Assessment of Dual Life Stage Antiplasmodial Activity of British Seaweeds
Abstract
:1. Introduction
2. Results and Discussion
| Seaweed Species | Code | Type | Family | Site of Collection | Time of Collection |
|---|---|---|---|---|---|
| Cladophora rupestris (L.) Kütz. | CR | Green | Cladophoraceae | Kimmeridge, Dorset | April 2007 |
| Codium fragile (Sur.) Hariot ssp. tomentosoides (van Goor) Silva | CFsT | Green | Codiaceae | Kimmeridge, Dorset | April 2007 |
| Ulva intestinalis L. | UI | Green | Ulvaceae | Kimmeridge, Dorset | April 2007 |
| Ulva lactuca L. | UL | Green | Ulvaceae | Kimmeridge, Dorset | April 2007 |
| Ascophyllum nodosum (L.) Le Jol. | AN | Brown | Fucaceae | Kimmeridge, Dorset | April 2007 |
| Fucus serratus L. | FS | Brown | Fucaceae | Kimmeridge, Dorset | April 2007 |
| Fucus vesiculosus L. | FV | Brown | Fucaceae | Kimmeridge, Dorset | April 2007 |
| Cystoseira baccata (S.G.Gmel.) P.C.Silva | CB | Brown | Sargassaceae | Kimmeridge, Dorset | April 2007 |
| Cystoseira tamariscifolia (Huds.) Papenf. | CT | Brown | Sargassaceae | Kimmeridge, Dorset | April 2007 |
| Sargassum muticum (Yendo) Fensholt | SM | Brown | Sargassaceae | Kimmeridge, Dorset | April 2007 |
| Laminaria digitata (Huds.) J.V. Lamour. | LD | Brown | Laminariaceae | Kimmeridge, Dorset | April 2007 |
| Saccorhiza polyschides (Lightf.) Batt. | SP | Brown | Phyllariaceae | Kimmeridge, Dorset | April 2007 |
| Pylaiella littoralis (L.) Kjellm. | PL | Brown | Acinetosporaceae | Emsworth, Hampshire | April 2007 |
| Leathesia difformis (L.) Aresch. | LD | Brown | Chordariaceae | Kimmeridge, Dorset | August 2007 |
| Dictyota dichotoma (Huds.) Lamour. | DD | Brown | Dictyotaceae | Hayling Island, Hampshire | July 2007 |
| Osmundea pinnatifida (Huds.) Stackh. | OP | Red | Rhodomelaceae | Kimmeridge, Dorset | April 2007 |
| Calliblepharis jubata (Good. et Woodw.) Kütz. | CJ | Red | Cystocloniaceae | Kimmeridge, Dorset | April 2007 |
| Ceramium virgatum Roth | CV | Red | Ceramiaceae | Kimmeridge, Dorset | April 2007 |
| Claviclonium ovatum (J.V.Lamour.) Kraft & Min-Thein | ClO | Red | Acrotylaceae | Kimmeridge, Dorset | April 2007 |
| Halopitys incurvus (Huds.) Batt. | HI | Red | Rhodomelaceae | Kimmeridge, Dorset | April 2007 |
| Corallina officinalis L. | CoO | Red | Corallinaceae | Seacombe, Dorset | April 2007 |
| Porphyra linearis Grev | PoL | Red | Bangiaceae | Seacombe, Dorset | April 2007 |
| Halurus equisetifolius (Lightf.) Kütz. | HE | Red | Wrangeliaceae | Kimmeridge, Dorset | August 2007 |
2.1. Blood Stage Antimalarial Activity and General Cytotoxicity of British Seaweeds
| Seaweed Species | Type | BS | LS | PfFabI | PfFabG | PfFabZ | L6 cells f |
|---|---|---|---|---|---|---|---|
| Cladophora rupestris | Green | 11.9 | 37.3 | >50 | >50 | 1.0 | >90 |
| Codium fragile ssp. tomentosoides | 11.8 | 34.6 | >50 | >50 | 13 | >90 | |
| Ulva intestinalis | 18.2 | >50 | n.t | n.t | n.t | >90 | |
| Ulva lactuca | 3.8 | 14.9 | 2.0 | >50 | 7.0 | >90 | |
| Ascophyllum nodosum | Brown | >50 | >50 | n.t | n.t | n.t | >90 |
| Fucus serratus | 17.6 | >50 | n.t | n.t | n.t | >90 | |
| Fucus vesiculosus | 15.7 | >50 | n.t | n.t | n.t | >90 | |
| Cystoseira baccata | 3.4 | 32.6 | 2.3 | 2.0 | 1.4 | >90 | |
| Cystoseira tamariscifolia | 3.3 | 49.4 | 37 | 2.8 | 1.3 | 62.5 | |
| Sargassum muticum | 18.2 | >50 | n.t | n.t | n.t | >90 | |
| Laminaria digitata | 17.6 | >50 | n.t | n.t | n.t | >90 | |
| Saccorhiza polyschides | 16.1 | >50 | n.t | n.t | n.t | >90 | |
| Pylaiella littoralis | >50 | >50 | n.t | n.t | n.t | >90 | |
| Leathesia difformis | >50 | >50 | n.t | n.t | n.t | >90 | |
| Dictyota dichotoma | >50 | >50 | n.t | n.t | n.t | >90 | |
| Osmundea pinnatifida | Red | 14.5 | 52.9 | >50 | >50 | 3.0 | >90 |
| Calliblepharis jubata | >50 | >50 | n.t | n.t | n.t | >90 | |
| Ceramium virgatum | 13.6 | 26.4 | 30 | 13 | 4.2 | >90 | |
| Claviclonium ovatum | >50 | >50 | >50 | >50 | 2.1 | >90 | |
| Halopitys incurvus | >50 | 28.8 | 2.4 | 15.9 | 2.9 | >90 | |
| Corallina officinalis L. | 8.6 | >50 | n.t | n.t | n.t | 88.6 | |
| Porphyra linearis | >50 | >50 | n.t | n.t | n.t | >90 | |
| Halurus equisetifolius | >50 | >50 | n.t | n.t | n.t | >90 | |
| Positive control | 0.056 a | 3.4 b | 0.014 c | 0.30 d | 0.03 d | 0.004 e,f |
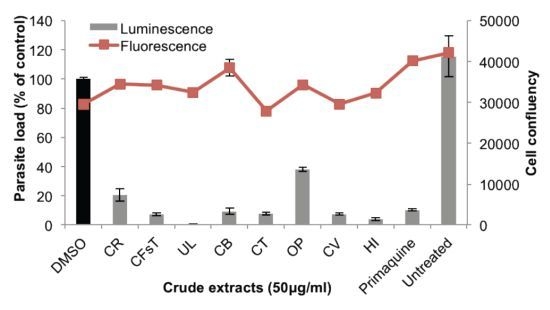
2.2. Liver Stage Activity of British Seaweeds
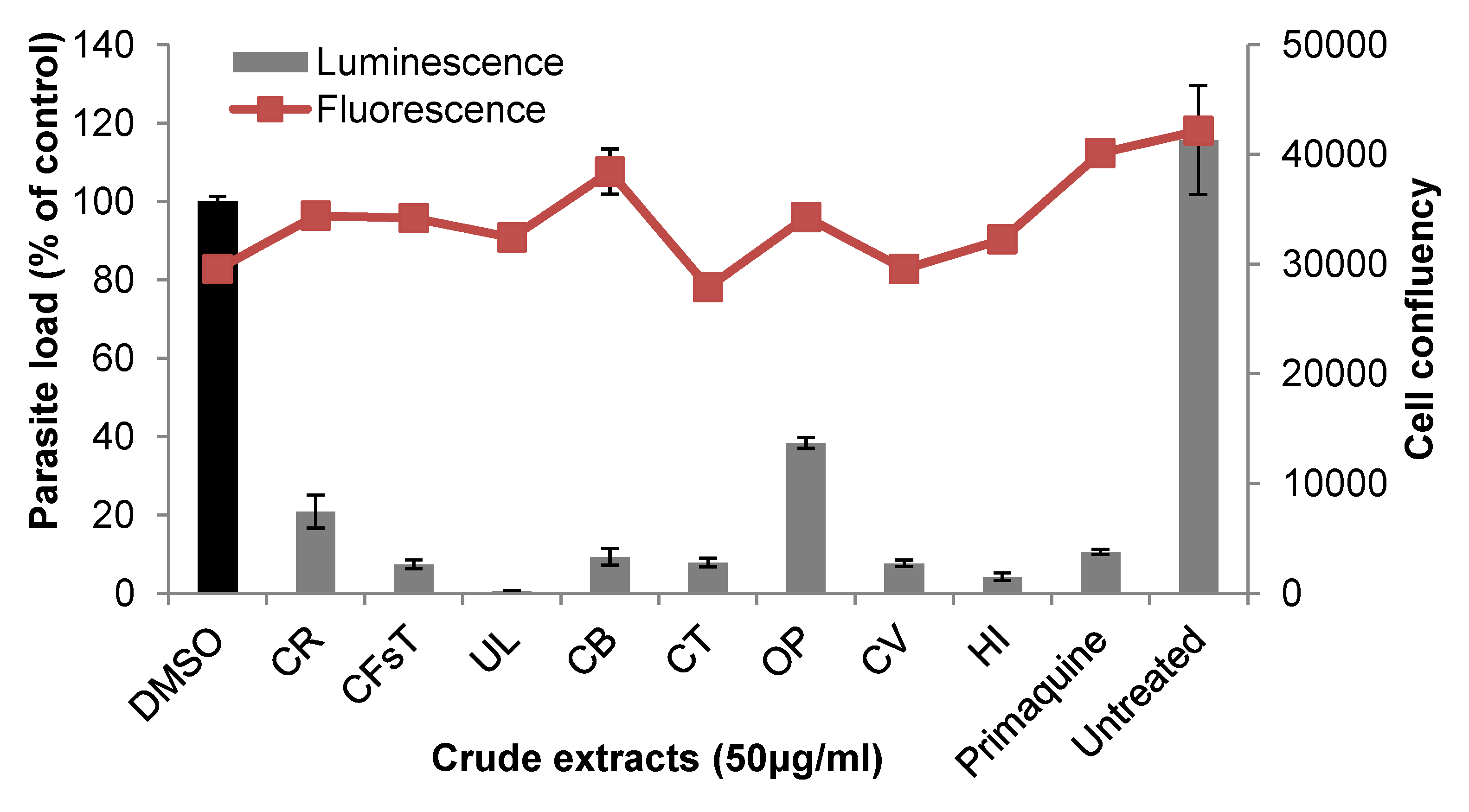
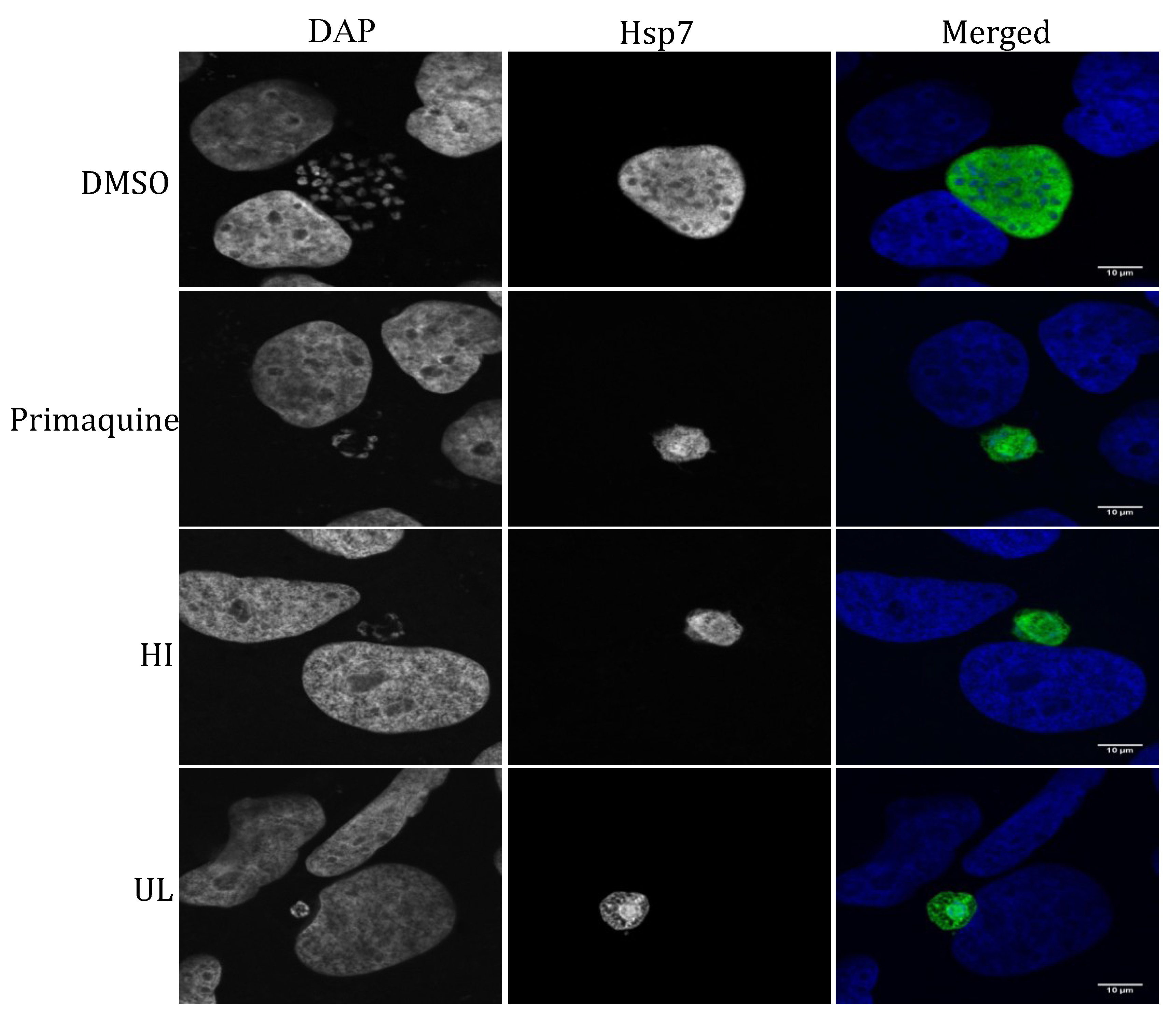
2.3. Plasmodial FAS-II Enzyme Inhibitory Activity of British Seaweeds
3. Experimental Section
3.1. Algal Material
3.2. Preparation of the Extracts
3.3. Blood Stage Antiplasmodial Activity
3.4. Liver Stage Antiplasmodial Activity
3.4.1. Culturing and Infection of Huh7 Cells with P. berghei ANKA Sporozoites
3.4.2. In Vitro Testing of Liver Stage Antiplasmodial Activity of Seaweed Crude Extracts
3.4.3. Immunofluorescence Detection of P. berghei Parasites in Extract- or Control-Treated Huh7 Cells
3.4.4. Cell Viability
3.5. Plasmodial FAS-II Enzyme Inhibition Assays
3.6. General Cytotoxicity against Mammalian Cells
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organization fact sheet. Available online: http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_factsheet.pdf (accessed on 6 August 2013).
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasit Vectors 2010, 3, 5. [Google Scholar] [CrossRef]
- Price, R.N.; Tjitra, E.; Guerra, C.A.; Yeung, S.; White, N.J.; Anstey, N.M. Vivax malaria: Neglected and not benign. Am. J. Trop. Med. Hyg. 2007, 77, 79–87. [Google Scholar]
- Prudencio, M.; Mota, M.M.; Mendes, A.M. A toolbox to study liver stage malaria. Trends Parasitol. 2011, 27, 565–574. [Google Scholar] [CrossRef]
- Derbyshire, E.R.; Prudencio, M.; Mota, M.M.; Clardy, J. Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc. Natl. Acad. Sci. USA 2012, 109, 8511–8516. [Google Scholar]
- Yu, M.; Kumar, T.R.; Nkrumah, L.J.; Coppi, A.; Retzlaff, S.; Li, C.D.; Kelly, B.J.; Moura, P.A.; Lakshmanan, V.; Freundlich, J.S.; et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 2008, 4, 567–578. [Google Scholar] [CrossRef]
- Vaughan, A.M.; O’Neill, M.T.; Tarun, A.S.; Camargo, N.; Phuong, T.M.; Aly, A.S.; Cowman, A.F.; Kappe, S.H. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009, 11, 506–520. [Google Scholar] [CrossRef]
- Surolia, N.; Surolia, A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 2001, 7, 167–173. [Google Scholar] [CrossRef]
- Tarun, A.S.; Peng, X.; Dumpit, R.F.; Ogata, Y.; Silva-Rivera, H.; Camargo, N.; Daly, T.M.; Bergman, L.W.; Kappe, S.H. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl. Acad. Sci. USA 2008, 105, 305–310. [Google Scholar] [CrossRef]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2008, 120, 92–97. [Google Scholar] [CrossRef]
- Baslow, M.H. Marine Pharmacology; Williams & Wilkins Co: Baltimore, MD, USA, 1969; pp. 69–71. [Google Scholar]
- Scheuer, P.J. Marine Natural Products, Chemical and Biological Perspectives, 3rd ed.; Academic Press: New York, NY, USA, 1980; pp. 100–106. [Google Scholar]
- Orhan, I.; Sener, B.; Brun, R.; Perozzo, R; Tasdemir, D. Turkish freshwater and marine macrophyte extracts show in vitro antiprotozoal activity and inhibit FabI, a key enzyme of Plasmodium falciparum fatty acid biosynthesis. Phytomedicine 2006, 13, 388–393. [Google Scholar] [CrossRef]
- Süzgeç-Selçuk, S.; Meriçli, A.H.; Güven, K.C.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Tasdemir, D. Evaluation of Turkish seaweeds for antiprotozoal, antimycobacterial and cytotoxic activities. Phytother. Res. 2011, 25, 778–783. [Google Scholar]
- Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of some British green algae. Phytother. Res. 2010, 24, 1095–1098. [Google Scholar]
- Allmendinger, A.; Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three British and Irish red algae. Phytother. Res. 2010, 24, 1099–1103. [Google Scholar]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.D.; Blunden, G.; Tasdemir, D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytother. Res. 2010, 24, 1724–1729. [Google Scholar] [CrossRef]
- Matile, H.; Pink, J.R.L. Plasmodium falciparum malaria parasite cultures and their use in immunology. In Immunological Methods; Lefkovits, I., Pernis, B., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 221–234. [Google Scholar]
- Tasdemir, D.; Lack, G.; Brun, R.; Ruedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium falciparum fatty acid biosynthesis: evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Vonthron-Sénécheau, C; Kaiser, M.; Devambez, I.; Vastel, A.; Mussio, I.; Rusig, A.M. Antiprotozoal activities of organic extracts from French marine seaweeds. Mar. Drugs 2011, 9, 922–933. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P. Seaweeds as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitol. Res. 2011, 109, 47–52. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Gokulakrishnan, R.; Venkatesan, M. In vitro antiplasmodial activity of ethanolic extracts of seaweed macroalgae against Plasmodium falciparum. Parasitol. Res. 2011, 108, 1411–1416. [Google Scholar] [CrossRef]
- Ravikumar, S.; Ramanathan, G.; Gnanadesigan, M.; Ramu, A.; Vijayakumar, V. In vitro antiplasmodial activity of methanolic extracts from seaweeds of South West Coast of India. Asian Pac. J. Trop. Med. 2011, 4, 862–865. [Google Scholar] [CrossRef]
- Elsayed, K.N.; Radwan, M.M.; Hassan, S.H.; Abdelhameed, M.S.; Ibraheem, I.B.; Ross, S.A. Phytochemical and biological studies on some Egyptian seaweeds. Nat. Prod. Commun. 2012, 7, 1209–1210. [Google Scholar]
- Afolayan, A.F.; Mann, M.G.; Lategan, C.A.; Smith, P.J.; Bolton, J.J.; Beukes, D.R. Antiplasmodial halogenated monoterpenes from the marine red alga Plocamium cornutum. Phytochemistry 2009, 70, 597–600. [Google Scholar] [CrossRef]
- Stout, E.P.; Cervantes, S.; Prudhomme, J.; France, S.; La Clair, J.J.; Le Roch, K.; Kubanek, J. Bromophycolide A targets heme crystallization in the human malaria parasite Plasmodium falciparum. ChemMedChem. 2011, 6, 1572–1577. [Google Scholar] [CrossRef]
- Stout, E.P.; Prudhomme, J.; Roch, K.L.; Fairchild, C.R.; Franzblau, S.G.; Aalbersberg, W.; Hay, M.E.; Kubanek, J. Unusual antimalarial meroditerpenes from tropical red macroalgae. Bioorg. Med. Chem. Lett. 2010, 20, 5662–5665. [Google Scholar] [CrossRef]
- Lin, A.S.; Stout, E.P.; Prudhomme, J.; Le Roch, K.; Fairchild, C.R.; Franzblau, S.G.; Aalbersberg, W.; Hay, M.E.; Kubanek, J. Bioactive bromophycolides R-U from the Fijian red alga Callophycus serratus. J. Nat. Prod. 2010, 73, 275–278. [Google Scholar] [CrossRef]
- Afolayan, A.F.; Bolton, J.J.; Lategan, C.A.; Smith, P.J.; Beukes, D.R. ucoxanthin, tetraprenylated toluquinone and toluhydroquinone metabolites from Sargassum heterophyllum inhibit the in vitro growth of the malaria parasite Plasmodium falciparum. Z. Naturforsch. C. 2008, 63, 848–852. [Google Scholar]
- Adams, Y.; Smith, S.L.; Schwartz-Albiez, R.; Andrews, K.T. Carrageenans inhibit the in vitro growth of Plasmodium falciparum and cytoadhesion to CD36. Parasitol Res. 2005, 97, 290–294. [Google Scholar] [CrossRef]
- Chen, J.H.; Lim, J.D.; Sohn, E.H.; Choi, Y.S.; Han, E.T. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol. Res. 2009, 104, 245–250. [Google Scholar] [CrossRef]
- Pancake, S.J.; Holt, G.D.; Mellouk, S.; Hoffman, S.L. Malaria sporozoites and circums-porozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 1992, 117, 1351–1357. [Google Scholar] [CrossRef]
- Hanson, K.K.; Ressurreiçao, A.S.; Buchholz, K.; Prudencio, M.; Herman-Ornelas, J.D.; Rebelo, M.; Beatty, W.L.; Wirth, D.F.; Hänscheid, T.; Moreira, R.; et al. Torins are potent antimalarials that block replenishment of Plasmodium liver stage parasitophorous vacuole membrane proteins. Proc. Natl. Acad. Sci. USA 2013, 110, E2838–E2847. [Google Scholar] [CrossRef]
- Waller, R.F.; Keeling, P.J.; Donald, R.G.; Striepen, B.; Handman, E.; Lang-Unnasch, N.; Cowman, A.F.; Besra, G.S.; Roos, D.S.; McFadden, G.I. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1998, 95, 12352–12357. [Google Scholar] [CrossRef]
- Janouskovec, J.; Horak, A.; Obornik, M.; Lukes, J.; Keeling, P.J. A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc. Natl. Acad. Sci. USA 2010, 107, 10949–10954. [Google Scholar] [CrossRef]
- Baschong, W.; Wittlin, S.; Inglis, K.A.; Fairlamb, A.H.; Croft, S.L.; Kumar, T.R.; Fidock, D.A.; Brun, R. Triclosan is minimally effective in rodent malaria models. Nat. Med. 2011, 17, 33–34. [Google Scholar] [CrossRef]
- Singh, A.P.; Surolia, N.; Surolia, A. Triclosan inhibit the growth of the late liver-stage of Plasmodium. IUBMB Life 2009, 61, 923–928. [Google Scholar] [CrossRef]
- Goodman, C.D.; McFadden, G.I. Targeting apicoplasts in malaria parasites. Expert Opin. Ther. Targets 2013, 17, 167–177. [Google Scholar] [CrossRef]
- Huber, W.; Koella, J.C. A comparison of the three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993, 55, 257–261. [Google Scholar] [CrossRef]
- Ploemen, I.H; Prudencio, M.; Douradinha, B.G.; Ramesar, J.; Fonager, J.; van Gemert, G.J.; Luty, A.J.; Hermsen, C.C.; Sauerwein, R.W.; Baptista, F.G.; et al. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One 2009, 4, e7881. [Google Scholar] [CrossRef]
- Rodrigues, T.; da Cruz, F.P.; Lafuente-Monasterio, M.J.; Gonçalves, D.; Ressurreiçao, A.S.; Sitoe, A.R.; Bronze, M.R.; Gut, J.; Schneider, G.; Mota, M.M.; et al. Quinolin-4(1H)-imines are potent antiplasmodial drugs targeting the liver stage of malaria. J. Med. Chem. 2013, 56, 4811–4815. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Spavieri, J.; Allmendinger, A.; Kaiser, M.; Itoe, M.A.; Blunden, G.; Mota, M.M.; Tasdemir, D. Assessment of Dual Life Stage Antiplasmodial Activity of British Seaweeds. Mar. Drugs 2013, 11, 4019-4034. https://doi.org/10.3390/md11104019
Spavieri J, Allmendinger A, Kaiser M, Itoe MA, Blunden G, Mota MM, Tasdemir D. Assessment of Dual Life Stage Antiplasmodial Activity of British Seaweeds. Marine Drugs. 2013; 11(10):4019-4034. https://doi.org/10.3390/md11104019
Chicago/Turabian StyleSpavieri, Jasmine, Andrea Allmendinger, Marcel Kaiser, Maurice Ayamba Itoe, Gerald Blunden, Maria M. Mota, and Deniz Tasdemir. 2013. "Assessment of Dual Life Stage Antiplasmodial Activity of British Seaweeds" Marine Drugs 11, no. 10: 4019-4034. https://doi.org/10.3390/md11104019
APA StyleSpavieri, J., Allmendinger, A., Kaiser, M., Itoe, M. A., Blunden, G., Mota, M. M., & Tasdemir, D. (2013). Assessment of Dual Life Stage Antiplasmodial Activity of British Seaweeds. Marine Drugs, 11(10), 4019-4034. https://doi.org/10.3390/md11104019