 2580-2601. https://doi.org/10.3390/md13052580
APA StyleChi, C.-F., Hu, F.-Y., Wang, B., Li, Z.-R., & Luo, H.-Y. (2015). Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle. Marine Drugs, 13(5), 2580-2601. https://doi.org/10.3390/md13052580








