Neuroprotective Effects of the Cultivated Chondrus crispus in a C. elegans Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
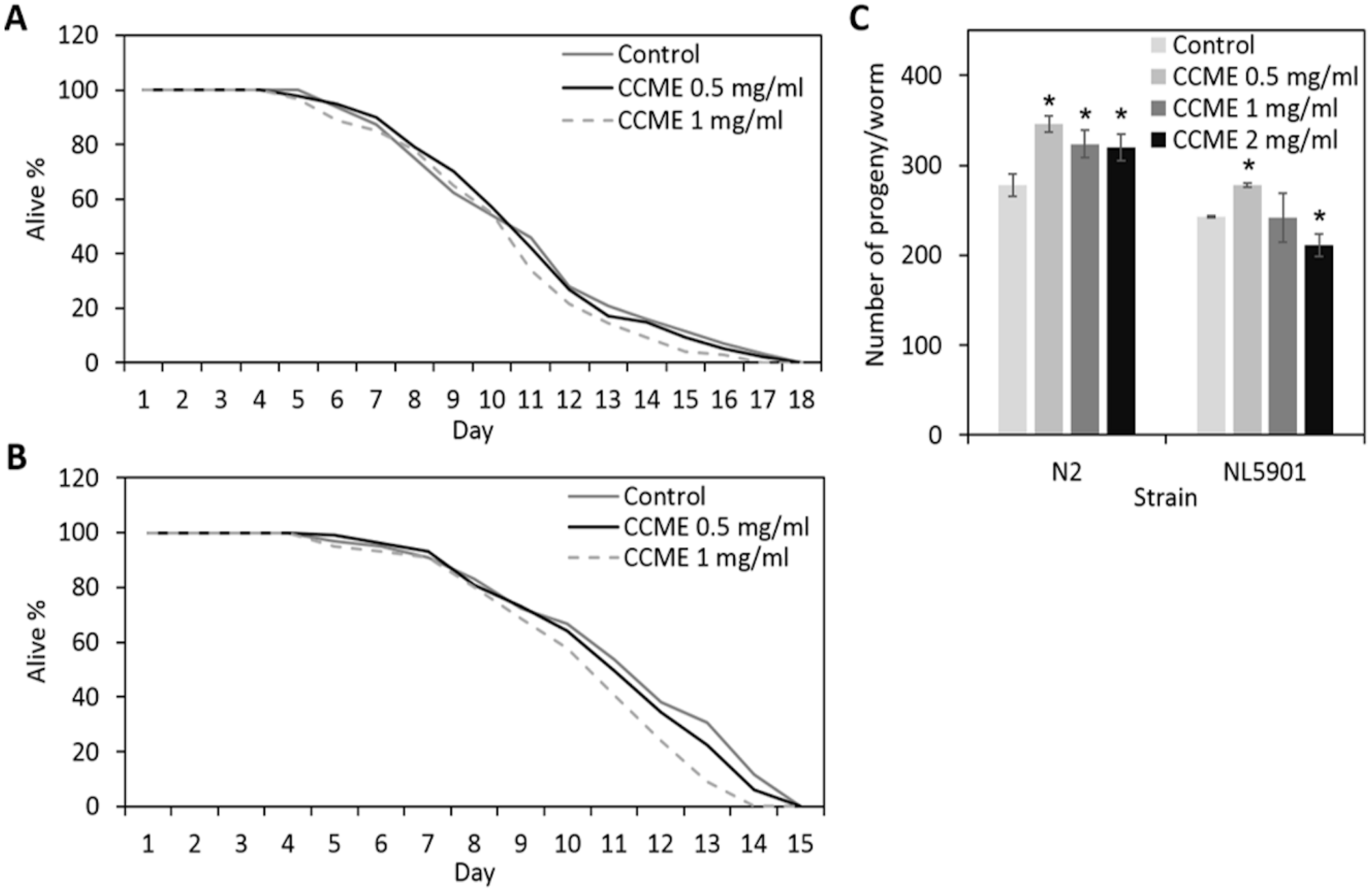
2.1. Effects of the Methanolic Extract of Chondrus crispus (CCME) on the General Health of C. elegans
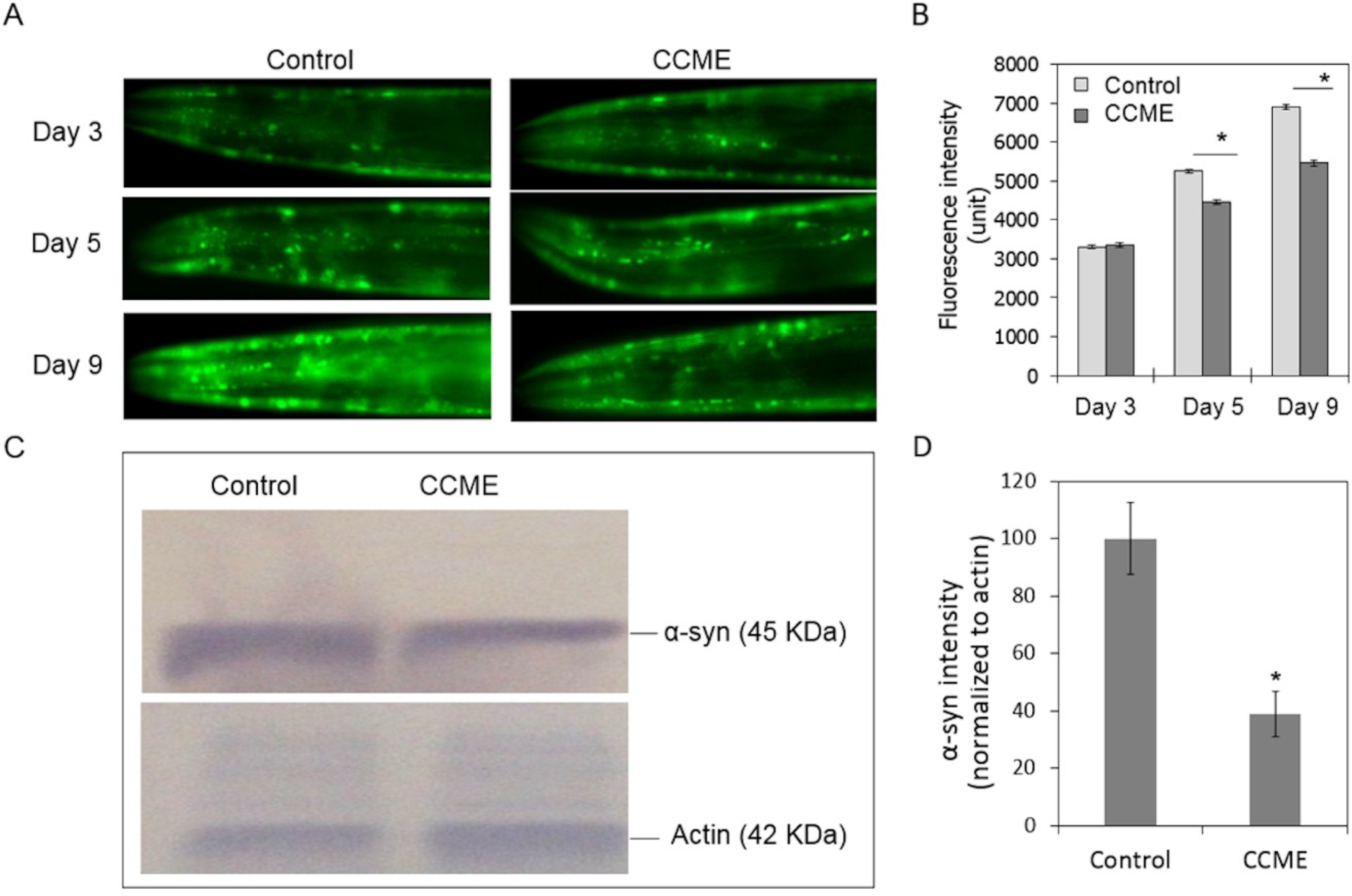
2.2. CCME Decreased α-syn Accumulation
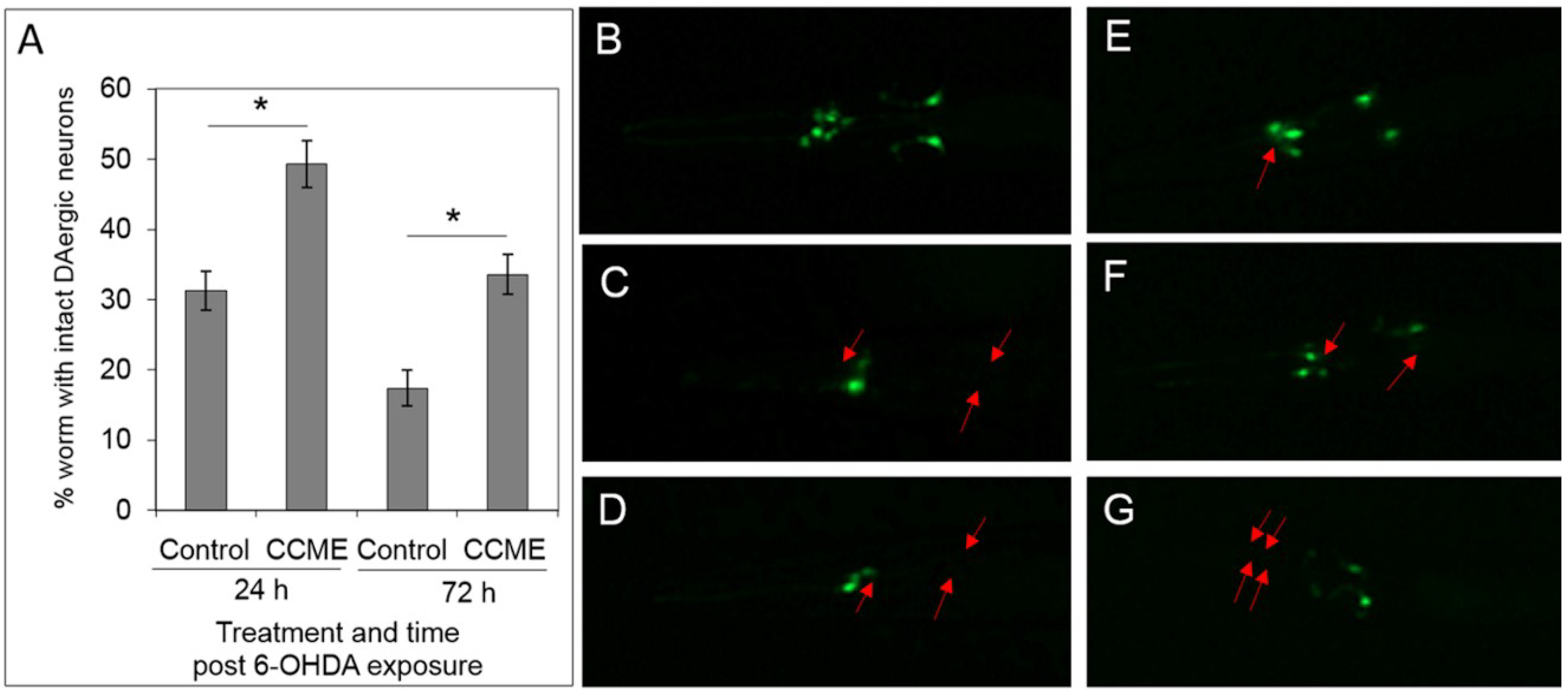
2.3. CCME Protected C. elegans from Drug-Induced DAergic Neuron Degeneration
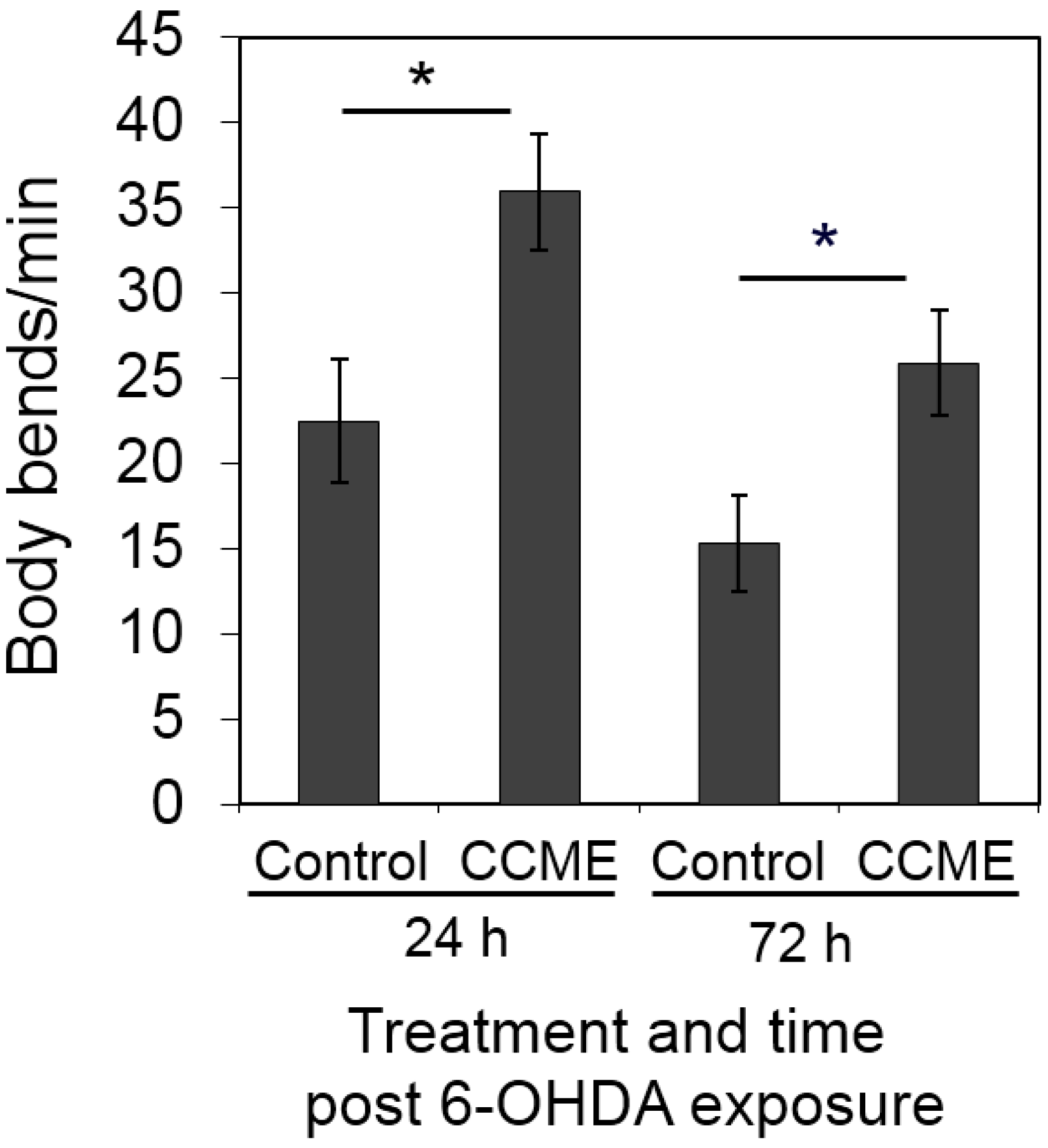
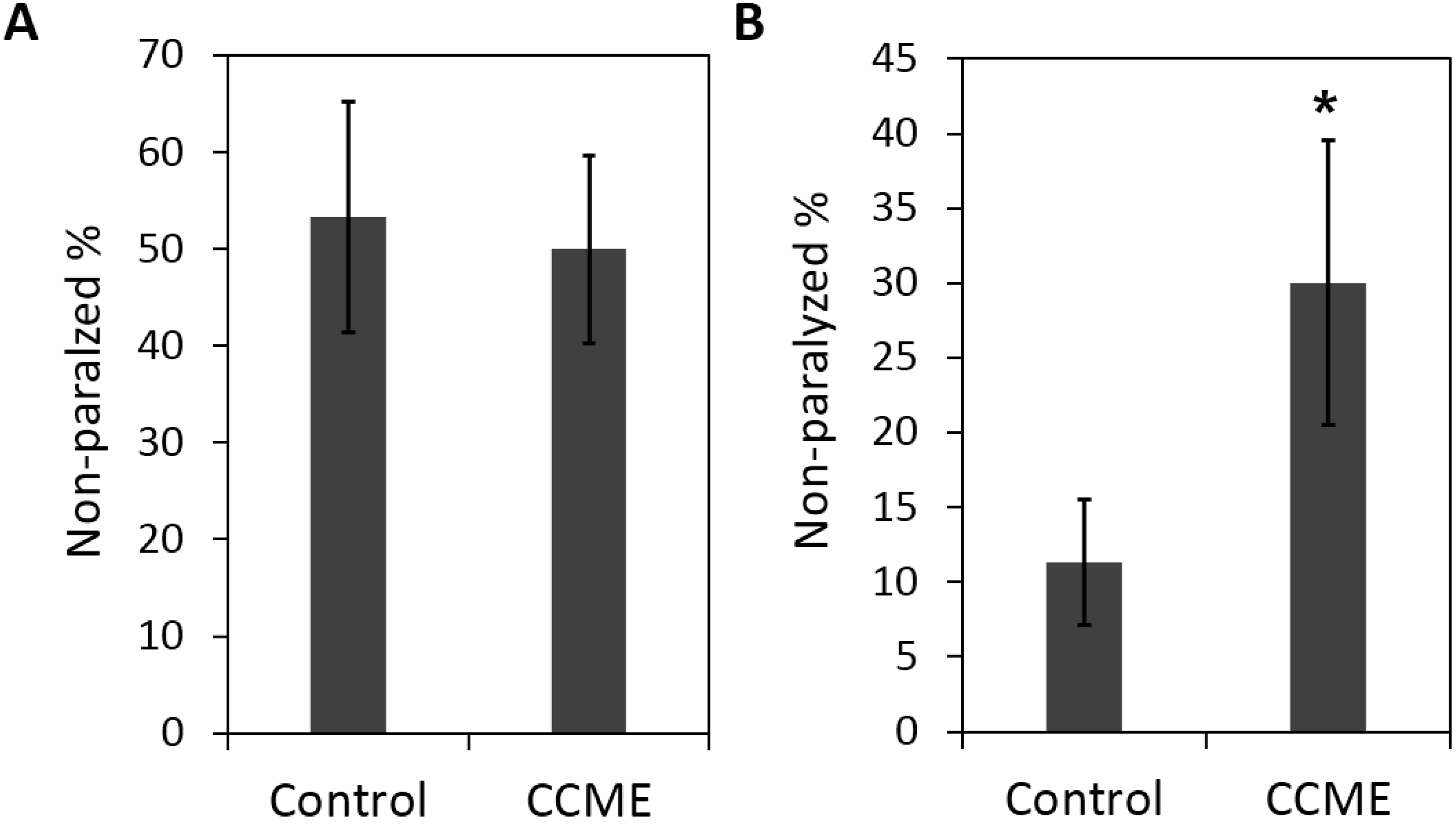
2.4. CCME Supplementation Prevented the Rapid Slow-down of Body Movement upon Treatment with 6-OHDA
2.5. The Decreased α-syn Accumulation by CCME Supplementation Was Associated with Enhanced Tolerance of Oxidative Stress, but not Heat Stress
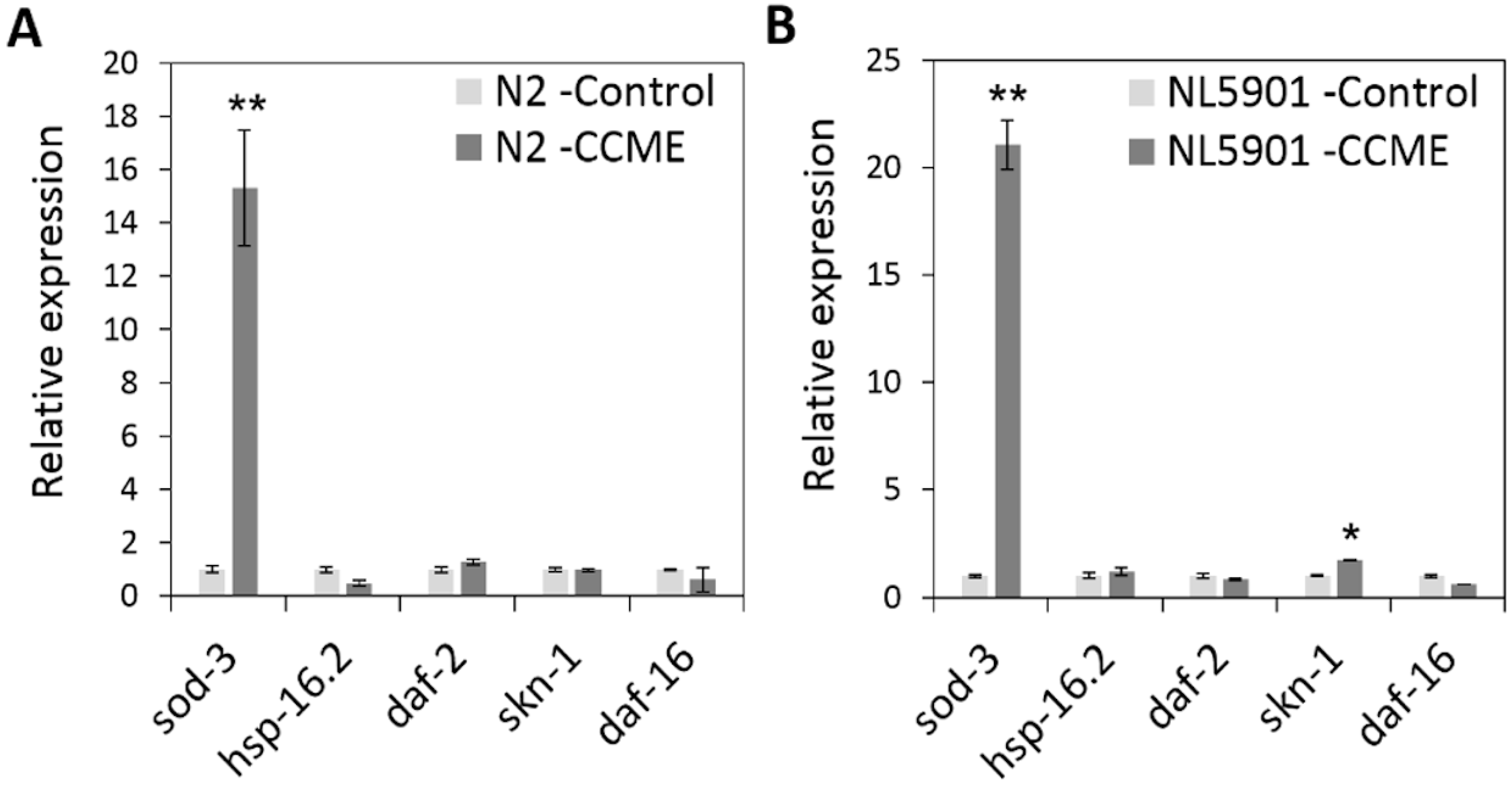
2.6. The Decreased α-syn Accumulation by CCME Was Associated with the Up-Regulation of the sod-3 and skn-1Genes
3. Discussion
4. Experimental Section
4.1. Seaweed Extract and Chemicals
4.2. C. elegans Strains and Maintenance
4.3. Phenotype Assays of C. elegans
4.3.1. Lifespan Assay
4.3.2. Brood Size Assay
4.3.3. Heat Stress Assay
4.3.4. Oxidative Stress Assay
4.3.5. α-syn Accumulation Assay
4.3.6. The 6-Hydroxydopamine-Induced DAergic Neuron Degeneration Assay
4.3.7. RNA Extraction and Quantification
4.3.8. Real-Time Quantitative PCR (qPCR)
4.3.9. Protein Extraction and Quantification
4.3.10. Western Blots
4.3.11. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bernheimer, H.; Birkmayer, W.; Hornykiewicz, O.; Jellinger, K.; Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973, 20, 415–455. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.; Trojanowski, J.Q. Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron 2006, 52, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Auluck, P.K.; Chan, H.Y.; Trojanowski, J.Q.; Lee, V.M.; Bonini, N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 2002, 295, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Gelwix, C.C.; Caldwell, K.A.; Caldwell, G.A. Torsin-mediated protection from cellular stress in the Dopaminergic neurons of Caenorhabditis elegans. J. Neurosci. 2005, 25, 3801–3812. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I. Neurobiology of the Caenorhabditis elegans genome. Science 1998, 282, 2028–2033. [Google Scholar] [CrossRef] [PubMed]
- van Ham, T.J.; Thijssen, K.L.; Breitling, R.; Hofstra, R.M.W.; Plasterk, R.H.A.; Nollen, E.A.A. C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet. 2008, 4, e1000027. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Hall, D.H.; Miller, D.M.; Blakely, R.D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 3264–3269. [Google Scholar] [CrossRef] [PubMed]
- Marvanova, M.; Nichols, C.D. Identification of neuroprotective compounds of Caenorhabditis elegans dopaminergic neurons against 6-OHDA. J. Mol. Neurosci. 2007, 31, 127–137. [Google Scholar] [PubMed]
- Kumar, C.S.; Ganesan, P.; Suresh, P.V.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds—A review. J. Food Sci. Technol. 2008, 45, 1–13. [Google Scholar]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Sangha, J.S.; Fan, D.; Banskota, A.H.; Stefanova, R.; Khan, W.; Hafting, J.; Craigie, J.; Critchley, A.T.; Prithiviraj, B. Bioactive components of the edible strain of red alga, Chondrus crispus, enhance oxidative stress tolerance in Caenorhabditis elegans. J. Func. Foods 2013, 5, 1180–1190. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Karutha, P.S.; Pandima, D.K. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar marine biosphere reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Lee, S.H.; Yong, L.; Kim, S.K. Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Curry, K.; Wu, Z.; Christen, Y.; Luo, Y. Ginkgo biloba extract EGb761 and Wisconsin Ginseng delay sarcopenia in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [PubMed]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B.; Jimena, S. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytotherap. Res. 2007, 21, 629–640. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Chonpathompikunlert, P.; Muchimapura, S.; Priprem, A.; Tankamnerdthai, O. Piperine, the potential functional food for mood and cognitive disorders. Food Chem. Toxicol. 2008, 46, 3106–3110. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Sangha, J.S.; Wally, O.; Banskota, A.H.; Stefanova, R.; Hafting, J.T.; Critchley, A.T.; Prithiviraj, B. Dalhousie University, Truro, Canada. A cultivated strain of a red seaweed (Chondrus crispus), suppresses β-amyloid-induced paralysis in Caenorhabditis elegans. 2015; Unpublished work. [Google Scholar]
- Li, Y.X.; Li, Y.; Lee, S.H.; Qian, Z.J.; Kim, S.J. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and d-isofloridoside from marine red alga Laurencia undulata. J. Agric. Food. Chem. 2010, 58, 578–586. [Google Scholar] [CrossRef]
- Kim, M.; Li, Y-X.; Dewapriya, P.; Ryu, B.; Kim, S-K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403. [Google Scholar] [CrossRef]
- Froger, N.; Moutsimilli, L.; Cadetti, L.; Jammoul, F.; Wang, Q.P.; Fan, Y.; Gaucher, D.; Rosolen, S.G.; Neveux, N.; Cynober, L.; et al. Taurine: The comeback of a neutraceutical in the prevention of retinal degenerations. Prog. Retin. Eye Res. 2014, 41, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Jin, H.; Nguyen, M.; Carr, J.; Lee, Y.J.; Hsu, C.C.; Faiman, M.D.; Schloss, J.V.; Wu, J.Y. Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J. Neurosci. Res. 2001, 66, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.R.; Dawson, R., Jr. Taurine is a weak scavenger of peroxynitrite and does not attenuate sodium nitroprusside toxicity to cells in culture. Amino Acids 2001, 20, 419–433. [Google Scholar] [CrossRef]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.E.; Martin, D.S.D.; Horrobin, D.F.; Lynch, M.A. Neuroprotective actions of eicosapentaenoic acid on lipopolysaccharide-induced dysfunction in rat hippocampus. J. Neurochem. 2004, 91, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Wang, B.G. Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.B.; Romanos, M.T.V.; de Souza, L.M.; Sassaki, G.; Barreto-Bergter, E. Glycolipids from macroalgae: potential biomolecules for marine biotechnology? Rev. Bras. Farmacogn. 2011, 21, 244–247. [Google Scholar] [CrossRef]
- Liu, J.; Hafting, J.; Critchley, A.T.; Banskota, A.H.; Prithiviraj, B. Components of the cultivated red seaweed Chondrus crispus enhance the immune response of Caenorhabditis elegans to Pseudomonas aeruginosa through the pmk-1, daf-2/daf-16, and skn-1 pathways. Appl. Environ. Microbiol. 2013, 79, 7343–7350. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new Millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.A.; Fowler, D.M.; Zhang, Q.; Nieva, J.; Powers, E.T.; Wentworth, P., Jr.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson’s disease. J. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Bhat, H.K. Superoxide dismutase 3 is induced by antioxidants, inhibits oxidative DNA damage and is associated with inhibition of estrogen-induced breast cancer. Carcinogenesis 2012, 33, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Blackwell, T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003, 17, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Vanduyn, N.; Settivari, R.; Wong, G.; Nass, R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol. Sci. 2010, 118, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Caito, S.; Slaughter, J.C.; Aschner, M. The role of skn-1 in methylmercury-induced latent dopaminergic neurodegeneration. Neurochem. Res. 2013, 38, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Banskota, A.H.; Critchley, A.T.; Hafting, J.; Prithiviraj, B. Neuroprotective Effects of the Cultivated Chondrus crispus in a C. elegans Model of Parkinson’s Disease. Mar. Drugs 2015, 13, 2250-2266. https://doi.org/10.3390/md13042250
Liu J, Banskota AH, Critchley AT, Hafting J, Prithiviraj B. Neuroprotective Effects of the Cultivated Chondrus crispus in a C. elegans Model of Parkinson’s Disease. Marine Drugs. 2015; 13(4):2250-2266. https://doi.org/10.3390/md13042250
Chicago/Turabian StyleLiu, Jinghua, Arjun H. Banskota, Alan T. Critchley, Jeff Hafting, and Balakrishnan Prithiviraj. 2015. "Neuroprotective Effects of the Cultivated Chondrus crispus in a C. elegans Model of Parkinson’s Disease" Marine Drugs 13, no. 4: 2250-2266. https://doi.org/10.3390/md13042250
APA StyleLiu, J., Banskota, A. H., Critchley, A. T., Hafting, J., & Prithiviraj, B. (2015). Neuroprotective Effects of the Cultivated Chondrus crispus in a C. elegans Model of Parkinson’s Disease. Marine Drugs, 13(4), 2250-2266. https://doi.org/10.3390/md13042250





