Hyperoxia Elevates Adrenic Acid Peroxidation in Marine Fish and Is Associated with Reproductive Pheromone Mediators
Abstract
:1. Introduction
2. Results
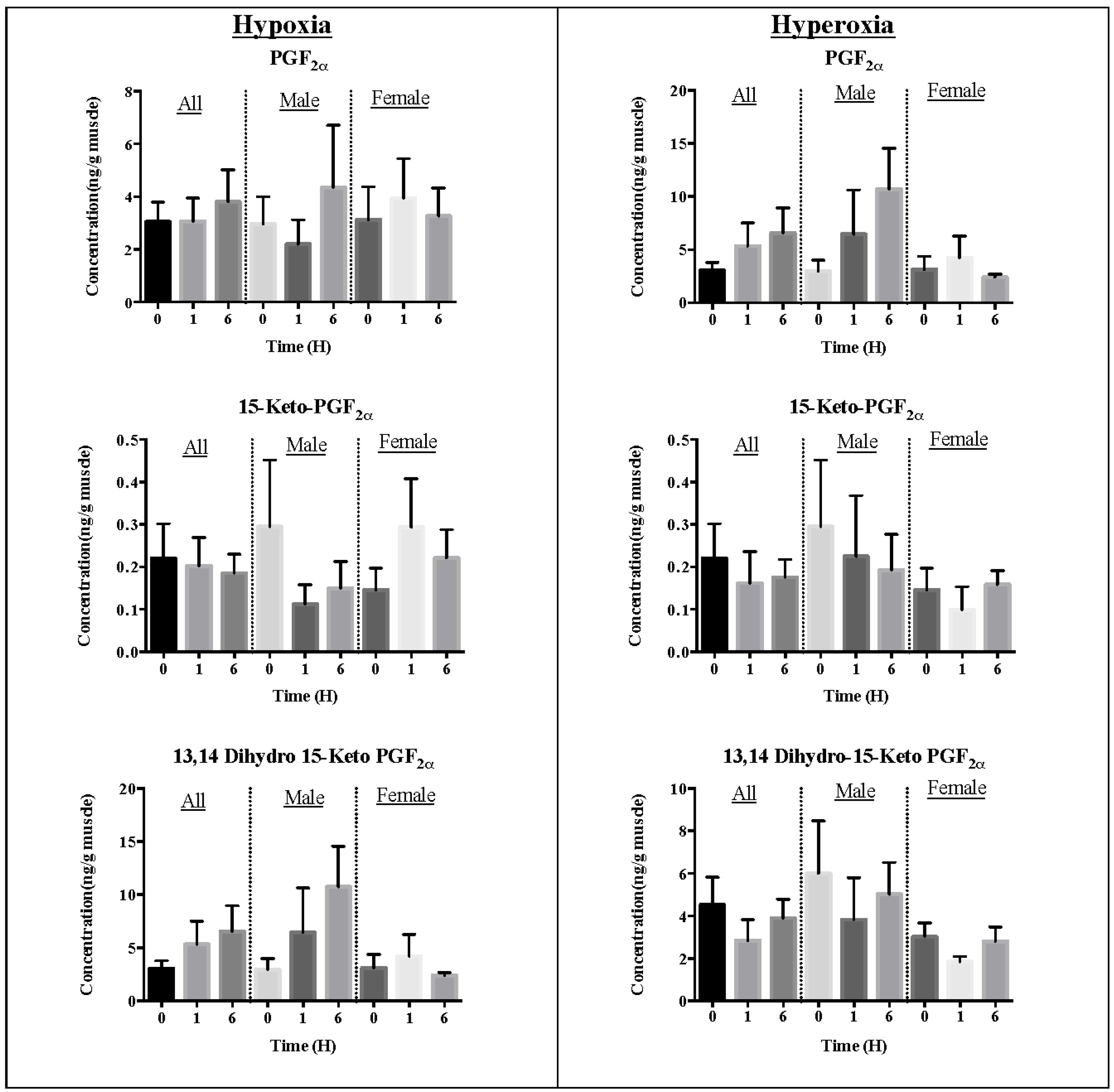
2.1. Effect of Oxidative Stress on Eicosanoid Pheromone Mediators
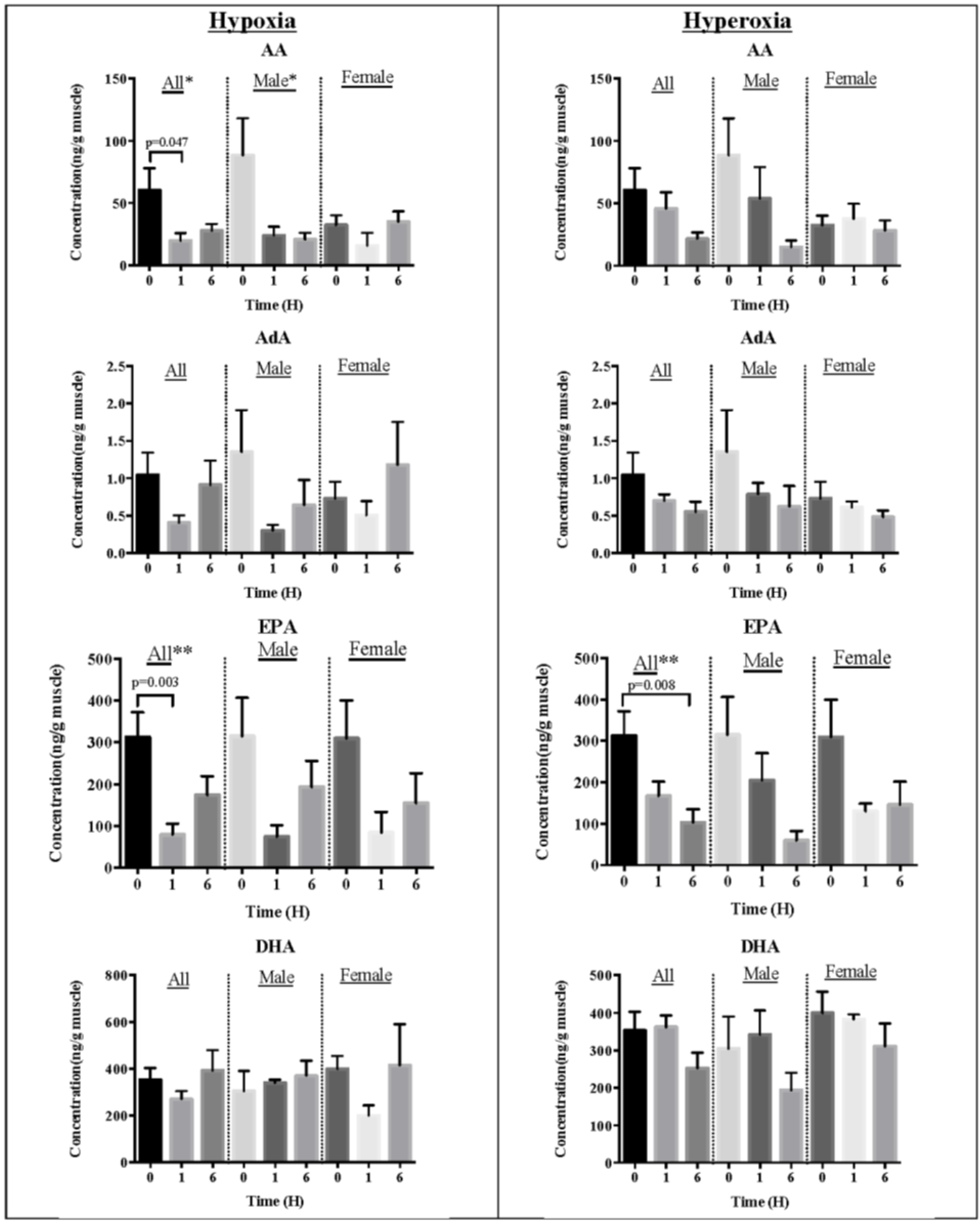
2.2. Effect of Oxidative Stress on Fatty Acids
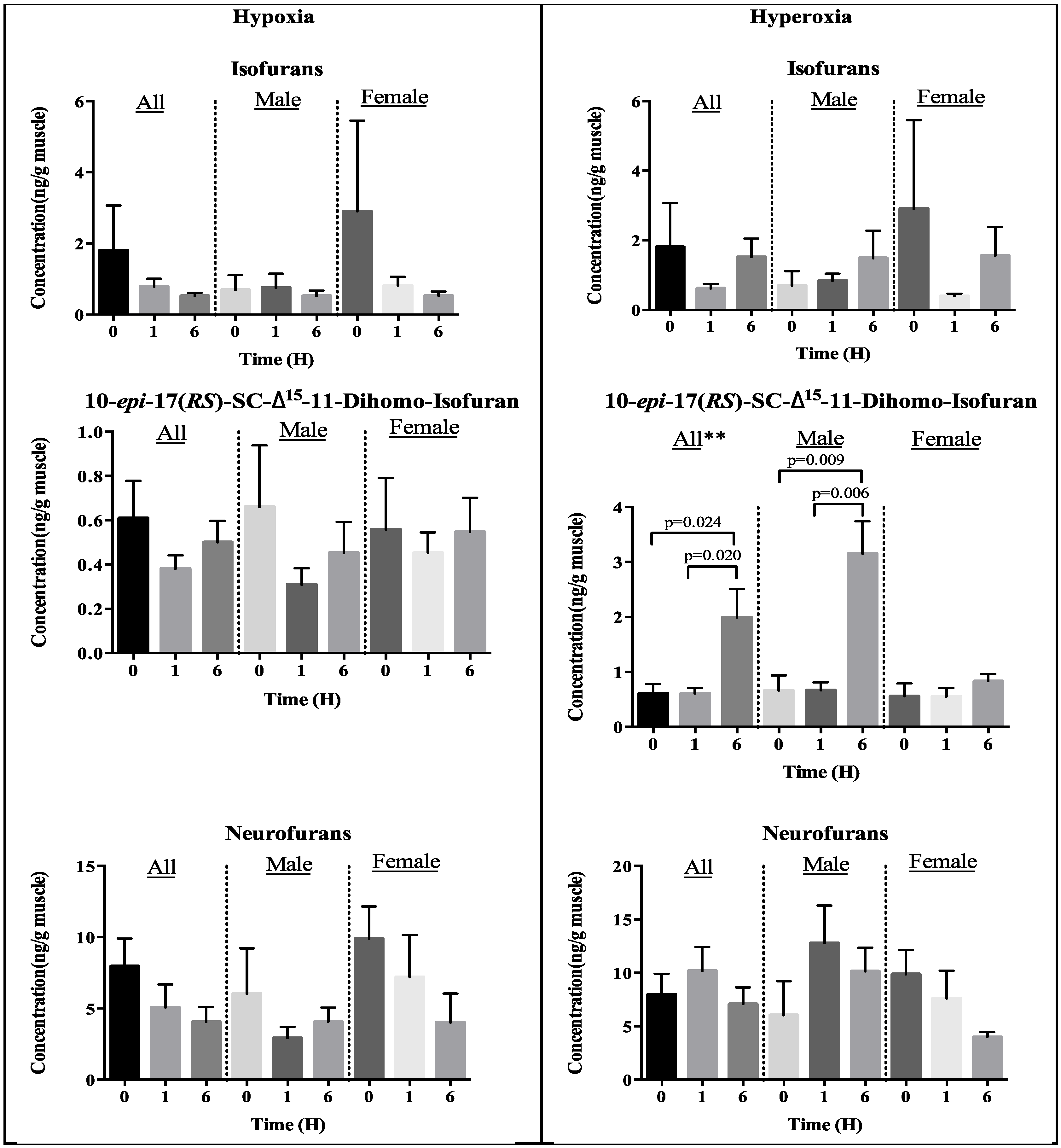
2.3. Effect of Oxidative Stress on Generation of Lipid Peroxidation Products
2.3.1. Enzyme-Independent
2.3.2. Enzyme-Dependent
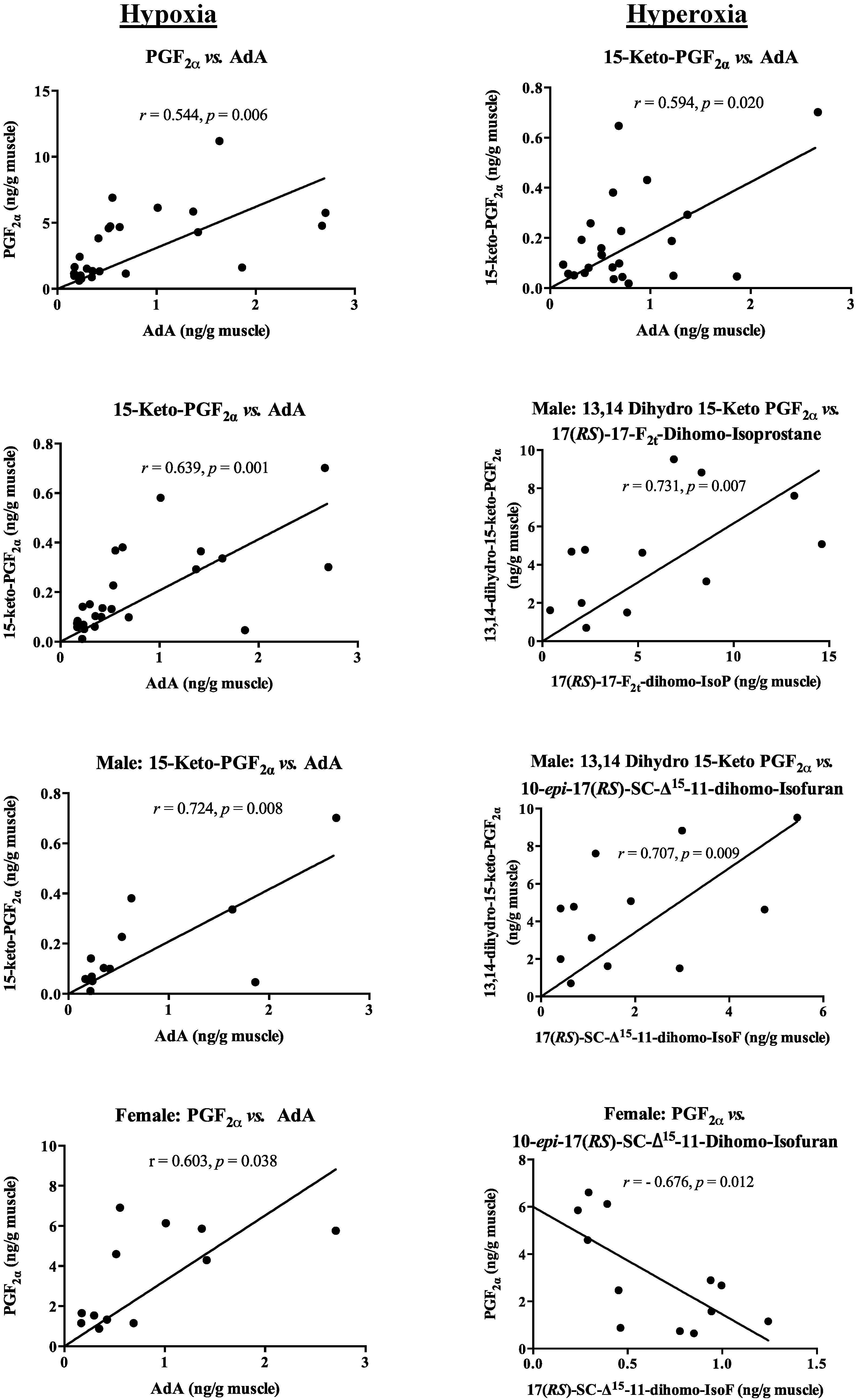
2.4. Correlation between PUFA and Oxidized Lipid Products, and Pheromone Mediators
| Normoxia | Hypoxia | Hyperoxia | ||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | 0 | 1 | 6 | p-trend | 1 | 6 | p-trend | |
| Arachidonic Acid | ||||||||
| 15-F2t-IsoP | All | 2.20 ± 0.76 | 2.44 ± 1.49 | 2.93 ± 0.90 | 0.727 | 1.26 ± 0.26 | 5.68 ± 2.24 | 0.078 |
| M | 1.51 ± 0.58 | 3.46 ± 2.90 | 2.33 ± 0.65 | 0.738 | 1.24 ± 0.39 | 7.24 ± 4.23 | 0.210 | |
| F | 2.88 ± 1.43 | 3.41 ± 1.40 | 3.54 ± 1.77 | 0.951 | 1.29 ± 0.40 | 4.12 ± 1.94 | 0.398 | |
| 2,3-dinor-15-F2t-IsoP+ | All | 1.89 ± 1.03 | 0.32 ± 0.06 | 0.76 ± 0.27 | 0.202 | 0.94 ± 0.25 | 0.63 ± 0.20 | 0.349 |
| M | 2.68 ± 2.13 | 0.27 ± 0.02 | 0.80 ± 0.32 | 0.394 | 1.24 ± 0.38 | 0.57 ± 0.20 | 0.505 | |
| F | 1.10 ± 0.14 | 0.36 ± 0.12 | 0.72 ± 0.47 | 0.258 | 0.64 ± 0.29 | 0.72 ± 0.47 | 0.504 | |
| 2,3-dinor-5,6-dihydro-15-F2t-IsoP+ | All | 19.43 ± 8.34 | 7.54 ± 1.39 | 7.96 ± 0.75 | 0.177 | 23.00 ± 8.27 | 13.84 ± 4.31 | 0.670 |
| M | 25.01 ± 16.30 | 0.27 ± 0.02 | 7.98 ± 1.44 | 0.384 | 29.00 ± 14.67 | 16.85 ± 8.58 | 0.816 | |
| F | 13.16 ± 6.34 | 7.13 ± 2.03 | 7.95 ± 0.73 | 0.440 | 17.00 ± 8.94 | 10.83 ± 2.61 | 0.803 | |
| Adrenic Acid | ||||||||
| 7(RS)-7-F2t-dihomo-IsoP | All | 0.98 ± 0.30 | 0.47 ± 0.09 | 0.50 ± 0.09 | 0.118 | 0.63 ± 0.15 | 0.56 ± 0.13 | 0.321 |
| M | 0.81 ± 0.43 | 0.49 ± 0.19 | 0.36 ± 0.09 | 0.537 | 0.68 ± 0.22 | 0.79 ± 0.17 | 0.952 | |
| F | 1.17 ± 0.46 | 0.45 ± 0.06 | 0.64 ± 0.13 | 0.218 | 0.59 ± 0.25 | 0.33 ± 0.11 | 0.198 | |
| 17(RS)-17-F2t-dihomo-IsoP | All | 3.44 ± 1.43 | 2.37 ± 0.37 | 3.05 ± 0.54 | 0.706 | 3.99 ± 0.87 | 5.78 ± 1.45 | 0.409 |
| M | 4.33 ± 2.97 | 2.43 ± 0.61 | 2.13 ± 0.34 | 0.646 | 4.95 ± 1.64 | 8.15 ± 2.31 | 0.501 | |
| F | 2.55 ± 0.42 | 2.31 ± 0.52 | 3.98 ± 0.83 | 0.173 | 3.02 ± 0.44 | 3.44 ± 0.86 | 0.604 | |
| Eicosapentaenoic Acid | ||||||||
| 8-F3t-IsoP | All | 2.95 ± 0.53 | 2.58 ± 0.57 | 2.37 ± 0.33 | 0.705 | 2.75 ± 0.64 | 3.73 ± 0.92 | 0.598 |
| M | 3.03 ± 0.90 | 2.51 ± 1.05 | 2.65 ± 0.63 | 0.912 | 3.77 ± 0.98 | 4.48 ± 1.58 | 0.701 | |
| F | 2.86 ± 0.70 | 2.65 ± 0.63 | 2.10 ± 0.23 | 0.626 | 1.74 ± 0.52 | 2.99 ± 1.04 | 0.492 | |
| Docosahexaenoic Acid | ||||||||
| 4(RS)-4-F4t-NeuroP | All | 61.07 ± 11.80 | 71.44 ± 28.17 | 33.77 ± 6.79 | 0.284 | 61.07 ± 11.80 | 77.33 ± 26.96 | 0.766 |
| M | 54.21 ± 7.74 | 73.51 ± 49.02 | 37.96 ± 12.63 | 0.706 | 59.27 ± 19.80 | 97.96 ± 50.16 | 0.580 | |
| F | 70.95 ± 6.41 | 69.37 ± 36.00 | 29.58 ± 6.63 | 0.347 | 62.87 ± 15.98 | 56.72 ± 24.35 | 0.845 | |
| 10-F4t-NeuroP | All | 1.54 ± 0.21 | 1.64 ± 0.50 | 0.94 ± 0.32 | 0.356 | 1.59 ± 0.25 | 1.01 ± 0.22 | 0.164 |
| M | 1.06 ± 0.13 | 0.94 ± 0.32 | 1.21 ± 0.59 | 0.894 | 1.35 ± 0.35 | 1.04 ± 0.44 | 0.769 | |
| F | 2.01 ± 0.22 ** | 2.34 ± 0.85 | 0.68 ± 0.27 | 0.119 | 1.85 ± 0.37 | 0.99 ± 0.16 | 0.049 | |
| Normoxia | Hypoxia | Hyperoxia | ||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | 0 | 1 | 6 | p-trend | 1 | 6 | p-trend | |
| Arachidonic Acid | ||||||||
| 5(S)-HETE | All | 41.90 ± 4.98 | 31.05 ± 11.44 | 39.10 ± 7.06 | 0.672 | 26.20 ± 4.98 | 49.40 ± 12.38 | 0.188 |
| M | 29.62 ± 2.17 | 16.19 ± 3.63 | 40.53 ± 8.07 | 0.029 | 30.10 ± 9.16 | 52.25 ± 16.99 | 0.312 | |
| F | 54.17 ± 12.12 | 45.91 ± 21.21 | 37.66 ± 12.87 | 0.771 | 22.29 ± 4.66 | 46.54 ± 20.53 | 0.294 | |
| 8(S)-HETE | All | 25.70 ± 5.66 | 26.08 ± 7.66 | 11.19 ± 1.93 | 0.126 | 30.79 ± 4.43 | 21.88 ± 3.96 | 0.425 |
| M | 26.41 ± 7.29 | 17.72 ± 7.72 | 10.84 ± 2.99 | 0.274 | 33.95 ± 7.62 | 25.92 ± 7.59 | 0.707 | |
| F | 25.00 ± 9.78 | 34.43 ± 12.94 | 11.54 ± 2.90 | 0.282 | 27.64 ± 5.18 | 17.85 ± 2.14 | 0.566 | |
| 12(S)-HETE | All | 161.96 ± 62.88 | 157.03 ± 50.60 | 69.20 ± 19.23 | 0.325 | 203.29 ± 48.19 | 180.55 ± 53.86 | 0.870 |
| M | 173.00 ± 90.36 | 127.50 ± 47.59 | 73.66 ± 26.64 | 0.538 | 246.30 ± 91.76 | 259.30 ± 94.76 | 0.756 | |
| F | 150.9 ± 101.00 | 186.50 ± 95.41 | 64.73 ± 31.65 | 0.579 | 160.30 ± 34.63 | 101.80 ± 20.51 | 0.783 | |
| 15(S)-HETE | All | 2.53 ± 0.29 | 13.00 ± 6.10 | 5.44 ± 2.55 | 0.160 | 1.79 ± 0.32 | 12.23 ± 4.85 * | 0.027 |
| M | 2.66 ± 0.40 | 127.50 ± 47.59 | 7.63 ± 5.09 | 0.543 | 1.75 ± 0.45 | 15.33 ± 8.74 | 0.781 | |
| F | 2.40 ± 0.48 | 186.50 ± 95.41 | 3.25 ± 1.08 | 0.332 | 1.82 ± 0.52 | 9.13 ± 5.18 | 0.209 | |
| Eicosapentaenoic Acid | ||||||||
| RvE1 | All | 0.32 ± 0.04 | 0.21 ± 0.07 | 0.33 ± 0.12 | 0.533 | 0.17 ± 0.03 | 0.23 ± 0.04 | 0.027 |
| M | 0.26 ± 0.04 | 0.12 ± 0.01 | 0.28 ± 0.16 | 0.446 | 0.13 ± 0.05 | 0.27 ± 0.05 | 0.142 | |
| F | 0.39 ± 0.07 | 0.30 ± 0.12 | 0.38 ± 0.20 | 0.894 | 0.20 ± 0.04 | 0.19 ± 0.05 | 0.045 | |
| Docosahexaenoic Acid | ||||||||
| RvD1 | All | 1.15 ± 0.15 | 0.71 ± 0.11 | 2.91 ± 1.69 | 0.265 | 0.83 ± 0.09 | 0.72 ± 0.15 | 0.073 |
| M | 1.02 ± 0.22 | 0.66 ± 0.12 | 1.88 ± 0.90 | 0.304 | 0.82 ± 0.11 | 0.69 ± 0.26 | 0.550 | |
| F | 1.28 ± 0.20 | 0.76 ± 0.20 | 3.95 ± 3.43 | 0.505 | 0.85 ± 0.15 | 0.75 ± 0.19 | 0.132 | |
2.5. Effect of Hypoxia-Hyperoxia on Reproductive Behavior and Discharge of Pheromone Mediators in Water
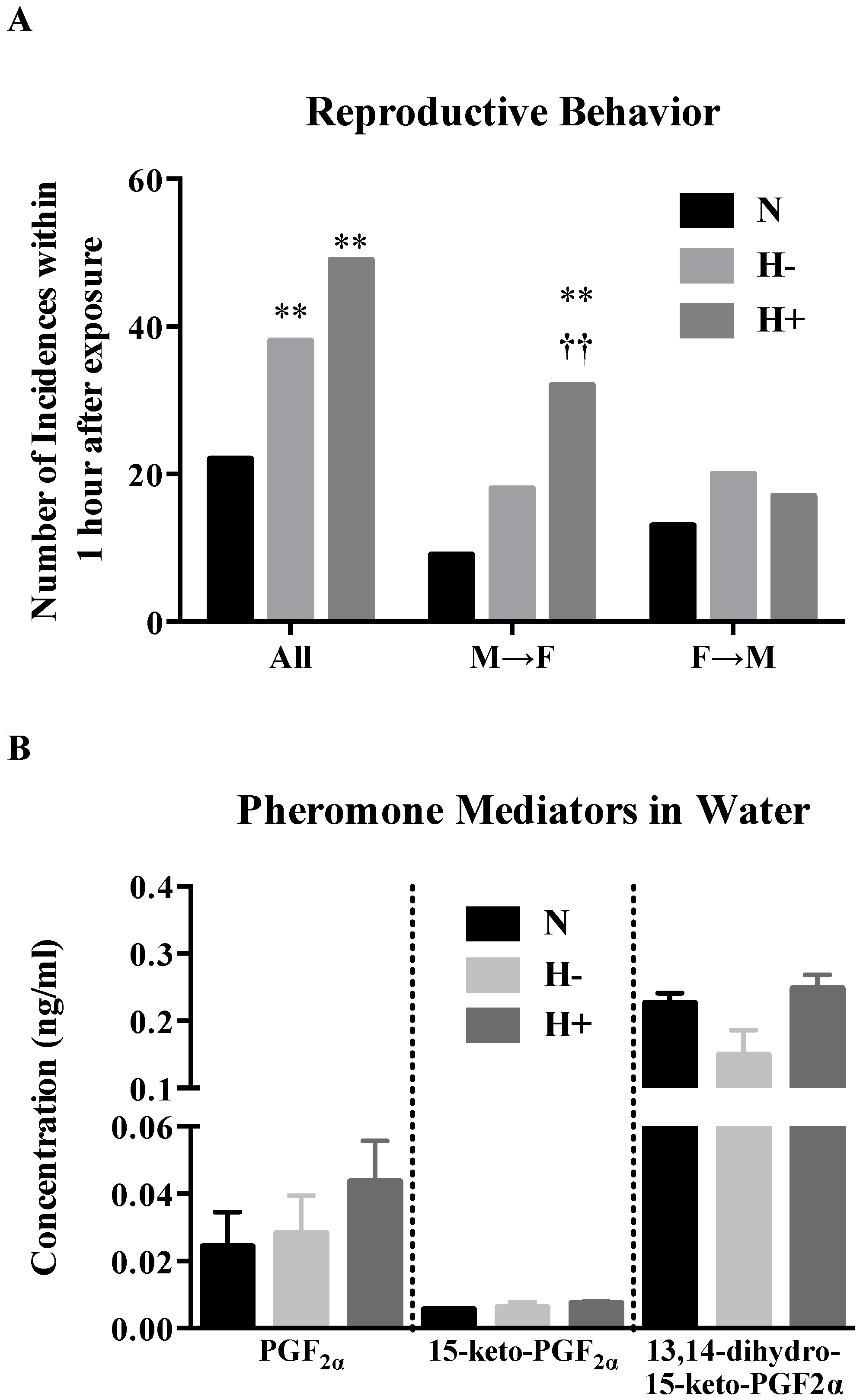
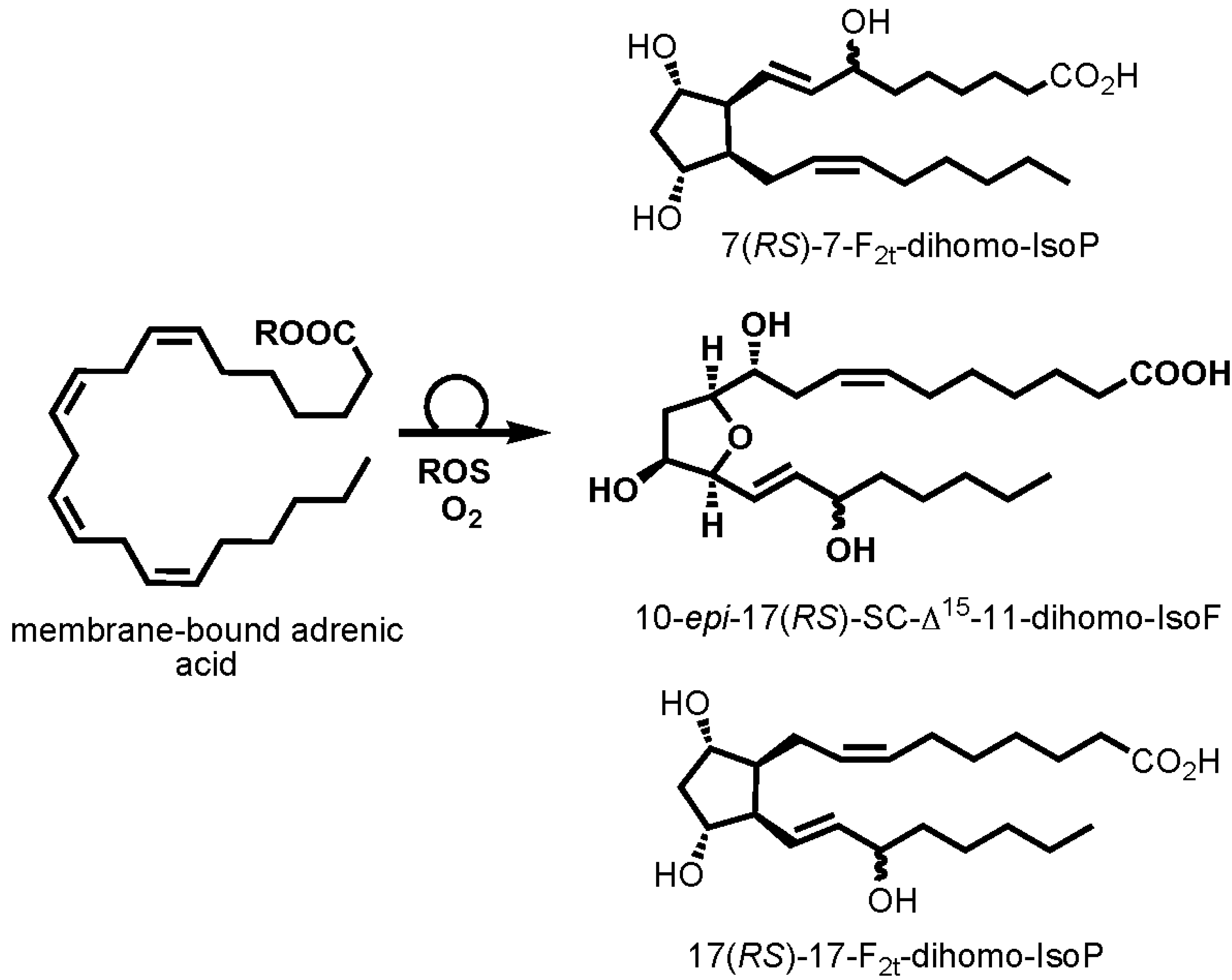
3. Discussion
4. Materials and Methods
4.1. Treatment of Medaka Fish
4.2. Sample Preparation for Lipid Extraction
4.3. Analysis of Oxidized Lipid Products of Fatty Acids
4.4. Pheromone Mediator Analysis
4.5. Reproductive Behavior
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Abbreviation
| 2,3-dinor-15-F2t-IsoP | 2,3-dinor-15-F2t-isoprostane |
| 2,3-dinor-5,6-dihydro-15-F2t-IsoP | 2,3-dinor-5,6-dihydro-15-F2t-isoprostane |
| 4(RS)-4-F4t-NeuroP | 4(RS)-4-F4t-neuroprostane |
| 7(RS)-7-F2t-dihomo-IsoP | 7(RS)-7-F2t-dihomo-isoprostane |
| 8-F3t-IsoP | 8-F3t-isoprostane |
| 10-F4t-NeuroP | 10-F4t-neuroprostane |
| 13,14-dihydro-15-keto-PGF2α | 13,14-dihydro-15-keto prostaglandin F2α |
| 15-F2t-IsoP | 15-F2t-isoprostane |
| 15-keto-PGF2α | 15-keto-prostaglandin F2α |
| 17(RS)-17-F2t-dihomo-IsoP | 17(RS)-17-F2t-dihomo-isoprostane |
| 10-epi-17(RS)-SC-Δ15-11-dihomo-IsoF | 10-epi-17(RS)-SC-Δ15-11-dihomo-isofuran |
| AA | arachidonic acid |
| AdA | adrenic acid |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| HETE | hydroxyeicosatetraenoic acid products |
| IsoFs | isofurans |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| LOX | lipoxygenase |
| NeuroFs | neurofurans |
| PGF2α | prostaglandin F2α |
| RvD1 | resolvin D1 |
| RvE1 | resolvin E1 |
Conflicts of Interest
References
- Wu, R.S.S. Hypoxia: From molecular responses to ecosystem responses. Mar. Poll Bull. 2002, 45, 35–45. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Met. 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Ann. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Ross, S.W.; Dalton, D.A.; Kramer, S.; Christensen, B.L. Physiological (antioxidant) responses of estuarine fishes to variability in dissolved oxygen. Comp. Biol. Physiol. Part. C 2001, 130, 289–303. [Google Scholar]
- Chung, M.L.S.; Lee, K.Y.; Lee, C.Y.J. Profiling of oxidized lipid products of marine fish under acute oxidative stress. Food Chem. Toxicol. 2013, 53, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.W.; Hara, T.J.; Stacey, N.E.; Goetz, F.W. F prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex pheromone in goldfish. Biol. Reprod. 1988, 39, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.W.; Stacey, N.E.; Chamberlain, K.J. Differing behavioral and endocrinological effects of two female sex Pheromones on male goldfish. Horm. Behav. 1989, 23, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Sorensen, P.W. Polar metabolites synergize the activity of prostaglandin F2α in a species-specific hormonal sex pheromone released by ovulated common carp. J. Chem. Ecol. 2011, 37, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Bjerselius, R.; Olsen, K.; Zheng, W. Behavioural and endocrinological responses of mature male goldfish to the sex pheromone 17alpha,20beta-dihydroxy-4-pregnen-3-one in the water. J. Exp. Biol. 1995, 198, 747–754. [Google Scholar]
- Wyatt, T.D. Pheromones and Animal Behavior: Communication by Smell and Taste; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Ono, Y.; Uematsu, T. Mating ethogram in Oryzias latipes. J. Fac. Sci. Hokkaido Univ. Ser. VI Zoo 1957, 13, 197–202. [Google Scholar]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; le Faouder, P.; Galano, J.M.; Lee, J.C.Y.; Durand, T. Non-Enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chrom. B 2014, 964, 65–78. [Google Scholar] [CrossRef]
- Milne, G.L.; Yin, H.; Hardy, K.D.; Davies, S.S.; Roberts, L.J., 2nd. Isoprostane generation and function. Chem. Rev. 2011, 111, 5973–5996. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Musiek, E.S.; Gao, L.; Porter, N.A.; Morrow, J.D. Regiochemistry of neuroprostanes generated from the peroxidation of docosahexaenoic acid in vitro and in vivo. J. Biol. Chem. 2005, 280, 26600–26611. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J., 2nd; Montine, T.J.; Markesbery, W.R.; Tapper, A.R.; Hardy, P.; Chemtob, S.; Dettbarn, W.D.; Morrow, J.D. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 1998, 273, 13605–13612. [Google Scholar] [CrossRef]
- Fessel, J.P.; Porter, N.A.; Moore, K.P.; Sheller, J.R.; Roberts, L.J., 2nd. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc. Natl. Acad. Sci. USA 2002, 99, 16713–16718. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J.P.; Roberts, L.J., 2nd. Isofurans: Novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension. Antiox. Redox Signal. 2005, 7, 202–209. [Google Scholar] [CrossRef]
- De La Torre, A.; Lee, Y.Y.; Oger, C.; Sangild, P.T.; Durand, T.; Lee, J.C.Y.; Galano, J.M. Synthesis, discovery, and quantitation of dihomo-isofurans: Biomarkers for in vivo adrenic acid peroxidation. Angew. Chemie Int. Ed. Engl. 2014, 53, 6249–6252. [Google Scholar] [CrossRef]
- Song, W.L.; Lawson, J.A.; Reilly, D.; Rokach, J.; Chang, C.T.; Giasson, B.; Fitzgerald, G.A. Neurofurans novel indices of oxidant stress derived from docosahexaenoic acid. J. Biol. Chem. 2008, 283, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Bocio, A.; Falco, G.; Llobet, J.M. Benefits and risks of fish consumption Part I. A quantitative analysis of the intake of omega-3 fatty acids and chemical contaminants. Toxicology 2007, 230, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Moussavi Nik, S.H.; Croft, K.; Mori, T.A.; Lardelli, M. The comparison of methods for measuring oxidative stress in zebrafish brains. Zebrafish 2014. [Google Scholar] [CrossRef]
- Welker, A.F.; Moreira, D.C.; Campos, E.G.; Hermes-Lima, M. Role redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Physiol. A 2013, 165, 384–404. [Google Scholar] [CrossRef]
- De la Torre, A.; Lee, Y.Y.; Mazzoni, A.; Guy, A.; Bultel-Ponce, V.; Durand, T.; Oger, C.; Lee, J.C.; Galano, J.M. Total syntheses and in vivo quantitation of novel neurofuran and dihomo-isofuran derived from docosahexaenoic acid and adrenic acid. Chem. Eur. J. 2015, 21, 2442–2446. [Google Scholar] [CrossRef] [PubMed]
- Konkel, A.; Schunck, W.H. Role of cytochrome P450 in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta 2011, 1814, 210–222. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, M.L.S.; Galano, J.-M.; Oger, C.; Durand, T.; Lee, J.C.-Y. Hyperoxia Elevates Adrenic Acid Peroxidation in Marine Fish and Is Associated with Reproductive Pheromone Mediators. Mar. Drugs 2015, 13, 2215-2232. https://doi.org/10.3390/md13042215
Chung MLS, Galano J-M, Oger C, Durand T, Lee JC-Y. Hyperoxia Elevates Adrenic Acid Peroxidation in Marine Fish and Is Associated with Reproductive Pheromone Mediators. Marine Drugs. 2015; 13(4):2215-2232. https://doi.org/10.3390/md13042215
Chicago/Turabian StyleChung, Ming Long Sirius, Jean-Marie Galano, Camille Oger, Thierry Durand, and Jetty Chung-Yung Lee. 2015. "Hyperoxia Elevates Adrenic Acid Peroxidation in Marine Fish and Is Associated with Reproductive Pheromone Mediators" Marine Drugs 13, no. 4: 2215-2232. https://doi.org/10.3390/md13042215
APA StyleChung, M. L. S., Galano, J.-M., Oger, C., Durand, T., & Lee, J. C.-Y. (2015). Hyperoxia Elevates Adrenic Acid Peroxidation in Marine Fish and Is Associated with Reproductive Pheromone Mediators. Marine Drugs, 13(4), 2215-2232. https://doi.org/10.3390/md13042215






