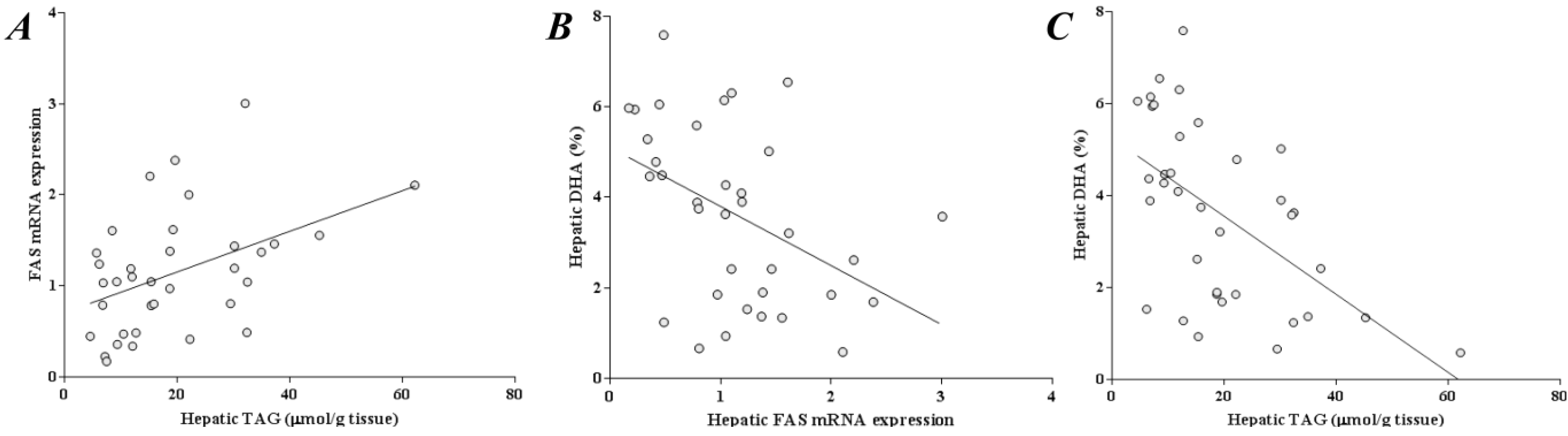
Dietary Docosahexaenoic Acid and Eicosapentaenoic Acid Influence Liver Triacylglycerol and Insulin Resistance in Rats Fed a High-Fructose Diet
Abstract
:1. Introduction
2. Results
2.1. Growth, Body and Tissue Weight and Metabolic Parameters
| Parameter | Fr | FrFO2 | FrFO5 | FrFO7 | C |
|---|---|---|---|---|---|
| Final body weight (g) | 614 ± 30 | 594 ± 24 | 588 ± 14 | 609 ± 22 | 558 ± 41 |
| Body weight gain (g) | 375 ± 30 | 346 ± 20 | 333 ± 13 | 350 ± 23 | 331 ± 34 |
| Food intake (g/day) | 26 ± 1 a,* | 28 ± 1 a,b | 29 ± 1 b | 28 ± 1 a,b | 31 ± 2 |
| Fructose intake (g/day) # | 16.25 ± 1.18 * | 17.35 ± 0.82 * | 18.45 ± 0.64 * | 17.83 ± 0.88 * | 1.56 ± 0.09 |
| Fish oil intake (g/day) ## | - | 0.59 ± 0.03 a | 1.59 ± 0.02 b | 2.26 ± 0.02 c | - |
| EPA+DHA (mg/day) | 7 ± 0.1 a | 128 ± 5 b,* | 328 ± 11 c,* | 440 ± 13 d,* | 8 ± 0.16 |
| EPA+DHA (mg/day/kg body weight) | 15 ± 2 a,* | 243 ± 28 b,* | 654 ± 56 c,* | 917 ± 61 d,* | 18 ± 1 |
| Serum Oxidative Stress Parameters | |||||
| FRAP (μmol/L) | 384.5 ± 19.2 a | 331.4 ± 11.1 a,b,* | 339.5 ± 6.86 a,b,* | 317.5 ± 20.6 b,* | 390.9 ± 20.24 |
| Hydroperoxides (μmol/L) | 40.3 ± 4.1 * | 51.8 ± 3.6 * | 50.1 ± 2.3 * | 53.5 ± 6.5 * | 30.6 ± 2.73 |
| TBARS (nmol/g protein) | 53.1 ± 4.6 | 75.7 ± 7.7 | 69.3 ± 4.0 | 65.8 ± 5.8 | 61.0 ± 7.06 |
| GSH (nmol/g protein) | 116.9 ± 8.4 | 116.8 ± 4.8 | 120.6 ± 6.0 * | 125.8 ± 7.8 * | 92.8 ± 8.53 |
| Liver Oxidative Stress Parameters | |||||
| GSH-Red (mU/mg protein) | 10.0 ± 3.5 | 8.1 ± 1.3 | 8.6 ± 1.0 | 13.9 ± 1.6 | 11.2 ± 2.78 |
| GSH-Px (mU/mg protein) | 111.8 ± 8.7 | 102.2 ± 17.0 | 70.5 ± 7.7 * | 106.6 ± 12.7 | 134.5 ± 27.1 |
| TBARS (nmol/g protein) | 409.3 ± 68.8 ª,b | 198.8 ± 29.1 a,* | 311.7 ± 48.1 a | 563.8 ± 65.6 b | 448.6 ± 101.8 |
| GSH (mmol/g protein) | 21.1 ± 1.8 | 16.4 ± 2.8 | 15.4 ± 1.6 | 22.5 ± 1.7 | 22.2 ± 4.2 |
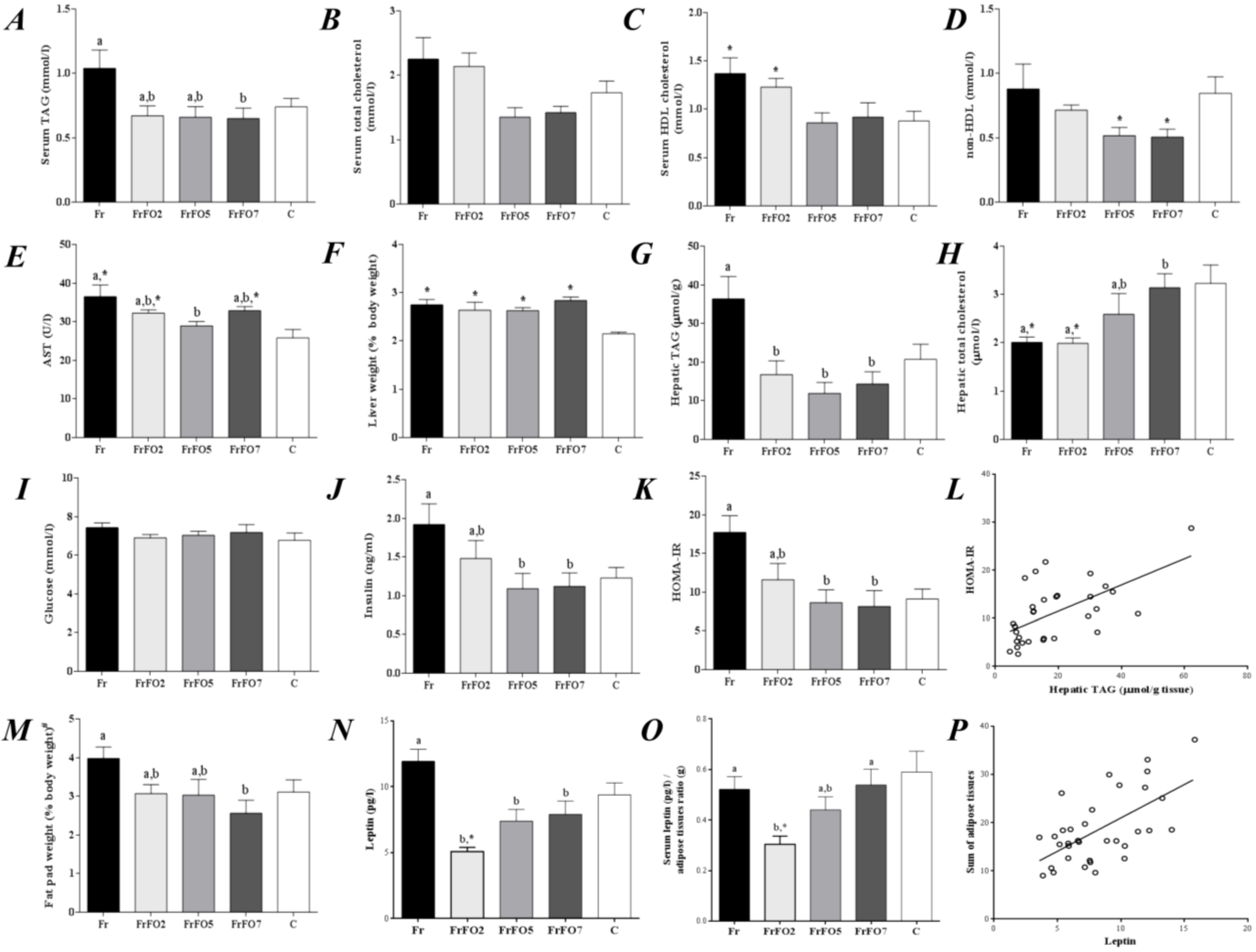
2.2. Oxidative Stress Markers
2.3. Hepatic Gene Expression
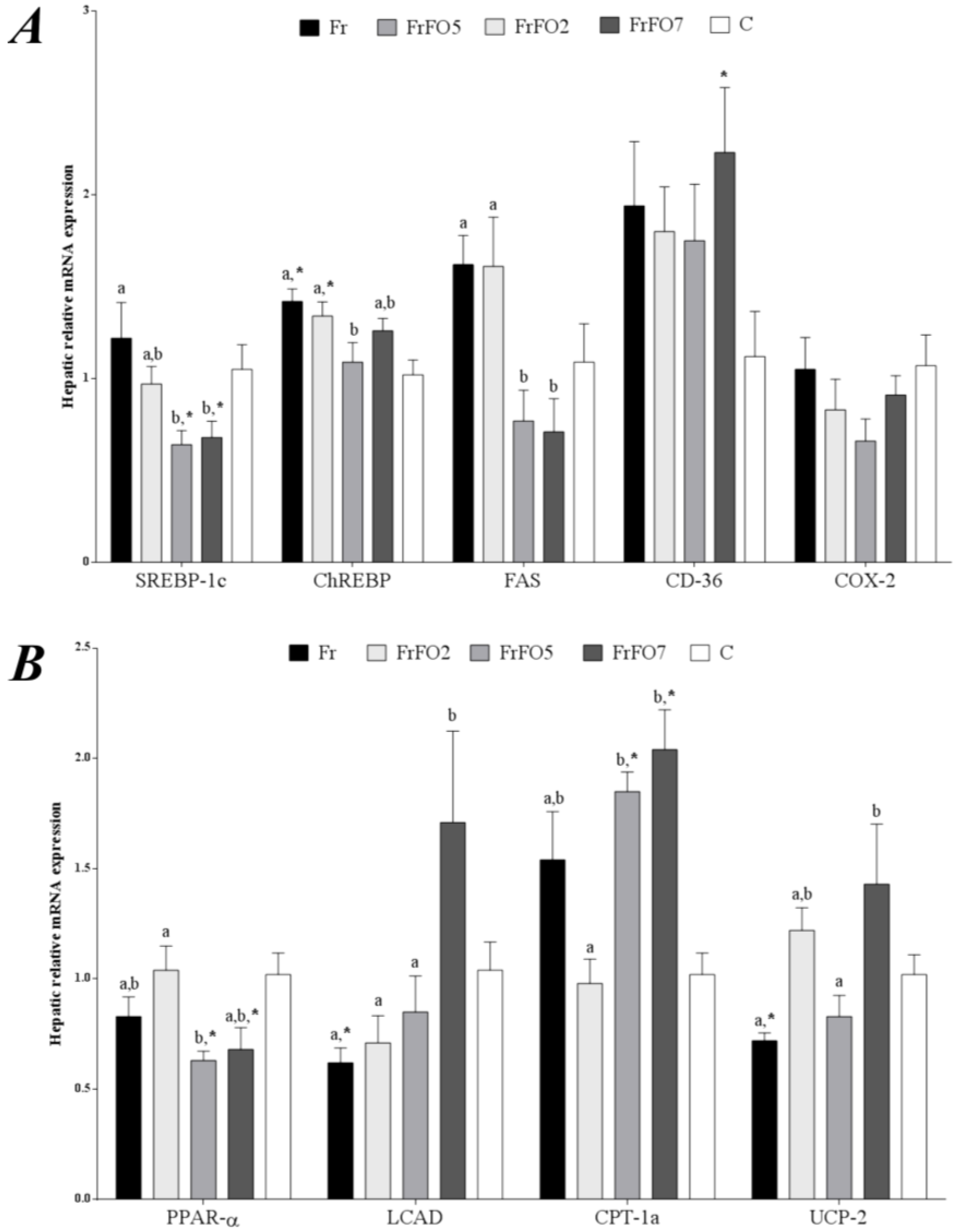
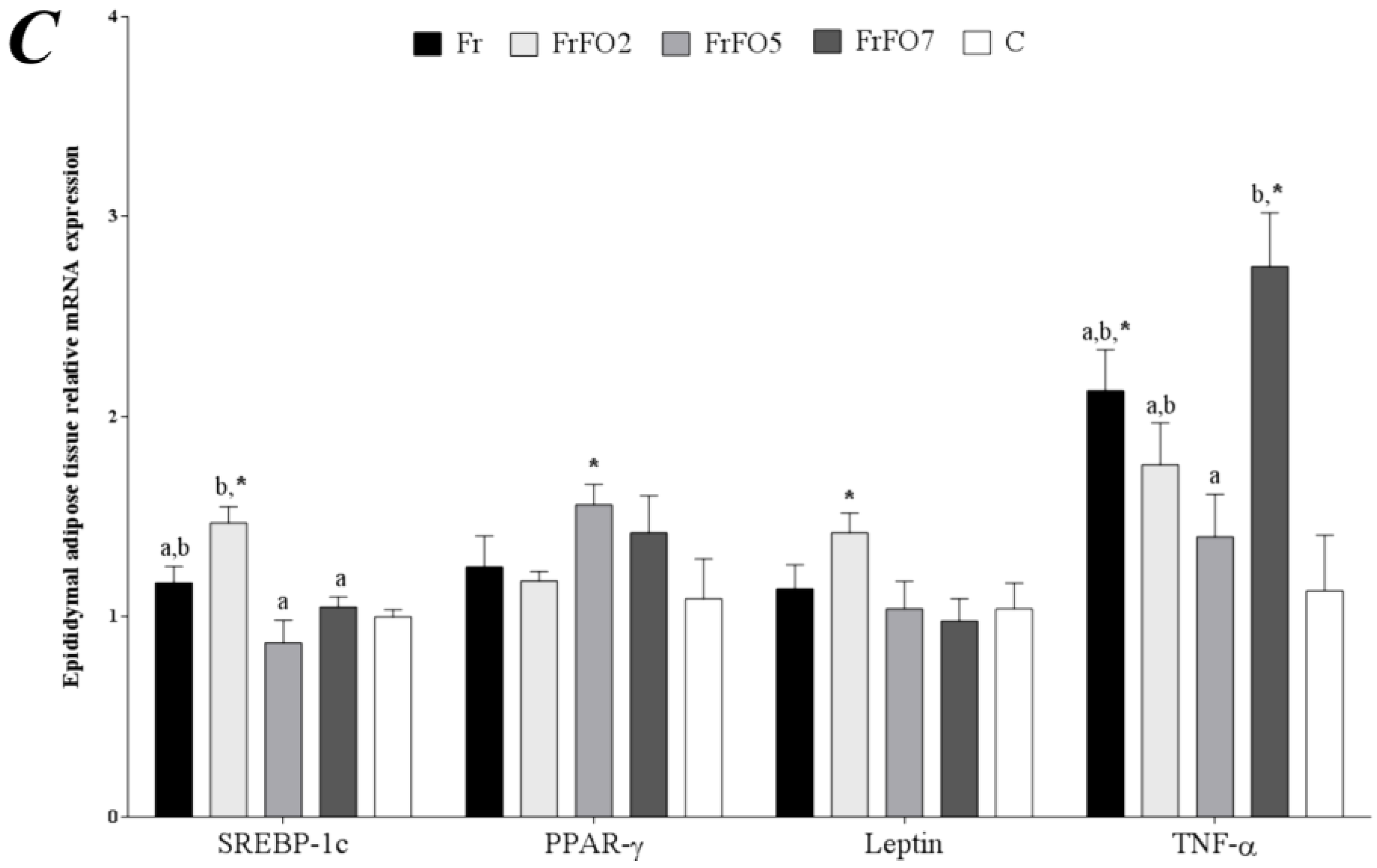
2.4. Adipose Tissue Gene Expression
2.5. Fatty acid Profile of Liver and Adipose Tissue
| Fatty acid | Fr | FrFO2 | FrFO5 | FrFO7 | C |
|---|---|---|---|---|---|
| Liver | |||||
| 14:0 | 0.9 ± 0.1 a,* | 0.6 ± 0.1 a,b | 0.5 ± 0.04 b | 0.7 ± 0.1 a,b | 0.5 ± 0.2 |
| 16:0 | 29.1 ± 1.0 a | 24.2 ± 1.3 b | 23.4 ± 0.6 b | 26.4 ± 0.7 a,b | 25.7 ± 3.4 |
| 18:0 | 12.9 ± 0.9 | 15.4 ± 1.0 | 14.6 ± 0.5 | 14.6 ± 1.3 | 14.3 ± 3.5 |
| Total SFA | 43.2 ± 0.4 a | 40.7 ± 0.8 a,b | 39.1 ± 0.4 b | 42.6 ± 0.8 a | 41.0 ± 3.3 |
| 16:1 | 5.1 ± 0.6 * | 3.5 ± 0.6 | 3.2 ± 0.5 | 4.6 ± 0.7 * | 1.9 ± 1.1 |
| 18:1n-9 | 23.0 ± 1.1 a,* | 17.0 ± 2.0 a,b | 14.8 ± 1.4 b | 16.3 ± 1.6 b | 16.5 ± 5.1 |
| Total MUFA | 28.3 ± 1.8 a,* | 20.7 ± 2.5 a,b | 18.3 ± 1.9 b | 24.6 ± 3.3 a,b | 18.5 ± 6.0 |
| 18:2n-6 | 11.6 ± 0.7 a,* | 14.1 ± 1.0 a,b,* | 16.0 ± 1.3 b,* | 11.0 ± 0.8 a,* | 21.9 ± 3.1 |
| 18:3n-3 | 0.4 ± 0.04 a,* | 0.5 ± 0.04 a,b,* | 0.8 ± 0.1 c | 0.7 ± 0.1 b,c,* | 0.8 ± 0.1 |
| 20:4n-6 | 8.1 ± 0.8 a | 13.1 ± 1.4 b | 11.1 ± 0.5 a,b | 8.6 ± 0.8 a | 10.4 ± 5.3 |
| 20:5n-3 | 0.1 ± 0.04 a | 0.9 ± 0.1 a,* | 2.9 ± 0.2 b,* | 3.8 ± 0.5 b,* | 0.2 ± 0.1 |
| 22:6n-3 | 1.6 ± 0.2 a | 3.9 ± 0.4 b,* | 5.3 ± 0.4 b,c,* | 5.4 ± 0.4 c,* | 1.3 ± 0.5 |
| Total PUFA | 22.1 ± 1.8 a,* | 33.1 ± 2.9 b | 37.1 ± 2.0 b | 30.1 ± 1.7 a,b | 35.2 ± 6.8 |
| n-6/n-3 | 9.7 ± 0.6 a,* | 5.2 ± 0.2 b,* | 3.1 ± 0.2 c,* | 2.1 ± 0.2 c,* | 14.3 ± 3.3 |
| Epididymal Adipose Tissue | |||||
| 14:0 | 1.20 ± 0.02 a | 1.3 ± 0.02 a,* | 1.6 ± 0.04 b,* | 2.1 ± 0.1 c,* | 1.5 ± 0.05 |
| 16:0 | 21.7 ± 0.3 | 21.3 ± 0.5 | 21.8 ± 0.6 | 23.0 ± 0.7 | 21.2 ± 1.20 |
| 18:0 | 2.1 ± 0.3 a | 2.5 ± 0.04 a,b | 2.7 ± 0.1 b | 2.6 ± 0.1 a,b | 2.5 ± 0.23 |
| Total SFA | 25.4 ± 0.4 a | 25.4 ± 0.5 a | 26.7 ± 0.6 a,b | 28.1 ± 0.7 b,* | 25.1 ± 1.36 |
| 16:1 | 5.0 ± 0.3 a | 5.1 ± 0.3 a | 5.2 ± 0.3 a | 6.7 ± 0.4 b | 5.3 ± 1.18 |
| 18:1n-9 | 27.7 ± 0.2 a,b,* | 28.3 ± 0.6 b,* | 26.8 ± 0.2 a,c,* | 25.5 ± 0.3 c | 25.4 ± 0.91 |
| Total MUFA | 32.9 ± 0.2 * | 33.6 ± 0.6 * | 32.3 ± 0.5 | 32.7 ± 0.5 | 31.0 ± 1.41 |
| 18:2n-6 | 32.5 ± 0.4 a,* | 31.9 ± 0.8 a,* | 30.4 ± 0.8 a,* | 25.9 ± 1.5 b,* | 36.1 ± 1.59 |
| 18:3n-3 | 2.8 ± 0.1 a | 2.6 ± 0.1 a | 2.5 ± 0.1 a,* | 2.0 ± 0.1 b,* | 3.0 ± 0.19 |
| 20:4n-6 | 0.52 ± 0.06 | 0.4 ± 0.05 | 0.39 ± 0.01 | 0.50 ± 0.05 | 0.42 ± 0.15 |
| 20:5n-3 | 0.04 ± 0.01 a | 0.13 ± 0.01 a,* | 0.39 ± 0.02 b,* | 0.98 ± 0.11 c,* | 0.04 ± 0.02 |
| 22:6n-3 | 0.11 ± 0.02 a | 0.28 ± 0.04 a,* | 0.88 ± 0.04 b,* | 2.26 ± 0.24 c,* | 0.06 ± 0.03 |
| Total PUFA | 36.3 ± 0.4 a,* | 35.7 ± 0.9 a,* | 34.82 ± 0.8 a,b,* | 31.9 ± 1.3 b,* | 39.9 ± 1.65 |
| n-6/n-3 | 11.4 ± 0.3 a | 10.8 ± 0.4 a,* | 8.18 ± 0.2 b,* | 5.3 ± 0.5 c,* | 12.0 ± 0.76 |
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Animals and Diets
| Fatty Acid | C and Fr | FrFO2 | FrFO5 | FrFO7 |
|---|---|---|---|---|
| 14:0 | 0.1 | 1.86 | 4.5 | 6.3 |
| 16:0 | 10.6 | 14.63 | 19.9 | 23.6 |
| 16:1 | ND | 1.92 | 4.8 | 6.7 |
| 17:0 | 0.1 | 0.33 | 0.7 | 0.9 |
| 17:1 | 0.1 | 0.29 | 0.6 | 0.9 |
| 18:0 | 2.9 | 3.64 | 4.6 | 5.3 |
| 18:1n-9 | 27.1 | 23.53 | 16.4 | 12.1 |
| 18:2n-6 | 52.0 | 42.65 | 25.1 | 14.3 |
| 18:3n-3 | 5.2 | 4.50 | 3.1 | 2.2 |
| 20:1n-9 | 0.3 | 0.21 | 0.1 | ND |
| 20:2 | 0.2 | 0.35 | 0.5 | 0.6 |
| 20:3n-6 | ND | 0.06 | 0.1 | 0.2 |
| 21:0 | ND | 0.05 | 0.1 | 0.2 |
| 20:3n-3 | ND | 0.02 | 0.01 | 0.1 |
| 20:4n-6 | ND | 0.41 | 1.0 | 1.4 |
| 20:5n-3 | 0.4 | 3.04 | 7.0 | 9.6 |
| 22:1n-9 | ND | 0.10 | 0.2 | 0.3 |
| 24:0 | 0.1 | 0.42 | 0.9 | 1.2 |
| 22:6n-3 | ND | 3.62 | 9.0 | 12.6 |
| Other | 0.9 | 4.27 | 9.3 | 12.6 |
| Total n-6 | 52.0 | 43.11 | 26.2 | 15.9 |
| Total n-3 | 5.6 | 11.17 | 19.1 | 24.5 |
| n-6/n-3 | 9.25 | 3.86 | 1.37 | 0.65 |
4.3. Biochemical Analyses
4.4. mRNA Extraction and Quantification
- SREBP-1c—F AGCACAGCAACCAGAAACTC, R AGGTTTCATGCCCTCCATAG
- ChREBP—F CTTCAAAGGCCTCAAGTTGC; R TTCCTCCGTTGCACATACTG
- FAS—F TCTGATCAGTGGCCTCCTTAAC, R CAGTGCTGAGATGTGGGAATAC
- PPAR-α—F GCAATGCACTGAACATCGAG, R TCTTGCAGCTTCGATCACAC
- LCAD—F AAACAGTCGCACACATCCAG, R CCAGACGTTTGGTTTCATGC
- CPT-1α—F TTGACTCTTTCGGCAAAGGC, R TCCTTGTAATGTGCGAGCTG
- CD-36—F TGGATGTGGAACCCATAACTGG, R TCCCAGTCTCATTTAGCCACAG
- COX-2—F CCAGTATCAGAACCGCATTG, R TGAGCAAGTCCGTGTTCAAG
- UCP-2—F ACAAGACCATTGCACGAGAG, R TGGCATTTCGGGCAACATTG
- Leptin—F CAAGCTGTGCCTATCCACAAAG, R ATGAAGTCCAAACCGGTGAC
- TNF-α—F TGCCTCAGCCTCTTCTCATTC, R TGGGAACTTCTCCTCCTTGTTG
- PPAR-γ—F TGCTTGTGAAGGATGCAAGG, R GCACTTCTGAAACCGACAGTAC
- 18S—F GATAAGCCCAAGCTCAATCG, R TTCTGGAGTAGCGGACATTG
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Taylor, D.S.; Yu-Poth, S.; Huth, P.; Moriarty, K.; Fishell, V.; Hargrove, R.L.; Zhao, G.; Etherton, T.D. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 2000, 71 (Suppl. S1), 179–188. [Google Scholar]
- Schaefer, E.J. Lipoproteins, nutrition, and heart disease. Am. J. Clin. Nutr. 2002, 75, 191–212. [Google Scholar] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Yaqoob, P. Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors 2009, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tsuboyama-Kasaoka, N.; Nakatani, T.; Ishii, M.; Tsutsumi, S.; Aburatani, H.; Ezaki, O. Fish oil feeding alters liver gene expressions to defend against PPARalpha activation and ROS production. Am. J. Physiol. Gastrointest Liver Physiol. 2002, 282. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, Y.B.; Chicco, A.G. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J. Nutr. Biochem. 2006, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Dietary alpha-linolenic acid and health-related outcomes: A metabolic perspective. Nutr. Res. Rev. 2006, 19, 26–52. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.J. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin. Liver Dis. 2004, 8, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Selassie, M.; Sinha, A.C. The epidemiology and aetiology of obesity: A global challenge. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.A.; Stanhope, K.L.; Graham, J.L.; Cummings, B.P.; Wang, W.; Saville, B.R.; Havel, P.J. Fructose-fed rhesus monkeys: A nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin. Transl. Sci. 2011, 4, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Dhawan, T.; Young, S.; Yong, W.H.; Boros, L.G.; Heaney, A.P. Fructose impairs glucose-induced hepatic triglyceride synthesis. Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- De Castro, G.S.; dos Santos, R.A.; Portari, G.V.; Jordao, A.A.; Vannucchi, H. Omega-3 improves glucose tolerance but increases lipid peroxidation and DNA damage in hepatocytes of fructose-fed rats. Appl. Physiol. Nutr. Metab. 2012, 37, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association; Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef]
- Scorletti, E.; Bhatia, L.; McCormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D.; WELCOME Study. Effects of purified eicosapentaenoic and docosahexaenoic acids in non-alcoholic fatty liver disease: Results from the WELCOME* study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef]
- Dentin, R.; Girard, J.; Postic, C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): Two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie 2005, 87, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Dentin, R.; Benhamed, F.; Pegorier, J.P.; Foufelle, F.; Viollet, B.; Vaulont, S.; Girard, J.; Postic, C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Invest. 2005, 115, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.E.; Chicco, A.; Lombardo, Y.B. Fish oil reverses the altered glucose transporter, phosphorylation, insulin receptor substrate-1 protein level and lipid contents in the skeletal muscle of sucrose-rich diet fed rats. Prostaglandins Leukot Essent Fatty Acids 2013, 88, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Lodhi, I.J.; Yin, L.; Malapaka, R.R.; Xu, H.E.; Turk, J.; Semenkovich, C.F. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell 2009, 138, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Dobrzyn, A.; Man, W.C.; Chu, K.; Sampath, H.; Kim, H.J.; Ntambi, J.M. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 2004, 279, 25164–25171. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Morgado, N.; Rigotti, A.; Valenzuela, A. Comparative effect of fish oil feeding and other dietary fatty acids on plasma lipoproteins, biliary lipids, and hepatic expression of proteins involved in reverse cholesterol transport in the rat. Ann. Nutr. Metab. 2005, 49, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cho, H.; OʼMalley, S.; Park, J.H.; Clarke, S.D. Dietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and -2 in three distinct stages and by different mechanisms. J. Nutr. 2002, 132, 3333–3339. [Google Scholar] [PubMed]
- Miller, M.; Ginsberg, H.N.; Schaefer, E.J. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am. J. Cardiol. 2008, 101, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Axen, K.V.; Axen, K. Very low-carbohydrate versus isocaloric high-carbohydrate diet in dietary obese rats. Obesity (Silver Spring) 2006, 14, 1344–1352. [Google Scholar] [CrossRef]
- Park, O.J.; Cesar, D.; Faix, D.; Wu, K.; Shackleton, C.H.; Hellerstein, M.K. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem. J. 1992, 282, 753–757. [Google Scholar] [PubMed]
- Zhang, Y.H.; An, T.; Zhang, R.C.; Zhou, Q.; Huang, Y.; Zhang, J. Very high fructose intake increases serum LDL-cholesterol and total cholesterol: A meta-analysis of controlled feeding trials. J. Nutr. 2013, 143, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.S.; Lombardo, Y.B.; Lacorte, J.M.; Chicco, A.G.; Rouault, C.; Slama, G.; Rizkalla, S.W. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289. [Google Scholar] [CrossRef]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Kim, H.J.; Kaburagi, Y.; Yasuda, K.; Ezaki, O. A low fish oil inhibits SREBP-1 proteolytic cascade, while a high-fish-oil feeding decreases SREBP-1 mRNA in mice liver: Relationship to anti-obesity. J. Lipid Res. 2003, 44, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, V.; Gao, L.; Morrow, J.D.; Chait, A.; Niswender, K.D.; Hasty, A.H. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J. Nutr. 2007, 137, 1776–1782. [Google Scholar] [PubMed]
- Yasuda, S.; Watanabe, S.; Kobayashi, T.; Okuyama, H. Effects of dietary unsaturated fatty acid and chronic carbon tetrachloride treatment on the accumulation of oxidation products, alpha-tocopherol and liver injury in mice. Biol. Pharm. Bull. 1998, 21, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Skuladottir, G.V.; Shi-Hua, D.; Brodie, A.E.; Reed, D.J.; Wander, R.C. Effects of dietary oils and methyl ethyl ketone peroxide on in vivo lipid peroxidation and antioxidants in rat heart and liver. Lipids 1994, 29, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Botham, K.M.; Zheng, X.; Napolitano, M.; Avella, M.; Cavallari, C.; Rivabene, R.; Bravo, E. The effects of dietary n-3 polyunsaturated fatty acids delivered in chylomicron remnants on the transcription of genes regulating synthesis and secretion of very-low-density lipoprotein by the liver: Modulation by cellular oxidative state. Exp. Biol. Med. (Maywood) 2003, 228, 143–151. [Google Scholar]
- Anderson, E.J.; Thayne, K.; Harris, M.; Carraway, K.; Shaikh, S.R. Aldehyde stress and up-regulation of Nrf2-mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n-3 polyunsaturated fatty acids. Biochem. J. 2012, 441, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Jude, S.; Bedut, S.; Roger, S.; Pinault, M.; Champeroux, P.; White, E.; le Guennec, J.Y. Peroxidation of docosahexaenoic acid is responsible for its effects on I TO and I SS in rat ventricular myocytes. Br. J. Pharmacol. 2003, 139, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Petursdottir, D.H.; Olafsdottir, I.; Hardardottir, I. Dietary fish oil increases tumor necrosis factor secretion but decreases interleukin-10 secretion by murine peritoneal macrophages. J. Nutr. 2002, 132, 3740–3743. [Google Scholar] [PubMed]
- Petursdottir, D.H.; Hardardottir, I. Dietary fish oil increases the number of splenic macrophages secreting TNF-alpha and IL-10 but decreases the secretion of these cytokines by splenic T cells from mice. J. Nutr. 2007, 137, 665–670. [Google Scholar] [PubMed]
- Wang, Y.C.; Kuo, W.H.; Chen, C.Y.; Lin, H.Y.; Wu, H.T.; Liu, B.H.; Chen, C.H.; Mersmann, H.J.; Chang, K.J.; Ding, S.T. Docosahexaenoic acid regulates serum amyloid A protein to promote lipolysis through down regulation of perilipin. J. Nutr. Biochem. 2010, 21, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.C.; Ding, S.T. N-3 polyunsaturated fatty acids regulate lipid metabolism through several inflammation mediators: Mechanisms and implications for obesity prevention. J. Nutr. Biochem. 2010, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Lewis, T.; Nichols, P.D.; McMeekin, T.A. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J. Microbiol. Methods 2000, 43, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Piroddi, M.; Annetti, C.; Aisa, C.; Floridi, E.; Floridi, A. Oxidative stress and reactive oxygen species. Contrib. Nephrol. 2005, 149, 240–260. [Google Scholar] [PubMed]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellmanʼs reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salim de Castro, G.; Deminice, R.; Cordeiro Simões-Ambrosio, L.M.; C. Calder, P.; A. Jordão, A.; Vannucchi, H. Dietary Docosahexaenoic Acid and Eicosapentaenoic Acid Influence Liver Triacylglycerol and Insulin Resistance in Rats Fed a High-Fructose Diet. Mar. Drugs 2015, 13, 1864-1881. https://doi.org/10.3390/md13041864
Salim de Castro G, Deminice R, Cordeiro Simões-Ambrosio LM, C. Calder P, A. Jordão A, Vannucchi H. Dietary Docosahexaenoic Acid and Eicosapentaenoic Acid Influence Liver Triacylglycerol and Insulin Resistance in Rats Fed a High-Fructose Diet. Marine Drugs. 2015; 13(4):1864-1881. https://doi.org/10.3390/md13041864
Chicago/Turabian StyleSalim de Castro, Gabriela, Rafael Deminice, Livia Maria Cordeiro Simões-Ambrosio, Philip C. Calder, Alceu A. Jordão, and Helio Vannucchi. 2015. "Dietary Docosahexaenoic Acid and Eicosapentaenoic Acid Influence Liver Triacylglycerol and Insulin Resistance in Rats Fed a High-Fructose Diet" Marine Drugs 13, no. 4: 1864-1881. https://doi.org/10.3390/md13041864
APA StyleSalim de Castro, G., Deminice, R., Cordeiro Simões-Ambrosio, L. M., C. Calder, P., A. Jordão, A., & Vannucchi, H. (2015). Dietary Docosahexaenoic Acid and Eicosapentaenoic Acid Influence Liver Triacylglycerol and Insulin Resistance in Rats Fed a High-Fructose Diet. Marine Drugs, 13(4), 1864-1881. https://doi.org/10.3390/md13041864