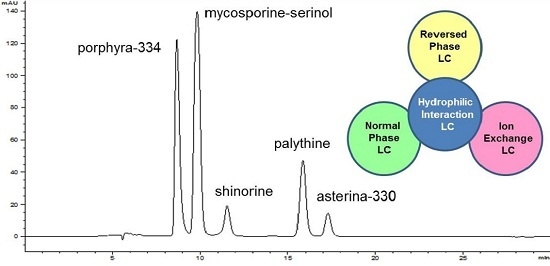
Analysis of Mycosporine-Like Amino Acids in Selected Algae and Cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a Novel MAA from the Red Alga Catenella repens
Abstract
:1. Introduction
2. Results
2.1. Method Development
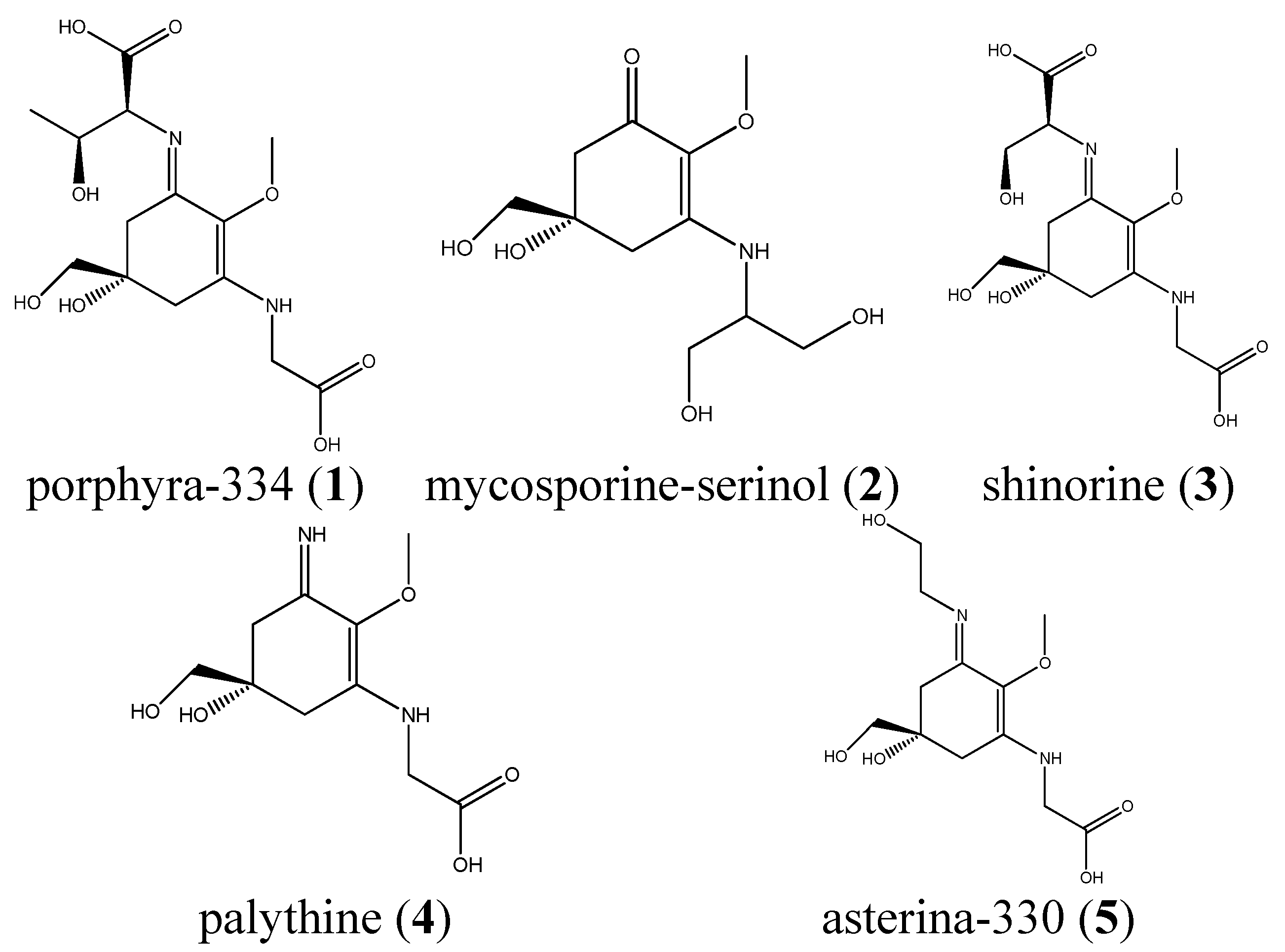
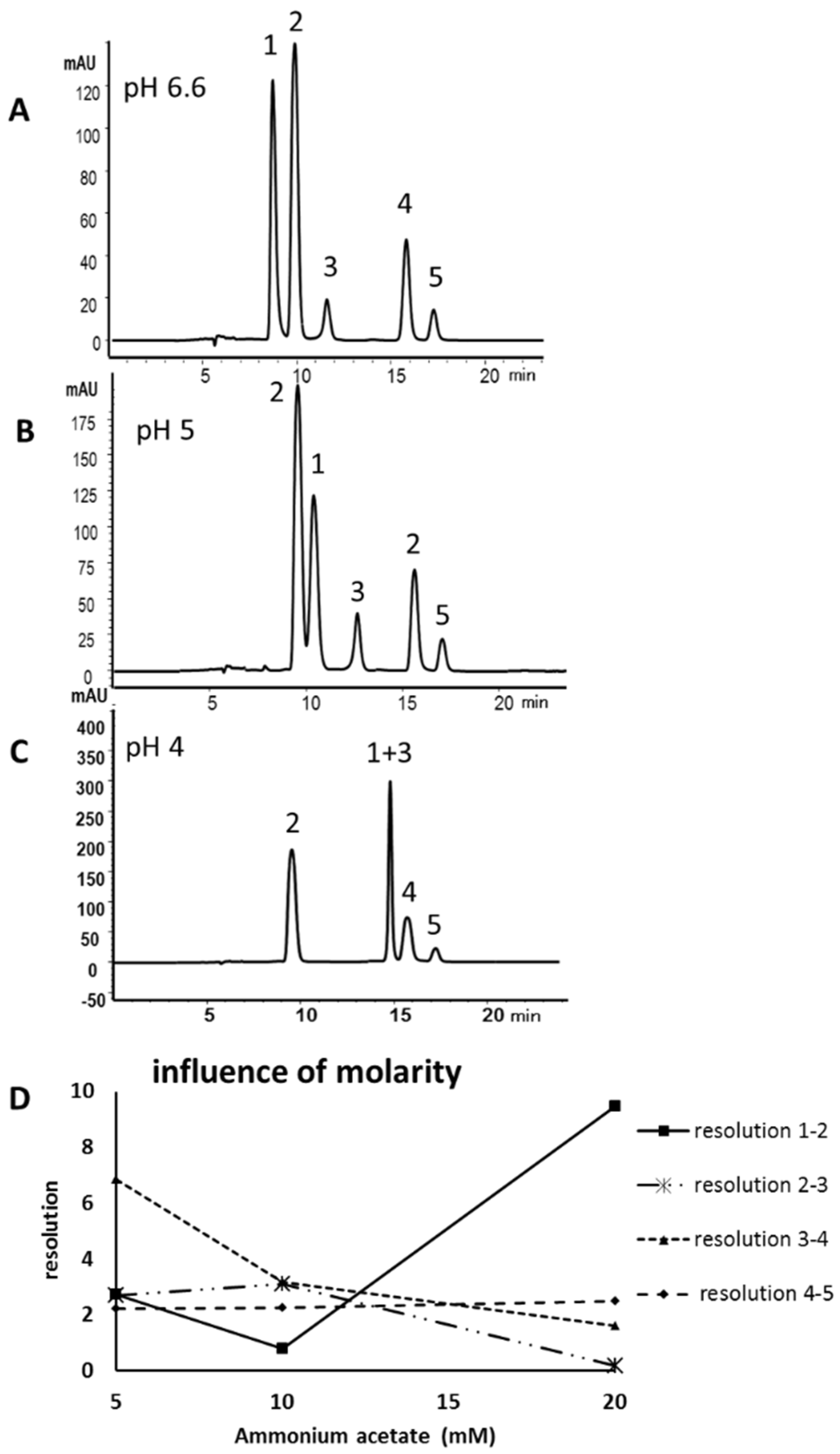
2.2. Validation
| Calibration Data for Porbphyra, Shinorine and Palythine | |||||||
|---|---|---|---|---|---|---|---|
| Substance | Regr. Equation | Corr. Coefficient | σ rel of Slope | Range (µg/mL) | LOD 1 (µg/mL) | LOQ 2 (µg/mL) | |
| 1 | y = 87.37x + 96.00 | R2 = 0.9999 | 0.36 | 250–3.91 | 0.30 | 0.91 | |
| 3 | y = 71.72x + 28.95 | R2 = 0.9991 | 0.59 | 125–1.96 | 0.16 | 0.48 | |
| 4 | y = 40.99x + 36.81 | R2 = 0.9999 | 0.18 | 250–3.91 | 0.43 | 1.31 | |
| Accuracy and Precision of the Assay | |||||||
| Accuracy 3 | Precision 4 | ||||||
| Substance | High Spike | Medium Spike | Low Spike | Day 1 | Day 2 | Day 3 | Intra-day |
| 1 | 104.14 | 104.13 | 102.75 | / | / | / | |
| 3 | 97.90 | 98.69 | 89.84 | / | / | / | |
| 4 | 102.36 | 96.77 | 101.02 | 44.79 (6.56) | 44.16 (1.67) | 49.95 (5.72) | 46.30 (5.57) |
2.3. Isolation of Catenelline from Catenella repens
| MAA m/z = 383 [M + H]+ | ||
|---|---|---|
| 13C | 1H | |
| 1 | 162.03 | - |
| 2 | 128.46 | - |
| 3 | 162.78 | - |
| 4 | 35.53 | 2.98 d (17.4) ; 2.91 d (17.5) |
| 5 | 73.74 | - |
| 6 | 36.16 | 2.93 d (17.4); 2.70 d (17.3) |
| 7 | 70.31 | 3.60 d |
| 8 | 62.14 | 3.66 s |
| 9 | 42.02 | 3.88 m |
| 10 | 52.54 | 3.27 dt (1.0, 6.5) |
| 11 | 63.39 | 4.33 dd (3.8, 6.7) |
| 12 | 177.37 | - |
| 13 | 65.40 | 3.98 m (6.4, 4.4) |
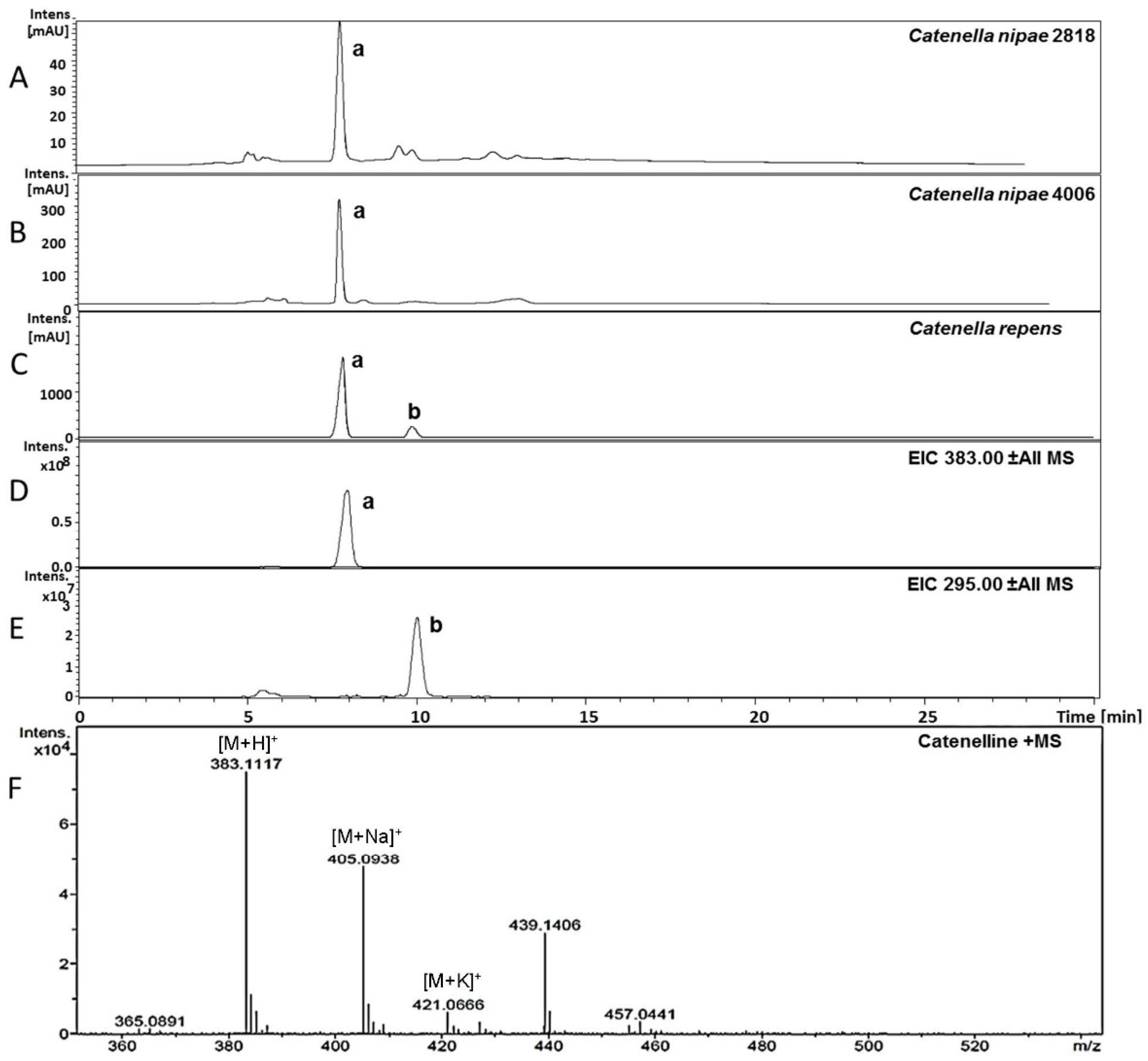
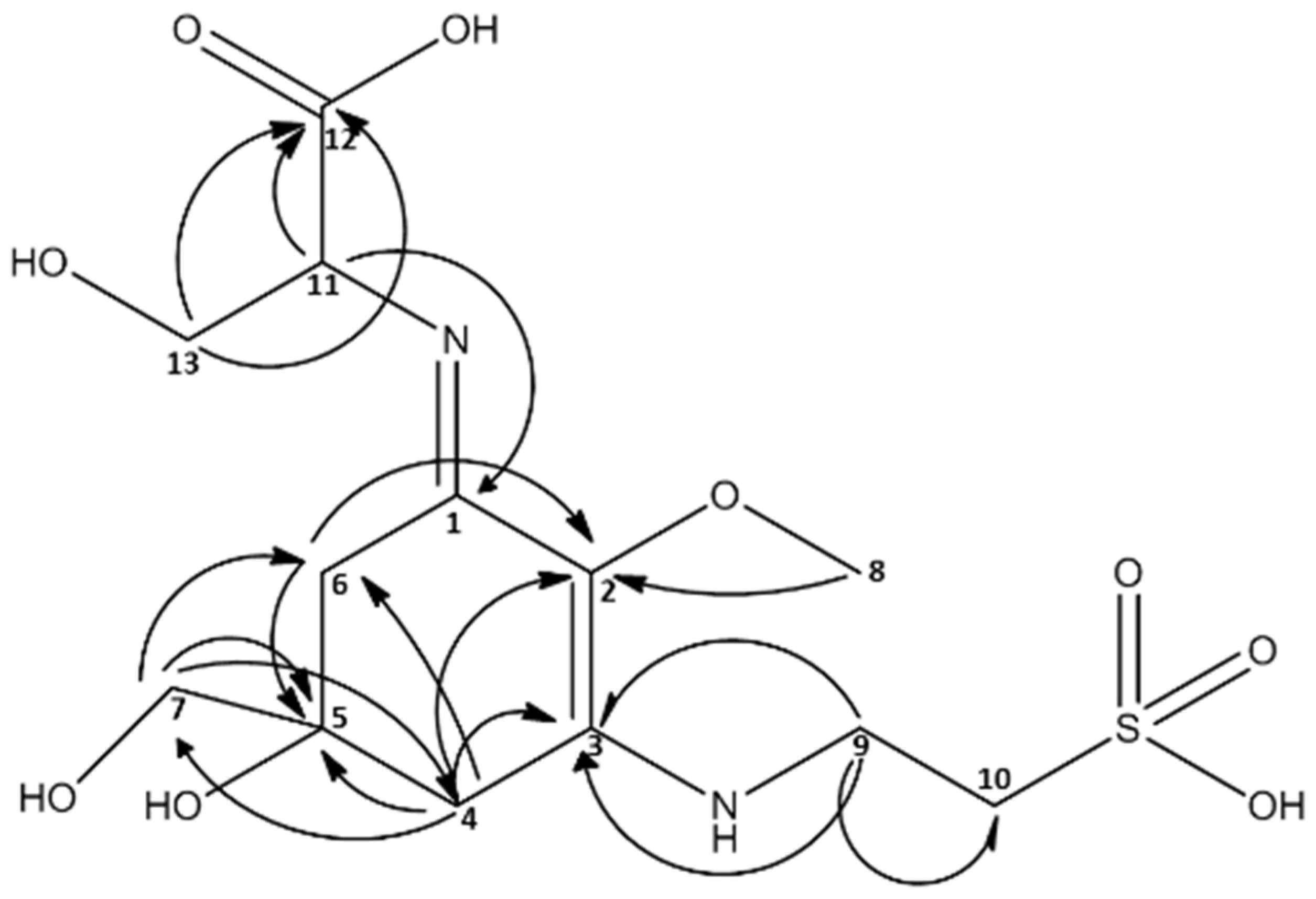
2.4. Quantitative Analysis of Samples
| Species | Origin of Sample |
|---|---|
| Marine algae | |
| Porphyra sp. | commercially available; Asia Express Food, LOT-Nr. 120516, Kampen, NL |
| Porphyra ssp. | commercially available; Porto Muinos, LOT-Nr. 2117543, Cambre, E |
| Palmaria palmata | commercially available; Irish Seaweeds, LOT-Nr. 5391513420184, Belfast, UK |
| Lichina pygmea | 1998, Millers landing, Victoria, Australia, collected and identified by U.K., University of Rostock, Germany, provided in 2013 |
| Catenella repens | 2002, Roscoff, Brittany, collected and identified by U.K., University of Rostock, Germany, provided in 2013 |
| Catenella nipae 2818 | 1987, Cowie Beach, Queensland, Australia, collected and identified by John West; since 2000 grown by U.K., University of Rostock, Germany and provided in 2013 |
| Catenella nipae 4006 | 1999, Maningrida, Arnhem Land, Northern Territory, Australia, collected and identified by John West; since 2000 grown by U.K., University of Rostock, Germany and provided in 2013 |
| Catenella caespitosa 2689 | 1984, La Parguera, Puerto Rico, collected and identified by John West; since 2000 grown by U.K., University of Rostock, Germany and provided in 2013 |
| Terrestrial algae | |
| Macrochloris multinucleata | EPSAG Culture Collection of Algae, University of Göttingen, Germany; Strain-Nr. 39.96 |
| Cyanobacteria | |
| Nostoc commune | Culture Collection of Autotrophic Organism, Třeboň, Czech Republic; isolated by Vinatzer 1975; strain-Nr. Innsbruck V157 |
| Calothrix sp. | Culture Collection of Autotrophic Organisms, Třeboň, Czech Republic; isolated by Zehnder 1977; strain-Nr. 034; GenBank: L05609.1 |
| Leptolyngbya foveolarum | Culture Collection of Autotrophic Organisms, Třeboň, Czech Republic; isolated by ZEHNDER 1965; strain-Nr. 081; GenBank: AM398970.1 |
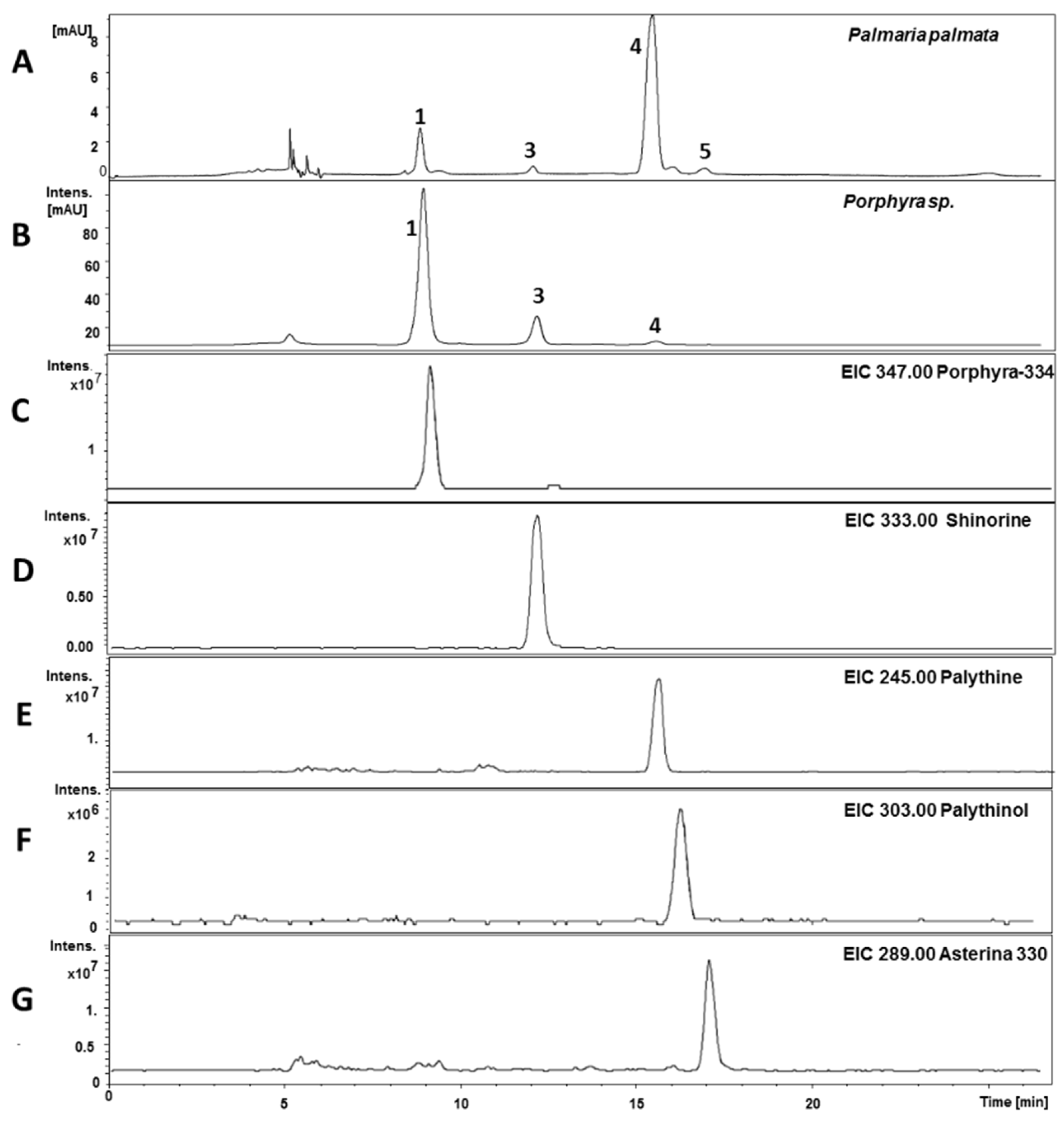
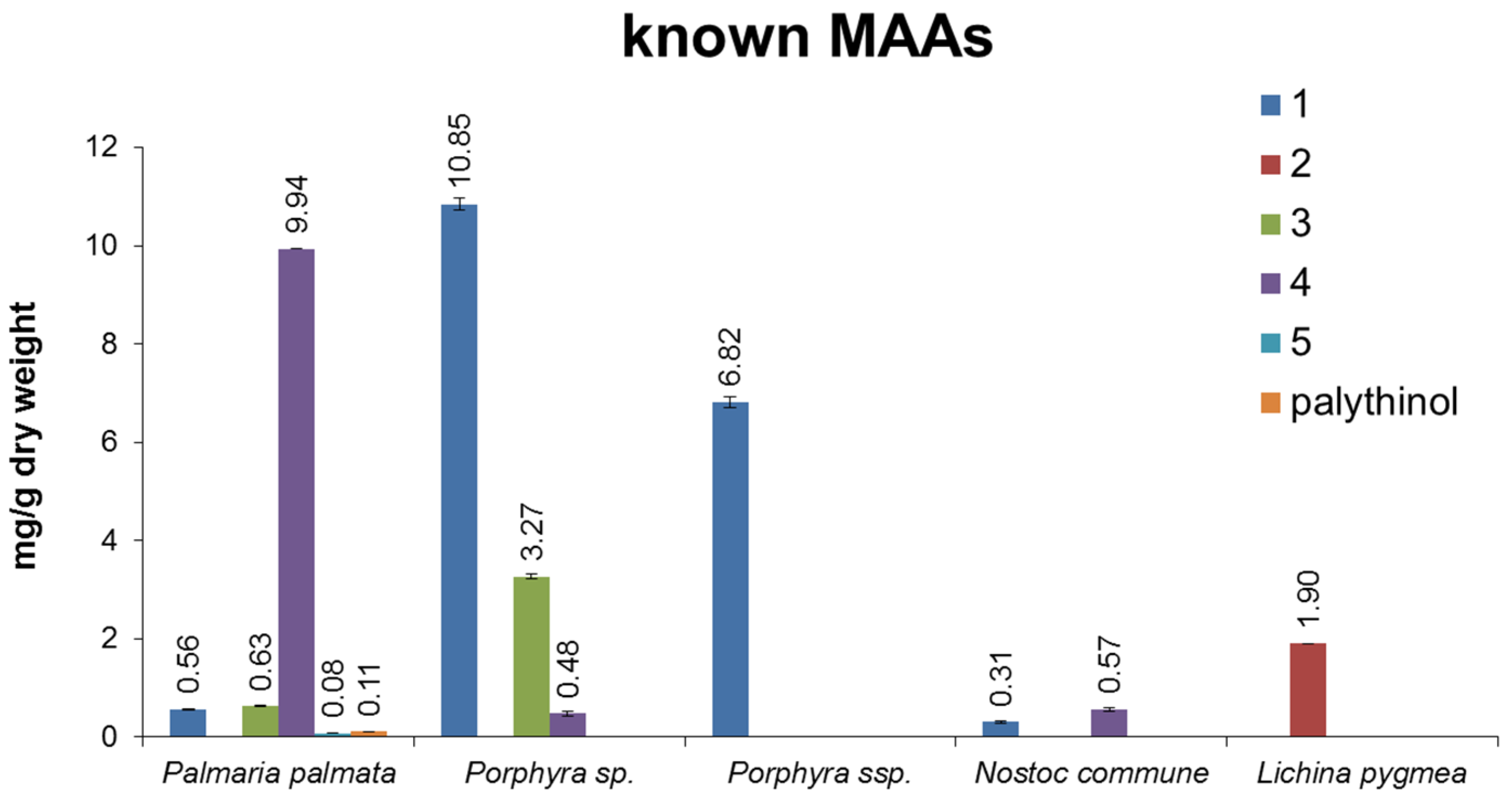
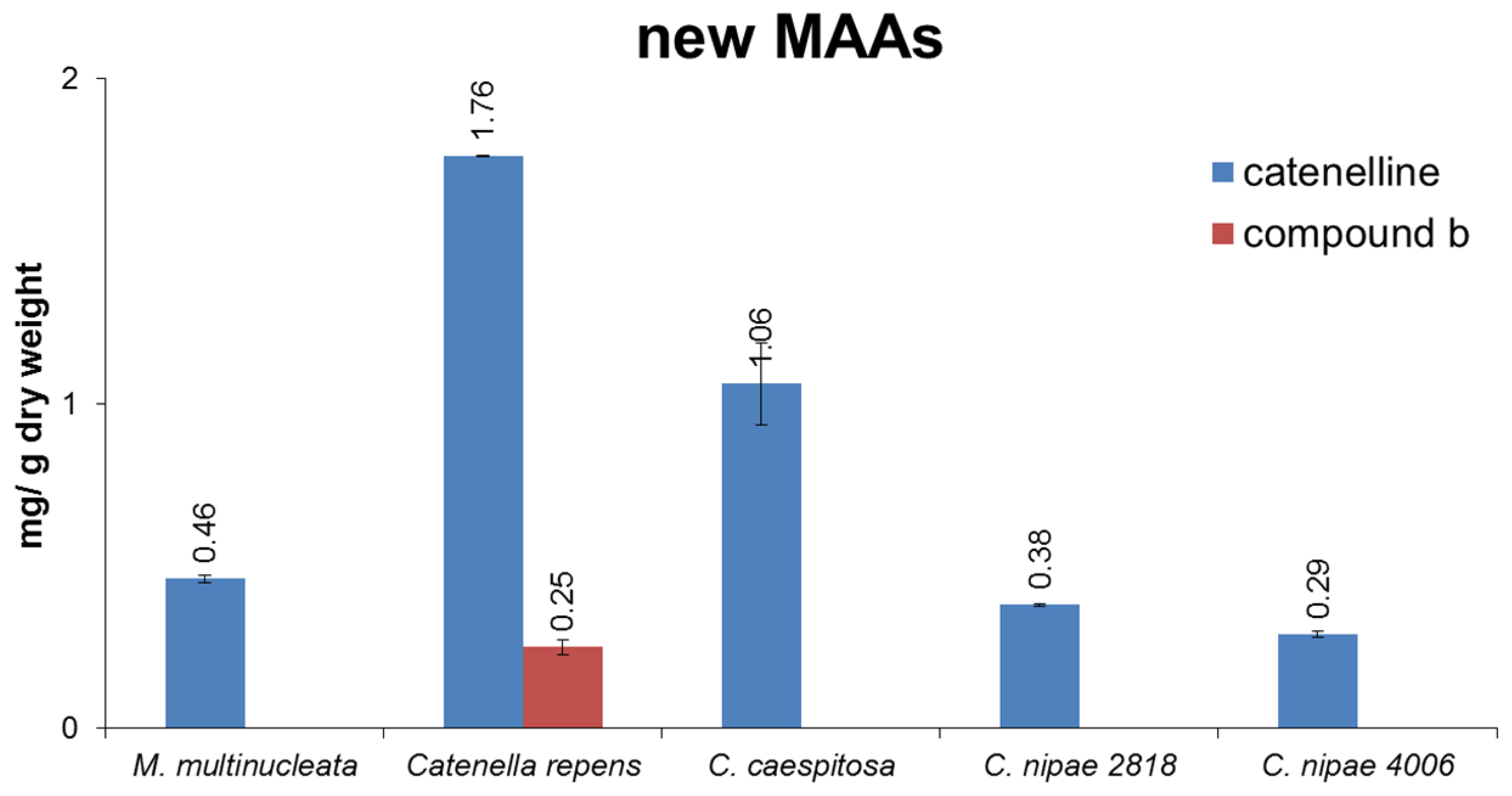
3. Discussion
4. Experimental
4.1. Reagents and Chemicals
4.2. Biological Material and Cultivation Methods
4.3. Isolation and Structural Analysis of MAAs
4.4. Sample Preparation
4.5. Analytical Conditions
4.6. Method Validation
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Körner, C. Climatic Stress. In Alpine Plant Life, 2nd ed.; Springer: Berlin, Germany, 2003; pp. 114–120. [Google Scholar]
- Larcher, W.C.; Kainmüller, C.; Wagner, J. Survival types of high mountain plants under extreme temperatures. Flora 2010, 205, 3–18. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. The high-energy radiation protectant extracellular sheath pigment scytonemin and its reduced counterpart in the cyanobacterium Scytonema sp. R77DM. Bioresour. Technol. 2014, 171, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U. Defence strategies of algae and cyanobacteria against solar ultraviolet radiation. In Algal Chemical Ecology, 1st ed.; Springer: Berlin, Germany, 2008; pp. 273–296. [Google Scholar]
- Flaim, G.; Obertegger, U.; Anesi, A.; Guella, G. Temperature-induced changes in lipid biomarkers and mycosporine-like amino acids in the psychrophilic dinoflagellate Peridinium aciculiferum. Freshw. Biol. 2014, 59, 985–997. [Google Scholar] [CrossRef]
- Karsten, U.; Bischof, K.; Hanelt, D.; Tüg, H.; Wiencke, C. The effect of ultraviolet radiation on photosynthesis and ultraviolet-absorbing substances in the endemic Arctic macroalga Devaleraea ramentacea (Rhodophyta). Physiol. Plant. 1999, 105, 58–66. [Google Scholar] [CrossRef]
- Takano, S.; Uemura, D.; Hirata, Y. Isolation and structure of 2 new amino-acids, palythinol and palythene, from zooanthid Palythoa tuberculosa. Tetrahedron Lett. 1978, 49, 4909–4912. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [PubMed]
- Whitehead, K.; Hedges, J.I. Analysis of mycosporine-like amino acids in plankton by liquid chromatography electrospray ionization mass spectrometry. Mar. Chem. 2002, 80, 27–39. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar. Biol. 2005, 146, 237–252. [Google Scholar] [CrossRef]
- Stochaj, W.R.; Dunlap, W.C.; Shick, J.M. Two new UV-absorbing mycosporine-like amino-acids from the sea-anemone Anthopleura elegantissima and the effects of zooxanthellae and spectral irradiance on chemical composition and content. Mar. Biol. 1994, 118, 149–156. [Google Scholar] [CrossRef]
- Volkmann, M.; Gorbushina, A.A. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol. Lett. 2006, 255, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Shick, J.M.; Dunlap, W.C.; Pearse, J.S.; Pearse, V.B. Mycosporine-like amino acid content in four species of sea anemones in the genus Anthopleura reflects phylogenetic but not environmental or symbiotic relationships. Biol. Bull. 2002, 203, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Med. 2015, 81, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Sawall, T.; West, J.; Wiencke, C. Ultraviolet sunscreen compounds in epiphytic red algae from mangroves. Hydrobiologia 2000, 432, 159–171. [Google Scholar] [CrossRef]
- Matsui, K.; Nazifi, E.; Kunita, S.; Wada, N.; Matsugo, S.; Sakamoto, T. Novel glycosylated mycosporine-like amino acids with radical scavenging activity from the cyanobacterium Nostoc commune. J. Photochem. Photobiol. B 2011, 105, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Heaton, J.; Smith, N.W. Advantages and disadvantages of HILIC: A brief overview. Chromatogr. Today 2012, 5, 44–47. [Google Scholar]
- McCusker, S.; Buff, P.R.; Yu, Z.; Fascetti, J.A. Amino acid content of selected plant, algae and insect species: A search for alternative protein sources for use in pet foods. J. Nutr. Sci. 2014, 3, e39. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H. In vitro analysis of taurine as anti-stress agent in tomato (Solanum lycopersicum)-Preliminary study. Adv. Exp. Med. Biol. 2015, 803, 75–85. [Google Scholar] [PubMed]
- Tevatia, R.; Allen, C.; Rudrappa, D.; White, D.; Clemente, T.E.; Cerutti, H.; Demirel, Y.; Blum, P. The taurine biosynthetic pathway of microalgae. Algal Res. 2015, 9, 21–26. [Google Scholar] [CrossRef]
- Dawes, C.J. Macroalgal diversity, standing stock and productivity in a northern mangalon the west coast of Florida. Nova Hedwig. 1996, 112, 525–535. [Google Scholar]
- Karsten, U.; Barrow, K.D.; Mostaert, A.S.; King, R.J. The osmotic significance of the heteroside floridoside in the mangrove alga Catenella nipae (Rhodophyta: Gigartinales) in Eastern Australia. Estuar. Coast. Shelf Sci. 1995, 40, 239–247. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Yamamoto, Y. Small-molecule antioxidants in marine organisms: Antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. B 1995, 112, 105–114. [Google Scholar] [CrossRef]
- Starr, R.C.; Zeikus, J.A. UTEX: The culture collection of algae at the University of Texas at Austin, 1993 list of cultures. J. Phycol. 1993, 29, 1–106. [Google Scholar] [CrossRef]
- La Barre, J.M.K.; Kornprobst, J.M. Outstanding Marine Molecules, 1st ed.; Wiley-Blackwell: Weinheim, Germany, 2014; pp. 387–430. [Google Scholar]
- Favre-Bonvin, J.; Arpin, N.; Brevard, C. Structure of mycosporine (P-310). Can. J. Chem. 1976, 54, 1105–1113. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of Mycosporine-Like Amino Acids in Selected Algae and Cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a Novel MAA from the Red Alga Catenella repens. Mar. Drugs 2015, 13, 6291-6305. https://doi.org/10.3390/md13106291
Hartmann A, Becker K, Karsten U, Remias D, Ganzera M. Analysis of Mycosporine-Like Amino Acids in Selected Algae and Cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a Novel MAA from the Red Alga Catenella repens. Marine Drugs. 2015; 13(10):6291-6305. https://doi.org/10.3390/md13106291
Chicago/Turabian StyleHartmann, Anja, Kathrin Becker, Ulf Karsten, Daniel Remias, and Markus Ganzera. 2015. "Analysis of Mycosporine-Like Amino Acids in Selected Algae and Cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a Novel MAA from the Red Alga Catenella repens" Marine Drugs 13, no. 10: 6291-6305. https://doi.org/10.3390/md13106291
APA StyleHartmann, A., Becker, K., Karsten, U., Remias, D., & Ganzera, M. (2015). Analysis of Mycosporine-Like Amino Acids in Selected Algae and Cyanobacteria by Hydrophilic Interaction Liquid Chromatography and a Novel MAA from the Red Alga Catenella repens. Marine Drugs, 13(10), 6291-6305. https://doi.org/10.3390/md13106291