Abstract
Marine natural products are a diverse, unique collection of compounds with immense therapeutic potential. This has resulted in these molecules being evaluated for a number of different disease indications including the neglected protozoan diseases, human African trypanosomiasis and Chagas disease, for which very few drugs are currently available. This article will review the marine natural products for which activity against the kinetoplastid parasites; Trypanosoma brucei brucei, T.b. rhodesiense and T. cruzi has been reported. As it is important to know the selectivity of a compound when evaluating its trypanocidal activity, this article will only cover molecules which have simultaneously been tested for cytotoxicity against a mammalian cell line. Compounds have been grouped according to their chemical structure and representative examples from each class were selected for detailed discussion.
1. Introduction
The trypanosomatid diseases human African trypanosomiasis (HAT) and Chagas disease account for over 19,000 deaths and the loss of over 100,000 disability adjusted life years (DALYs) annually [1,2]. The etiological agents of the disease are kinetoplastid parasites of the genus Trypanosoma. Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense are responsible for HAT, while infection with Trypanosoma cruzi is the causative agent of Chagas disease. Both diseases rely on insect vectors for their transmission; tsetse flies (Glossina spp.) are the vectors for HAT, whereas a number of Triatoma bug species transmit T. cruzi [3,4]. HAT is prevalent throughout 36 sub-Saharan African countries whilst Chagas disease primarily occurs in Southern parts of North America, and South America [5,6].
Initially, inoculation of the parasites into human hosts results in acute disease. In HAT, this is characterized by the presence of the parasites in the vasculature and lymphatic systems. Patients experience fever, nausea, headaches and lymphedema [7]. Without treatment the parasites penetrate the blood brain barrier (BBB) and invade the central nervous system (CNS) initiating chronic or CNS stage disease. CNS stage disease manifests as mental disturbances, anxiety, hallucinations and a characteristic disruption of the sleep-wake cycle [7,8,9,10]. Without treatment the disease is considered fatal [11].
In contrast to HAT, acute Chagas disease is often asymptomatic and as such is not often diagnosed [12]. Approximately one third of infected individuals go on to develop the chronic form of the disease which can remain asymptomatic for 10 to 30 years [12]. The chronic stage can manifest as cardiac or cardiodigestive disorders (megacolon, megaeosphagus), or a combination of these [13]. Chagas related heart disease is one of the major causes of morbidity and mortality in endemic areas [14].
Despite the morbidity and mortality inflicted by HAT and Chagas disease, very few effective drugs are currently available (Figure 1). Acute T.b. gambiense and T.b. rhodesiense infections are treated with pentamidine and suramin, respectively [15]. CNS T.b. rhodesiense infections are treated with melarsoprol, while T.b. gambiense infections are treated with either eflornithine or a nifurtimox/eflornithine combination therapy (NECT) [15]. However, none of these treatments are ideal. Melarsoprol is extremely toxic, resulting in the death of 5% of all patients to whom the drug is administered, and eflornithine has a complicated, protracted administration schedule requiring 56 slow intravenous (i.v.) infusions over 14 days [16,17]. The development of NECT reduced the administration schedule of eflornithine to 14 i.v. infusions over seven days, plus oral nifurtimox every eight hours for 10 days [18,19]. However, NECT is not ideal as parenteral administration is still required and patients must be hospitalized for the duration of treatment. Acute and chronic Chagas diseases are treated with either nifurtimox or benznidazole. Both drugs have lengthy administration schedules requiring bi- or tri-daily administration for 60 to 90 days [20]. Patients frequently experience vomiting, nausea, hepatic intolerance, convulsions and skin disease manifestations [21]. The unpleasant side effects experienced by patients, coupled with administration schedules, result in many patients failing to complete the treatment regimes [22,23].
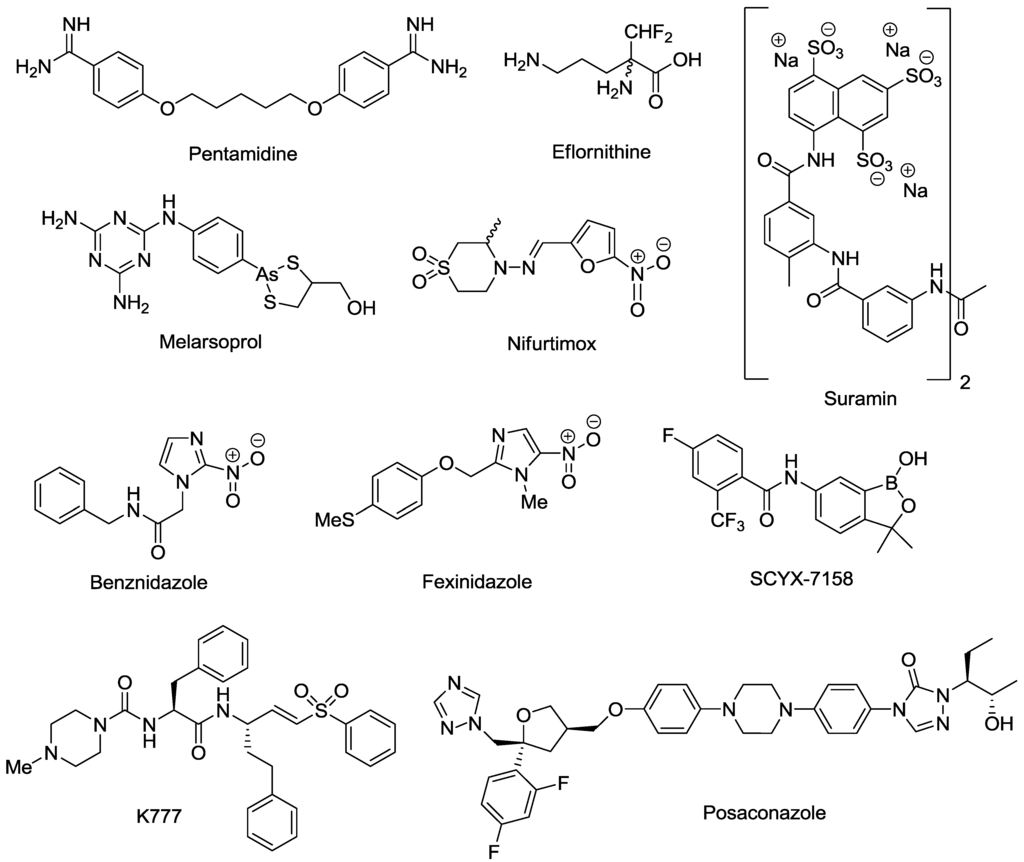
Figure 1.
Drugs currently registered and in development for the treatment of human African trypanosomiasis (HAT) and Chagas disease.
The paucity of safe, effective and easily administrable drugs for HAT and Chagas disease is partly due to a lack of interest by large pharmaceutical companies. HAT and Chagas disease primarily affect poor, disadvantaged people, with limited access to health care and very little means to pay for drugs. Consequently, there is little incentive for pharmaceutical companies to invest in the research and development of new compounds for these disease indications. It has only been in the last decade, with the establishment of non-for-profit organizations such as the Drugs for Neglected Diseases initiative (DNDi) and the Bill and Melinda Gates Foundation, that substantial investment and progress has been made in drug discovery for HAT and Chagas disease. As a result, one compound, fexinidazole, is now in phase II/III clinical trials for HAT, while a second compound, SCYX-7158, is in phase I clinical trials [24,25]. In addition, during the past five years numerous drug targets have been identified and validated in T.b. brucei which are discussed in detail in a recent review [26]. Promising targets described include, the enzymes S-adenosylmethionine decarboxylase (AdoMetDC) [27,28], N-myristoyltransferase (NMT) [29,30] and trypanothione synthetase-amidase (TrySyn) [31]. For Chagas disease, K777 is currently in pre-clinical trials [32], whilst clinical trials with posaconazole are due for completion in 2013 [33]. Target identification studies have indicated that cysteine protease is the target of K777, thus validating further development of this class of inhibitors. Posaconazole inhibits T. cruzi sterol 14α-demethylase (CYP51) [34], and research continues to identify further inhibitors of this specific target [35,36,37]. Azole antifungals with CYP51 activity have previously entered clinical trials, however, have not demonstrated curative activity [38]. Few validated targets have been identified against T. cruzi and studies to determine new targets will be of benefit for Chagas disease research. Cloning of recombinant proteins based on the identified genome sequence could facilitate this process [39]. The mitochondria and mitochondrial metabolism [40] have been identified as potential sources of new targets for T. cruzi drug discovery research, as well as enzymes involved in pentose phosphate and thymidine synthesis [41].
Non-for profit organizations have highlighted the plight of HAT and Chagas disease patients and have provided the financial resources required for new therapeutics to be identified and developed. However, numerous problems still exist which impede drug development for HAT and Chagas disease. A large proportion of the molecules identified by phenotypic high-throughput screening (HTS) campaigns have undesirable chemical properties and biological characteristics, which makes them unsuitable for further development. Structure activity relationship (SAR) studies are frequently undertaken in order to improve a molecule’s physiochemical properties, but this often results in a significant loss of trypanocidal activity. In the last five years, multiple drug targets have been identified in T. brucei spp. and T. cruzi. However, the targets are often inaccessible and it is difficult to develop small molecule inhibitors, which are capable of reaching and interacting with the target. Target-based screening can be utilized to identify potent inhibitors of targets but often the molecules lack trypanocidal activity when subsequently screened against the whole parasite, as they are unable to penetrate the parasites and reach the intracellular target.
The high attrition rate associated with drug discovery and development and the difficulties encountered, means that there still exists a critical need to identify novel compounds for HAT and Chagas disease. Natural products including, marine organisms and metabolites, are one potential source from which unique trypanocidal compounds could be identified.
Natural products are attractive chemical starting points for drug discovery. They have been investigated for a number of different disease indications and biological targets resulting in the identification of both lead molecules and drugs suitable for entry into the drug discovery pipeline. Between 1981 and 2010 natural products and synthetic small molecules either derived from a natural product or based on a natural product, pharmacophore, accounted for over 50% of new chemical entities [42]. Research into the chemistry, pharmacology and therapeutic potential of marine natural products began with the development of self-contained breathing apparatus (SCUBA) in the 1960s and has continued to progress and develop with thousands of compounds now identified [43]. The first marine natural product to be registered by the United States (US) Food and Drugs Administration (FDA) was cytarabine (1β-arabinofuranosylcytosine), a chemotherapeutic agent, in 1969. Since then six other marine natural product based drugs have been approved by the FDA; vidarabine (anti-cancer and anti-viral), ziconotide (an analgesic agent), eribulin mesylate (anti-cancer), brentuximab vedotin (for the treatment of Hodgkin’s lymphoma and large cell lymphoma) and the omega-3-ethyl ester preparations, lovaza and vascepa (triglyceride lowering agents). In addition, one further compound, trabectedin (anti-cancer), has been approved by the European Medicines Agency (EMA).
Cytarabine (1β-arabinofuranosylcytosine) and vidarabine (adenine arabinoside) (Figure 2) are synthetic pyrimidine and purine nucleosides, respectively, developed from nucleosides isolated from the Caribbean sponge Tethya crypta [44,45]. Cytarabine is used for the treatment of acute myeloid and lymphocytic leukemia, while vidarabine was approved in 1976 for the treatment of acute keratoconjunctivitis and recurrent epithelial keratitis caused by Herpes simplex viruses [46,47,48]. The therapeutic effects of cytarabine and vidarabine are thought to arise due to inhibition of DNA polymerase and DNA synthesis [49,50].
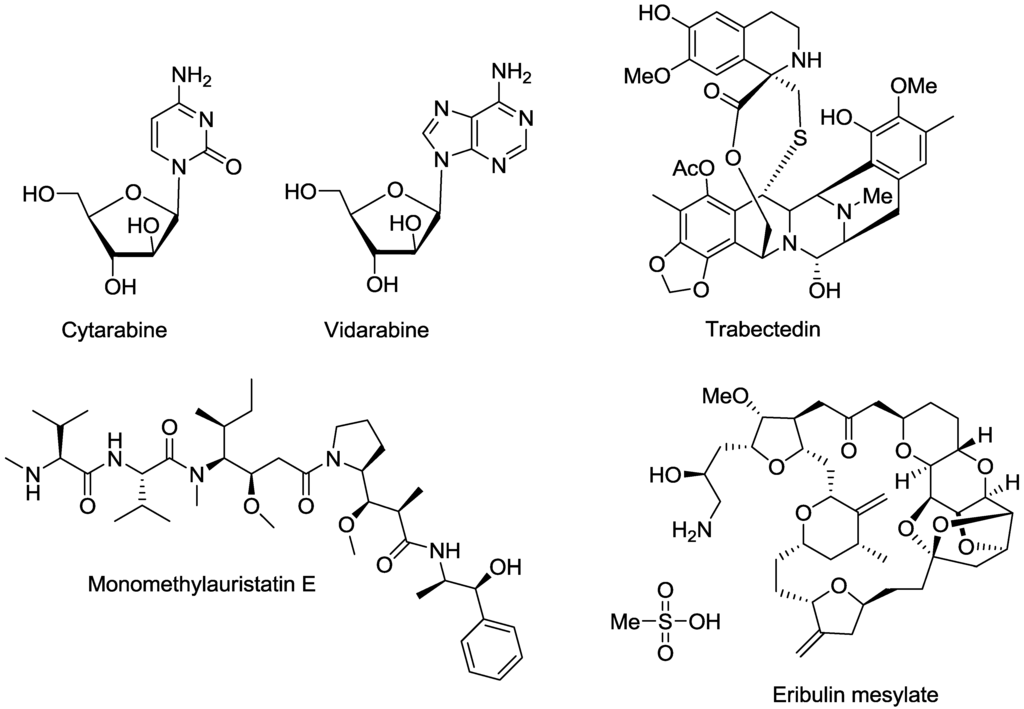
Figure 2.
Examples of small molecule-based marine natural products or their derivatives which have received Food and Drug Administration (FDA) or European Medicines Agency (EMA) approval.
Twenty-eight years after the registration of vidarabine, ziconotide, a synthetic equivalent of a peptide originally isolated from the venom of the cone snail Conus magus, was approved by the FDA [51]. The drug is a powerful analgesic due to its ability to selectively and specifically block N-type voltage sensitive calcium channels and is used to manage chronic pain in cancer and AIDS patients [52]. Also in 2004, lovaza, the first drug containing the fish derived omega-3-ethyl fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) was approved for the reduction of triglyceride levels in severe hypertriglyceridemia [53]. This was followed by the registration of vascepa, containing only EPA, in 2012 [54]. Omega-3-ethyl fatty acids are found in all fish species but are most abundant in oily fish, such as salmon, mackerel and herring [55]. The mechanism of action (MOA) for the hypotriglyceridemic effect of omega-3-ethyl fatty acids is not fully understood but has been attributed to the suppression of hepatic lipogenesis, an increase in fatty β-oxidation and down regulation of hepatic nuclear factor-4α (HNF-4α) [56,57,58,59,60]. In 2010, eribulin mesylate, a synthetic macro-cyclic ketone analogue of halichondrin B, a molecule isolated from the marine sponge Halichondria okadai, received FDA approval for the treatment of metastatic breast cancer [61]. Eribulin induces cell death by inhibiting microtubule growth and sequestering tubulin into nonproductive aggregates [62,63,64]. Brentuximab vedotin, a CD30 specific antibody-drug conjugate received FDA approval for the treatment of Hodgkin’s lymphoma in 2011. Brentuximab vedotin is composed of monomethylauristatin E (MMAE), a synthetic analogue of the marine natural product dolastatin 10 conjugated with the chimeric anti-CD30 monoclonal antibody, SGN-30 [65]. Dolastatin 10 was originally isolated from the Indian Ocean sea hare Dolabella auricularia in 1987 [66]. MMAE is an anti-tubulin agent which binds to tubulin and prevents microtubule polymerization leading to G2-M phase growth arrest and apoptosis [67]. Trabectedin (ecteinascidin) (Figure 2) has been approved in the European Union (EU) by the EMA. The compound was isolated from the ascidian Ecteinascidia turbinata and is an anti-cancer agent used in the treatment of soft tissue sarcoma and platinum-sensitive ovarian cancer [68]. The MOA of trabectedin is not fully elucidated, however, the compound has been shown to bind to the minor groove of DNA and interact with different binding proteins of the Nucleotide Excision Repair System (NERS) [69,70,71,72]. In addition to the marine natural products which have received regulatory approval and progressed to the market, numerous molecules are currently in clinical development [73].
To date, no marine natural products or derivatives have entered pre-clinical development specifically for trypanosomatid diseases. However, numerous marine natural products which exhibit anti-trypanosomal activity have been reported in the literature.
In this article, the natural products isolated from marine sources for which activity against the protozoan parasites; T.b. brucei, T.b. rhodesiense and T. cruzi has been reported, is reviewed. The majority of the compounds have been identified through phenotypic screening campaigns, which have recently been reviewed in detail [74,75]. It should be noted that although T.b. brucei primarily infects domestic mammals and antelopes and is not the human infective subspecies responsible for HAT, it is frequently used in early drug discovery screening campaigns to identify active compounds [76,77]. Compounds active against T.b. brucei would ultimately be evaluated against the human infective forms of the parasite, T.b. rhodesiense and T.b. gambiense. The bloodstream form of T. brucei spp. is used in phenotypic screening assays, as this is the clinically relevant form of the parasite (Figure 3A). In T. cruzi infection, the amastigote and the trypomastigote life cycle stages are both found within the human host (Figure 3B). All lifecycle stages of T. cruzi can be used in assays to evaluate the activity of compounds. However, activity against the amastigote form of the parasite has been deemed to be of primary importance in many assays, with activity against the trypomastigote stage also considered favorable or necessary [78,79,80]. Herein, only activity against the human infective forms, namely amastigotes and trypomastigotes are considered. Many assay formats used in T. cruzi research are based on the method by Buckner et al., whereby compounds are added two hours after addition of T. cruzi β-galactosidase transfected trypomastigotes to host cells [81]. Cells are incubated for seven days before detection of released trypomastigotes via lysis of cells and detection of β-galactosidase activity. This assay may affect both host cell infection and/ or development of amastigotes. The T. cruzi assays discussed in this article are based on this assay format, unless a modification is discussed.
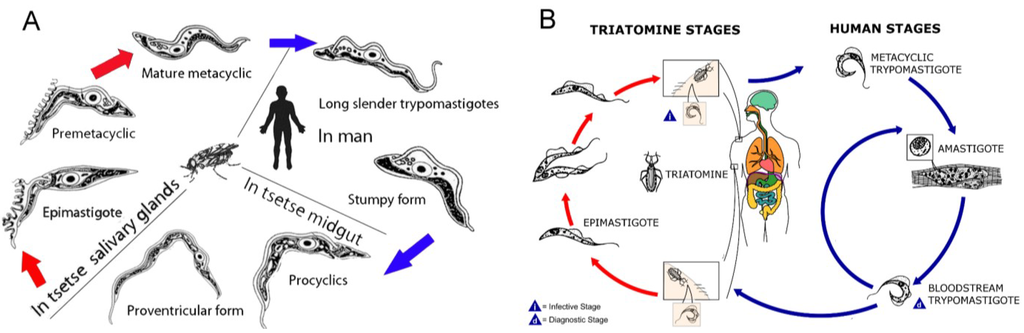
Figure 3.
(A) The life cycle of T. brucei spp. (modified content from [82]). (B) The life cycle of T. cruzi (modified content from [83]).
In drug discovery screening campaigns for HAT and Chagas disease, a compound is only classed as a “hit”, if it has an IC50 < 10 µM [84,85,86,87]. Compounds with an IC50 ≥ 10 µM would not be considered suitable for progression along the drug discovery pipeline and would only be used as tools or probes. In this review, the trypanocidal activity of compounds is described according to their IC50 values and are defined as: IC50 < 10 µM = promising trypanocidal activity, 10 µM ≤ IC50 < 20 µM = moderate activity, 20 µM ≤ IC50 < 30 µM = marginal activity, 30 µM ≤ IC50 < 40 µM = limited activity, IC50 ≥ 40 µM = no activity/inactive. When evaluating the activity of compounds against a human pathogen or disease target it is important that the cytotoxicity of the compound is also investigated against a mammalian cell line to allow the selectivity index (SI) of the compound to be determined. The SI for selecting compounds with anti-trypanosomal activity is the ratio of the IC50 value obtained for mammalian cells divided by the IC50 against trypanosome species. We have considered herein that an SI < 10 suggests that the compound may be exerting a generally toxic effect. If the SI is ≥10, the compound is considered to have some selective activity against the parasite. However, a significantly greater SI is required in order for molecules to progress along the drug discovery pipeline and eventually into clinical studies.
This article will focus on compounds which have an IC50 < 40 µM against T.b. brucei, T.b. rhodesiense or T. cruzi and which have also been evaluated against a mammalian cell line. Compounds have been grouped according to their chemical structures into three categories; terpenes, polyketides and xanthones, and alkaloids. Representative examples for each category are discussed in terms of their trypanocidal activity and SI. To allow the activity of compounds to be compared independently of their molecular weight, all literature values have been converted into micromolar concentrations (µM).
2. Marine Natural Products with Reported in Vitro Activity against the Trypanosome Species T. cruzi, T. brucei or T.b. rhodesiense
2.1. Terpenes
The marine sponges Spongia sp. and Ircinia sp. collected from the Turkish coastline of the Aegean Sea yielded a series of linear furanoterpenes and meroterpenes, as well as di- and tri-terpenes all of which were assessed for growth inhibitory activity against a series of protozoan parasites (Figure 4) [88]. 4-hydroxy-3-tetraprenylphenylacetic acid (1) was the most active and selective molecule with an IC50 value of 1.4 µM against T.b. rhodesiense and a selectivity index (SI) of >150, versus mammalian L6 rat skeletal muscle cells. The related structure heptaprenyl-p-quinol (2) possessing a longer isoprene chain and a hydroquinone terminal unit showed promising activity against T.b. rhodesiense with an IC50 value of 5.9 µM, however had no selectivity with an almost equivalent IC50 value of 4.4 µM observed against L6 cells. Demethylfurospongin-4 (3) was selectively active against T.b. rhodesiense with an IC50 value of 11.8 µM and an SI > 18. The diterpene 11β-acetoxyspongi-12-en-16-one (4) exhibited moderate activity against T.b. rhodesiense with an IC50 value of 11.5 µM but had no selectivity with an IC50 of 9.2 µM against L6 cells [88]. A number of trypanocidal molecules with varying degrees of activity have been identified from Agelas sp. marine sponges. The sterol 24-ethyl-cholest-5α-7-en-3-β-ol (5) isolated from the n-hexane extract of the Turkish sponge Agelas oroides showed limited activity against T.b. rhodesiense with an IC50 value of 34.2 µM [89]. Compound 5 was inactive against both T. cruzi (IC50 > 72 µM) and L6 cells (IC50 > 217 µM). These authors used the T. cruzi β-galactosidase assay to estimate compound activity [81].
A series of steroidal saponins characterized by a 2-hydroxycyclopentenone ring D and a glucuronic acid substituent at C-3 isolated from the Caribbean sponge Pandaros acanthifolium have demonstrated wide-ranging biological activity, including inhibition of both T.b. brucei and T. cruzi. Notably, pandaroside G methyl ester (6) had sub-micromolar activity against both T.b. rhodesiense and T. cruzi with IC50 values of 0.038 and 0.77 µM, respectively [90]. However, the molecule was not specific for T.b. rhodesiense or T. cruzi as it also inhibited mammalian L6 cells with an IC50 value of 0.22 µM, suggesting the natural product was generally toxic. Related steroidal saponins, the acanthifolisides, were also isolated as minor components from the same sponge collection [91]. Acanthifolioside E (7) showed moderate activity against T. cruzi, with an IC50 value of 10.6 µM, and marginal activity against T.b. rhodesiense, with an IC50 of 27.4 µM. In contrast, the trisaccharide acanthifolioside F methyl ester (8) had promising activity against T.b. rhodesiense with an IC50 value of 6.4 µM but only displayed marginal activity against T. cruzi, IC50 = 22.2 µM. Both compounds showed pan-panel activity against a series of other protozoa, as well as low SI values (<3) against mammalian L6 cells.
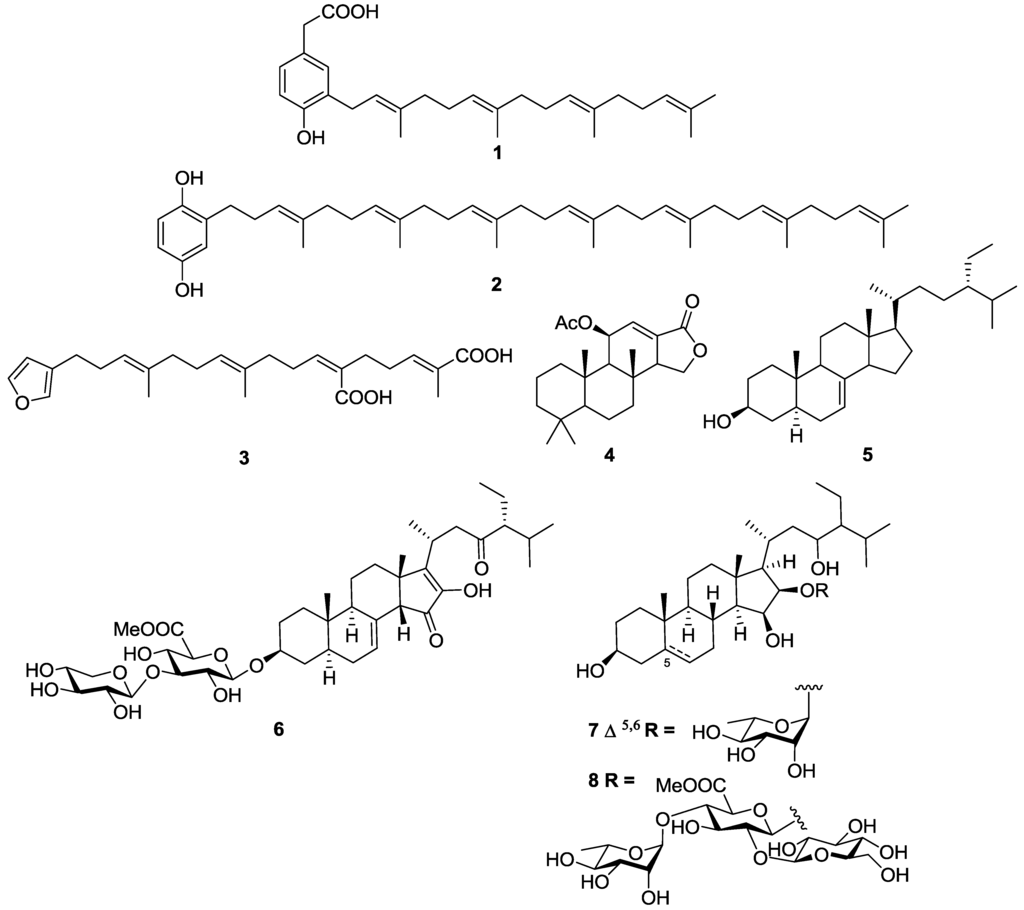
Figure 4.
The structure of terpenes of marine origin which have exhibited activity against T.b. rhodesiense (1–8) and T. cruzi (6–8).
2.2. Polyketides and Xanthones
A series of marine-derived polyketide endoperoxides have shown potent activity and good selectivity against trypanosomes (Figure 5). Plakortide P (9) isolated from a Brazilian collection of the sponge Plakortis angulospiculatus inhibited T. cruzi with an IC50 value of 6.3 μM but had a poor SI of 7 [92]. These authors utilized the soluble tetrazolium salt, MTT, to detect the metabolic activity of host cell-free trypomastigotes. 11,12-didehydro-13-oxo-plakortide Q (10) and 10-carboxy-11,12,13,14-tetranor-plakortide Q (11) isolated from an Australian collection of the sponge Plakortis sp. showed activity against T.b. brucei with IC50 values of 0.049 and 0.940 μM, respectively, and favorable selectivity indices, with compound 10 displaying a SI of 105 times and compound 11 <88 times over the human embryonic kidney cells, HEK-293 [93]. Interestingly, a substitution of the enone functionality in 10 with that of a carboxylic acid group in 11 resulted in a 20-fold reduction of activity against T.b. brucei. Related structures, manadoperoxides and peroxyplakoric ester B3 isolated from the Indonesian sponge Plakortis cfr. lita were also found to inhibit T.b. rhodesiense at low micro-molar concentrations [94]. Manadoperoxides B (12), C (13), F (14), H (15), I (16), and K (17) exhibited IC50 values of 0.0088, 2.2, 2, 1, 0.17, and 0.2 μM respectively, with favorable selectivity indices of > 3000, >15, >13, >27, >161 and >115, against human mammary epithelial cells (HMEC). Manadoperoxide G (18) as well as the peroxyplakoric ester B3 (19) were demonstrated to have moderate activity against T.b. rhodesiense with IC50 values of 5.6 and 11 µM, but exhibited very poor selectivity (< 5). The availability of ten structurally related analogues of manadoperoxide B gave an insight into the structure-activity relationship for this chemical class of compounds, suggesting that both the polarity of the side-chain and the presence of a C-4 methyl substituent were crucial for trypanocidal activity.
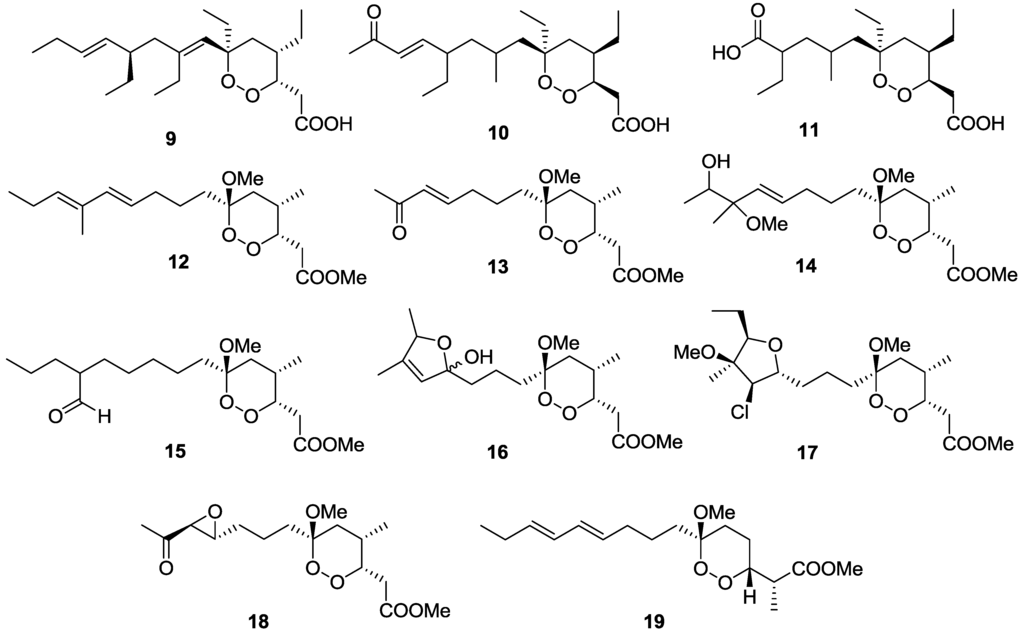
Figure 5.
The structure of endoperoxide polyketides of marine origin for which activity against T. cruzi (9), T.b. brucei (10–11), and T.b. rhodesiense (12–19) has been described.
Tetronic acid-containing tetromycin B (20) as well as tetromycins 1 (21), and 3 (22) isolated from Streptomyces axinellae Po1001 cultivated from the Mediterranean sponge Axinella polypoides, showed limited activity against T.b. brucei with IC50 values of 34, 32, and 30 µM, respectively (Figure 6) [95]. Compounds 20 and 23 had poor selectivity (SI < 2) against 293T kidney cells, with the most selective compound 21, having a SI > 3. Three new heterocyclic-substituted xanthone analogues (23–25) were isolated from the fungus Chaetomium sp. which was obtained from an algal species collected in Greece [96]. Of the series, compound 23 was the most active and selective for T.b. rhodesiense with an IC50 of 13.3 µM and a SI of 13 versus L6 cells. In contrast, the molecule had marginal activity against T. cruzi with an IC50 value >28 µM. Compound 25 had the greatest activity and selectivity against T. cruzi with an IC50 value of 3.8 µM and SI of 31, while 24 exhibited a similar activity against both parasites with IC50 values of 25 and 19 µM against T. cruzi and T.b. brucei, respectively, and a SI > 10 [96].
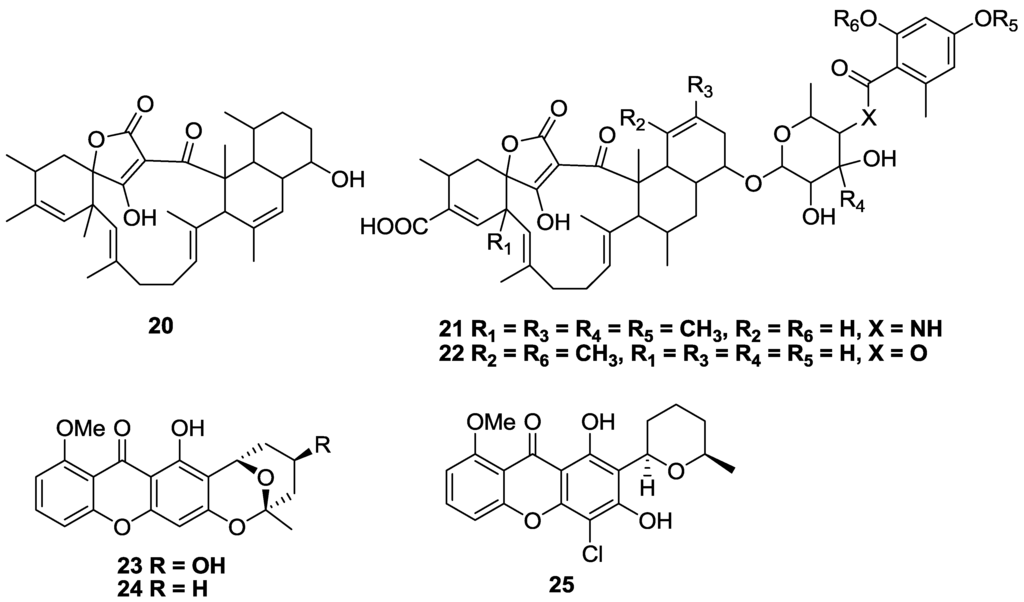
Figure 6.
The structure of tetromycins (20–22) and xanthone analogues (23–25) of marine origin for which activity against T.b. brucei (20–22), T.b. rhodesiense (23–24), T. cruzi (23–25) has been described.
2.3. Alkaloids
A number of indole-, bromopyrrole-, and purine-based alkaloids have shown a range of anti-trypanosomal activity (Figure 7). An indole alkaloid tryptophol (26) isolated from the Turkish, Aegean Sea sponge Ircinia spinulosa [97] showed broad-spectrum inhibitory activity against a panel of parasitic protozoa, including T.b. rhodesiense with an IC50 value of 36.6 µM, while showing no significant toxicity against L6 cells (SI > 11) [88]. Three other indole alkaloids sourced from the marine bacterium Bacillus pumilus, isolated from a Panamanian collection of the black coral Anthiphates sp., namely 3-formylindole (27), 3-hydroxyacetylindole (28) and N-acetyl-β-oxotryptamine (29) showed marginal activity against T. cruzi (in a modification of the β-galactosidase method, whereby trypomastigotes are washed off before addition of compound to infected host cells) with IC50 values of 26.9, 20.6 and 19.4 µM, respectively, although the selectivity of the compounds was very poor (SI < 4) [98]. A New Zealand collection of the ascidian Pseudodistoma opacum yielded three alkylguanidine-substituted β-carboline alkaloids, opacalines A–C [99]. Opacaline A (30) and the N-hydroxy analogue opacaline B (31) showed marginal inhibition of T.b. rhodesiense with IC50 values of 30 and 27 µM, but had poor selectivity (<5). Compound 32, a synthetically-prepared de-bromo analogue of 30 had improved activity against T.b. rhodesiense with an IC50 value of 12 µM and a slightly higher SI of 7 versus mammalian L6 cells [99].
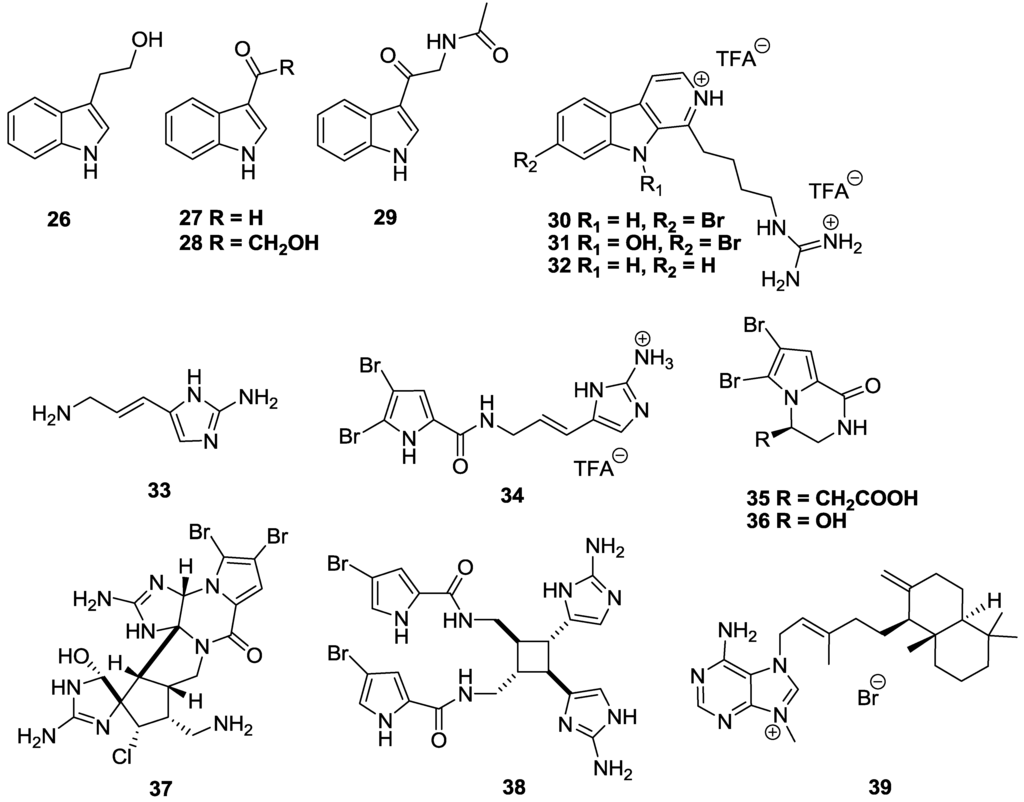
Figure 7.
The structure of indole-, bromopyrrole-, and purine-based alkaloids of marine origin which have shown activity against T.b. rhodesiense (26, 30–38), T. cruzi (27–29, 33, 39) and T.b. brucei (39).
The Turkish sponge, Agelas oroides collected in the Northern Aegean Sea yielded a series of bromopyrrole derivatives [89]. Moderate activity against both T.b. rhodesiense and T. cruzi with IC50 values of 17 and 18 µM, respectively, was observed for amino-1-(aminoimidazoyl)-prop-1-ene (33). However, the molecule displayed significant cytotoxicity towards L6 cells with an IC50 value of 5 µM. Oroidin trifluoroacetate salt (34) inhibited T.b. rhodesiense growth with an IC50 value of 25 µM, with no activity demonstrated against T. cruzi (IC50 > 62 µM) and L6 cells (IC50 = 157 µM). Bromopyrroles 35–38 sourced from another study of Turkish sponges belonging to the genera Agelas and Axinella displayed a range of activities against T.b. rhodesiense and T. cruzi, utilising an assay where T. cruzi trypomastigotes were washed off infected host cells before addition of compound [100]. The alkaloid longamide B (35) obtained from Agelas dispar [101] was active against T.b. rhodesiense, IC50 = 4.3 µM and displayed moderate cytotoxicity against L6 cells with an IC50 of 28 µM [100]. The compound displayed no activity against T. cruzi (IC50 > 94 µM). The hydroxyl analogue, longamide A (36) isolated from Agelas longissima [102] was over sixty-times less active against T.b. rhodesiense (IC50 > 290 µM) suggesting the importance of the carboxymethyl substituent for trypanocidal activity [100]. The oroidin dimer dibromopalau’amine, extracted from Axinella verrucosa [103], (37) exhibited sub-micromolar selective activity against T.b. rhodesiense with an IC50 value of 0.8 µM and a SI of 10 compared with mammalian L6 cells [100]. As with previous bromoryrroles, the compound had no activity against T. cruzi with an IC50 value of 119 µM. A second oroidin dimer, sceptrin (38), obtained from Agelas sceptrum [104] also showed selective activity against T.b. rhodesiense with an IC50 value of 15.7 µM and again no activity against T. cruzi (IC50 = 97 µM) or the mammalian L6 cell line (IC50 > 145 µM) [100]. Synthetically prepared agelasine D (39) a bicyclic diterpenoid purine, originally isolated from the Okinawan sea sponge Agelas nakamurai [105], inhibited both T.b. brucei and T. cruzi growth with IC50 values of 1.8 and 9 µM, respectively [106]. However, the selectivity of 39 was poor with an SI of <7 against MRC-5 human fetal lung fibroblasts cells.
Two brominated β-phenyl ethylamine-based alkaloids, convolutamines I (40) and J (41), were reported from a Tasmanian bryozoan Amathia tortusa with IC50 values against T.b. brucei of 1.1 and 13.7 µM, respectively (Figure 8) [107]. However, only convolutamine I (40) had a favorable SI of 18 against HEK-293 cells, with convolutamine J demonstrating cytotoxicity (SI > 3). As part of a HTS screen of a pre-fractionated natural product library to identify inhibitors of T.b. brucei, two cinnamoyl amino acids, iotrochotamides A (42) and B (43), were reported from an Australian marine sponge Iotrochota sp. [108]. Compounds 42 and 43 showed low micromolar activity against T.b. brucei with IC50 values of 3.4 and 4.7 µM, respectively, while exhibiting mild cytotoxicity against, HEK-293 with 85% inhibition at 50 µM for 42 and 100% inhibition at 70 µM for 43. Decahydroquinoline alkaloids lepadins D–F (44–46), were reported from a Great Barrier Reef collection of an ascidian Didemnum sp. [109]. Compounds 45 and 46 exhibited selective sub-micromolar activity against T.b. rhodesiense with IC50 values of 0.9 and 0.55 µM, respectively, and selectivity indices >40 versus mammalian L6 cells. Lepadins also displayed activity against T. cruzi, with IC50 values of 5.2 and 6.2 µM reported for 45 and 46, but the SI was only 7 [109]. The presence of the 2E-octenoic acid ester functionality in 45 and 46 was concluded to be essential for the anti-trypanosomal activity of the series as the hydroxyl analogue 44 was observed to be over 20-fold less active against T.b. rhodesiense (IC50 = 19 µM) and was inactive against T. cruzi (IC50 = 125 µM). A synthetic preparation of a 3-alkylpyridinium alkaloid, viscosamine (47), originally isolated from the Arctic sponge Haliclona viscosa [110], displayed sub-micromolar, selective activity against T.b. brucei with an IC50 of 0.41 µM and SI of 63 against HEK-293 [111]. The pentacyclic bis-indole alkaloid fascaplysin (48) isolated from a Fijian collection of the sponge Hyrtios cf. erecta exhibited wide-ranging biological activity, including potent, selective activity against T.b. rhodesiense with an IC50 value of 0.46 μM and SI of 15 versus L6 cells [112]. Pyridoacridines ascididemnin (49) and 12-deoxyascididemnin (50), isolated from an Australian ascidian Polysyncraton echinatum also displayed selective sub-micromolar activity against T.b. brucei with IC50 values of 0.032 and 0.077 μM, respectively, and selectivity indices >45, against HEK-293 [113]. Eilatin (51) an analogue of ascididemnin was over 40 fold less active against T.b. brucei with an IC50 of 1.33 µM [113].
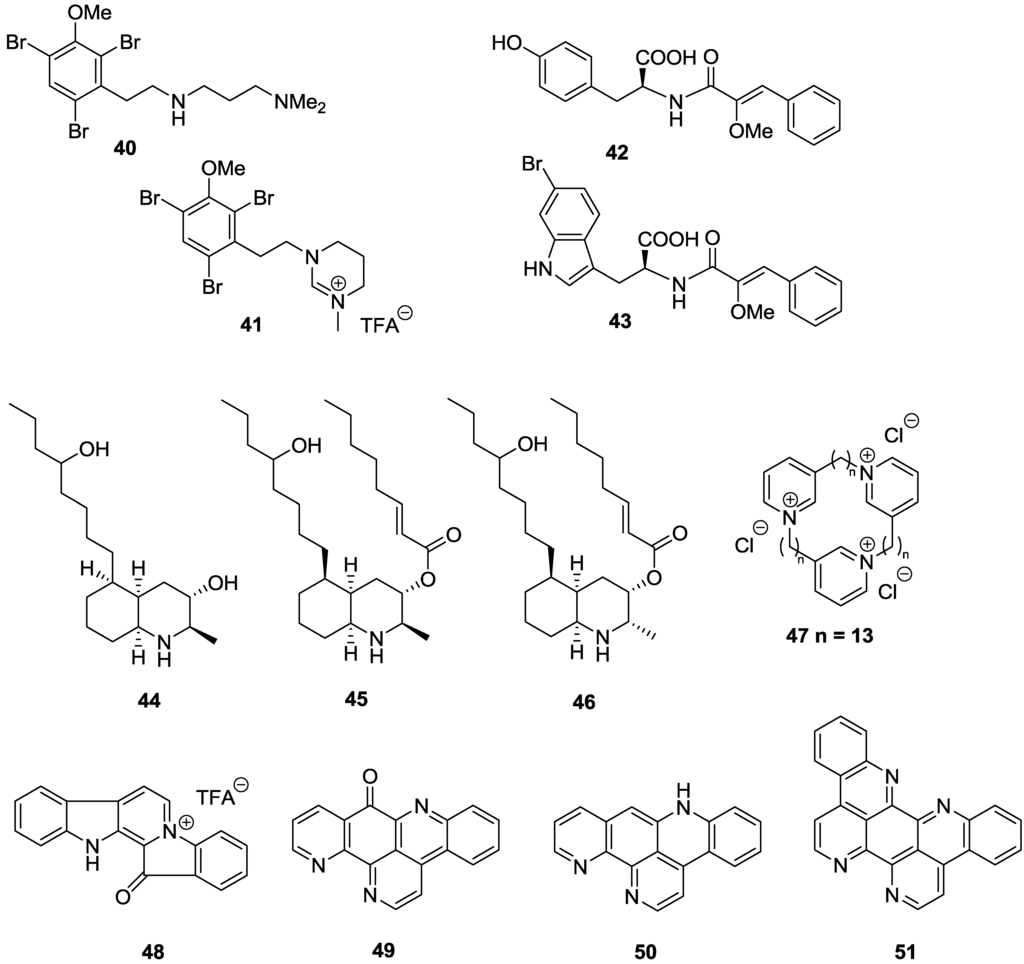
Figure 8.
The structure of alkaloids of marine origin for which activity against T.b. brucei (40–43, 47, 49–51), T.b. rhodesiense (44–46, 48) and T. cruzi (45, 46) has been described.
A series of dimethylthio (52), spiro-pentacyclic (53) and fused penta- and hexacyclic diketopiperazines (54–56) isolated from the marine-derived fungus Aspergillus fumigatus sourced from a Vanuatu sediment showed varying activity against T.b. brucei with IC50 values of 8.5, 5.7, 12.9, 6.4 and 19.5 µM, respectively [114] (Figure 9). The cytotoxicity of the compounds also varied with compounds 52 and 55 having a SI > 10, while 53, 54 and 56 were considerably cytotoxic with SI < 8. A dimethylthio (57) and two disulfide diketopiperazines, verticilin B (58) and chaetocin (59) were isolated from the marine fungus Nectria inventa which was obtained from a dredge sample of deep-water Californian sediment [114]. Compound 57 had low micromolar, selective activity against T.b. brucei with an IC50 of 5.9 µM and SI of 16, while verticilin B (58) and chaetocin (59) exhibited potent, sub-micromolar activity against T.b. brucei with IC50 values of 0.007 and 0.002 μM, respectively [114]. However, the molecules exhibited pronounced cytotoxicity against Jurkat T Lymphocytes (IC50 < 0.6 µM) preventing further evaluation of their therapeutic potential.
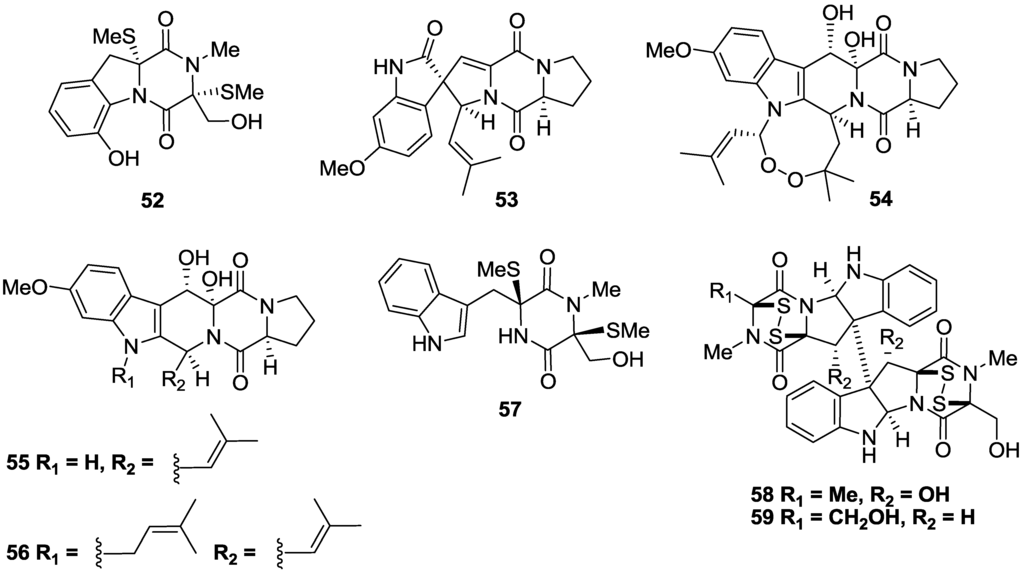
Figure 9.
The structure of diketopiperazines of marine origin which have shown activity against T.b. brucei (52–59).
Two cyclic hexapeptides, venturamides A (60) and B (61) were isolated from the Panamanian collection of the marine cyanobacterium Oscillatoria sp. [115] (Figure 10). The two compounds showed moderate activity against T. cruzi with IC50 values of 14.6 and 15.8 µM, respectively, and mild cytotoxicity to mammalian Vero (monkey kidney epithelial) cells with IC50 values of 86 and 56 µM, respectively, and thus an SI of < 6. Related cyclic peptides aerucyclamides B (62) and C (63) isolated from the cyanobacterium Microrcystis aeruginosa also displayed anti-trypanosomal activity with IC50 values of 15.9 and 9.2 µM, respectively, reported for T.b. rhodesiense [116]. Aerucyclamide C had a SI of 12 against L6 cells, whilst the SI of 62 was lower at 8. In a study using natural products as chemical probes to identify the molecular targets of small molecules, two linear peptides, almiramides B (64) and C (65) extracted from a Panamanian collection of the marine cyanobacterium Lyngbya majuscula were found to be low micromolar inhibitors of T.b. brucei with IC50 values of 6 and 3 µM, respectively [117]. Almiramide C displayed a SI of 11 compared to Vero cells while the SI for almiramide B was slightly lower at 9. Moreover, through a series of target based affinity probes, and fluorescence site localisation imaging studies, the compounds were shown to disrupt glycosome function in the parasite. Glycolysis is an essential pathway in trypanosomatids, and glycosomal enzymes have been identified as a potential drug target in trypanosomes [118].
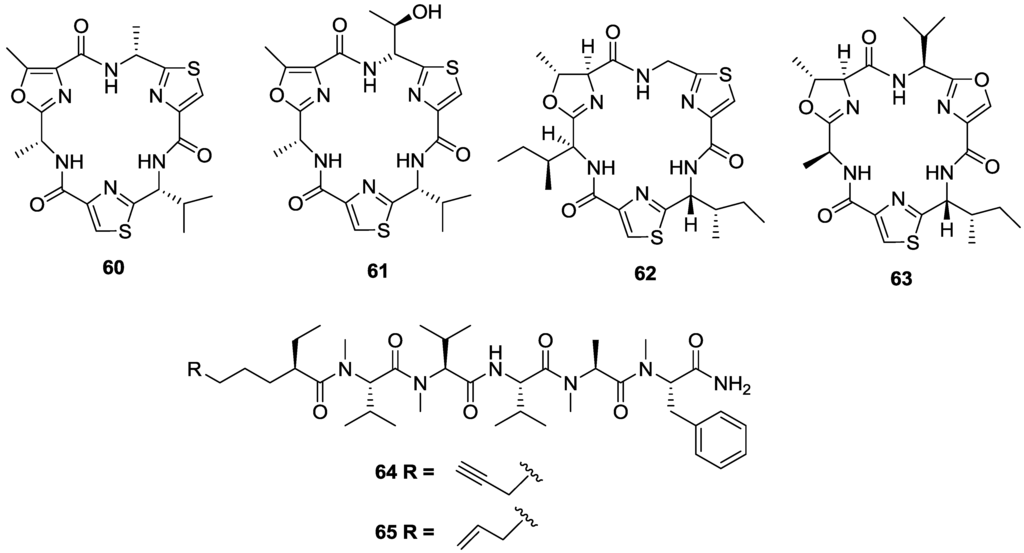
Figure 10.
The structure of peptides of marine origin for which activity has been reported against T. cruzi (60–61), T.b. rhodesiense (62–63) and T.b. brucei (64–65).
3. Conclusions
A large number of structurally diverse marine natural products have been identified with trypanocidal activity. The manadoperoxides isolated from the marine sponge Plakortis cfr. lita are the most promising compounds for HAT. Manadoperoxide B (12) was the most active and selective molecule of the series exhibiting sub-micromolar activity against T.b. rhodesiense whilst highly selective against mammalian cells [94]. This compound was also demonstrated to possess anti-malarial activity, however, it is reported to be more than 700-fold less active against Plasmodium falciparum (D10) than T.b. rhodesiense [119]. As manadoperoxide B has sub-micromolar activity against T.b. rhodesiense and is not cytotoxic, one would anticipate that the physiochemical properties of the molecule, together with the biological activity are being investigated further to ensure the molecule possesses the required characteristics to meet the final target product profile.
The heterocyclic-substituted xanthone analogue 25 isolated from the marine fungus Chaetomium sp. was the most active and selective, marine derived compound for T. cruzi [96]. However, xanthones have been reported to have activity against multiple organisms and disease indications through interacting with a plethora of enzymes and targets [120]. This promiscuous activity may prevent further development of the compounds for Chagas disease.
In the last decade numerous molecules, both natural and synthetic, have been identified with trypanocidal activity. However, only two, have entered pre-clinical development for HAT. Furthermore, despite the identification of new targets and a multitude of in vitro and in vivo studies having been conducted, candidates for Chagas disease have failed to progress to the advanced stages of clinical development. Many of the molecules identified with potent trypanocidal activity, cannot be developed further as they possess unsuitable and undesirable structural and pharmacokinetic properties. This highlights the need to continue to explore other avenues for new chemical entities, whilst reviewing the approaches currently undertaken and the potential reasons for the lack of success. Evaluation of the current in vitro assays used to identify new compounds, in particular the life cycle stage for Chagas disease, is warranted. This is particularly true for the in vivo models where the parasite strain, administration route and duration of the study can impact on the outcomes.
Marine natural products have provided the pharmaceutical industry with many incredibly potent compounds—some developed into therapeutics whilst others providing valuable insights into the biology of disease and desired attributes of the compounds required to ameliorate it. Whilst compounds isolated from this source have yet to progress to pre-clinical development for trypanosomatid diseases, collectively the improvements to the in vitro assays used to identify them, the in vivo models used to evaluate them, and the methodology required for isolating them could change this situation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Pepin, J.; Meda, H.A. The epidemiology and control of human African trypanosomiasis. Adv. Parasitol. 2001, 49, 71–132. [Google Scholar] [CrossRef]
- Zeledon, R.; Rabinovich, J.E. Chagas disease: An ecological appraisal with special emphasis on its insect vectors. Annu. Rev. Entomol. 1981, 26, 101–133. [Google Scholar] [CrossRef]
- Simarro, P.P.; Diarra, A.; Ruiz Postigo, J.A.; Franco, J.R.; Jannin, J.G. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: The way forward. PLoS Negl. Trop. Dis. 2011, 5, e1007. [Google Scholar] [CrossRef]
- Moncayo, A.; Silveira, A.C. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem. Inst. Oswaldo Cruz 2009, 104 (Suppl. 1), 17–30. [Google Scholar]
- Apted, F.I.C.; Mulligan, H.W. Clinical manifestations and diagnosis of sleeping sickness. In The African Trypanosomiases; George Allen and Unwin LTD: London, UK, 1970; pp. 661–683. [Google Scholar]
- Atouguia, J.M.; Kennedy, P.G.E.; Davis, L.E. Neurological aspects of human African trypanosomiasis. In Infectious Diseases of the Nervous System; Davis, L.E., Kennedy, P.G.E., Eds.; Butterworth-Heinemann: Oxford, UK, 2000; pp. 321–372. [Google Scholar]
- Galfand, M. Transitory neurological signs in sleeping sickness. Trans. R. Soc. Trop. Med. Hyg. 1947, 41, 255–258. [Google Scholar] [CrossRef]
- Lundkvist, G.B.; Kristensson, K.; Bentivoglio, M. Why trypanosomes cause sleeping sickness. Physiology 2004, 19, 198–206. [Google Scholar] [CrossRef]
- Human African trypanosomiasis (sleeping sickness). World Health Organisation Fact Sheet 259. Available online: http://www.who.int/mediacentre/factsheets/fs259/en/ (accessed on 9 September 2013).
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Rassi, A.; Rezende, J.M.; Luquetti, A.O. Clinical phases and forms of Chagas disease. In American Trypanosomiasis (Chagas Disease). One Hundred Years of Research, 1st ed.; Telleria, J., Tibayrenc, M., Eds.; Elsevier: Burlington, MA, USA, 2010; pp. 709–741. [Google Scholar]
- Munoz-Saravia, S.G.; Haberland, A.; Wallukat, G.; Schimke, I. Chronic Chagas heart disease: A disease on its way to becoming a worldwide health problem: Epidemiology, etiopathology, treatment, pathogenesis and laboratory medicine. Heart Fail. Rev. 2012, 17, 45–64. [Google Scholar] [CrossRef]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- Pepin, J.; Milord, F. The treatment of human African trypanosomiasis. Adv. Parasitol. 1994, 33, 1–47. [Google Scholar] [CrossRef]
- Milord, F.; Pepin, J.; Loko, L.; Ethier, L.; Mpia, B. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 1992, 340, 652–655. [Google Scholar] [CrossRef]
- Priotto, G.; Kasparian, S.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Karunakara, U. Nifurtimox-eflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: A randomized clinical trial in Congo. Clin. Infect. Dis. 2007, 45, 1435–1442. [Google Scholar] [CrossRef]
- Priotto, G.; Kasparian, S.; Mutombo, W.; Ngouama, D.; Ghorashian, S.; Arnold, U.; Ghabri, S.; Baudin, E.; Buard, V.; Kazadi-Kyanza, S.; et al. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: A multicentre, randomised, phase III, non-inferiority trial. Lancet 2009, 374, 56–64. [Google Scholar] [CrossRef]
- Apt, W. Current and developing therapeutic agents in the treatment of Chagas disease. Drug Des. Devel. Ther. 2010, 4, 243–253. [Google Scholar] [CrossRef]
- Castro, J.A.; Diaz de Toranzo, E.G. Toxic effects of nifurtimox and benznidazole, two drugs used against American trypanosomiasis (Chagas disease). Biomed. Environ. Sci. 1988, 1, 19–33. [Google Scholar]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and safety of nifurtimox in patients with chronic Chagas disease. Clin. Infect. Dis. 2010, 51, 69–75. [Google Scholar] [CrossRef]
- Hasslocher-Moreno, A.M.; Do Brasil, P.E.; De Sousa, A.S.; Xavier, S.S.; Chambela, M.C.; Sperandio Da Silva, G.M. Safety of benznidazole use in the treatment of chronic Chagas disease. J. Antimicrob. Chemother. 2012, 67, 1261–1266. [Google Scholar] [CrossRef]
- Kaiser, M.; Bray, M.A.; Cal, M.; Bourdin Trunz, B.; Torreele, E.; Brun, R. Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness. Antimicrob. Agents Chemother. 2011, 55, 5602–5608. [Google Scholar] [CrossRef]
- Jacobs, R.T.; Nare, B.; Wring, S.A.; Orr, M.D.; Chen, D.; Sligar, J.M.; Jenks, M.X.; Noe, R.A.; Bowling, T.S.; Mercer, L.T.; et al. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl. Trop. Dis. 2011, 5, e1151. [Google Scholar] [CrossRef]
- Jacobs, R.T.; Nare, B.; Phillips, M.A. State of the art in African trypanosome drug discovery. Curr. Top. Med. Chem. 2011, 11, 1255–1274. [Google Scholar] [CrossRef]
- Barker, R.H., Jr.; Liu, H.; Hirth, B.; Celatka, C.A.; Fitzpatrick, R.; Xiang, Y.; Willert, E.K.; Phillips, M.A.; Kaiser, M.; Bacchi, C.J.; et al. Novel S-adenosylmethionine decarboxylase inhibitors for the treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 2009, 53, 2052–2058. [Google Scholar] [CrossRef]
- Bacchi, C.J.; Barker, R.H.; Rodriguez, A.; Hirth, B.; Rattendi, D.; Yarlett, N.; Hendrick, C.L.; Sybertz, E. Trypanocidal activity of 8-methyl-5′-[(Z)-4-aminobut-2-enyl](methylamino)adenosine (Genz-644131), an adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 2009, 53, 3269–3272. [Google Scholar] [CrossRef]
- Price, H.P.; Menon, M.R.; Panethymitaki, C.; Goulding, D.; McKean, P.G.; Smith, D.F. Myristoyl-CoA: Protein N-myristoyltransferase, an essential enzyme and potential drug target in kinetoplastid parasites. J. Biol. Chem. 2003, 278, 7206–7214. [Google Scholar]
- Frearson, J.A.; Brand, S.; McElroy, S.P.; Cleghorn, L.A.T.; Smid, O.; Stojanovski, L.; Price, H.P.; Guther, M.L.S.; Torrie, L.S.; Robinson, D.A.; et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature 2010, 464, 728–732. [Google Scholar] [CrossRef]
- Wyllie, S.; Oza, S.L.; Patterson, S.; Spinks, D.; Thompson, S.; Fairlamb, A.H. Dissecting the essentiality of the bifunctional trypanothione synthetase-amidase in Trypanosoma brucei using chemical and genetic methods. Mol. Microbiol. 2009, 74, 529–540. [Google Scholar] [CrossRef]
- Clayton, J. Chagas disease: Pushing through the pipeline. Nature 2010, 465, S12–S15. [Google Scholar] [CrossRef]
- A Study of the Use of Oral Posaconazole (POS) in the Treatment of Asymptomatic Chronic Chagas Disease. Clinical Trials. Available online: http://clinicaltrials.gov/show/NCT01377480 (accessed on 23 September 2013).
- Chen, C.K.; Leung, S.S.; Guilbert, C.; Jacobson, M.P.; McKerrow, J.H.; Podust, L.M. Structural characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei bound to the antifungal drugs posaconazole and fluconazole. PLoS Negl. Trop. Dis. 2010, 4, e651. [Google Scholar] [CrossRef]
- Gunatilleke, S.S.; Calvet, C.M.; Johnston, J.B.; Chen, C.K.; Erenburg, G.; Gut, J.; Engel, J.C.; Ang, K.K.; Mulvaney, J.; Chen, S.; et al. Diverse inhibitor chemotypes targeting Trypanosoma cruzi CYP51. PLoS Negl. Trop. Dis. 2012, 6, e1736. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Villalta, F.; Waterman, M.R. Targeting Trypanosoma cruzi sterol 14alpha-demethylase (CYP51). Adv. Parasitol. 2011, 75, 65–87. [Google Scholar] [CrossRef]
- Soeiro Mde, N.; de Souza, E.M.; da Silva, C.F.; Batista Dda, G.; Batista, M.M.; Pavao, B.P.; Araujo, J.S.; Aiub, C.A.; da Silva, P.B.; Lionel, J.; et al. In vitro and in vivo studies of the antiparasitic activity of sterol 14alpha-demethylase (CYP51) inhibitor VNI against drug-resistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 2013, 57, 4151–4163. [Google Scholar] [CrossRef]
- Buckner, F.S. Sterol 14-demethylase inhibitors for Trypanosoma cruzi infections. Adv. Exp. Med. Biol. 2008, 625, 61–80. [Google Scholar] [CrossRef]
- Clayton, J. The promise of T. cruzi genomics. Nature 2010, 465, S16–S17. [Google Scholar] [CrossRef]
- Lisvane Silva, P.; Mantilla, B.S.; Barison, M.J.; Wrenger, C.; Silber, A.M. The uniqueness of the Trypanosoma cruzi mitochondrion: Opportunities to identify new drug target for the treatment of Chagas disease. Curr. Pharm. Des. 2011, 17, 2074–2099. [Google Scholar] [CrossRef]
- Soeiro, M.N.; de Castro, S.L. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin. Ther. Targets 2009, 13, 105–121. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. The isolation of a new thymine pentoside from sponges. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Swift, A.N. Contributions to the study of marine products. Component acids of lipids of sponges. J. Org. Chem. 1951, 16, 1206–1221. [Google Scholar] [CrossRef]
- O’Day, D.M.; Poirier, R.H.; Jones, D.B.; Elliott, J.H. Vidarabine therapy of complicated Herpes simplex keratitis. Am. J. Ophthalmol. 1976, 81, 642–649. [Google Scholar]
- Pavan-Langston, D.; Hess, F. Ocular and systemic antiviral activity of vidarabine. Compr. Ther. 1977, 3, 42–48. [Google Scholar]
- Mori, J.; Tsubokura, M.; Kami, M. Cytarabine dose for acute myeloid leukemia. N. Engl. J. Med. 2011, 364, 2166–2167. [Google Scholar] [CrossRef]
- Fox, B.W. Pharmacology and chemistry of some inhibitors of herpes replication. J. Antimicrob. Chemother. 1977, 3, 23–32. [Google Scholar] [CrossRef]
- Gedik, C.M.; Collins, A.R. The mode of action of 1-beta-d-arabinofuranosylcytosine in inhibiting DNA repair; New evidence using a sensitive assay for repair DNA synthesis and ligation in permeable cells. Mutat. Res. 1991, 254, 231–237. [Google Scholar] [CrossRef]
- Olivera, B.M.; Gray, W.R.; Zeikus, R.; McIntosh, J.M.; Varga, J.; Rivier, J.; De Santos, V.; Cruz, L.J. Peptide neurotoxins from fish-hunting cone snails. Science 1985, 230, 1338–1343. [Google Scholar]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef]
- Lovaza Drug Details. Food and Drug Administration Approved Products. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails (accessed on 18 September 2013).
- Vascepa. Food and Drug Administration Orange Book: Approved drug products with therapeutic equivalence evaluations. Available online: http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=202057&TABLE1=OB_Rx (accessed on 18 September 2013).
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef]
- Nestel, P.J.; Connor, W.E.; Reardon, M.F.; Connor, S.; Wong, S.; Boston, R. Suppression by diets rich in fish oil of very low-density lipoprotein production in man. J. Clin. Invest. 1984, 74, 82–89. [Google Scholar] [CrossRef]
- Sanders, T.A.B.; Sullivan, D.R.; Reeve, J.; Thompson, G.R. Triglyceride-lowering effect of marine polyunsaturates in patients with hypertriglyceridemia. Arteriosclerosis 1985, 5, 459–465. [Google Scholar] [CrossRef]
- Bordin, P.; Bodamer, O.A.F.; Venkatesan, S.; Gray, R.M.; Bannister, P.A.; Halliday, D. Effects of fish oil supplementation on apolipoprotein B100 production and lipoprotein metabolism in normolipidaemic males. Eur. J. Clin. Nutr. 1998, 52, 104–109. [Google Scholar]
- Madsen, L.; Rustan, A.C.; Vaagenes, H.; Berge, K.; Dyroy, E.; Berge, R.K. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 1999, 34, 951–963. [Google Scholar] [CrossRef]
- Davidson, M.H. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 27–33. [Google Scholar] [CrossRef]
- Hirata, Y.; Uemura, D. Halichondrins—Antitumor polyether macrolides from a marine sponge. Pure Appl. Chem. 1986, 58, 701–710. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Towle, M.J.; Cheng, H.S.; Kawamura, T.; TenDyke, K.; Liu, D.; Kishi, Y.; Yu, M.J.; Littlefield, B.A. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004, 64, 5760–5766. [Google Scholar] [CrossRef]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef]
- Dabydeen, D.A.; Burnett, J.C.; Bai, R.L.; Verdier-Pinard, P.; Hickford, S.J.H.; Pettit, G.R.; Blunt, J.W.; Munro, M.H.G.; Gussio, R.; Hamel, E. Comparison of the activities of the truncated halichondrin B analog NSC 707389 (E7389) with those of the parent compound and a proposed binding site on tubulin. Mol. Pharmacol. 2006, 70, 1866–1875. [Google Scholar] [CrossRef]
- Francisco, J.A.; Cerveny, C.G.; Meyer, D.L.; Mixan, B.J.; Klussman, K.; Chace, D.F.; Rejniak, S.X.; Gordon, K.A.; DeBlanc, R.; Toki, B.E. cAC10-vcMMAE, an anti-CD30–monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 2003, 102, 1458–1465. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tuinman, A.A.; Boettner, F.E.; Kizu, H.; Schmidt, J.M.; Baczynskyj, L.; Tomer, K.B.; Bontems, R.J. The isolation and structure of a remarkable marine animal antineoplastic constituent: Dolastatin 10. J. Am. Chem. Soc. 1987, 109, 6883–6885. [Google Scholar] [CrossRef]
- Deng, C.; Pan, B.; O’Connor, O.A. Brentuximab vedotin. Clin. Cancer Res. 2013, 19, 22–27. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Holt, T.G.; Fregeau, N.L.; Stroh, J.G.; Keifer, P.A.; Sun, F.; Li, L.H.; Martin, D.G. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4512–4515. [Google Scholar] [CrossRef]
- Zewail-Foote, M.; Hurley, L.H. Differential rates of reversibility of ecteinascidin 743-DNA covalent adducts from different sequences lead to migration to favored bonding sites. J. Am. Chem. Soc. 2001, 123, 6485–6495. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Pourquier, P.; Zimonjic, D.B.; Nakayama, K.; Emmert, S.; Ueda, T.; Urasaki, Y.; Kanzaki, A.; Akiyama, S.; Popescu, N.; et al. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat. Med. 2001, 7, 961–966. [Google Scholar] [CrossRef]
- Soares, D.G.; Escargueil, A.E.; Poindessous, V.; Sarasin, A.; De Gramont, A.; Bonatto, D.; Henriques, J.A.P.; Larsen, A.K. Replication and homologous recombination repair regulate DNA double-strand break formation by the antitumor alkylator ecteinascidin 743. Proc. Natl. Acad. Sci. USA. 2007, 104, 13062–13067. [Google Scholar] [CrossRef]
- Herrero, A.B.; Martin-Castellanos, C.; Marco, E.; Gago, F.; Moreno, S. Cross-talk between nucleotide excision and homologous recombination DNA repair pathways in the mechanism of action of antitumor trabectedin. Cancer Res. 2006, 66, 8155–8162. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Jones, A.J.; Avery, V.M. Whole-organism high-throughput screening against Trypanosoma brucei brucei. Exp. Opin. Drug Discov. 2013, 8, 495–507. [Google Scholar]
- Sykes, M.L.; Avery, V.M. Approaches to protozoan drug discovery: Phenotypic screening. J. Med. Chem. 2013, in press. [Google Scholar]
- Stevens, J.; Brisse, S. Systematics of trypanosomes of medical and veterinary importance. In The Trypanosomiases; Maudlin, I., Holmes, P.H., Miles, M.A., Eds.; CABI Publishing: Trowbridge, UK, 2004; pp. 1–23. [Google Scholar]
- Pink, R.; Hudson, A.; Mouries, M.A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Chennamaneni, N.K.; Arif, J.; Buckner, F.S.; Gelb, M.H. Isoquinoline-based analogs of the cancer drug clinical candidate tipifarnib as anti-Trypanosoma cruzi agents. Bioorg. Med. Chem. Lett. 2009, 19, 6582–6584. [Google Scholar] [CrossRef]
- Romanha, A.J.; Castro, S.L.; Soeiro Mde, N.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Ennes-Vidal, V.; Menna-Barreto, R.F.; Santos, A.L.; Branquinha, M.H.; d’Avila-Levy, C.M. Effects of the calpain inhibitor MDL28170 on the clinically relevant forms of Trypanosoma cruzi in vitro. J. Antimicrob. Chemother. 2010, 65, 1395–1398. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar]
- Vickerman, K. Developmental cycles and biology of pathogenic trypanosomes. Br. Med. Bull. 1985, 41, 105–114. [Google Scholar]
- Da Silva, A.J.; Moser, M. Trypanosomiasis, American (Chagas disease, Trypanosoma cruzi). Center for Disease Control and Prevention: Public Health Image Library (PHIL). Available online: http://phil.cdc.gov/phil/details.asp (accessed on 14 October 2013).
- Nwaka, S.; Hudson, A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006, 5, 941–955. [Google Scholar] [CrossRef]
- Dardonville, C.; Fernandez-Fernandez, C.; Gibbons, S.L.; Jagerovic, N.; Nieto, L.; Ryan, G.; Kaiser, M.; Brun, R. Antiprotozoal activity of 1-phenethyl-4-aminopiperidine derivatives. Antimicrob. Agents Chemother. 2009, 53, 3815–3821. [Google Scholar] [CrossRef]
- Jones, D.C.; Hallyburton, I.; Stojanovski, L.; Read, K.D.; Frearson, J.A.; Fairlamb, A.H. Identification of a κ-opioid agonist as a potent and selective lead for drug development against human African trypanosomiasis. Biochem. Pharmacol. 2010, 80, 1478–1486. [Google Scholar] [CrossRef]
- Sykes, M.L.; Baell, J.B.; Kaiser, M.; Chatelain, E.; Moawad, S.R.; Ganame, D.; Ioset, J.R.; Avery, V.M. Identification of compounds with anti-proliferative activity against Trypanosoma brucei brucei strain 427 by a whole cell viability based HTS campaign. PLoS Negl. Trop. Dis. 2012, 6, e1896. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs 2010, 8, 47–58. [Google Scholar] [CrossRef]
- Tasdemir, D.; Topaloglu, B.; Perozzo, R.; Brun, R.; O’Neill, R.; Carballeira, N.M.; Zhang, X.; Tonge, P.J.; Linden, A.; Ruedi, P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg. Med. Chem. 2007, 15, 6834–6845. [Google Scholar] [CrossRef]
- Regalado, E.L.; Tasdemir, D.; Kaiser, M.; Cachet, N.; Amade, P.; Thomas, O.P. Antiprotozoal steroidal saponins from the marine sponge Pandaros acanthifolium. J. Nat. Prod. 2010, 73, 1404–1410. [Google Scholar] [CrossRef]
- Regalado, E.L.; Jimenez-Romero, C.; Genta-Jouve, G.; Tasdemir, D.; Amade, P.; Nogueiras, C.; Thomas, O.P. Acanthifoliosides, minor steroidal saponins from the Caribbean sponge Pandaros acanthifolium. Tetrahedron 2011, 67, 1011–1018. [Google Scholar] [CrossRef]
- Kossuga, M.H.; Nascimento, A.M.; Reimao, J.Q.; Tempone, A.G.; Taniwaki, N.N.; Veloso, K.; Ferreira, A.G.; Cavalcanti, B.C.; Pessoa, C.; Moraes, M.O.; et al. Antiparasitic, antineuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J. Nat. Prod. 2008, 71, 334–339. [Google Scholar] [CrossRef]
- Feng, Y.J.; Davis, R.A.; Sykes, M.; Avery, V.M.; Camp, D.; Quinn, R.J. Antitrypanosomal cyclic polyketide peroxides from the Australian marine sponge Plakortis sp. J. Nat. Prod. 2010, 73, 716–719. [Google Scholar] [CrossRef]
- Chianese, G.; Fattorusso, E.; Scala, F.; Teta, R.; Calcinai, B.; Bavestrello, G.; Dien, H.A.; Kaiser, M.; Tasdemir, D.; Taglialatela-Scafati, O. Manadoperoxides, a new class of potent antitrypanosomal agents of marine origin. Org. Biomol. Chem. 2012, 10, 7197–7207. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Buback, V.; Gulder, T.A.M.; Bugni, T.S.; Reppart, J.; Bringmann, G.; Ireland, C.M.; Schirmeister, T.; Hentschel, U. New tetromycin derivatives with anti-trypanosomal and protease inhibitory activities. Mar. Drugs 2011, 9, 1682–1697. [Google Scholar] [CrossRef]
- Pontius, A.; Krick, A.; Kehraus, S.; Brun, R.; Konig, G.M. Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J. Nat. Prod. 2008, 71, 1579–1584. [Google Scholar] [CrossRef]
- Erdogan, I.; Sener, B.; Higa, T. Tryptophol, a plant auxin isolated from the marine sponge Ircinia spinulosa. Biochem. Syst. Ecol. 2000, 28, 793–794. [Google Scholar] [CrossRef]
- Martinez-Luis, S.; Gomez, J.F.; Spadafora, C.; Guzman, H.M.; Gutierrez, M. Antitrypanosomal alkaloids from the marine bacterium Bacillus pumilus. Molecules 2012, 17, 11146–11155. [Google Scholar] [CrossRef]
- Chan, S.T.S.; Pearce, A.N.; Page, M.J.; Kaiser, M.; Copp, B.R. Antimalarial β-carbolines from the New Zealand ascidian Pseudodistoma opacum. J. Nat. Prod. 2011, 74, 1972–1979. [Google Scholar] [CrossRef]
- Scala, F.; Fattorusso, E.; Menna, M.; Taglialatela-Scafati, O.; Tierney, M.; Kaiser, M.; Tasdemir, D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar. Drugs 2010, 8, 2162–2174. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Taglialatela-Scafati, O. Novel bromopyrrole alkaloids from the sponge Agelas dispar. J. Nat. Prod. 1998, 61, 122–125. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Mangoni, A.; Taglialatelascafati, O. Longamide and 3,7-dimethylisoguanine, 2 novel alkaloids from the marine sponge Agelas longissima. Tetrahedron Lett. 1995, 36, 7893–7896. [Google Scholar]
- Aiello, A.; D’Esposito, M.; Fattorusso, E.; Menna, M.; Muller, W.E.G.; Perovic-Ottstadt, S.; Schroder, H.C. Novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella verrucosa. Bioorg. Med. Chem. 2006, 14, 17–24. [Google Scholar] [CrossRef]
- Walker, R.P.; Faulkner, D.J.; Van Engen, D.; Clardy, J. Sceptrin, an antimicrobial agent from the sponge Agelas sceptrum. J. Am. Chem. Soc. 1981, 103, 6772–6773. [Google Scholar] [CrossRef]
- Wu, H.; Nakamura, H.; Kobayashi, J.; Kobayashi, M.; Ohizumi, Y.; Hirata, Y. Structures of agelasines, diterpenes having a 9-methyladeninium chromophore isolated from the Okinawan marine sponge Agelas nakamurai hoshino. Bull. Chem. Soc. Jpn. 1986, 59, 2495–2504. [Google Scholar] [CrossRef]
- Vik, A.; Proszenyak, A.; Vermeersch, M.; Cos, P.; Maes, L.; Gundersen, L.L. Screening of agelasine D and analogs for inhibitory activity against pathogenic protozoa; Identification of hits for visceral leishmaniasis and Chagas disease. Molecules 2009, 14, 279–288. [Google Scholar] [CrossRef]
- Davis, R.A.; Sykes, M.; Avery, V.M.; Camp, D.; Quinn, R.J. Convolutamines I and J, antitrypanosomal alkaloids from the bryozoan Amathia tortusa. Bioorg. Med. Chem. 2011, 19, 6615–6619. [Google Scholar] [CrossRef]
- Feng, Y.J.; Davis, R.A.; Sykes, M.L.; Avery, V.M.; Quinn, R.J. Iotrochamides A and B, antitrypanosomal compounds from the Australian marine sponge Iotrochota sp. Bioorg. Med. Chem. Lett. 2012, 22, 4873–4876. [Google Scholar] [CrossRef]
- Wright, A.D.; Goclik, E.; Koenig, G.M.; Kaminsky, R. Lepadins D–F: Antiplasmodial and antitrypanosomal decahydroquinoline derivatives from the tropical marine tunicate Didemnum sp. J. Med. Chem. 2002, 45, 3067–3072. [Google Scholar] [CrossRef]
- Volk, C.A.; Kock, M. Viscosamine: The first naturally occurring trimeric 3-alkyl pyridinium alkaloid. Org. Lett. 2003, 5, 3567–3569. [Google Scholar] [CrossRef]
- Rodenko, B.; Al-Salabi, M.I.; Teka, I.A.; Ho, W.; El-Sabbagh, N.; Ali, J.A.M.; Ibrahim, H.M.S.; Wanner, M.J.; Koomen, G.; De Koning, H.P. Synthesis of marine-derived 3-alkylpyridinium alkaloids with potent antiprotozoal activity. ACS Med. Chem. Lett. 2011, 2, 901–906. [Google Scholar] [CrossRef]
- Kirsch, G.; Konig, G.M.; Wright, A.D.; Kaminsky, R. A new bioactive sesterterpene and antiplasmodial alkaloids from the marine sponge Hyrtios cf. erecta. J. Nat. Prod. 2000, 63, 825–829. [Google Scholar] [CrossRef]
- Feng, Y.J.; Davis, R.A.; Sykes, M.L.; Avery, V.M.; Carroll, A.R.; Camp, D.; Quinn, R.J. Antitrypanosomal pyridoacridine alkaloids from the Australian ascidian Polysyncraton echinatum. Tetrahedron Lett. 2010, 51, 2477–2479. [Google Scholar] [CrossRef]
- Watts, K.R.; Ratnam, J.; Ang, K.H.; Tenney, K.; Compton, J.E.; McKerrow, J.; Crews, P. Assessing the trypanocidal potential of natural and semi-synthetic diketopiperazines from two deep water marine-derived fungi. Bioorg. Med. Chem. 2010, 18, 2566–2574. [Google Scholar]
- Linington, R.G.; Gonzalez, J.; Urena, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H. Venturamides A and B: Antimalarial constituents of the Panamanian marine cyanobacterium Oscillatoria sp. J. Nat. Prod. 2007, 70, 397–401. [Google Scholar] [CrossRef]
- Portmann, C.; Blom, J.F.; Kaiser, M.; Brun, R.; Juttner, F.; Gademann, K. Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A: Heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J. Nat. Prod. 2008, 71, 1891–1896. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Knudsen, G.M.; Helbig, C.; De Muylder, G.; Mascuch, S.M.; Mackey, Z.B.; Gerwick, L.; Clayton, C.; McKerrow, J.H.; Linington, R.G. Examination of the mode of action of the almiramide family of natural products against the kinetoplastid parasite Trypanosoma brucei. J. Nat. Prod. 2013, in press. [Google Scholar]
- Verlinde, C.L.; Hannaert, V.; Blonski, C.; Willson, M.; Perie, J.J.; Fothergill-Gilmore, L.A.; Opperdoes, F.R.; Gelb, M.H.; Hol, W.G.; Michels, P.A. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug Resist. Updat. 2001, 4, 50–65. [Google Scholar] [CrossRef]
- Fattorusso, C.; Persico, M.; Calcinai, B.; Cerrano, C.; Parapini, S.; Taramelli, D.; Novellino, E.; Romano, A.; Scala, F.; Fattorusso, E.; et al. Manadoperoxides A–D from the Indonesian sponge Plakortis cfr. simplex. Further insights on the structure-activity relationships of simple 1,2-dioxane antimalarials. J. Nat. Prod. 2010, 73, 1138–1145. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Barbary, M.A.; El-Ghorab, D.M.H.; Bohlin, L.; Borg-Karlson, A.K.; Goransson, U.; Verpoorte, R. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).