Abstract
New eunicellin-type hirsutalins N–R (1–5), along with two known eunicellins, (6 and 7) were isolated from the soft coral Cladiella hirsuta. The structures of the metabolites were determined by extensive spectroscopic analysis. Cytotoxic activity of compounds 1–7 against the proliferation of a limited panel of cancer cell lines was measured. The in vitro anti-inflammatory activity of compounds 1–7 was evaluated by measuring their ability in suppressing superoxide anion generation and elastase release in fMLP/CB-induced human neutrophils.
1. Introduction
The chemical investigations on soft corals of the genus Cladiella and Klyxum [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] have afforded several eunicellin-based diterpenoids, of which many have been shown to exhibit interesting bioactivities [8,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Our recent chemical study of a Taiwanese soft coral Cladiella hirsuta has led to the discovery of 13 eunicellin-based diterpenoids hirsutalins A–M [29,30] and seven steroids hirsutosterols A–G [31] some of which have been found to possess cytotoxic [29] and anti-inflammatory activities [29,30]. In this paper we further report the isolation of five new eunicellin-based compounds, hirsutalins N–R (Chart 1), along with two known compounds, (1R*,2R*,3R*,6S*,7S*,9R*,10R*,14R*)-3-butanoyloxycladiell-11(17)-en-6,7-diol (6) [6], and hirsutalin E (7) [29] from C. hirsuta (Chart 2). The structures of new compounds were determined by extensive spectroscopic analysis. Cytotoxicity of 1–7 against a limited panel of cancer cell lines and their anti-inflammatory activity, determined by their ability to inhibit the generation of super oxide anion and elastase release in N-formyl-methionyl-leucylphenylalanine/cytochalasin B(fMLP/CB)-induced human neutrophiles, were studied in order to discover bioactive compounds for future new drug development.
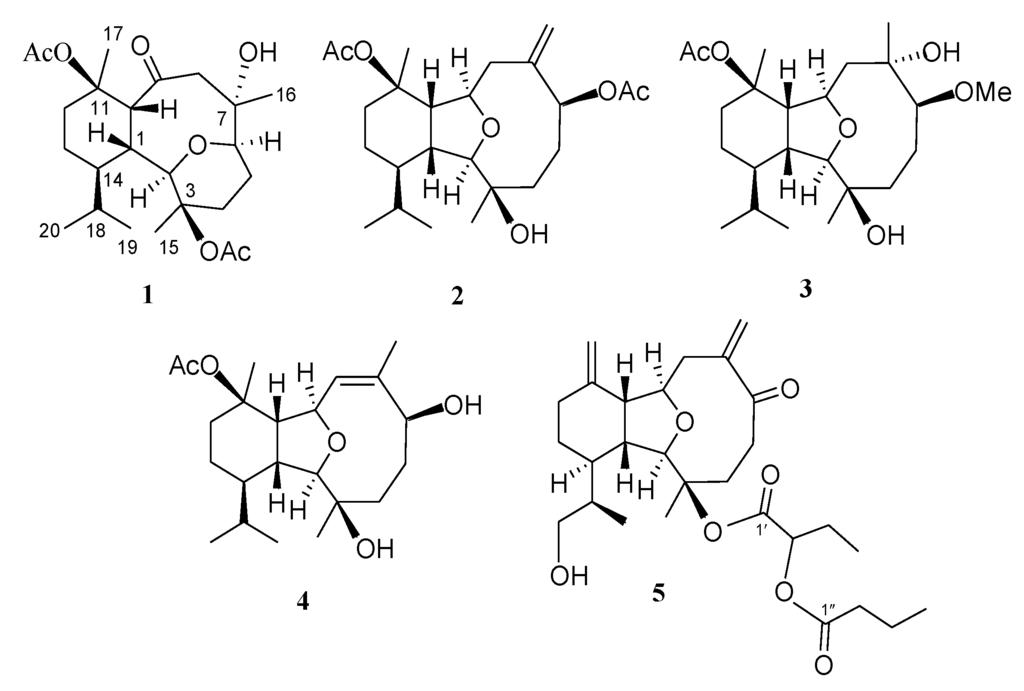
Chart 1.
Structures of metabolites 1–5.
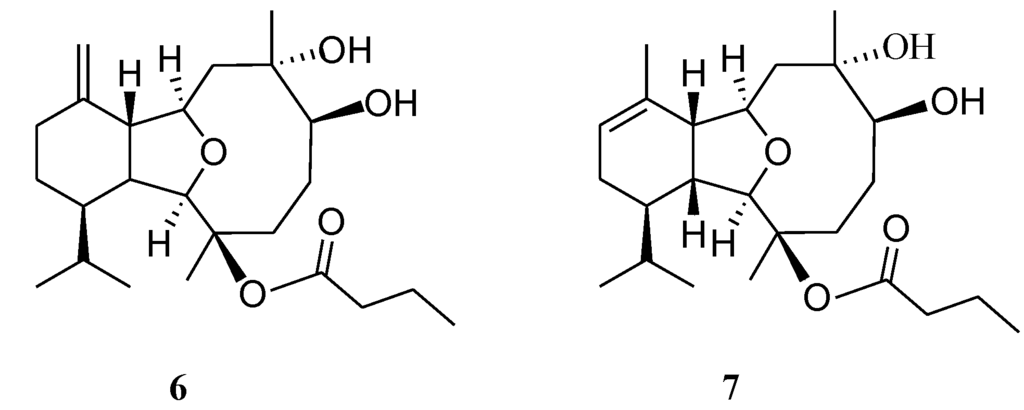
Chart 2.
Structures of metabolites 6 and 7.
2. Results and Discussion
Hirsutalin N (1) was isolated as a colorless oil. The HRESIMS (m/z 461.2518) of 1 established a molecular formula of C24H38O7. The IR spectrum of 1 showed the presence of hydroxy and carbonyl groups from absorptions at 3451 and 1733 cm−1, respectively. The 13C NMR of 1 exhibited 24 carbon signals as expected which were found to be similar to these of a known metabolite hirsutalin I (8, Chart 3) [30], the difference being that the hydroxymethyl group attached at C-18 in hirsutalin I was replaced by a methyl group in 1. This was confirmed by 1H NMR spectrum of 1 which shows the presence of two isopropyl methyls at δ 0.73 (d, J = 7.2 Hz) and 0.97 (d, J = 7.2 Hz) (Table 1). Also, NMR data revealed that the n-butanoyloxy group at C-3 in 8 was replaced by an acetoxy group in 1. Key HMBC correlations from H-2 to C-6; H-1, H2-8, and H-10 to C-9; H3-15 to C-2, C-3 and C-4; H3-16 to C-6, C-7 and C-8; H3-17 to C-10, C-11 and C-12; and both H3-19 and H3-20 to C-14 and C-18, permitted the assembly of the carbon skeleton of 1. Based on above results and HMBC correlations (Figure 1), the planar structure of 1 was established. Further, comparison of the NOE correlations of 1 (Figure 2) with those of hirsutalin I, the relative configuration of 1 was thus determined to be the same.

Table 1.
NMR spectroscopic data for hirsutalins N–P (1–3).
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| Position | δC, mult. a,b | δH ( J in Hz) c | δC, mult. a,b | δH ( J in Hz) c | δC, mult. a,b | δH ( J in Hz) c |
| 1 | 49.6, CH | 2.55, dd (12.0, 4.4) | 41.4, CH | 2.25, m | 41.9, CH | 2.18, m |
| 2 | 78.0, CH | 3.80, s | 91.3, CH | 3.56, s | 90.8, CH | 3.56, s |
| 3 | 81.3, C | - | 74.0, C | - | 74.7, C | - |
| 4 | 27.7, CH2 | 1.36, m | 34.9, CH2 | 1.75, m | 41.0, CH2 | 1.83, m |
| - | 2.92, dd (11.8, 4.4) | - | - | - | - | |
| 5 | 20.6, CH2 | 1.34, m | 32.0, CH2 | 1.99, m | 25.7, CH2 | 1.98, m |
| - | 1.66, m | - | - | - | - | |
| 6 | 80.4, CH | 3.82, dd (11.4, 6.0) | 76.4, CH | 5.19, dd (12.0, 6.0) | 90.8, CH | 4.07, m |
| 7 | 85.4, C | - | 149.0, C | - | 76.6, C | - |
| 8 | 49.5, CH2 | 2.00, d (12.0) | 41.4, CH2 | 3.12, dd (13.6, 6.0) | 47.0, CH2 | 1.73, m |
| - | 2.78, d (12.0) | - | 2.47, d (13.6) | - | 2.30, dd (12.8, 11.6) | |
| 9 | 211.4, C | - | 78.3, CH | 4.09, dd (11.2, 6.0) | 75.6, CH | 4.07, m |
| 10 | 55.2, CH | 4.14, dd (4.4, 2.0) | 46.4, CH | 2.95, dd (11.2, 7.2) | 54.4, CH | 2.82, t (7.6) |
| 11 | 83.3, C | - | 82.3, C | - | 82.9, C | - |
| 12 | 31.4, CH2 | 2.10, m | 32.5, CH2 | 1.43, m | 30.5, CH2 | 1.38, m |
| - | 2.24, m | - | 2.24, m | - | 2.40, m | |
| 13 | 19.3, CH2 | 1.61, m | 18.2, CH2 | 1.34, m | 17.7, CH2 | 1.20, m |
| - | 1.25, m | - | 1.45, m | - | 1.40, m | |
| 14 | 36.5, CH | 1.98, m | 42.8, CH | 1.20, m | 42.6, CH | 1.22, m |
| 15 | 23.6, CH3 | 1.53, s | 27.4, CH3 | 1.19, s | 30.3, CH3 | 1.16, s |
| 16 | 22.9, CH3 | 1.13, s | 118.3, CH2 | 5.29, s | 23.8, CH3 | 1.16, s |
| - | - | - | 5.53, s | - | - | |
| 17 | 24.3, CH3 | 1.45, s | 25.5, CH3 | 1.52, s | 24.5, CH3 | 1.46, s |
| 18 | 27.2, CH | 1.87, m | 27.9, CH | 1.80, m | 29.1, CH | 1.71, m |
| 19 | 14.5, CH3 | 0.73, d (7.2) | 15.0, CH3 | 0.78, d (6.8) | 15.0, CH3 | 0.78, d (6.8) |
| 20 | 21.7, CH3 | 0.97, d (7.2) | 21.8, CH3 | 0.94, d (6.8) | 21.8, CH3 | 0.94, d (6.8) |
| 3-OAc | 22.4, CH3 | 2.00, s | - | - | - | - |
| 169.7, C | - | - | - | - | - | |
| 11-OAc | 22.3, CH3 | 2.19, s | 22.6, CH3 | 2.00, s | 22.6, CH3 | 2.00, s |
| 170.1, C | - | 170.3, C | - | 170.2, C | - | |
| 6-OAc | - | - | 21.4, CH3 | 1.99, s | - | - |
| - | - | 170.5, C | - | - | - | |
| 6-OMe | - | - | - | - | 57.1, CH3 | 3.37, s |
a Spectra recorded at 100 MHz in CDCl3; b multiplicity deduced from DEPT; c spectra recorded at 400 MHz in CDCl3.
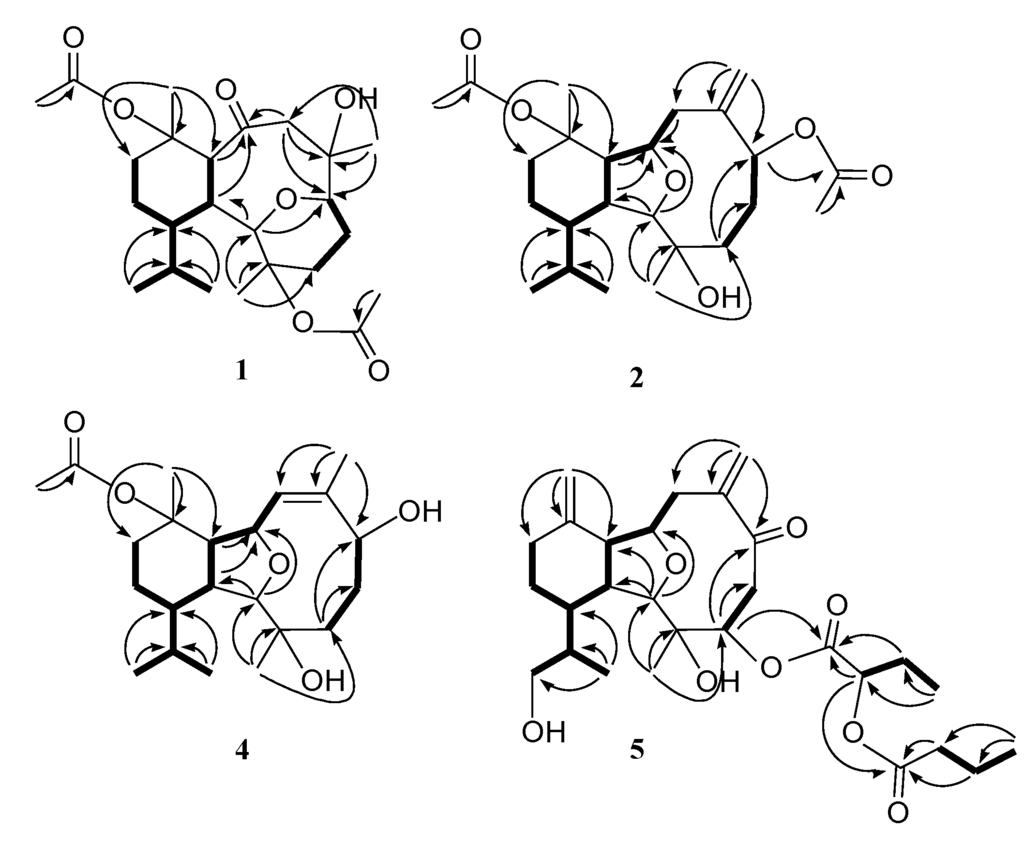
Figure 1.
COSY and HMBC correlations for 1, 2, 4 and 5.
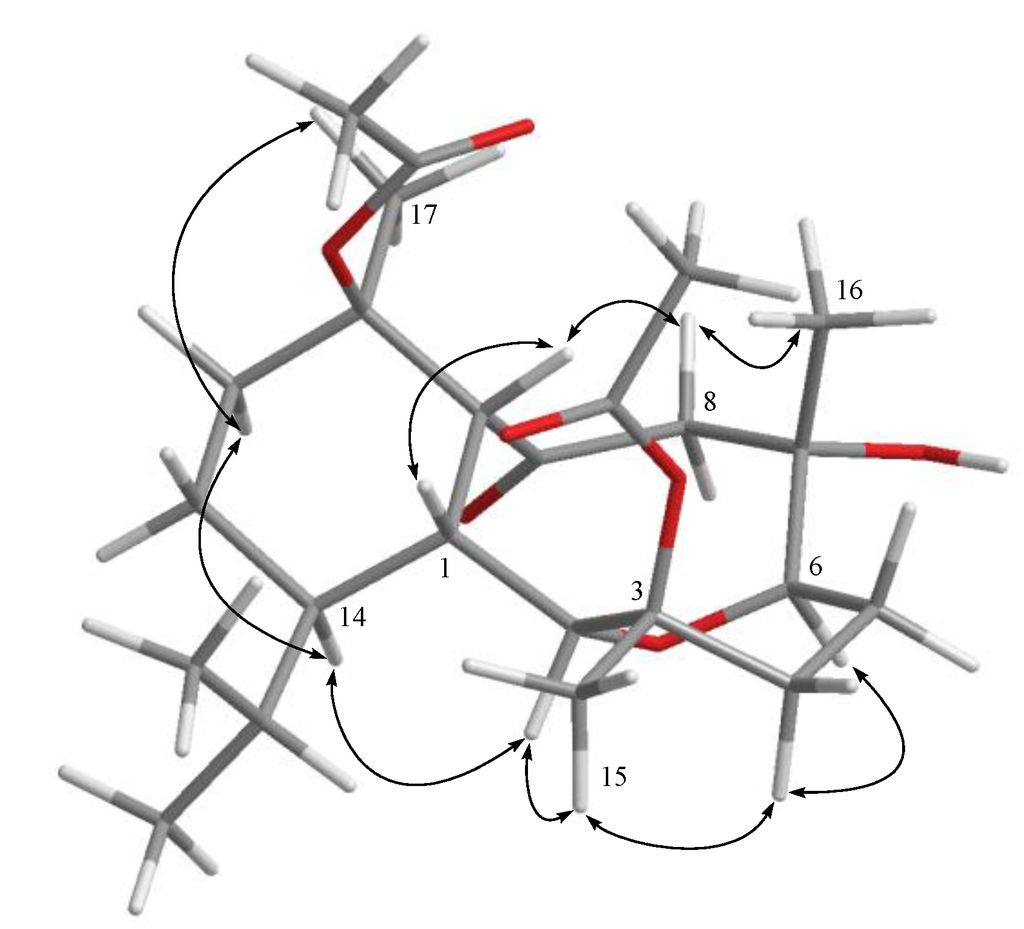
Figure 2.
Key NOESY correlations for 1.
Hirsutalin O (2) was also afforded as a colorless oil. Compound 2 has a molecular formula C24H38O6, as determined by HRESIMS. In comparing NMR data of 2 with those of the known compound simplexin A (9, Chart 3) [11], it was found that the n-butanoyloxy group at C-3 and the hydroxy group at C-6 in simplexin A (9) were replaced by a hydroxy group and acetoxy group in 2, respectively, as confirmed by the downfield shift of C-3 (δC 81.3) of 1, relative to that of 2 (δC 74.0), and the HMBC connectivity from H-6 (δ 5.19) to the carbonyl carbon resonating at δ 170.5 (C) (Table 1). The relative configuration of 2 was confirmed to be the same as that of 9 by analysis of NOE correlations (Figure 3).
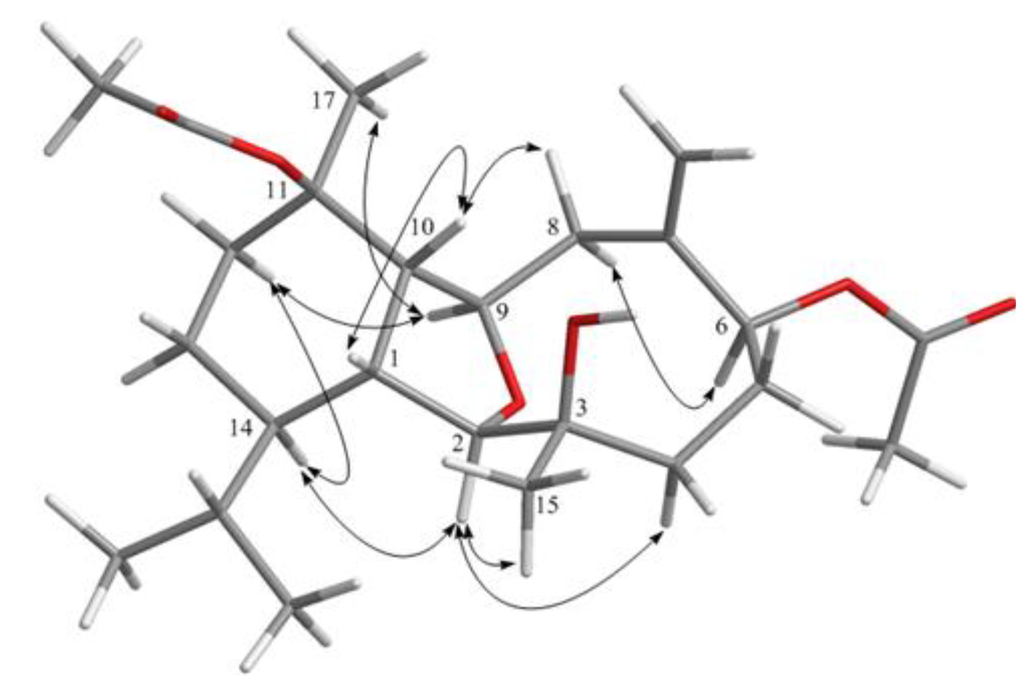
Figure 3.
Key NOESY correlations for 2.
The new eunicellin, hirsutalin P (3), has a molecular formula C23H40O6 as determined by HRESIMS. The spectroscopic data (IR, 1H NMR, and 13C NMR) of 3 were similar to those of a known one, klysimplex G (10, Chart 3) [12], except that the acetoxy group at C-3 and the hydroxy group at C-6 in 10 were replaced by a hydroxy group and methoxy group, respectively, in 3. The similar 1H NMR data and the analysis of NOE correlations of 3 further revealed the same relative configuration of both compounds. Thus, the structure of 3 was established.
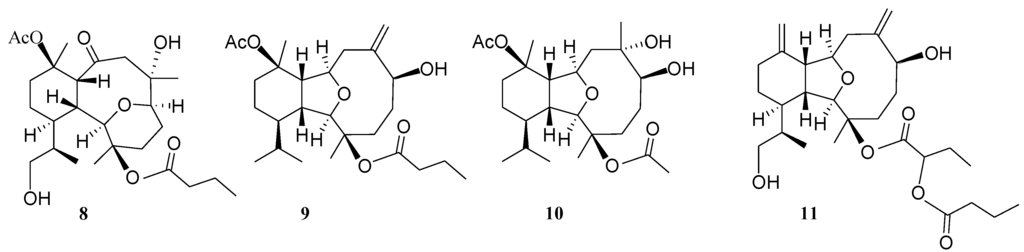
Figure 6.
Structures of known compounds 8–11.
Hirsutalin Q (4) was obtained as a colorless oil and exhibited a molecular formula C22H36O5. IR absorptions of 4 showed the presence of hydroxy and carbonyl groups at 3421 and 1724 cm−1, respectively. The NMR spectroscopic data revealed the presence of a trisubstituted double bond (δH 5.28, s, 1H; δC 128.4, CH and 139.4, C) (Table 2). One ester carbonyl (δC 170.2) was assigned from the 13C NMR spectrum and was HMBC correlated with an acetate methyl (δH 1.99 s). The chemical shift of H3-15 at δ 1.18 indicated the presence of a hydroxy group substitution at C-3, the same as that in compounds 2 and 3. The presence of an acetoxy group at C-11 could be seen from the more downfield shift of H3-17 (δ 1.53), in comparison with that of H3-15 (δ 1.18). The planar structure of metabolite 1 was elucidated by analysis of COSY and HMBC correlations (Figure 1). The Z geometry of the double bond at C-7 and C-8 was evidenced by the presence of NOE correlation between H-8 and H3-16. In the NOESY spectrum of 4, observation of the NOE correlation between H-1 with H-10 suggested that H-1 and H-10 are β-oriented. Also, correlations between H-2 with both H-14 and H3-15; H-9 with both H-14 and H3-17; and H-6 with H3-15 suggested that all of H-2, H-6, H-9, H-14, H3-15 and H3-17 are α-oriented. Thus, the structure of diterpenoid 4 was established.
A structurally-related metabolite, hirsutalin R (5), was also isolated as a colorless oil with a molecular formula of C28H42O7. Two ester carbonyl carbons (δC 169.0 and 173.5) were correlated in the HMBC spectrum with the methine proton (H-2′, δH 4.76 t, J = 6.8 Hz) of a 2-butyryloxybutanoate unit. Moreover, the 13C NMR spectroscopic data (Table 2) of 5 showed the presence of two 1, 1-disubstituted carbon–carbon double bonds (δC 147.7 (C) and 118.4 (CH2); 145.2 (C) and 111.6 (CH2)). Comparison of the NMR data of 5 with those of hirsutalin C (11, Chart 3) [29] revealed that the only difference between both compounds is the replacement of the hydroxy group in hirsutalin C by a ketone (δC 206.5) at C-6 in 5. The absolute configuration of hirsutalin A [29] and hirsutalin J [30] have been completely assigned based on NOE correlations and Mosher’s method. Compounds 1–5 are likely in the same enantiomeric series as hirsutalin A and hirsutalin J, based on a shared biosynthetic pathway. Thus, these compounds are suggested to possess the absolute configurations as shown in formula 1–5.

Table 2.
NMR spectroscopic data for hirsutalins Q and R (4 and 5).
| 4 | 5 | |||
|---|---|---|---|---|
| Position | δC, mult. a,b | δH ( J in Hz) c | δC, mult. a,b | δH ( J in Hz) c |
| 1 | 40.9, CH | 2.35, m | 45.0, CH | 2.25, m |
| 2 | 90.8, CH | 3.57, s | 90.8, CH | 3.69, s |
| 3 | 74.7, C | - | 86.0, C | - |
| 4 | 37.2, CH2 | 1.83, m; | 32.2, CH2 | 2.12, m |
| 5 | 25.7, CH2 | 1.81, m | 36.4, CH2 | 2.68, m |
| - | 1.90, m | - | 2.28, m | |
| 6 | 70.6, CH | 5.48, d (8.8) d | 206.5, CH | - |
| 7 | 139.4, C | - | 147.7, C | - |
| 8 | 128.4, CH | 5.28, s | 37.3, CH2 | 3.22, dd (13.2, 5.6) |
| - | - | - | 2.34, m | |
| 9 | 78.6, CH | 4.47, d (6.0) | 78.4, CH | 4.08, m |
| 10 | 54.9, CH | 2.70, t (7.2) | 48.8, CH | 3.08, dd (9.6, 7.6) |
| 11 | 83.0, C | - | 145.2 , C | - |
| 12 | 30.4, CH2 | 1.32, m | 31.2, CH2 | 2.08, m |
| - | 1.52, m | - | 2.27, m | |
| 13 | 18.4, CH2 | 1.35, m | 25.9, CH2 | 1.10, m |
| - | 1.45, m | - | 1.65, m | |
| 14 | 42.1, CH | 1.26, m | 37.5, CH | 1.66, m |
| 15 | 27.7, CH3 | 1.18, s | 22.7, CH3 | 1.48, s |
| 16 | 17.9, CH3 | 1.79, s | 118.4, CH2 | 5.27, s |
| - | - | - | 5.62, s | |
| 17 | 23.7, CH3 | 1.53, s | 111.6, CH2 | 4.72, s |
| - | - | - | 4.85, s | |
| 18 | 29.2, CH | 1.72, m | 36.4, CH | 1.78, m |
| 19 | 16.5, CH3 | 0.83, d (7.2) | 16.3, CH3 | 0.79, d (7.2) |
| 20 | 21.9, CH3 | 0.96, d (7.2) | 66.4, CH2 | 3.52, d (7.2) |
| 11-OAc | 22.6, CH3 | 1.99, s | - | - |
| 170.2, C | - | - | - | |
| 2-butanoyloxybutanoate | - | - | - | - |
| 1′ | - | - | 169.0, C | - |
| 2′ | - | - | 73.6, CH | 4.76, t (6.8) |
| 3′ | - | - | 24.5, CH2 | 1.83, m |
| 4′ | - | - | 9.7, CH3 | 1.03, t (7.2) |
| 1′′ | - | - | 173.5, C | - |
| 2′′ | - | - | 35.8, CH2 | 2.40, m |
| 3′′ | - | - | 18.3, CH2 | 1.66, m |
| 4′′ | - | - | 13.6, CH3 | 0.98, t (7.2) |
a Spectra recorded at 100 MHz in CDCl3; b Multiplicity deduced from DEPT; c Spectra recorded at 400 MHz in CDCl3.
Cytotoxicity of compounds 1–7 against the proliferation of a limited panel of cancer cell lines, including P388 (murine leukemia), K562 (human erythro myeloblastoid leukemia), A549 (human lung adenocarcinoma), and HT-29 (human colon adenocarcinoma), was evaluated. Compound 5 was found to exhibit cytotoxicity toward P388 and K562 cell lines with IC50 values of 13.8 and 36.3 μM (Table 3). Compound 7 displayed cytotoxicity toward A549 cell line with IC50 value of 37.2 μM. Other metabolites were found to be inactive against the four cancer cells. The neutrophil pro-inflammatory responses to compounds 1–7 were evaluated by suppressing N-formyl-methionyl-leucyl-phenylalanine/cytochalasin B (fMLP/CB)-induced superoxide anion (O2•−) generation and elastase release in human neutrophils, as shown in Table 4. At a concentration of 10 μg/mL, none of compounds were able to significantly reduce the expression of superoxide anion generation, relative to the control cells stimulated with fMLP/CB only. At the same concentration, compound 1 was found to significantly inhibit the elastase release (31.7% ± 3.2% inhibition) in the same fMLP/CB-stimulated neutrophils.

Table 3.
Cytotoxicity (IC50 μM) of compounds 5 and 7.
| Compound | P388 | K562 | HT-29 | A-549 |
|---|---|---|---|---|
| 5 | 13.8 | 36.3 | (–) a | (–) |
| 7 | (–) | (–) | (–) | 37.2 |
| 5-Fluorouracil | 8.5 | 24.6 | 20.8 | 38.5 |
a IC50 > 40 μM.

Table 4.
Effect of compounds 1–7 on superoxide anion generation and elastase release in fMLP/CB-induced human neutrophils at 10 μg/mL.
| Compounds | Superoxide Anion | Elastase Release | |||
|---|---|---|---|---|---|
| IC50 (μg/mL) a | Inhibition % | IC50 (μg/mL) a | Inhibition % | ||
| 1 | >10 | 1.0 ± 5.5 | >10 | 31.7 ± 3.2 | *** |
| 2 | >10 | 9.6 ± 5.5 | >10 | 11.5 ± 5.0 | - |
| 3 | >10 | 1.7 ± 0.7 | >10 | 17.9 ± 6.9 | * |
| 4 | >10 | 6.1 ± 2.6 | >10 | 6.4 ± 2.4 | - |
| 5 | >10 | 6.5 ± 2.9 | >10 | 13.6 ± 4.9 | * |
| 6 | >10 | 1.0 ± 1.9 | >10 | 6.1 ± 5.6 | - |
| 7 | >10 | 4.2 ± 3.8 | >10 | 3.1 ± 6.9 | - |
Percentage of inhibition (Inh %) at 10 μM concentration. Results are presented as mean ± S.E.M. (n = 3 or 4). * p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control value. a Concentration necessary for 50% inhibition (IC50).
3. Experimental Section
3.1. General Experimental Procedures
Silica gel (230–400 mesh, Merck, Darmstadt, Germany) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254, 0.2 mm) were used for analytical TLC. High-performance liquid chromatography was performed on a Hitachi L-7100 HPLC apparatus with a Hitachi L-2455 HPLC apparatus (Hitachi Ltd., Tokyo, Japan) with a Supelco C18 column (250 × 21.2 mm, 5 μm). NMR spectra were recorded on a Varian 400MR FT-NMR instrument (Varian Inc, Palo Alto, CA, USA) at 400 MHz for 1H and 100 MHz for 13C in CDCl3. LRMS and HRMS were obtained by ESI on a Bruker APEX II mass spectrometer (Bruker, Bremen, Germany). Optical rotations were measured on a JASCO P-1020 polarimeter. IR spectra were recorded on a JASCO FT/IR-4100 infrared spectrophotometer (Japan Spectroscopic Corporation, Tokyo, Japan).
3.2. Animal Material
The animal Cladiella hirsuta was collected by hand using SCUBA off the coast of Sianglu Islet (23°32' N, 119°38' E) in the region of Penghu Islands, in June 2008, at a depth of 10 m, and was stored in a freezer until extraction. A voucher sample (PI-20080610-17) was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The frozen bodies of C. hirsuta (3.1 kg, wet wt) were sliced and exhaustively extracted with acetone (3 × 10 L). The organic extract was concentrated to an aqueous suspension and was partitioned between ethyl acetate (EtOAc) and H2O. The EtOAc layer was dried with anhydrous Na2SO4. After removal of solvent in vacuo, the residue (32.8 g) was subjected to column chromatography on silica gel and eluted with EtOAc in n-hexane (0%–100% of EtOAc, gradient) and further with MeOH in EtOAc of increasing polarity to yield 25 fractions. Fraction 18, eluting with n-hexane–EtOAc (1:1), was rechromatographed over a Sephadex LH-20 column using acetone as the mobile phase to afford four subfractions (A1–A4). Subfractions A3 and A4 were combined and separated by reversed-phase HPLC (MeOH–H2O, 3:1 and 2:1) to afford compounds 4 (1.8 mg), 5 (1.4 mg), 6 (27.7 mg) and 7 (5.6 mg), respectively. Fraction 19, eluting with n-hexane–EtOAc (1:2), was rechromatographed over a Sephadex LH-20 column, using acetone as the mobile phase, to afford four subfractions (B1–B4). Subfractions B2 and B3 were combined and separated by reversed-phase HPLC (acetonitrile–H2O, 3:1 and 2:1) to afford compounds 1 (9.2 mg), 2 (4.0 mg), and 3 (1.8 mg), respectively.
Hirsutalin N (1): colorless oil; [α]25D −98 (c 0.54, CHCl3); IR (neat) vmax 3451 and 1733 cm−1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 1; ESIMS m/z 461 [M + Na]+; HRESIMS m/z 461.2518 [M + Na]+(calcd for C24H38O7Na, 461.2515) (Supplementary Information, Figures S1–S3).
Hirsutalin O (2): colorless oil; [α]25D −128 (c 0.68, CHCl3); IR (neat) vmax 3482 and 1729 cm−1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 1; ESIMS m/z 445 [M + Na]+; HRESIMS m/z 445.2564 [M + Na]+(calcd for C24H38O6Na, 445.2566) (Supplementary Information, Figures S4–S6).
Hirsutalin P (3): colorless oil; [α]25D +27 (c 0.54, CHCl3); IR (neat) vmax 3426 and 1730 cm−1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 1; ESIMS m/z 435 [M + Na]+; HRESIMS m/z 435.2720 [M + Na]+(calcd for C23H40O6Na, 435.2722) (Supplementary Information, Figures S7–S9).
Hirsutalin Q (4): colorless oil; [α]25D +12 (c 0.51, CHCl3); IR (neat) vmax 3421 and 1724 cm−1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 2; ESIMS m/z 403 [M + Na]+; HRESIMS m/z403.2457 [M + Na]+(calcd for C22H36O5Na, 403.2460) (Supplementary Information, Figures S10–S12).
Hirsutalin R (5): yellow oil; [α]25D −18 (c 0.54, CHCl3); IR (neat) vmax 3437 and 1740 cm−1; 13C and 1H NMR data (400 MHz; CDCl3), see Table 2; ESIMS m/z 513 [M + Na]+; HRESIMS m/z 513.2831 [M + Na]+(calcd for C28H42O7Na, 513.2828) (Supplementary Information, Figures S13–S15).
3.4. Cytotoxicity Testing
Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of compounds 1–7 were performed using the Alamar Blue assay [32,33].
3.5. In Vitro Anti-Inflammatory Assay
Human neutrophils were obtained using dextran sedimentation and Ficoll centrifugation. Measurements of superoxide anion generation and elastase release were carried out according to previously described procedures. [34,35]. LY294002, a phosphatidylinositol-3-kinase inhibitor, was used as a positive control for inhibition of superoxide anion generation and elastase release with IC50 0.6 ± 0.1 and 1.2 ± 0.3 μg/mL [36].
4. Conclusions
Five new eunicellin-type compounds, hirsutalins N–R (1–5) and two known eunicellin-type compounds (6 and 7), were discovered from the soft coral C. hirsuta. Compound 5 displayed cytotoxicity against the proliferation of P388 and K562 cancer cells possibly due to the presence of the α,β-unsaturated ketone group. Compound 1 was found to effectively inhibit the elastase release in FMLP/CB-induced human neutrophils.
Supplementary Files
Acknowledgments
This research was supported by grants from the National Science Council (100-2320-B-110-001-MY2), NSYSU-KMU JOINT RESEARCH PROJECT (NSYSUKMU 02C030117) and Aim for the Top University Program (02C030205) from Ministry of Education of Taiwan, awarded to J.-H. Sheu.
Author Contributions
Jyh-Horng Sheu designed the whole experiment and contributed to manuscript preparation. Tzu-Zin Huang and Bo-Wei Chen carried out the experiment and wrote the manuscript. Chiung-Yao Huang and Tsong-Long Hwang performed and analyzed the bioassay. Chang-Feng Dai identified the soft coral.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kazlauskas, R.; Murphy, P.T.; Wells, R.J.; Schönholzer, P. Two new diterpenes related to eunicellin from a Cladiella species (soft coral). Tetrahedron Lett. 1977, 18, 4643–4646. [Google Scholar] [CrossRef]
- Hochlowski, J.E.; Faulkner, D.J. A diterpene related to cladiellin from a Pacific soft coral. Tetrahedron Lett. 1980, 21, 4055–4056. [Google Scholar] [CrossRef]
- Uchio, Y.; Nakatani, M.; Hase, T.; Kodama, M.; Usui, S.; Fukazawa, Y. A new eunicellin-based diterpene from an Okinawan soft coral, Cladiella sp. Tetrahedron Lett. 1989, 30, 3331–3332. [Google Scholar] [CrossRef]
- Uchio, Y.; Kodama, M.; Usui, S.; Fukazawa, Y. Three new eunicellin-based diterpenoids from an Okinawan Cladiella species of soft coral. Tetrahedron Lett. 1992, 33, 1317–1320. [Google Scholar] [CrossRef]
- Sarma, N.S.; Chavakula, R.; Rao, I.N. Crystal and molecular structure of sclerophytin F methyl ether from the soft coral Cladiella krempfi. J. Nat. Prod. 1993, 56, 1977–1980. [Google Scholar] [CrossRef]
- Rao, C.B.; Rao, D.S.; Satyanarayana, C.; Rao, D.V.; Kassühlke, K.E.; Faulkner, D.J. New cladiellane diterpenes from the soft coral Cladiella australis of the Andaman and Nicobar Islands. J. Nat. Prod. 1994, 57, 574–580. [Google Scholar] [CrossRef]
- Rao, D.S.; Sreedhara, C.; Rao, D.V.; Rao, C.B. Two new cladiellane diterpenes from the soft coral Cladiella australis of the Indian Ocean. Ind. J. Chem. Sect. B 1994, 33B, 198–199. [Google Scholar]
- Yamada, K.; Ogata, N.; Ryu, K.; Miyamoto, T.; Komori, T.; Higuchi, R. Bioactive terpenoids from octocorallia. 3. A new eunicellin-based diterpenoid from the soft coral Cladiella sphaeroides. J. Nat. Prod. 1997, 60, 393–396. [Google Scholar] [CrossRef]
- Chill, L.; Berrer, N.; Benayahu, Y.; Kashman, Y. Eunicellin diterpenes from two Kenyan soft corals. J. Nat. Prod. 2005, 68, 19–25. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Wu, M.-H.; Wang, G.-H.; Wu, Y.-C.; Sheu, J.-H. Eunicellin-based diterpenoids, australins A−D, isolated from the soft coral Cladiella australis. J. Nat. Prod. 2005, 68, 1051–1055. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Chen, B.-W.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Simplexins A–I, eunicellin-based diterpenoids from soft coral Klyxum simplex. J. Nat. Prod. 2009, 72, 994–1000. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wu, Y.-C.; Chiang, M.Y.; Su, J.-H.; Wang, W.-H.; Fan, T.-Y.; Sheu, J.-H. Eunicellin-based diterpenes from the soft coral Klyxum simplex. Tetrahedron 2009, 65, 7016–7022. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Sung, P.-J.; Sheu, J.-H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010, 8, 2363–2366. [Google Scholar] [CrossRef]
- Hassan, H.M.; Khanfar, M.A.; Elnagar, A.Y.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.A.; Hifnawy, M.S.; El Sayed, K.A. Pachycladins A−E, prostate cancer invasion and migration inhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachyclados. J. Nat. Prod. 2010, 73, 848–853. [Google Scholar] [CrossRef]
- Williams, D.E.; Amlani, A.; Dewi, A.S.; Patrick, B.O.; van Ofwegen, L.; Mui, A.L.-F.; Andersen, R.J. Australin E isolated from the soft coral Cladiella sp. collected in Pohnpei activates the inositol 5-phosphatase SHIP1. Aust. J. Chem. 2010, 63, 895–900. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Hwang, T.-L.; Weng, C.-F.; Li, J.-J.; Fang, L.-S.; Wang, W.-H.; Wu, Y.-C.; Sung, P.-Y. Cladielloides A and B: New eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar. Drugs 2010, 8, 2936–2945. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Tsai, C.-W.; Wang, W.-H.; Wen, Z.-H.; Hsieh, C.-H.; Sung, P.-J.; Wu, Y.-C.; Sheu, J.-H. Klysimplexins I–T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2011, 9, 834–844. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Manzo, E.; Mollo, E.; Mattia, C.A.; Tedesco, C.; Irace, C.; Guo, Y.-W.; Li, X.-B.; Cimino, G.; Gavagnin, M. Tritoniopsins A–D, cladiellane-based diterpenes from the South China Sea nudibranch Tritoniopsis elegans and its prey Cladiella krempfi. J. Nat. Prod. 2011, 74, 1902–1907. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Kuo, Y.-H.; Li, J.-J.; Hwang, T.-L.; Fang, L.-S.; Wang, W.-H.; Sheu, J.-H.; Sung, P.-J. Cladieunicellins A–E, new eunicellins from an Indonesian soft coral Cladiella sp. Chem. Pharm. Bull. 2011, 59, 353–358. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Lu, Y.; Chen, B.-W.; Huang, C.-Y.; Wen, Z.-H.; Kuo, Y.-H.; Sheu, J.-H. Simplexins J–O, eunicellin-based diterpenoids from a Dongsha Atoll soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011, 84, 626–632. [Google Scholar] [CrossRef]
- Hsu, F.-J.; Chen, B.-W.; Wen, Z.-H.; Huang, C.-Y.; Dai, C.-F.; Su, J.-H.; Wu, Y.-C.; Sheu, J.-H. Klymollins A−H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle. J. Nat. Prod. 2011, 74, 2467–2471. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2011, 9, 2036–2045. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Su, Y.-D.; Chang, Y.-C.; Lu, M.-C.; Weng, C.-F.; Su, J.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellins from an Indonesian octocoral Cladiella sp. Mar. Drugs 2011, 9, 934–943. [Google Scholar] [CrossRef]
- Lin, M.-C.; Chen, B.-W.; Huang, C.-Y.; Dai, C.-F.; Hwang, T.-L.; Sheu, J.-H. Eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2013, 76, 1661–1667. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, C.-Y.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Cytotoxic and anti-inflammatory eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 788–799. [Google Scholar] [CrossRef]
- Chen, T.-H.; Lu, M.-C.; Chang, Y.-C.; Su, Y.-D.; Chen, Y.-H.; Lin, N.-C.; Fang, L.-S.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellin-based diterpenoids from a Formosan soft coral Cladiella sp. Mar. Drugs 2013, 11, 4585–4593. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Tai, C.-J.; Huang, T.-L.; Sheu, J.-H. Krempfielins J–M, new eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 2741–2750. [Google Scholar] [CrossRef]
- Cai, Y.-S.; Yao, L.-G.; Di Pascale, A.; Irace, C.; Mollo, E.; Taglialatela-Scafati, O.; Guo, Y.-W. Polyoxygenated diterpenoids of the eunicellin-type from the Chinese soft coral Cladiella krempfi. Tetrahedron 2013, 69, 2214–2219. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chang, S.-M.; Huang, C.-Y.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins A−H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010, 73, 1785–1791. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wang, S.-Y.; Huang, C.-Y.; Chen, S.-L.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins I–M, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. Tetrahedron 2013, 69, 2296–2301. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chang, S.-M.; Huang, C.-Y.; Su, J.-H.; Wen, Z.-H.; Wu, Y.-C.; Sheu, J.-H. Hirsutosterols A–G, polyoxygenated steroids from a Formosan soft coral Cladiella hirsuta. Org. Biomol. Chem. 2011, 9, 3272–3278. [Google Scholar]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Wang, C.-C.; Kuo, Y.-H.; Huang, H.-C.; Wu, Y.-C.; Kuo, L.-M.; Wu, Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Leu, Y.-L.; Kao, S.-H.; Tang, M.-C.; Chang, H.-L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006, 41, 1433–1441. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Tai, C.-J.; Huang, T.-L.; Sheu, J.-H. Krempfielins N–P, new anti-flammatory eunicellins from a Taiwanese soft coral Cladiella krempfi. Mar. Drugs 2014, 12, 1148–1156. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).