Exploiting the Nephrotoxic Effects of Venom from the Sea Anemone, Phyllodiscus semoni, to Create a Hemolytic Uremic Syndrome Model in the Rat
Abstract
:1. Introduction
| Classification | Type of envenomation | References | |
|---|---|---|---|
| Phylum | Genus, Species | ||
| Cnidaria | |||
| Jellyfishes | |||
| Portuguese man-of-war (Physalia physalis) | sting | [15] | |
| Irukandji jellyfish (Carukia barnesi, Malo kingi) | sting | [15,16] | |
| Mauve stinger (Pelagia noctiluca) | sting | [15] | |
| Box jellyfish (Chironex fleckeri) | sting | [13] | |
| Chesapeake Bay sea nettle (Chrysaora quinquecirrha) | sting | [15] | |
| Sea wasp (Chiropsalmus quadrigatus) | sting | [14] | |
| Fire coral (Millepora alcicornis) | sting | [11,12] | |
| Sea anemones | |||
| The Hell’s Fire sea anemone (Actinodendron plumosum) | sting | [10,20] | |
| Night sea anemone (Phyllodiscus semoni) | sting | [18,21] | |
| Haddon’s carpet anemone (Stichodactyla haddoni) | sting | [22] | |
| Snakelock’s anemone (Anemonia sulcata (=Anemonia viridis)) | sting | [23] | |
| Condylactis sp. | sting | [24] | |
| Echinodermata | |||
| Sea urchins | |||
| Flower sea urchin (Toxopneustes pileolus) | sting | [25] | |
| Purple sea urchin (Paracentrotus lividus) | sting | [26] | |
| Sea star | |||
| Crown-of-Thorns starfish (Acanthaster planci (Linnaeus)) | sting | [27,28] | |
| Mollusca | |||
| Cone shells (Conidae) | sting | [29,30] | |
| Blue-ringed octopus (Hapalochlaena) | bite | [31,32] | |
| Shellfish poisoning by brevetoxins and domoic acid | food | [19] | |
| Chordata | |||
| Stone fish, lion fish, scorpionfish (Scorpaernidae) | sting | [20,33,34,35] | |
| Stingray (Dasyatidae) | sting | [36] | |
| Weeverfish (Trachinus) | sting | [37] | |
| Striped eel catfish (Plotosus lineatus, Plotosus japonicus) | sting | [38] | |
| Globe fishes (Tetraodontidae) | food | [19] | |
| Hydrophiidae | |||
| Hydorophis, Laticauda , Pelamis | bite | [39,40] | |
| Organisms | Agents | Targets | References |
|---|---|---|---|
| (A) Extracted agents | |||
| Jellyfish | |||
| Chiropsalmus quadrigatus | CqTX | glioma cells | [52] |
| Chrysaora quinquecirrha | Sea nettle nematocyst venom (SNV) | cancer cells | [70] |
| Starfish | |||
| Crown-of-Thorns starfish | extracts | breast cancer cells | [53] |
| Sponge | |||
| Callyspongia truncate | callystatin A | cancer cells | [71] |
| Discodermia dissoluta | (+)-Discodermolide | cancer cells | [57] |
| Dysidea arenaria | arenastatin A | cancer cells | [71] |
| Hyrtios altum | altohyrtin A | cancer cells | [71] |
| Petrosia sp. | dideoxpetrasynol A | melanoma cells | [51] |
| Spirastrella spinispirulifera, Hyrtios erecta | Spongistatin 1 | cancer cells, leukemia | [72] |
| Sea anemone | |||
| Actineria villosa | MACPF | cancer cells | [55] |
| Actinia equina | EqTX-II | glioblastoma cells | [54] |
| Anemonia viridis | ATX-II | antiarrthymia | [65] |
| Anthropleura elegantissima | APETx2 | inflammation, postoperative pain | [60,61,62,63] |
| Bunodosoma caissarum | Bc2 | glioblastoma cells | [54] |
| Radianthus macrodactylus | PTX-A | cancer cells | [59] |
| Stichodactyla helianthus | sticholysin I (StI) | cancer cells | [56] |
| Stichodactyla helianthus | ShK | T lymphocyte proliferation, Autoimmune diseases | [66,67] |
| (B) Derivatives of extracted agents | |||
| Sponge | |||
| Discodermia dissolute | (+)-Discodermolide-paclitaxel hybrids | cancer cells | [73] |
| Dysidea arenaria | analogoue of arenastatin A | cancer cells | [58] |
| Sea anemone | |||
| Stichodactyla helianthus | StI W111C | cancer cells | [74] |
| Stichodactyla helianthus | ShK analogues | autoimmune diseases | [75] |
| Cone shell | |||
| Conus magus | Ziconotide (a derivative of conotoxin) | non-opioid intrathecal therapy | [68,69] |
2. Acute Kidney Injuries Induced by Natural Venoms
| Organisms | Type of renal injuries/pathology | Human or animal models (References) | ||
|---|---|---|---|---|
| 1. Land envenomation | ||||
| (1) Biting | ||||
| Snakes: viper (Viperidea) and cobra (Elapidae) | ||||
| Habu snakes (Trimeresurus) | Mesangial proliferative glomerulonephritis, mesangial injuries | [84,85,91,92] | ||
| Mamushi snake (Gloydius blomhoffii) | ATN * with hemolysis | [92] | ||
| Tiger snake (Notechis scutatus) | TMA **, ATN with rhabdomyolysis | [82,93] | ||
| “Fer-de-Lance” pit viper (Botherops lanceolatus) | TMA | [83] | ||
| Bothrops (B.)jararaca, B. jarararacussu, B.moojeni | Renal cortical necrosis | [1,94,95] | ||
| Brazilian rattlesnake (Crotalus durissus) | Rhabdomyolysis and hemolysis related renal injuries | [96,97,98] | ||
| Russell’s viper (Vipera russellii) | Cortical necrosis, ATN with rhabdomyolysis, mesangiolysis | [2,99] | ||
| Lansberg’s pit viper (Porthidium lansbergii) | ATN, glomerular and tubular changes | [100,101] | ||
| Taipan (Oxyuranus scutellatus) | HUS *** | [81] | ||
| Spider | ||||
| Brown recluse spider (Loxosceles intermedia) | Hemolysis and rhabdomyolysis related renal injuries, glomerulonephritis | [4,102] | ||
| (2) Sting | ||||
| Honey Bee (Apis mellifera) | ATN with hemolysis and rhabdomyolysis, renal ischemia | [103] | ||
| Hornet (Vespa crabro) | ATN with hemolysis and rhabdomyolysis | [104] | ||
| Wasp (Vespa magnifica) | ATN with hemolysis and rhabdomyolysis, or by direct toxic effects | [105,106] | ||
| Iranian scorpion (Hemiscorpius lepturus) | HUS | [107,108] | ||
| Lonomia caterpillars (Lonomia obliqua) | Hemodynamic changes and disseminated intravascular coagulation related renal injuries | [8,109] | ||
| (3) Food poison | ||||
| Mushroom | ||||
| Cortinarius sp. | Chronic interstitial nephritis | [110,111] | ||
| Amanita (A.) phylloides, A. proxima, A. smithiana, A. pseudoporphyria, A. boudierim, A. gracilior, A. echinocephala | ATN, acute interstitial nephritis | [112,113,114,115,116] | ||
| Lepiota sp. | Acute renal failure (no detail pathology) | [117] | ||
| Squirting cucumber (Ecbalium Elaterium) | Renal failure (no detail pathology) | [118] | ||
| Herb | ||||
| Chinese herb (Aristolochia sp.) | Chinese harb nephropathy, ATN, tubulointerstitial nephritis | [119,120] | ||
| 2. Marine envenomation | ||||
| (1) Biting | ||||
| Sea snakes (Hydrophis cyanocinctus, Laticauda semifasciata) | ATN, renal ischemia | [39,89,90,121] | ||
| (2) Sting | ||||
| Lionfish (genus Pterois) | ATN | [122] | ||
| Jelly fishes | ||||
| Portuguese man-of-war (Physalia physalis) | ATN with hemolysis | [86,123] | ||
| Box-jellyfish (Chirodropids) | ATN | [124] | ||
| Fire coral (Millepora species) | Minimal change nephrotic syndrome | [12] | ||
| Sea anemone (Phyllodiscus semoni, Condylactis sp.) | ATN, TMA, renal ischemia | [18,24,76] | ||
| (3) Food poisons | ||||
| Puffer (Globe) fish (Lagocephalus, Lactoria) | ATN with rhabdomyolysis, renal ischemia | [88,125,126] | ||
3. Envenomation by Sea Anemones including P. semoni and the Acute Kidney Injuries
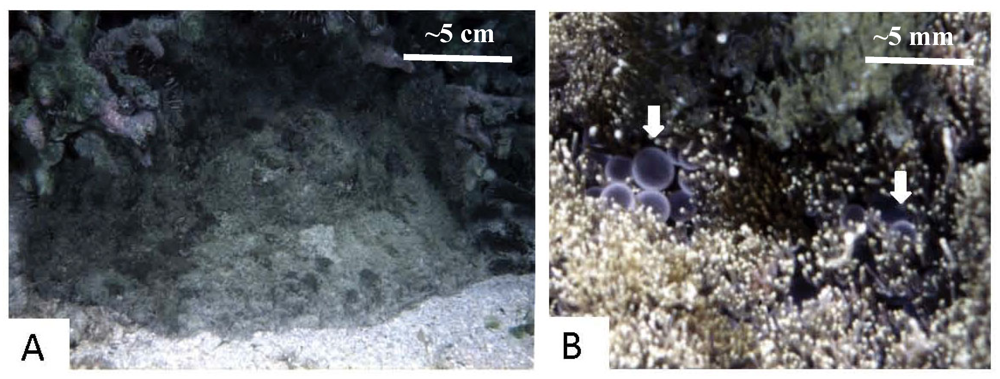
4. Thrombotic Microangiopathy, Renal Pathology and Renal Function after Exposure of Rats to Venom of P. semoni, PsTX-T
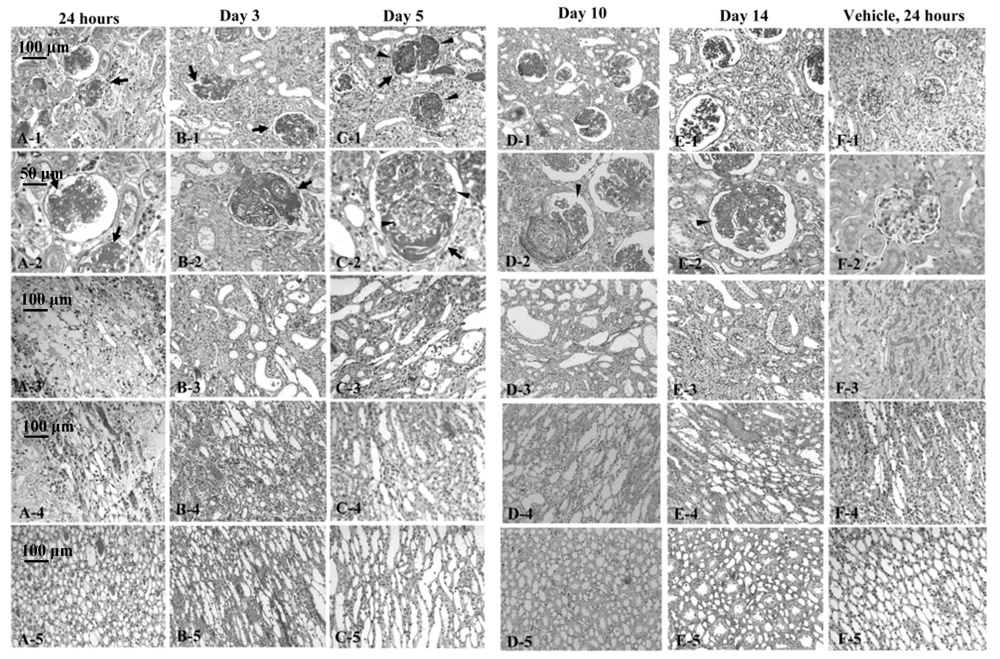
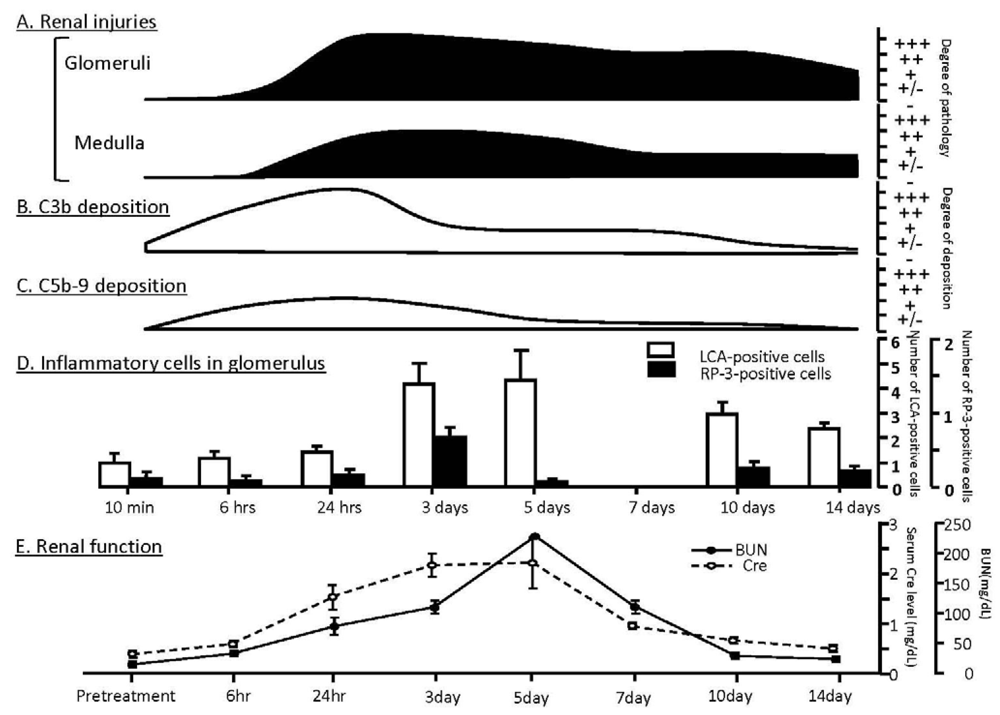
| 1. Infection-related | ||
| Bacteria | ||
| Escherichia coli (O157:H7, O104:H4, etc.), Shigella dysenteriae type 1, Salmonella typhi, Salmonella pneumonia, Campylobacter jejuni, Yersinia pseudotuberculosis, Pseudomonas sp., Bacteroides sp., Mycobacterium tuberculosis | ||
| Virus | ||
| Rubella, Coxsackievirus, Echoviruses, Influenza virus, Epstein-Barr virus, Rotaviruses, Cytomegalovirus, Human immunodeficiency virus | ||
| 2. Drug-related | ||
| Immunosuppressant and chemotherapy | ||
| Cyclosporine, Tacrolimus, OKT3, Dopidogrel, Valacyclovir, Cyclosporine, Mitomycin C, Cisplatin, Daunorubicin, Cytosine arabinoside, Methyl CCNU, Chlorozotocin, Zinostatin, Deoxycoformycin, Gemcitabine | ||
| Other drugs | ||
| Oral contraceptives, Quinine, Penicillin, Penicillamine, Metronidazole, Ticlopidine, Clopidogrel | ||
| 3. Toxins | ||
| Carbon monoxide, Bee sting, Arsenic poisoning, Snake bites, Iodine, etc. | ||
| 4. ADAMTS 13 * related TTP | ||
| Deficiency of ADAMTS 13 activity, Inhibitor of ADAMS 13 (antibody to ADAMS 13) | ||
| 5. Abnormalities of complement components and complement regulators (aHUS) | ||
| Mutations in complement regulators/components (factor H, factor I, factor B, C3, CD46) | ||
| Anti-factor H autoantibodies, etc. | ||
| 6. Secondary | ||
| Malignant neoplasm | ||
| Transplantation | (conditioning for hematopoietic stem cell transplantation, GVHD **, chronic transplant rejection) | |
| Autoimmune disease | ||
| Systemic lupus erythematosus, Scleroderma renal crisis, Antiphospholipid antibody syndrome, Polyarteritis nodosa, Primary glomerulopathies (MPGN ***, etc.), malignant nephrosclerosis with malignant hypertension | ||
| 7. Other reasons | ||
| Pregnancy or postpartum | ||
| Radiation | ||
5. Impairment of Complement Regulator Expression and Enhanced Complement Deposition in Kidney after Exposure of PsTX-T in Rat
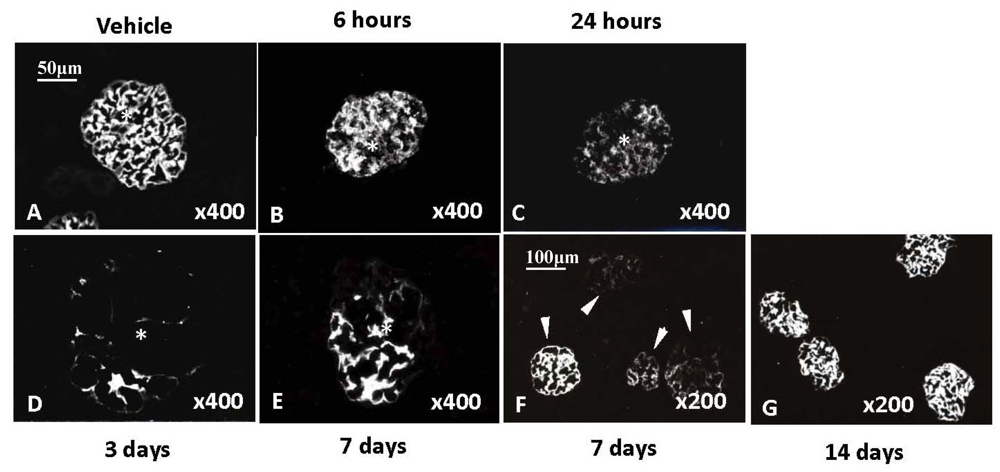
6. Thrombotic Microangiopathy in Kidney, HUS, aHUS and Impairment of Complement Regulation
7. Conclusion and Future
Acknowledgments
References
- Amaral, C.F.; Da Silva, O.A.; Goody, P.; Miranda, D. Renal cortical necrosis following Bothrops jararaca and B. jararacussu snake bite. Toxicon 1985, 23, 877–885. [Google Scholar] [CrossRef]
- Ratcliffe, P.J.; Pukrittayakamee, S.; Ledingham, J.G.; Warrell, D.A. Direct nephrotoxicity of Russell’s viper venom demonstrated in the isolated perfused rat kidney. Am. J. Trop. Med. Hyg. 1989, 40, 312–319. [Google Scholar]
- Yamamoto, C.; Tsuru, D.; Oda-Ueda, N.; Ohno, M.; Hattori, S.; Kim, S.T. Flavoxobin, a serine protease from Trimeresurus flavoviridis (habu snake) venom, independently cleaves Arg726–Ser727 of human C3 and acts as a novel, heterologous C3 convertase. Immunology 2002, 107, 111–117. [Google Scholar] [CrossRef]
- Luciano, M.N.; da Silva, P.H.; Chaim, O.M.; dos Santos, V.L.; Franco, C.R.; Soares, M.F.; Zanata, S.M.; Mangili, O.C.; Gremski, W.; Veiga, S.S. Experimental evidence for a direct cytotoxicity of Loxosceles intermedia (brown spider) venom in renal tissue. J. Histochem. Cytochem. 2004, 52, 455–467. [Google Scholar] [CrossRef]
- Tambourgi, D.V.; de F Fernandes Pedrosa, M.; van den Berg, C.W.; Gonçalves-de-Andrade, R.M.; Ferracini, M.; Paixão-Cavalcante, D.; Morgan, B.P.; Rushmere, N.K. Molecular cloning, expression, function and immunoreactivities of members of a gene family of sphingomyelinases from Loxosceles venom glands. Mol. Immunol. 2004, 41, 831–840. [Google Scholar] [CrossRef]
- Klsbister, G.; Fan, H.W. Spider bite. Lancet 2011, 378, 2039–2047. [Google Scholar] [CrossRef]
- Bertazzi, D.T.; de Assis-Pandochi, A.I.; Azzolini, A.E.; Talhaferro, V.L.; Lazzarini, M.; Arantes, E.C. Effect of Tityus serrulatus scorpion venom and its major toxin, TsTX-I, on the complement system in vivo. Toxicon 2003, 41, 501–508. [Google Scholar] [CrossRef]
- Burdmann, E.A.; Antunes, I.; Saldanha, L.B.; Abdulkader, R.C. Severe acute renal failure induced by the venom of Lonomia caterpillars. Clin. Nephrol. 1996, 46, 337–339. [Google Scholar]
- Whittington, C.M.; Tapenfuss, A.T.; Locke, D.P.; Mardis, E.R.; Wilson, R.K.; Abubucker, S.; Mitreva, M.; Wong, E.S.; Hsu, A.L.; Kuchel, P.W.; et al. Novel venom gene discovery in the platypus. Genome Biol. 2010, 11, R95. [Google Scholar] [CrossRef]
- Haddad, V., Jr.; Lupi, O.; Lonza, J.P.; Tyring, S.K. Tropical dermatology: Marine and aquatic dermatology. J. Am. Acad. Dermatol. 2009, 61, 733–750. [Google Scholar] [CrossRef]
- Wittle, L.W.; Middlebrook, R.E.; Lane, C.E. Isolation and partial purification of a toxin from Millepora alcicornis. Toxicon 1971, 9, 327–331. [Google Scholar] [CrossRef]
- Ramesh Prasad, G.V.; Vincent, L.; Hamilton, R.; Lim, K. Minimal change disease in association with fire coral (Millepora species) exposure. Am. J. Kidney Dis. 2006, 47, e15–e16. [Google Scholar] [CrossRef]
- Williamson, J. Classification (with Description and Medical Implications of Seven Venomous Jellyfish). In Some Australian Marine Stings, Envenomations and Poisonings; Williamson, J., Ed.; Surf Life Saving Foundation: Brisbane, Australia, 1981; pp. 1–26. [Google Scholar]
- Nagai, H.; Takuwa-Kuroda, K.; Nakao, M.; Oshiro, N.; Iwanaga, S.; Nakajima, T. A novel protein toxin from the deadly box jellyfish (sea wasp, habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem. 2002, 66, 97–102. [Google Scholar] [CrossRef]
- Auerbach, P.S. Marine envenomations. N. Engl. J. Med. 1991, 325, 486–493. [Google Scholar] [CrossRef]
- Fenner, P.; Carney, I. The Irukanji syndrome. A devastating syndrome caused by a north Australian jellyfish. Aust. Fam. Physician 1999, 28, 1131–1137. [Google Scholar]
- Lim, Y.L.; Kumarasinghe, S.P.W. Cutaneous injuries from marine animals. Singap. Med. J. 2007, 48, e25. [Google Scholar]
- Mizuno, M.; Nishikawa, K.; Yuzawa, Y.; Kanie, T.; Mori, H.; Araki, Y.; Hotta, N.; Matsuo, S. A case report of acute renal failure following a sting presumedly by a sea anemone. Am. J. Kidney Dis. 2000, 36, E10. [Google Scholar] [CrossRef]
- Isbister, G.K.; Kiernan, M.C. Neurotoxic marine poisoning. Lancet Neurol. 2005, 4, 219–228. [Google Scholar] [CrossRef]
- Brush, D.E. Marine Envenomations. In Goldfrank’s Toxicologic Emergencies, 8th; Flomenbaum, N.E., Goldfrank, L.R., Hoffman, R.S., Howland, M.A., Lewin, N.A., Nelson, L.S., Eds.; McGraw-Hill Medical: New York, NY, USA, 2006; pp. 1629–1642. [Google Scholar]
- Nakamoto, M.; Uezato, H. Stings of box-jellyfish and sea anemones. Clin. Dermatol. 1998, 52, 29–33. [Google Scholar]
- Nagata, K.; Hide, M.; Tanaka, T.; Ishii, K.; Izawa, M.; Sairenji, T.; Tomita, K.; Shimizu, E. Anaphylactic shock caused by exposure to sea anemones. Allergol. Int. 2006, 55, 181–184. [Google Scholar] [CrossRef]
- Maretic, Z.; Russell, F.E. Stings by the sea anemone Anemonia sulcata in the Adriatic Sea. Am. J. Trop. Med. Hyg. 1983, 32, 891–896. [Google Scholar]
- Garcia, P.J.; Schein, R.M.; Burnett, J.W. Fulminant hepatic failure from a sea anemone sting. Ann. Intern. Med. 1994, 120, 665–666. [Google Scholar]
- De la Torre, C.; Toribio, J. Sea-urchin granuloma: Histologic profile. A pathologic study of 50 biopsies. J. Cutan. Pathol. 2001, 28, 223–228. [Google Scholar] [CrossRef]
- Nassab, R.; Rayatt, S.; Peart, F. The management of hand injuries caused by sea urchin spines. J. Hand Surg. Eur. 2005, 30, 432–433. [Google Scholar] [CrossRef]
- Lin, B.; Norris, R.L.; Auerbach, P.S. A case of elevated liver function tests after crown-of-thorns (Acanthaster planci) envenomation. Wilderness Environ. Med. 2008, 19, 275–279. [Google Scholar] [CrossRef]
- Shiroma, N.; Noguchi, K.; Matsuzaki, T.; Ojiri, Y.; Hirayama, K.; Sakanashi, M. Haemodynamic and haematologic effects of Acanthaster planci venom in dogs. Toxicon 1994, 32, 1217–1225. [Google Scholar] [CrossRef]
- Barbier, J.; Lamthanh, H.; Le Gall, F.; Favreau, P.; Benoit, E.; Chen, H.; Gilles, N.; Ilan, N.; Heinemann, S.H.; Gordon, D.; et al. A delta-conotoxin from Conus ermineus venom inhibits inactivation in vertebrate neuronal Na+ channels, but not in skeletal and cardiac muscles. J. Biol. Chem. 2004, 279, 4680–4685. [Google Scholar]
- Vianna Braga, M.C.; Konno, K.; Portaro, F.C.; de Freitas, J.C.; Yamane, T.; Olivera, B.M.; Pimenta, D.C. Mass spectrometric and high performance liquid chromatography profiling of the venom of the Brazilian vermivorous mollusk Conus regius: Feeding behavior and identification of one novel conotoxin. Toxicon 2005, 45, 113–122. [Google Scholar] [CrossRef]
- Flachsenberger, W.A. Respiratory failure and lethal hypotension due to blue-ringed octopus and tetrodotoxin envenomation observed and counteracted in animal models. J. Toxicol. Clin. Toxicol. 1986, 24, 485–502. [Google Scholar] [CrossRef]
- Cavazzoni, E.; Lister, B.; Sargent, P.; Schibler, A. Blue-ringed octopus (Hapalochlaena sp.) envenomation of a 4-year-old boy: A case report. Clin. Toxicol. 2008, 46, 760–761. [Google Scholar] [CrossRef]
- Kizer, K.W.; Mckinney, H.E.; Auerbach, P.S. Scorpaenidae envenomation. A five-year poison center experience. JAMA 1985, 253, 807–810. [Google Scholar] [CrossRef]
- Haddad, V., Jr.; Martins, I.A.; Makyama, H.M. Injuries caused by scorpionfishes (Scorpaena plumieri Bloch, 1789 and Scorpaena brasiliensis Cuvier, 1829) in the Southwestern Atlantic Ocean (Brazilian coast): Epidemiologic, clinic and therapeutic aspects of 23 stings in humans. Toxicon 2003, 42, 79–83. [Google Scholar] [CrossRef]
- Hahn, S.T.; O’Connor, J.M. An investigation of the biological activity of bullrout (Nothesthes robusa) venom. Toxicon 2000, 38, 79–89. [Google Scholar] [CrossRef]
- Clark, R.F.; Girard, R.H.; Rao, D.; Ly, B.T.; Davis, D.P. Stingray envenomation: A retrospective review of clinical presentation and treatment in 119 cases. J. Emerg. Med. 2007, 33, 33–37. [Google Scholar] [CrossRef]
- Borondo, J.C.; Sanz, P.; Noque, S.; Pocela, J.L.; Garrido, P.; Valverde, J.L. Fatal weeverfish sting. Hum. Exp. Toxicol. 2000, 20, 118–119. [Google Scholar]
- Shiomi, K.; Takamiya, M.; Yamanaka, H.; Kikuchi, T.; Konno, K. Hemolytic, lethal and edema-forming activities of the skin secretion from the oriental catfish (Plotosus lineatus). Toxicon 1986, 24, 1015–1018. [Google Scholar] [CrossRef]
- Tu, A.T. Biotoxicology of sea snake venoms. Ann. Emerg. Med. 1987, 16, 1023–1028. [Google Scholar] [CrossRef]
- Tamiya, N.; Yagi, T. Studies on sea snake venom. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 41–52. [Google Scholar] [CrossRef]
- Das, T.; Bhattacharya, S.; Halder, B.; Biswas, A.; Das Gupta, S.; Gomes, A.; Gomes, A. Cytotoxic and antioxidant property of a purified fraction (NN-32) of Indean Naja naja venom on Ehrlich ascites carcinoma in BALB/c mice. Toxicon 2011, 57, 1065–1072. [Google Scholar] [CrossRef]
- Rodrigues, F.G.; Petretski, J.H.; Kanashiro, M.M.; Lemos, L.; de Silva, W.D.; Kipnis, T.L. The complement system is involved in acute inflammation but not in the hemorrhage produced by a Bothrops atrox snake venom low molecular mass proteinase. Mol. Immunol. 2004, 40, 1149–1156. [Google Scholar] [CrossRef]
- Pickering, R.J.; Wolfson, M.R.; Good, R.A.; Gewurz, H. Passive hemolysis by serum and cobra venom factor: A new mechanism inducing membrane damage by complement. Proc. Natl. Acad. Sci. USA 1969, 62, 521–527. [Google Scholar] [CrossRef]
- Drake, W.P.; Pokorney, D.R.; Kopyta, L.P.; Mardiney, M.R., Jr. In vivo decomplementation of guinea pigs with cobra venom factor and anti-C3 serum: Anoalysis of the requirement of C3 and C5 for the mediation of endotoxin-induced death. Biomedicine 1976, 25, 91–94. [Google Scholar]
- Mizuno, M.; Nishikawa, K.; Goodfellow, R.M.; Piddlesden, S.J.; Morgan, B.P.; Matsuo, S. The effects of functional suppression of a membrane-bound complement regulatory protein, CD59, in the synovial tissue in rats. Arthritis Rhreum. 1997, 40, 527–533. [Google Scholar] [CrossRef]
- Mizuno, M.; Nishikawa, K.; Okada, N.; Matsuo, S.; Ito, K.; Okada, H. Inhibition of a membrane complement regulatory protein by a monoclonal antibody induces acute lethal shock in rats primed with lipopolysaccharide. J. Immunol. 1999, 162, 5477–5482. [Google Scholar]
- Mizuno, M.; Ito, Y.; Hepburn, N.; Mizuno, T.; Noda, Y.; Yuzawa, Y.; Harris, C.L.; Morgan, B.P.; Matsuo, S. Zymosan, but not lipopolysaccharide, triggers severe and progressive peritoneal injury accompanied by complement activation in a rat peritonitis model. J. Immunol. 2009, 183, 1403–1412. [Google Scholar] [CrossRef]
- Gorsuch, W.B.; Guikema, B.J.; Fritzinger, D.C.; Vogel, C.W.; Stahl, G.L. Humanized cobra venom factor decreases myocardial ischemia-reperfusion injury. Mol. Immunol. 2009, 47, 506–510. [Google Scholar] [CrossRef]
- Vogel, C.W.; Fritzinger, D.C. Cobra venom factor: Structure, function, and humanization for therapeutic complement depletion. Toxicon 2010, 56, 1198–1222. [Google Scholar] [CrossRef]
- Chaim, O.M.; Trevisan-Silva, D.; Chaves-Moreira, D.; Wille, A.C.M.; Ferrer, V.P.; Matsubara, F.H.; Mangili, O.C.; da Silveira, R.B.; Gremski, L.H.; Gremski, W.; et al. Brown Spider (Loxosceles genus) Venom Toxins: Tools for Biological Purposes. Toxins 2011, 3, 309–344. [Google Scholar] [CrossRef]
- Choi, H.J.; Bae, S.J.; Kim, N.D.; Jung, J.H.; Choi, Y.H. Induction of apoptosis by dideoxypetrosynol A, a polyacetylene from the sponge Petrosia sp., in human skin melanoma cells. Int. J. Mol. Med. 2004, 14, 1091–1096. [Google Scholar]
- Sun, L.K.; Yoshii, Y.; Hyodo, A.; Tsurushima, H.; Saito, A.; Harakuni, T.; Li, Y.P.; Nozaki, M.; Morine, N. Apoptosis induced by box jellyfish (Chiropsalmus quadrigatus) toxin in glioma and vascular endothelial cell lines. Toxicon 2002, 40, 441–446. [Google Scholar] [CrossRef]
- Mutee, A.F.; Salhimi, S.M.; Ghazali, F.C.; Al-Hassan, F.M.; Lim, C.P.; Ibrahim, K.; Asmawi, M.Z. Apoptosis induced in human breast cancer cell line by Acanthaster planci starfish extract compared to tamoxifen. African J. Pharm. Pharmacol. 2012, 6, 129–134. [Google Scholar]
- Soletti, R.C.; de Faria, G.P.; Vernal, J.; Terenzi, H.; Anderluh, G.; Borges, H.L.; Moura-Neto, V.; Gabilan, N.H. Potentiation of anticancer-drug cytotoxicity by sea anemone pore-formimg proteins in human glioblastoma cells. Anticancer Drugs 2008, 19, 517–525. [Google Scholar] [CrossRef]
- Oshiro, N.; Kobayashi, C.; Iwanaga, S.; Nozaki, M.; Namikoshi, M.; Spring, J.; Nagai, H. A new membrane-attack complex/perforin (MACPF) domain lethal toxin from the nematocyst venom of the Okinawan sea anemone Actineria villosa. Toxicon 2004, 43, 225–228. [Google Scholar] [CrossRef]
- Tejuca, M.; Díaz, I.; Figueredo, R.; Roque, L.; Pazos, F.; Martínez, D.; Iznaga-Escobar, N.; Pérez, R.; Alvarez, C.; Lanio, M.E. Construction of an immunotoxin with the pore forming protein StI and/or C5, a monoclonal antibody against a colon cancer cell line. Int. Immunopharmacol. 2004, 4, 731–744. [Google Scholar] [CrossRef]
- De Souza, M.V. (+)-Discodermolide: A marine natural product against cancer. Sci. World J. 2004, 4, 415–436. [Google Scholar] [CrossRef]
- Murakami, N.; Tamura, S.; Koyama, K.; Sugimoto, M.; Maekawa, R.; Kobayashi, M. New analogue of arenastatin A, a potent cytotoxic spongean depsipeptide, with anti-tumor activity. Bioorg. Med. Chem. Lett. 2004, 14, 2597–2601. [Google Scholar] [CrossRef]
- Fedorov, S.; Dyshlovoy, S.; Monastyrnaya, M.; Shubina, L.; Leychenko, E.; Kozlovskaya, E.; Jin, J.O.; Kwak, J.Y.; Bode, A.M.; Dong, Z.; Stonik, V. The anticancer effects of actinoporin RTX-A from the sea anemone Heteractis crispa (=Radianthus macrodactylus). Toxicon 2010, 55, 811–817. [Google Scholar]
- Blanchard, M.G.; Rash, L.D.; Kellenberger, S. Inhibition of voltage-gated Na+ currents in sensory neurons by the sea anemone toxin APETx2. Br. J. Pharmacol. 2012, 165, 2167–2177. [Google Scholar] [CrossRef]
- Deval, E.; Noël, J.; Lay, N.; Alloui, A.; Diochot, S.; Friend, V.; Jodar, M.; Lazdunski, M.; Lingueglia, E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO. J. 2008, 27, 3047–3055. [Google Scholar] [CrossRef]
- Deval, E.; Noël, J.; Gasull, X.; Delaunay, A.; Alloui, A.; Frined, V.; Eschalier, A.; Lazdunski, M.; Lingueglia, E. Acid-sensing ion channels in postoperative pain. J. Neurosci. 2011, 31, 6059–6066. [Google Scholar]
- Karczewski, J.; Spencer, R.H.; Garsky, V.M.; Liang, A.; Leitl, M.D.; Cato, M.J.; Cook, S.P.; Kane, S.; Urban, M.O. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br. J. Pharmacol. 2010, 161, 950–960. [Google Scholar] [CrossRef]
- Shimizu, W.; Antzelevitch, C. Cellular and ionic basis for T-wave alternans under long-QT conditions. Circulation 1999, 99, 1499–1507. [Google Scholar] [CrossRef]
- Platou, E.S.; Refsum, H.; Hotvedt, R. Class III antiarrhythmic action linked with positive inotropy: Antiarrhythmic, electrophysiological, and hemodynamic effects of the sea-anemone polypeptide ATX II in the dog heart in situ. J. Cardiovasc. Pharmacol. 1986, 8, 459–465. [Google Scholar] [CrossRef]
- Beeton, C.; Smith, B.J.; Sabo, J.K.; Crossley, G.; Nugent, D.; Khaytin, I.; Chi, V.; Chandy, K.G.; Pennington, M.W.; Norton, R.S. The D-diastereomer of ShK toxin selectively blocks voltage-gated K+ channels and inhibits T lymphocyte proliferation. J. Biol. Chem. 2008, 283, 988–997. [Google Scholar]
- Chi, V.; Pennington, M.W.; Norton, R.S.; Tarcha, E.J.; Londono, L.M.; Sims-Fahey, B.; Upadhyay, S.K.; Lakey, J.T.; Iadonato, S.; Wulff, H.; Beeton, C.; et al. Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon 2011, 59, 529–546. [Google Scholar]
- Kapural, L.; Lokey, K.; Leong, M.S.; Fiekowsky, S.; Stanton-Hicks, M.; Sapienza-Crawford, A.J.; Webster, L.R. Intrathecal ziconitide for complex regional pain syndrome: Seven case reports. Pain Pract. 2009, 9, 296–303. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577. [Google Scholar]
- Balamurugan, E.; Reddy, B.V.; Menon, V.P. Antitumor and antioxidant role of Chrysaora quinquecirrha (sea nettle) nemotocyst venom peptide Ehrlich ascites carcinoma in Swiss Albino mice. Mol. Cell. Biochem. 2010, 338, 69–76. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kitagawa, I. Marine spongean cytotoxins. J. Nat. Toxins 1999, 8, 249–258. [Google Scholar]
- Schyschka, L.; Rudy, A.; Jeremias, I.; Barth, N.; Pettit, G.R.; Vollmar, A.M. Spongistatin 1: A new chemosensitizing marine compound that degrades XIAP. Leukemia 2008, 22, 1737–1745. [Google Scholar] [CrossRef]
- Smith, A.B.; Sugasawa, K.; Atasoylu, O.; Yang, C.P.; Horwitz, S.B. Design and synthesis of (+)-discodermolide-paclitaxel hybrids leading to enhanced biological activity. J. Med. Chem. 2011, 54, 6319–6327. [Google Scholar]
- Pentón, D.; Pérez-Barzaga, V.; Diaz, I.; Reytor, M.L.; Campos, J.; Fando, R.; Calvo, L.; Cilli, E.M.; Morera, V.; Castellanos-Serra, L.R.; et al. Validation of a mutant of the pore-forming toxin sticholysin-I for the construction of proteinase-activated immunotoxins. Protein Eng. Des. Sel. 2011, 24, 485–493. [Google Scholar] [CrossRef]
- Norton, R.S.; Pennington, M.W.; Wulff, H. Pottasium channel blockade by the sea anemone toxin ShK for the treatment of multiple sclerosis and other autoimmune diseases. Curr. Med. Chem. 2004, 11, 3041–3052. [Google Scholar]
- Mizuno, M.; Nozaki, M.; Morine, N.; Suzuku, N.; Nishikawa, K.; Morgan, B.P.; Matsuo, S. A protein toxin from the sea anemone Phyllodiscus semoni targets the kidney and causes a renal injury resembling haemolytic uremic syndrome. Am. J. Pathol. 2007, 171, 402–414. [Google Scholar] [CrossRef]
- Zimmerman, S.E.; Yong, L.C. Nephrotoxicity of notexin in experimental mice. Exp. Toxicol. Pathol. 1995, 47, 149–155. [Google Scholar] [CrossRef]
- Abuelo, J.G. Renal failure caused by chemicals, foods, plants, animal venoms, and misuse of drugs. An overview. Arch. Intern. Med. 1990, 150, 505–510. [Google Scholar] [CrossRef]
- Sitprija, V. Animal toxins and the kidney. Nat. Clin. Pract. Nephrol. 2008, 4, 616–627. [Google Scholar] [CrossRef]
- Juckett, G.; Hancox, J.G. Venomous snakebites in the united states: Management review and update. Am. Fam. Physician 2002, 65, 1367–1374. [Google Scholar]
- Cobcroft, R.G.; Williams, A.; Cook, D.; Williams, D.J.; Masci, P. Hemolytic uremic syndrome following taipan envenomation with response to plasmapheresis. Pathology 1997, 29, 399–402. [Google Scholar] [CrossRef]
- Casamento, A.J.; Isbister, G.K. Thrombotic microangiopathy in two tiger snake envenomations. Anaesth. Intensive Care 2011, 39, 1124–1127. [Google Scholar]
- Malbranque, S.; Piercecchi-Marti, M.D.; Thomas, L.; Barbey, C.; Courcier, D.; Bucher, B.; Ridarch, A.; Smadja, D.; Warrell, D.A. Fatal diffuse thrombotic microangiopathy after a bite by the “Fer-de-Lance” pit viper (Botherops lanceolatus) of Martinique. Am. J. Trop. Med. Hyg. 2008, 78, 856–861. [Google Scholar]
- Kubo, A.; Iwano, M.; Kobayashi, Y.; Kyoda, Y.; Isumi, Y.; Maruyama, N.; Samejima, K.; Dohi, Y.; Minamino, N.; Yonemasu, K. In vivo effects of Habu snake venom on cultured mesangial cells. Nephron 2002, 92, 665–672. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hiraiwa, N.; Yoshiki, A.; Ohnishi, M.; Kusakabe, M. Tenascin-C expression and splice variant in Habu snake venom-induced glomerulonephritis. Exp. Mol. Pathol. 2002, 72, 186–195. [Google Scholar] [CrossRef]
- Guess, H.A.; Saviteer, P.L.; Morris, C.R. Hemolysis and acute renal failure following a Portuguese man-of-war sting. Pediatrics 1982, 70, 979–981. [Google Scholar]
- Deekajorndech, T.; Kingwatanakul, P.; Wananukul, S.; Deekajorn, T. Acute renal failure in a child with jelly fish contact dermatitis. J. Med. Assoc. Thail. 2004, 87, S292–S294. [Google Scholar]
- Nakashima, R.; Nakata, Y.; Kameoka, M.; Hayashi, N.; Watanabe, K.; Yagi, K. Case of tetrodotoxin intoxication in a uremic patient. Chudoku Kenkyu 2007, 20, 141–145. [Google Scholar]
- Sitprija, V.; Sribhibhadh, R. Haemodialysis in poisoning by sea-snake venom. Br. Med. J. 1971, 3, 218. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Abdelbaki, Y.Z.; Tu, A.T. Nephrotoxic action of rattlesnake and sea snake venoms: An electron-microscopic study. J. Pathol. 1976, 118, 75–81. [Google Scholar] [CrossRef]
- Masuda, Y.; Shimizu, A.; Mori, T.; Ishiwata, T.; Kitamura, H.; Ohashi, R.; Ishizaki, M.; Asano, G.; Sugisaki, Y.; Yamanaka, N. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am. J. Pathol. 2001, 159, 599–608. [Google Scholar] [CrossRef]
- Yasunaga, H.; Horiguchi, H.; Kuwabara, K.; Hashimoto, H.; Matsuda, S. Short report: Venomous snake bites in Japan. Am. J. Trop. Med. Hyg. 2011, 84, 135–136. [Google Scholar] [CrossRef]
- Hood, V.L.; Johnson, J.R. Acute renal failure with myoglobinuria after tiger snake bite. Med. J. Aust. 1975, 18, 638–641. [Google Scholar]
- Burdmann, E.A.; Woronik, V.; Prado, E.B.; Abdulkader, R.C.; Saldanha, L.B.; Barreto, O.C.; Marcondes, M. Snakebite-induced acute renal failure: An experimental model. Am. J. Trop. Med. Hyg. 1993, 48, 82–88. [Google Scholar]
- Barbosa, P.S.; Havt, A.; Facó, P.E.; Sousa, T.M.; Bezerra, I.S.; Fonteles, M.C.; Toyama, M.H.; Marangoni, S.; Novello, J.C.; Monteiro, H.S. Renal toxicity of Bothrops moojeni snake venom and its main myotoxins. Toxicon 2002, 40, 1427–1435. [Google Scholar] [CrossRef]
- Azevedo-Marques, M.M.; Cupo, P.T.; Coimbra, M.; Hering, S.E.; Rossi, M.A.; Laure, C.J. Myonecrosis, myoglobulinuria and acute renal failure induced by South Amerian rattesnake (Crotalus durissus terrificus) envenomation in Brazil. Toxicon 1985, 23, 631–636. [Google Scholar] [CrossRef]
- Martins, A.M.; Toyama, M.H.; Havt, A.; Novello, J.C.; Marangoni, S.; Fonteles, M.C.; Monteiro, H.S. Determination of Crotalus durissus cascavella venom components that induce renal toxicity in isolated rat kidneys. Toxicon 2002, 40, 1165–1171. [Google Scholar] [CrossRef]
- Pinho, F.M.O.; Zanetta, D.M.T.; Burdmann, E.A. Acute renal failure after Crotalus durissus snakebite: A prospective survey on 100 patients. Kidney Int. 2005, 67, 659–667. [Google Scholar] [CrossRef]
- Willinger, C.C.; Thamaree, S.; Schramek, H.; Gstraunthaler, G.; Pfaller, W. In vitro nephrotoxicity of Russell’s viper venom. Kidney Int. 1995, 47, 518–528. [Google Scholar] [CrossRef]
- Otero, R.; Gutiérrez, J.; Beatriz Mesa, M.; Duque, E.; Rodríguez, O.; Luis Arango, J.; Gómez, F.; Toro, A.; Cano, F.; María Rodríguez, L.; et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 2002, 40, 1107–1114. [Google Scholar] [CrossRef]
- Vargas, A.; Finol, H.; Girón, M.; Scannone, H.; Fernández, I.; Rodriguez-Acosta, A. Effects of Lansberg’s Hognose pit vipers (Porthidium lansbergii hutmanni) venom on renal ultrastructure in experimental mice. Acta Sci. Vet. 2011, 39, 941. [Google Scholar]
- Lung, J.M.; Mallory, S.B. A child with spider bite and glomerulonephritis: A diagnostic challenge. Int. J. Dermatol. 2000, 39, 287–289. [Google Scholar] [CrossRef]
- Vetter, R.S.; Visscher, P.K.; Camazine, S. Mass envenomations by honey bees and wasps. West. J. Med. 1999, 170, 223–227. [Google Scholar]
- Xuan, B.H.; Mai, H.L.; Thi, M.T.; Nguyen, H.N.; Rabenou, R.A. Swarming hornet attacks: Shock and acute kidney injury—a large case series from Vietnam. Nephrol. Dial. Transplant. 2010, 25, 1146–1150. [Google Scholar]
- Vikrant, S.; Pandey, D.; Machhan, P.; Gupta, D.; Kaushal, S.S.; Grover, N. Wasp envenomation-induced acute renal failure: A report of three cases. Nephrology 2005, 10, 548–552. [Google Scholar] [CrossRef]
- Vachvanichsanong, P.; Dissaneewate, P. Acute renal failure following wasp sting in children. Eur. J. Pediatr. 2009, 168, 991–994. [Google Scholar] [CrossRef]
- Pipelzadeh, M.H.; Jalali, A.; Taraz, M.; Pourabbas, R.; Zaremirakabadi, A. An epidermiological and clinical study on scorpionism by the Iranian scorpion Hemiscorpius lepturus. Toxicon 2007, 50, 984–992. [Google Scholar] [CrossRef]
- Valavi, E.; Ansari, M.J. Hemolytic uremic syndrome following Hemiscorius lepturns (scorpion) sting. J. Nephrol. 2008, 18, 166–168. [Google Scholar]
- Gamborgi, G.P.; Metcalf, E.B.; Barros, E.J. Acute renal failure provoked by toxin from caterpillars of the species Lonomia obliqua. Toxicon 2006, 47, 68–74. [Google Scholar] [CrossRef]
- Frank, H.; Zilker, T.; Kirchmair, M.; Eyer, F.; Haberl, B.; Tuerkoglu-Raach, G.; Wessely, M.; Gröne, H.J.; Heemann, U. Acute renal failure by ingestion of Cortinarius species confounded with psychoactive mushrooms: A case series and literature survey. Clin. Nephrol. 2009, 71, 557–562. [Google Scholar]
- Calviño, J.; Romero, R.; Pintos, E.; Novoa, D.; Güimil, D.; Cordal, T.; Mardaras, J.; Arcocha, V.; Lens, X.M.; Sanchez-Guisande, D. Voluntary ingestion of Cortinarius mushrooms leading to chronic interstitial nephritis. Am. J. Nephrol. 1998, 18, 565–569. [Google Scholar] [CrossRef]
- Garrouste, C.; Hémery, M.; Boudat, A.M.; Kamar, N. Amanita phalloides poisoning-induced end-stage renal failure. Clin. Nephrol. 2009, 71, 571–574. [Google Scholar]
- Courtin, P.; Gallardo, M.; Berrouba, A.; Drouet, G.; de Haro, L. Renal failure after ingestion of Amanita proxima. Clin. Toxicol. 2009, 47, 906–908. [Google Scholar] [CrossRef]
- West, P.L.; Lindgren, J.; Horowitz, B.Z. Amanita smithiana mushroom ingestion: A case of delayed renal failure and literature review. J. Med. Toxicol. 2009, 5, 32–38. [Google Scholar] [CrossRef]
- Iwafuchi, Y.; Morita, T.; Kobayashi, H.; Kasuga, K.; Ito, K.; Nakagawa, O.; Kunisada, K.; Miyazaki, S.; Kamimura, A. Delayed onset acute renal failure associated with Amanita pseudoporphyria Hongo ingestion. Intern. Med. 2003, 42, 78–81. [Google Scholar] [CrossRef]
- Kirchmair, M.; Carrilho, P.; Pfab, R.; Haberi, B.; Felgueiras, J.; Carvalho, F.; Cardoso, J.; Melo, I.; Vinhas, J.; Neuhauser, S. Amanita poisonings resulting in acute, reversible renal failure: New cases, new toxic Amanita mushrooms. Nephrol. Dial. Transplant. 2012, 27, 1380–1386. [Google Scholar] [CrossRef]
- Paydas, S.; Kocak, R.; Erturk, F.; Erken, E.; Zaksu, H.S.; Gurcay, A. Poisoning due to amatoxin-containing Lepiota species. Br. J. Clin. Pract. 1990, 44, 450–453. [Google Scholar]
- Vlachos, P.; Kanitsakis, N.N.; Kokonas, N. Fatal cardiac and renal failure due to Ecbalium elaterium (squirting cucumber). J. Toxicol. Clin. Toxicol. 1994, 32, 737–738. [Google Scholar] [CrossRef]
- Martinez, M.C.; Nortier, J.; Vereerstraeten, P.; Vanherweghem, J.L. Progression rate of Chinese herb nephropathy: Impact of Aristolochia fangchi ingested dose. Nephrol. Dial. Transplant. 2002, 17, 408–412. [Google Scholar] [CrossRef]
- Liu, M.C.; Maruyama, S.; Mizuno, M.; Morita, Y.; Hanaki, S.; Yuzawa, Y.; Matsuo, S. The nephrotoxicity of Aristolochia manshuriensis in rats is attributable to its aristolochic acids. Clin. Exp. Nephrol. 2003, 7, 186–194. [Google Scholar] [CrossRef]
- Ali, S.A.; Alam, J.M.; Abbasi, A.; Zaidi, Z.H.; Stoeva, S.; Voelter, W. Sea snake Hydrophis cyanocinctus venom. II. Histopathological changes, induced by a myotoxic phospholipase A2 (PLA2-H1). Toxicon 2000, 38, 687–705. [Google Scholar] [CrossRef]
- Balasubashini, M.S.; Karthigayan, S.; Somasundaram, S.T.; Balasubramanian, T.; Viswanathan, P.; Menon, V.P. In vivo and in vitro characterization of the biochemical and pathological changes induced by lionfish (Pterios volitans) venom in mice. Toxicol. Mech. Methods 2006, 16, 525–531. [Google Scholar] [CrossRef]
- Spielman, F.J.; Bowe, E.A.; Watson, C.B.; Klein, E.F.J. Acute renal failure as a result of Physalia physalis sting. South. Med. J. 1982, 75, 1425–1426. [Google Scholar] [CrossRef]
- Fenner, P.J.; Lippmann, J.; Gershwin, L.A. Fatal and nonfatal severe jellyfish stings in Thai waters. J. Travel Med. 2010, 17, 133–138. [Google Scholar] [CrossRef]
- Saoudi, M.; Allagui, M.S.; Abdelmouleh, A.; Jamoussi, K.; Feki, A.E. Protective effects of aqueous extract of Artemisia campestris against puffer fish Lagocephalus lagocephalus extract-induced oxidative damage in rats. Exp. Toxicol. Pathol. 2010, 62, 601–605. [Google Scholar] [CrossRef]
- Shinzato, T.; Furuse, A.; Nishino, T.; Abe, K.; Kanda, T.; Maeda, T.; Kohno, S. Cowfish (Umisuzume, Lactoria diaphana) poisoning with rhabdomyolysis. Intern. Med. 2008, 47, 853–856. [Google Scholar] [CrossRef]
- Hansen, P.A.; Halstead, B.W. The venomous sea anemone Actinodendron plumosum haddon of South Vietnam. Micronessica 1971, 7, 123–136. [Google Scholar]
- Massmanian, A.; Valcuende Cavero, F.V.; Ramirez Bosca, A.R.; Castells Rodellas, A.C. Sea anemone dermatitis. Contact Dermat. 1988, 18, 169–170. [Google Scholar] [CrossRef]
- Macek, P.; Lebez, D. Kinetics of hemolysis induced by equinatoxin, a cytolytic toxin from the sea anemone Actinia equina: Effect of some ions and pH. Toxicon 1981, 19, 233–240. [Google Scholar] [CrossRef]
- Bunc, M.; Drevensek, G.; Budihna, M.; Suput, D. Effects of equinatoxin II from Actinia equina (L.) on isolated rat heart: The role of direct cardiotoxic effects in equinatoxin II lethality. Toxicon 1999, 37, 109–123. [Google Scholar] [CrossRef]
- Wang, L.; Ou, J.; Peng, L.; Zhong, X.; Du, J.; Liu, Y.; Huang, Y.; Liu, W.; Zhang, Y.; Dong, M.; et al. Functional expression and characterization of four novel neurotoxins from sea anemone Anthopleura sp. Biochem. Biophys. Res. Commun. 2004, 313, 163–170. [Google Scholar]
- Huerta, V.; Morera, V.; Guanche, Y.; Chinea, G.; González, L.J.; Betancourt, L.; Martínez, D.; Alvarez, C.; Lanio, M.E.; Besada, V. Primary structure of two cytolysin isoforms from Stichodactyla helianthus differing in their hemolytic activity. Toxicon 2001, 39, 1253–1256. [Google Scholar] [CrossRef]
- Goudet, C.; Ferrer, T.; Galán, L.; Artiles, A.; Batista, C.F.V.; Possani, L.D.; Alvarez, J.; Aneiros, A.; Tytgat, J. Characterization of two Bunodosoma granulifera toxins active on cardiac channels. Br. J. Pharmacol. 2001, 134, 1195–1206. [Google Scholar] [CrossRef]
- Sanchez, J.; Bruhn, T.; Aneiros, A.; Wachter, E.; Béress, L. A simple biochemical method in the search for bioactive polypeptides in a sea anemone (Anemonia sulcata). Toxicon 1996, 34, 1361–1366. [Google Scholar] [CrossRef]
- Matins, R.D.; Alves, R.S.; Martins, A.M.; Evangelista, J.S.; Evangelista, J.J.; Ximenes, R.M.; Toyama, M.H.; Toyama, D.O.; Souza, A.J.; Orts, D.J.; et al. Purification and chracterization of the biological effects of phorpholipase A2 from sea anemone Bunodosoma caissarum. Toxicon 2009, 54, 413–420. [Google Scholar] [CrossRef]
- Nagai, H.; Oshiro, N.; Takuwa-Kuroda, K.; Iwanaga, S.; Nozaki, M.; Nakajima, T. Novel proteinaceous toxins from the nematocyst venom of the Okinawan sea anemone Phyllodiscus semoni Kwietniewski. Biochem. Biophys. Res. Commun. 2002, 294, 760–763. [Google Scholar] [CrossRef]
- Nagai, H.; Oshiro, N.; Takuwa-Kuroda, K.; Iwanaga, S.; Nozaki, M.; Nakajima, T. A new polypeptide toxin from the nematocyst venom of an Okinawa sea anemone Phyllodiscus semoni (Japanese name“unbachi-isoginchaku”). Biosci. Biotechnol. Biochem. 2002, 66, 2621–2625. [Google Scholar] [CrossRef]
- Kerr, H.; Richards, A. Complement-mediated injury and protection of endothelium: Lessons from atypical haemolytic uraemic syndrome. Immunology 2012, 217, 195–203. [Google Scholar]
- Kanso, A.A.; Abou Hassan, N.M.; Badr, K.F. Microvasular and Macrovascular Diseases of the Kidney. In Brenner and Rector’s The kidney; Brenner, B.M., Ed.; Saunders Elsevier: Philadelphia, PA, USA, 2007; pp. 1147–1173. [Google Scholar]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic profile of Shiga-toxin-produsing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef]
- Clark, W.F. Thrombotic microangiopathy: Current knowledge and outcomes with plasma exchange. Semin. Dial. 2012, 25, 214–219. [Google Scholar] [CrossRef]
- George, J.N.; Terrell, D.R.; Vesely, S.K.; Kremer Hovinga, J.A.; Lämmle, B. Thrombotic microangiopathic syndromes associated with drugs, HIV infection, hematopoietic stem cell transplantation and cancer. Presse Med. 2012, 41, e177–e188. [Google Scholar]
- Loirat, C.; Frémeaux-Bacchi, V. Atypical hemolytic uremic syndrome. Orphanet J. Rare Dis. 2011, 6, 60. [Google Scholar] [CrossRef]
- De Goicoechiea Jorge, E.; Harris, C.L.; Esparza-Gordillo, J.; Carreras, L.; Arranz, E.A.; Garrido, C.A.; López-Trascasa, M.; Sánchez-Corral, P.; Morgan, B.P.; de Rodríguez Córdobam, S. Gain-of function mutaions in complement factor B are associated with atypical hemolytic uremic symdrome. Proc. Natl. Acad. Sci. USA 2007, 104, 240–245. [Google Scholar]
- Rock, G.A.; Shumak, K.H.; Buskard, N.A.; Blanchette, V.S.; Kelton, J.G.; Nair, R.C.; Spasoff, R.A. The Canadian Apheresis Study Group. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenia purpura. N. Engl. J. Med. 1991, 325, 393–397. [Google Scholar]
- Kelly, R.J.; Hill, A.; Arnold, L.M.; Brooksbank, G.L.; Richards, S.J.; Cullen, M.; Mitchell, L.D.; Cohen, D.R.; Gregory, W.M.; Hillmen, P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: Sustained efficacy and improved survival. Blood 2011, 117, 6786–6792. [Google Scholar] [CrossRef]
- Lapeyraque, A.L.; Frémeaux-Bacchi, V.; Robitaille, P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr. Nephrol. 2011, 26, 621–624. [Google Scholar] [CrossRef]
- Caprioli, J.; Noris, M.; Brioschi, S.; Pianetti, G.; Castelletti, F.; Bettinaglio, P.; Mele, C.; Bresin, E.; Cassis, L.; Gamba, S.; et al. Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 2006, 108, 1267–1279. [Google Scholar]
- Loirat, C.; Fremeaux-Bacchi, V. Hemolytic uremic syndrome recurrence after renal transplantation. Pediatr. Transplant. 2008, 12, 619–629. [Google Scholar] [CrossRef]
- Al-Akash, S.I.; Almond, P.S.; Savell, V.J.; Gharaybeh, S.I.; Hogue, C. Eculizumab induces long-term remission in recurrent post-transplant HUS associated with C3 gene mutation. Pediatr. Nephrol. 2011, 26, 613–619. [Google Scholar] [CrossRef]
- Hodgkins, K.S.; Bobrowski, A.E.; Lane, J.C.; Langman, C.B. Clinical grand rounds: Atypical hemolytic uremic syndrome. Am. J. Nephrol. 2012, 35, 394–400. [Google Scholar] [CrossRef]
- Mizuno, M.; Morgan, B.P. The possibilities and pitfalls for anti-complement therapies in inflammatory diseases. Curr. Drug Targets Inflamm. Allergy 2004, 3, 85–94. [Google Scholar]
- Mizuno, M.; Morgan, B.P. An update on the roles of the complement system in autoimmune diseases and the therapeutic possibilities of anti-complement agents. Curr. Drug Ther. 2011, 6, 35–50. [Google Scholar] [CrossRef]
- Mizuno, M. A review of current knowledge of the complement system and the therapeutic opportunities in inflammatory arthritis. Curr. Med. Chem. 2006, 13, 1707–1717. [Google Scholar] [CrossRef]
- Luzzatto, L.; Gianfaldoni, G. Recent advances in biological and clinical aspects of paroxysmal nocturnal hemoglobinuria. Int. J. Hematol. 2006, 84, 104–112. [Google Scholar] [CrossRef]
- Bowen, T.; Cicardi, M.; Bork, K.; Zuraw, B.; Frank, M.; Ritchie, B.; Farkas, H.; Varga, L.; Zingale, L.C.; Binkley, K.; et al. Hereditary angiodema: A current state-of-the-art review, VII: Canadian Hungarian 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of Hereditary Angioedema. Ann. Allergy Asthma Immunol. 2008, 100, S30–S40. [Google Scholar] [CrossRef]
- Samples Availability: Limitedly available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mizuno, M.; Ito, Y.; Morgan, B.P. Exploiting the Nephrotoxic Effects of Venom from the Sea Anemone, Phyllodiscus semoni, to Create a Hemolytic Uremic Syndrome Model in the Rat. Mar. Drugs 2012, 10, 1582-1604. https://doi.org/10.3390/md10071582
Mizuno M, Ito Y, Morgan BP. Exploiting the Nephrotoxic Effects of Venom from the Sea Anemone, Phyllodiscus semoni, to Create a Hemolytic Uremic Syndrome Model in the Rat. Marine Drugs. 2012; 10(7):1582-1604. https://doi.org/10.3390/md10071582
Chicago/Turabian StyleMizuno, Masashi, Yasuhiko Ito, and B. Paul Morgan. 2012. "Exploiting the Nephrotoxic Effects of Venom from the Sea Anemone, Phyllodiscus semoni, to Create a Hemolytic Uremic Syndrome Model in the Rat" Marine Drugs 10, no. 7: 1582-1604. https://doi.org/10.3390/md10071582
APA StyleMizuno, M., Ito, Y., & Morgan, B. P. (2012). Exploiting the Nephrotoxic Effects of Venom from the Sea Anemone, Phyllodiscus semoni, to Create a Hemolytic Uremic Syndrome Model in the Rat. Marine Drugs, 10(7), 1582-1604. https://doi.org/10.3390/md10071582





