Marinopyrrole Derivatives as Potential Antibiotic Agents against Methicillin-Resistant Staphylococcus aureus (III)
Abstract
:1. Introduction
2. Results and Discussion
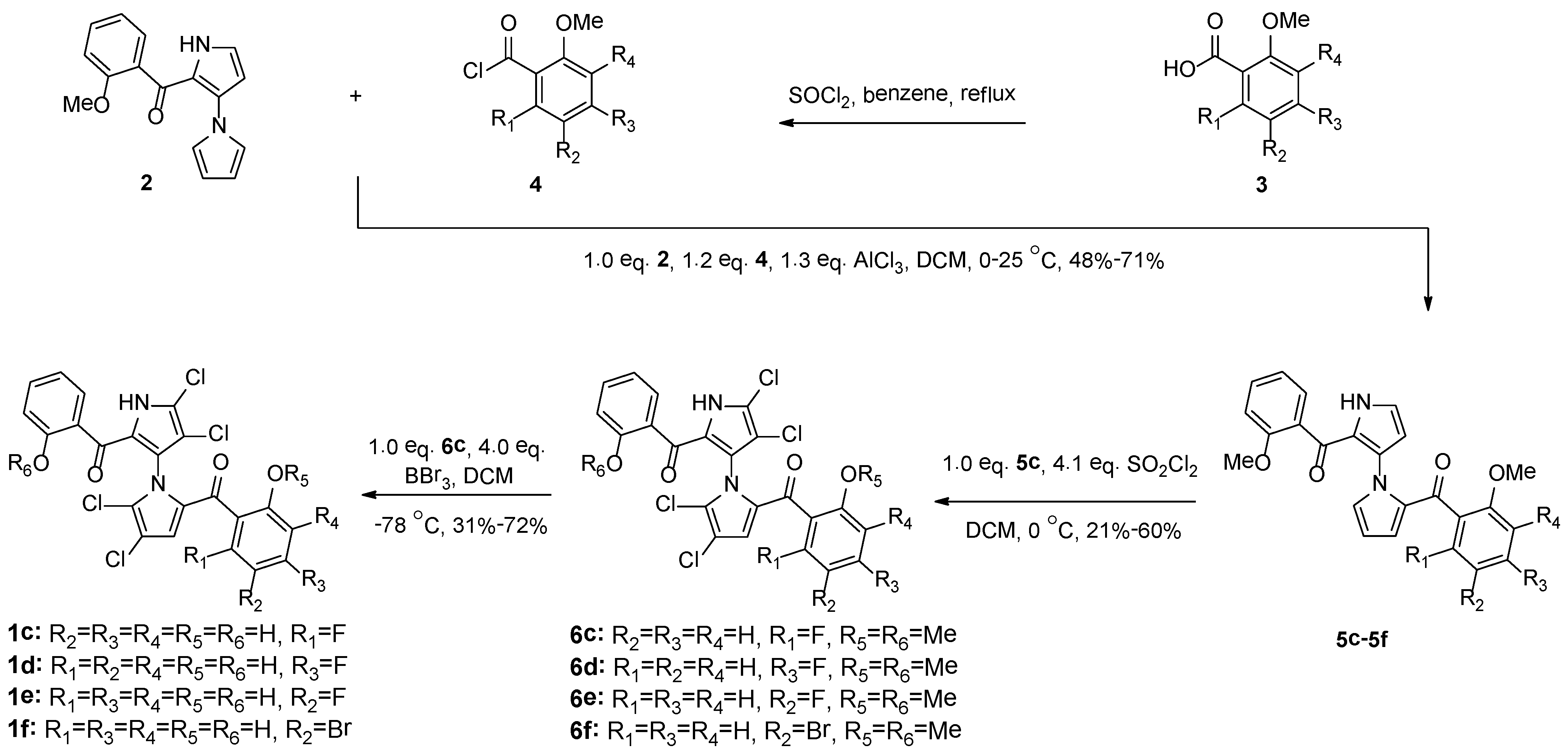
2.1. Synthesis and Structural Activity Relationships of Non-Symmetrical Marinopyrrole Derivatives
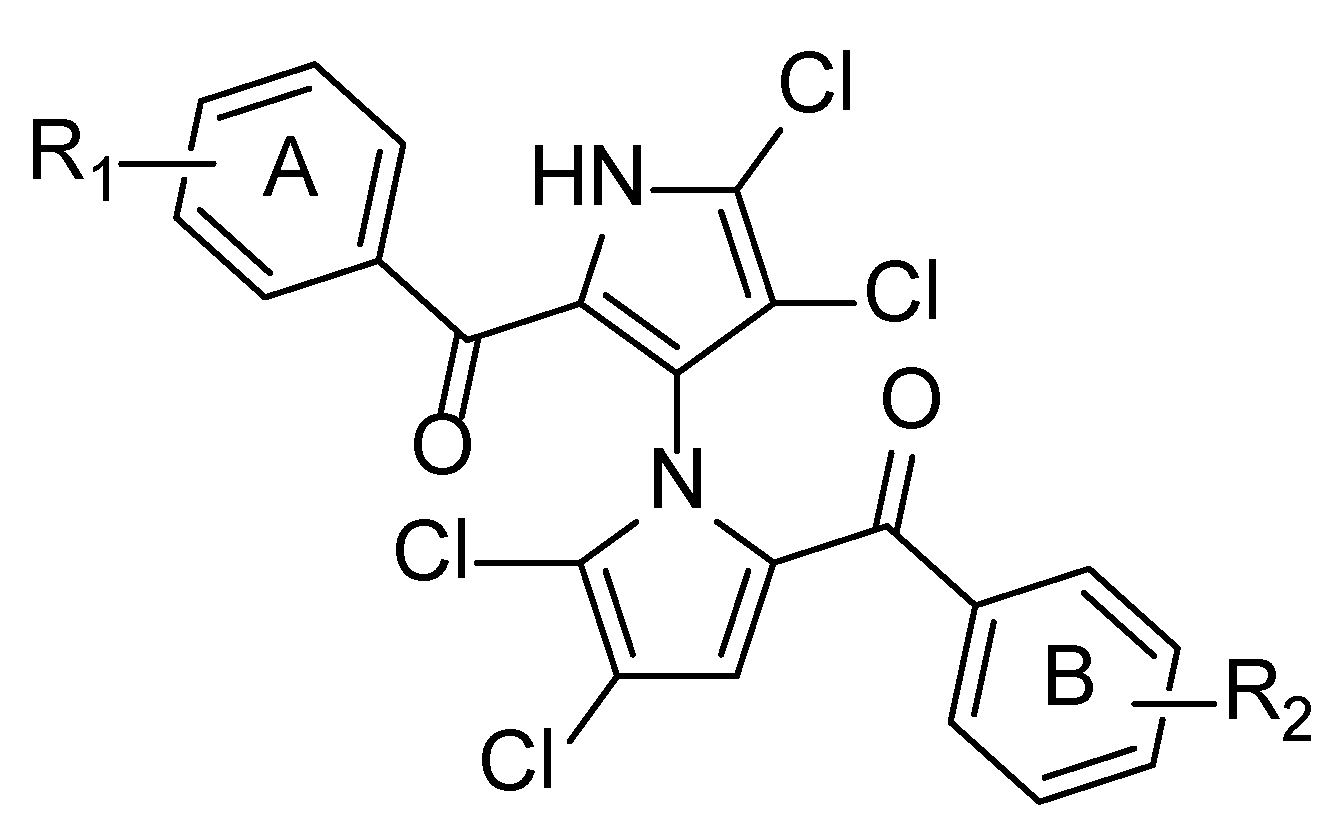
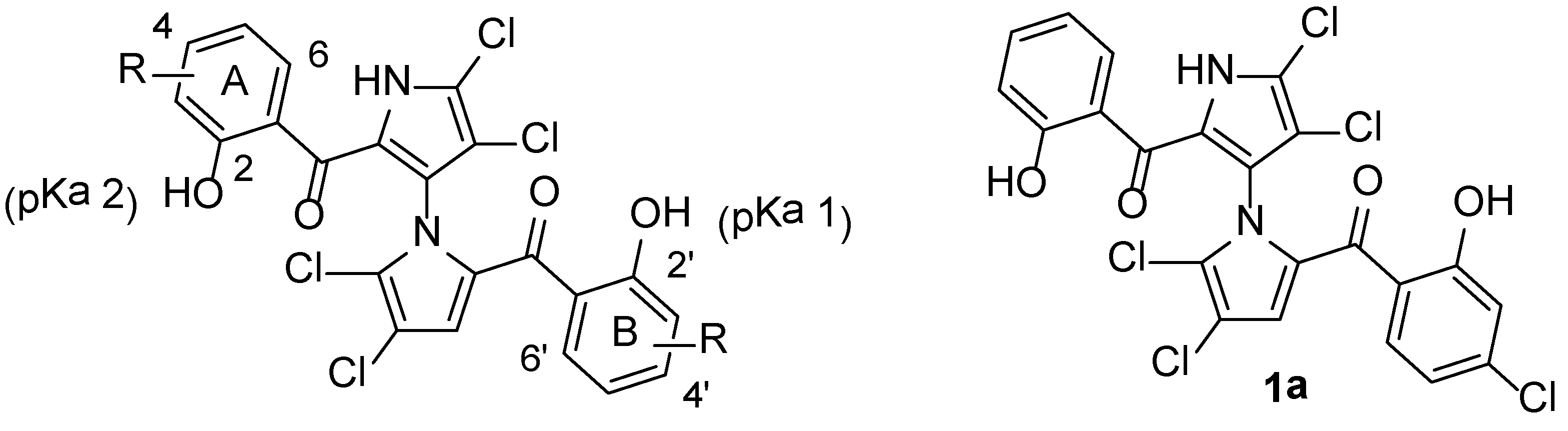
| Compound | MW f | pKa 1 a | pKa 2 a | Clog p a | THB c | THB + 20% Serum c |
|---|---|---|---|---|---|---|
| 1 (parent) | 510.15 | 7.8 b | 8.4 b | 5.6 b | 0.75 d | 94–188 d |
| 1a d | 544.60 | 7.3 | 8.2 | 6.1 | 0.19–0.39 d | 12.5–25 d |
| 1c | 528.14 | 7.2 | 8.2 | 5.7 | 3.13 | ND e |
| 1d | 528.14 | 7.0 | 8.1 | 5.7 | 0.78 | ND |
| 1e | 528.14 | 7.6 | 8.3 | 5.7 | 0.19–0.78 | 25–50 |
| 1f | 589.05 | 7.5 | 8.2 | 6.4 | 1.56 | ND |
| Vancog | 1485.72 | 0.85–1.7 | 0.85–1.7 |
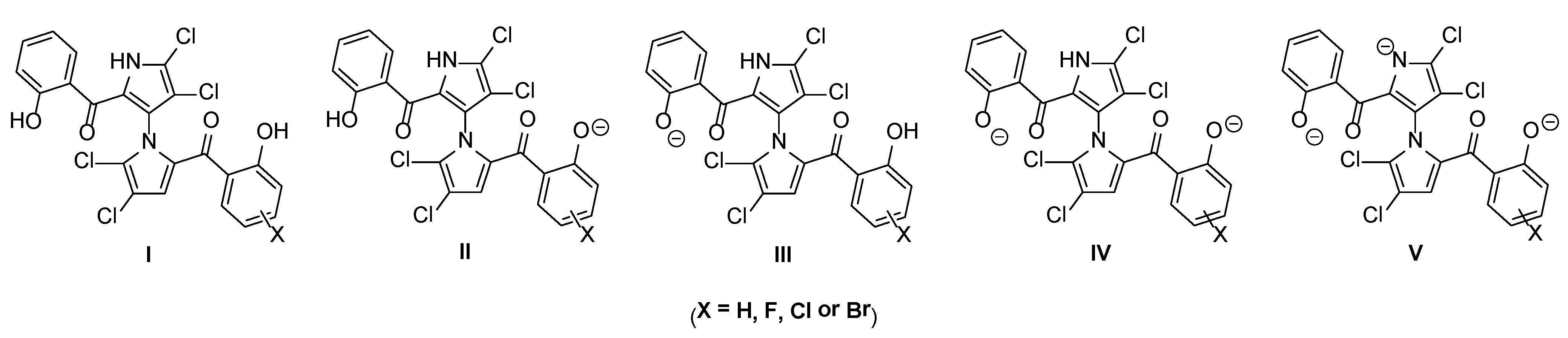
| Microspecies | 1 | 1a | 1c | 1d | 1e | 1f |
|---|---|---|---|---|---|---|
| I | 85.3/68.5/29.9 a | 63.2/38.4/9.7 | 61.2/36.3/9.0 | 50.3/26.7/5.9 | 79.8/59.4/21.3 | 75.3/52.7/16.7 |
| II | 7.1/14.3/24.8 | 29.2/44.4/45.0 | 31.2/46.5/45.7 | 42.0/56.1/48.8 | 6.6/12.4/17.6 | 17.1/30.0/38.0 |
| III | 7.1/14.3/24.8 | 5.2/8.0/8.1 | 5.1/7.6/7.4 | 4.2/5.6/4.8 | 12.5/23.4/33.4 | 6.2/11.0/13.9 |
| IV | 0.6/3.0/20.5 | 2.4/8.0/37.2 | 2.6/9.7/37.9 | 3.5/11.7/40.4 | 1.0/4.9/27.6 | 1.4/6.3/31.4 |
| V | 0.0/0.0/0.01 | 0.0/0.0/0.01 | 0.0/0.0/0.01 | 0.0/0.0/0.01 | 0.0/0.0/0.02 | 0.0/0.0/0.01 |
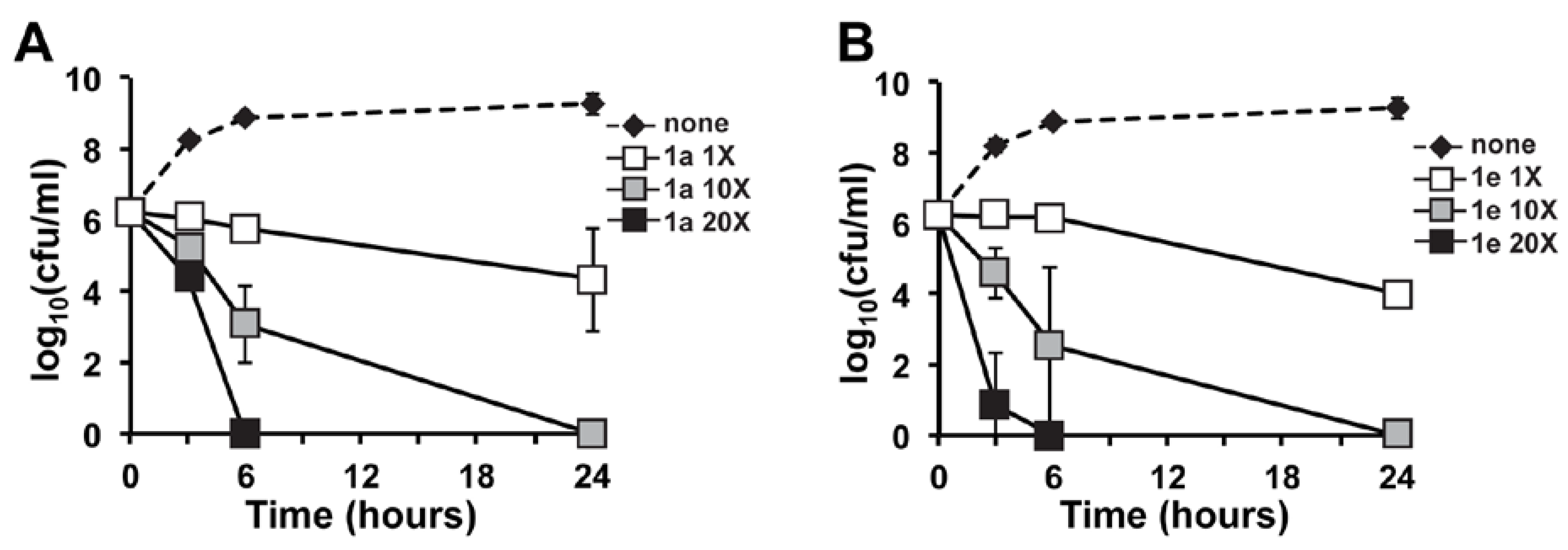
2.2. In Vitro Time-Kill of Marinopyrrole Derivative 1e
3. Experimental Section
3.1. Synthesis of Compounds 5c–6f
3.2. Synthesis of Compounds 1c–1f
3.3. In Vitro Antibacterial Assays
3.4. In Vitro Time-Kill Analysis
4. Conclusions
Acknowledgments
Author Contributions
References
- Liu, Y.; Haste, N.M.; Thienphrapa, W.; Nizet, V.; Hensler, M.; Li, R. Marinopyrrole derivatives as potential antibiotic agents against methicillin-resistant Staphylococcus aureus (I). Mar. Drugs 2012, 10, 953–962. [Google Scholar] [CrossRef]
- Yamanaka, K.; Ryan, K.S.; Gulder, T.A.; Hughes, C.C.; Moore, B.S. Flavoenzyme-catalyzed atropo-selective n,c-bipyrrole homocoupling in marinopyrrole biosynthesis. J. Am. Chem. Soc. 2012, 134, 12434–12437. [Google Scholar] [CrossRef]
- Cheng, P.; Clive, D.L.; Fernandopulle, S.; Chen, Z. Racemic marinopyrrole B by total synthesis. Chem. Commun. 2013, 49, 558–560. [Google Scholar] [CrossRef]
- Clive, D.L.J.; Cheng, P. The marinopyrroles. Tetrahedron 2013, 69, 5067–5078. [Google Scholar] [CrossRef]
- Pan, L.; Cheng, C.; Song, H. Optimization of synthetic method of marinopyrrole A derivatives. Chem. J. Chin. Univ. 2012, 33, 1476–1480. [Google Scholar]
- Cheng, C.; Liu, Y.; Song, H.; Pan, L.; Li, J.; Qin, Y.; Li, R. Marinopyrrole derivatives as potential antibiotic agents against methicillin-resistant Staphylococcus aureus (II). Mar. Drugs 2013, 11, 2927–2948. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Y.; Balasis, M.E.; Simmons, N.L.; Li, J.; Song, H.; Pan, L.; Qin, Y.; Nicolaou, K.C.; Sebti, S.M.; et al. Cyclic marinopyrrole derivatives as disruptors of Mcl-1 and Bcl-xL binding to Bim. Mar. Drugs 2014, 12, 1335–1348. [Google Scholar] [CrossRef]
- Cheng, C.; Pan, L.; Chen, Y.; Song, H.; Qin, Y.; Li, R. Total synthesis of (±)-marinopyrrole a and its library as potential antibiotic and anticancer agents. J. Comb. Chem. 2010, 12, 541–547. [Google Scholar] [CrossRef]
- Li, R.; Cheng, C.; Balasis, M.E.; Liu, Y.; Garner, T.P.; Daniel, K.G.; Li, J.; Qin, Y.; Gavathiotis, E.; Sebti, S.M. Design of marinopyrrole derivatives selective for Mcl-1 or Bcl-xL and dual inhibitors of Mcl-1 and Bcl-xL binding to Bim. 2014; Unpublished work. [Google Scholar]
- Suga, T.; Ishii, T.; Iwatsuki, M.; Yamamoto, T.; Nonaka, K.; Masuma, R.; Matsui, H.; Hanaki, H.; Omura, S.; Shiomi, K. Aranorosin circumvents arbekacin-resistance in MRSA by inhibiting the bifunctional enzyme AAC(6′)/APH(2″). J. Antibiot. 2012, 65, 527–529. [Google Scholar] [CrossRef]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J.; Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef]
- Como-Sabetti, K.; Harriman, K.H.; Buck, J.M.; Glennen, A.; Boxrud, D.J.; Lynfield, R. Community-associated methicillin-resistant Staphylococcus aureus: Trends in case and isolate characteristics from six years of prospective surveillance. Public Health Rep. 2009, 124, 427–435. [Google Scholar]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Deleo, F.R.; Otto, M.; Kreiswirth, B.N.; Chambers, H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010, 375, 1557–1568. [Google Scholar] [CrossRef]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Invest. 2003, 111, 1265–1273. [Google Scholar] [CrossRef]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840–1846. [Google Scholar] [CrossRef]
- New Research Estimates MRSA Infections Cost U.S. Hospitals $3.2 Billion to $4.2 Billion Annually. Available online: http://www.infectioncontroltoday.com/news/2005/05/new-research-estimates-mrsa-infections-cost-u-s-h.aspx (accessed on 18 January 2014).
- Jarvis, W.R.; Jarvis, A.A.; Chinn, R.Y. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at United States health care facilities, 2010. Am. J. Infect. Control. 2012, 40, 194–200. [Google Scholar] [CrossRef]
- Zhao, K.; Reiner, J.; Xie, W. FDA new drug approvals in 2000. Front. Biotechnol. Pharm. 2001, 2, 329–349. [Google Scholar]
- Eisenstein, B.I. Lipopeptides, focusing on daptomycin, for the treatment of Gram-positive infections. Expert Opin. Investig. Drugs 2004, 13, 1159–1169. [Google Scholar] [CrossRef]
- FDA Approves Teflaro for Bacterial Infections. FDA News & Events, 10/29/2010. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm231594.htm (accessed on 18 January 2014).
- Hiramatsu, K.; Hanaki, H.; Ino, T.; Yabuta, K.; Oguri, T.; Tenover, F.C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 1997, 40, 135–136. [Google Scholar] [CrossRef]
- Aguado, J.M.; San-Juan, R.; Lalueza, A.; Sanz, F.; Rodriguez-Otero, J.; Gomez-Gonzalez, C.; Chaves, F. High vancomycin MIC and complicated methicillin-susceptible Staphylococcus aureus bacteremia. Emerg. Infect. Dis. 2011, 17, 1099–1102. [Google Scholar] [CrossRef]
- Dhand, A.; Sakoulas, G. Reduced vancomycin susceptibility among clinical Staphylococcus aureus isolates (“the MIC Creep”): Implications for therapy. F1000 Med. Rep. 2012, 4. [Google Scholar] [CrossRef]
- Bauer, K.A. Daptomycin resistance following vancomycin failure in invasive methicillin-resistant Staphylococcus aureus bacteremia. Proceedings of Interscience Conference on Antimicrobial Agents and Chemotherapy.
- Jones, R.N.; Fritsche, T.R.; Sader, H.S.; Ross, J.E. LEADER surveillance program results for 2006: An activity and spectrum analysis of linezolid using clinical isolates from the United States (50 medical centers). Diagn. Microbiol. Infect. Dis. 2007, 59, 309–317. [Google Scholar] [CrossRef]
- Sanchez Garcia, M.; De la Torre, M.A.; Morales, G.; Pelaez, B.; Tolon, M.J.; Domingo, S.; Candel, F.J.; Andrade, R.; Arribi, A.; Garcia, N.; et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 2010, 303, 2260–2264. [Google Scholar] [CrossRef]
- Dolgin, E. Sequencing of superbugs seen as key to combating their spread. Nat. Med. 2010, 16. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Simmons, N.L.; Chen, J.S.; Haste, N.M.; Nizet, V. Total synthesis and biological evaluation of marinopyrrole A and analogues. Tetrahedron Lett. 2011, 52, 2041–2043. [Google Scholar] [CrossRef]
- Haste, N.M.; Hughes, C.C.; Tran, D.N.; Fenical, W.; Jensen, P.R.; Nizet, V.; Hensler, M.E. Pharmacological Properties of the Marine Natural Product Marinopyrrole A against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 3305–3312. [Google Scholar] [CrossRef]
- Chin, J.N.; Rybak, M.J.; Cheung, C.M.; Savage, P.B. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 1268–1273. [Google Scholar] [CrossRef]
- Credito, K.; Lin, G.; Appelbaum, P.C. Activity of daptomycin alone and in combination with rifampin and gentamicin against Staphylococcus aureus assessed by time-kill methodology. Antimicrob. Agents Chemother. 2007, 51, 1504–1507. [Google Scholar] [CrossRef]
- Ueda, Y.; Kanazawa, K.; Eguchi, K.; Takemoto, K.; Eriguchi, Y.; Sunagawa, M. In vitro and in vivo antibacterial activities of SM-216601, a new broad-spectrum parenteral carbapenem. Antimicrob. Agents Chemother. 2005, 49, 4185–4196. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Haste, N.M.; Thienphrapa, W.; Li, J.; Nizet, V.; Hensler, M.; Li, R. Marinopyrrole Derivatives as Potential Antibiotic Agents against Methicillin-Resistant Staphylococcus aureus (III). Mar. Drugs 2014, 12, 2458-2470. https://doi.org/10.3390/md12052458
Liu Y, Haste NM, Thienphrapa W, Li J, Nizet V, Hensler M, Li R. Marinopyrrole Derivatives as Potential Antibiotic Agents against Methicillin-Resistant Staphylococcus aureus (III). Marine Drugs. 2014; 12(5):2458-2470. https://doi.org/10.3390/md12052458
Chicago/Turabian StyleLiu, Yan, Nina M. Haste, Wdee Thienphrapa, Jerry Li, Victor Nizet, Mary Hensler, and Rongshi Li. 2014. "Marinopyrrole Derivatives as Potential Antibiotic Agents against Methicillin-Resistant Staphylococcus aureus (III)" Marine Drugs 12, no. 5: 2458-2470. https://doi.org/10.3390/md12052458
APA StyleLiu, Y., Haste, N. M., Thienphrapa, W., Li, J., Nizet, V., Hensler, M., & Li, R. (2014). Marinopyrrole Derivatives as Potential Antibiotic Agents against Methicillin-Resistant Staphylococcus aureus (III). Marine Drugs, 12(5), 2458-2470. https://doi.org/10.3390/md12052458




