Vitamin D-Loaded Chitosan Nanostructures for Bone Regeneration: A Combined In Vitro and In Vivo Evaluation in an Osteoporotic Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CS-Based NS
2.3. In Vitro Characterization of the NS
Structural Analysis
2.4. Biological Evaluation
2.4.1. Cell Culture
2.4.2. Cytotoxicity Assay (MTT)
2.4.3. Cell Viability (Alamar Blue)
2.4.4. Mineralization Assay (Alizarin Red Staining)
2.4.5. Vitamin D3 Release Assay
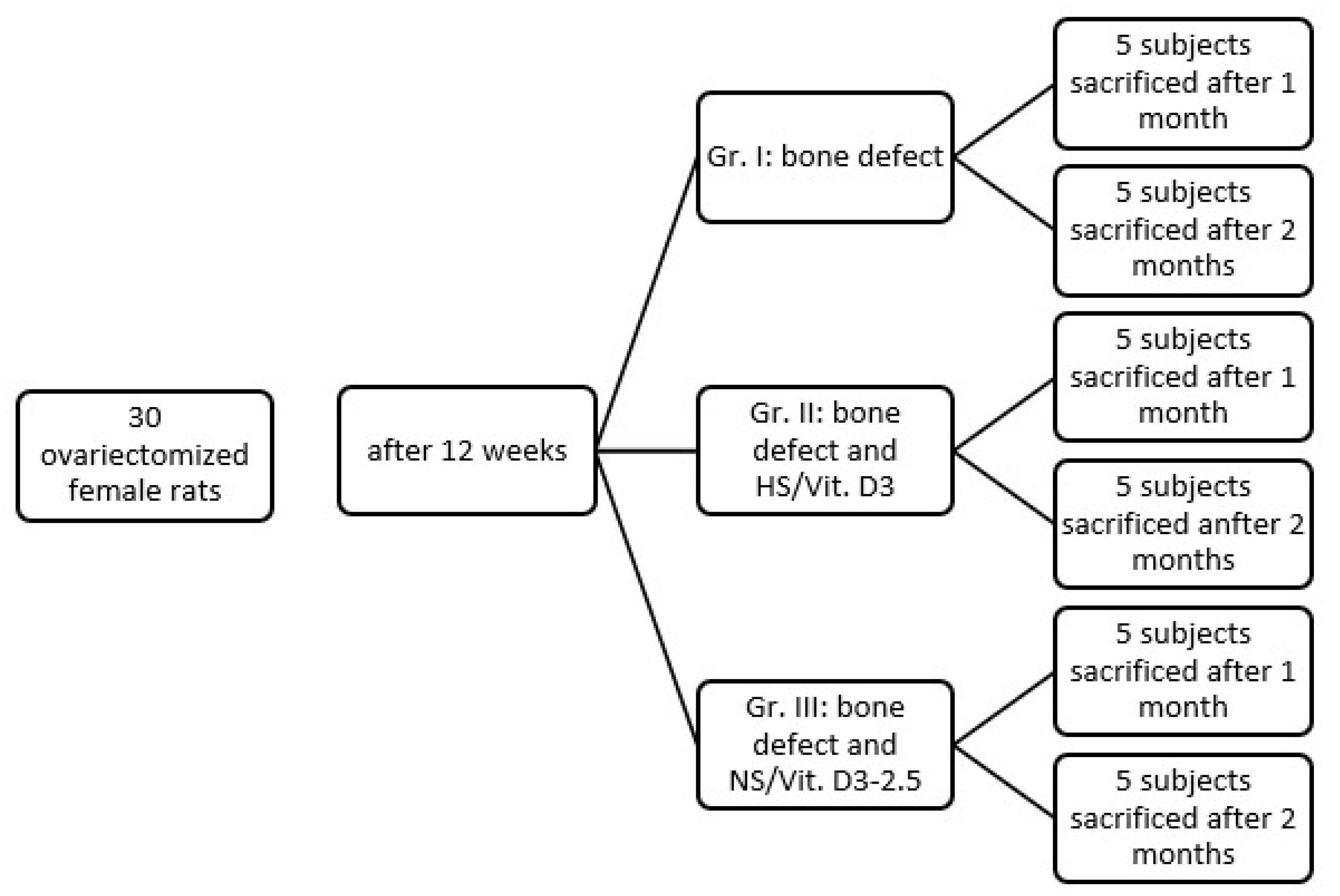
2.5. In Vivo Experimental Design
2.5.1. Ethical Statement and Animal Housing
2.5.2. Study Groups
- Group I (Control): Ovariectomy (OVX) + Bone Defect + No Treatment (Clot only).
- Group II (HS/Vit. D3): OVX + Bone Defect + Hemostatic Sponge soaked in 6.25 ng/mL Vit. D3 (the value of 6.25 ng/mL represents the equivalent of 2.5 µL).
- Group III (NS/Vit. D3-2.5): OVX + Bone Defect + NS/Vit. D3-6.25 ng/mL.
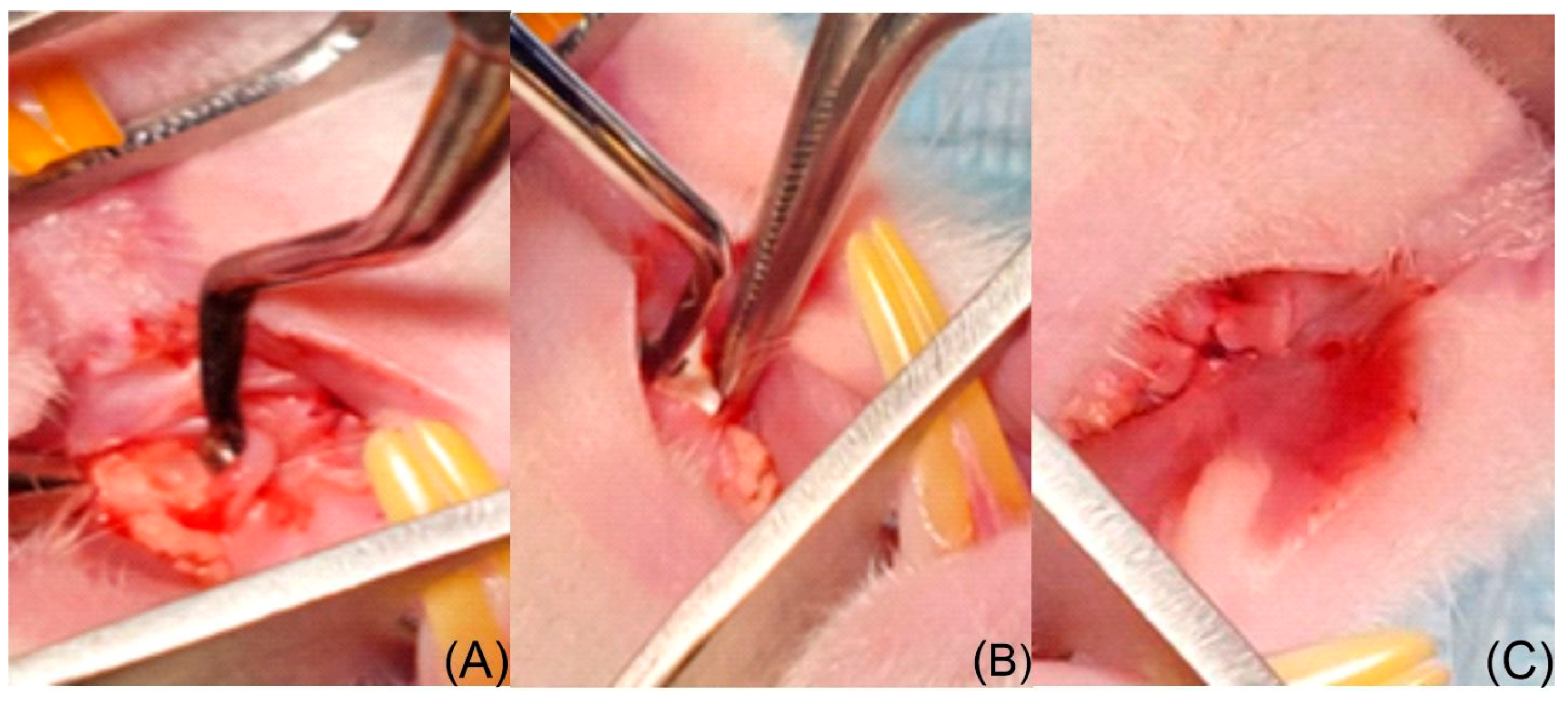
2.5.3. Surgical Protocol
2.5.4. Humane Endpoints and Euthanasia
2.6. Histological and Morphometric Analysis
2.6.1. Sample Processing
2.6.2. Microscopic Assessment
2.6.3. Morphometry and Histological Scoring
- Bone Architecture: Presence of osteoporosis, bone bridging, trabecular thickness, and the ratio of immature to mature bone.
- Cellular Response: Presence and localization of osteoblasts, osteocytes, osteoclasts, and vascularization.
- Graft Interaction: Inflammation, presence of granulation tissue, graft degradation, and replacement by new bone.
2.7. Statistical Analysis
3. Results
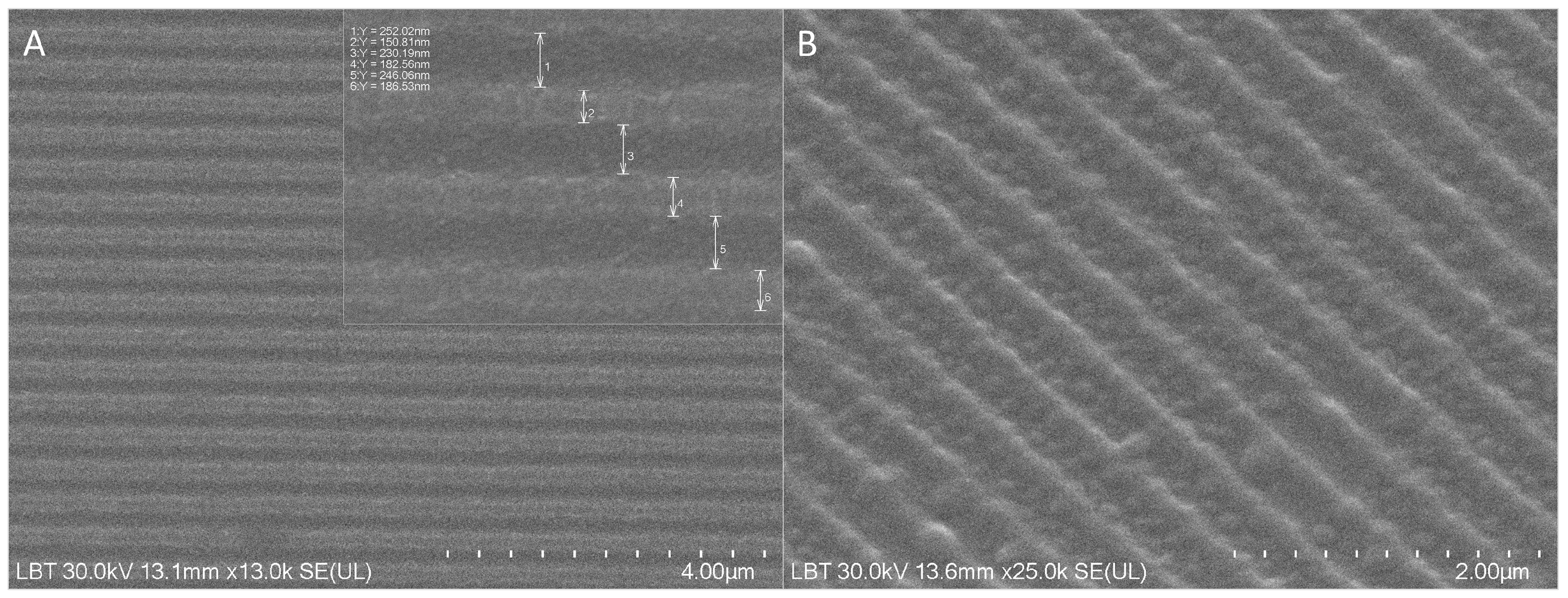
3.1. Structural Characterization of the NS
3.2. In Vitro Biological Assessment
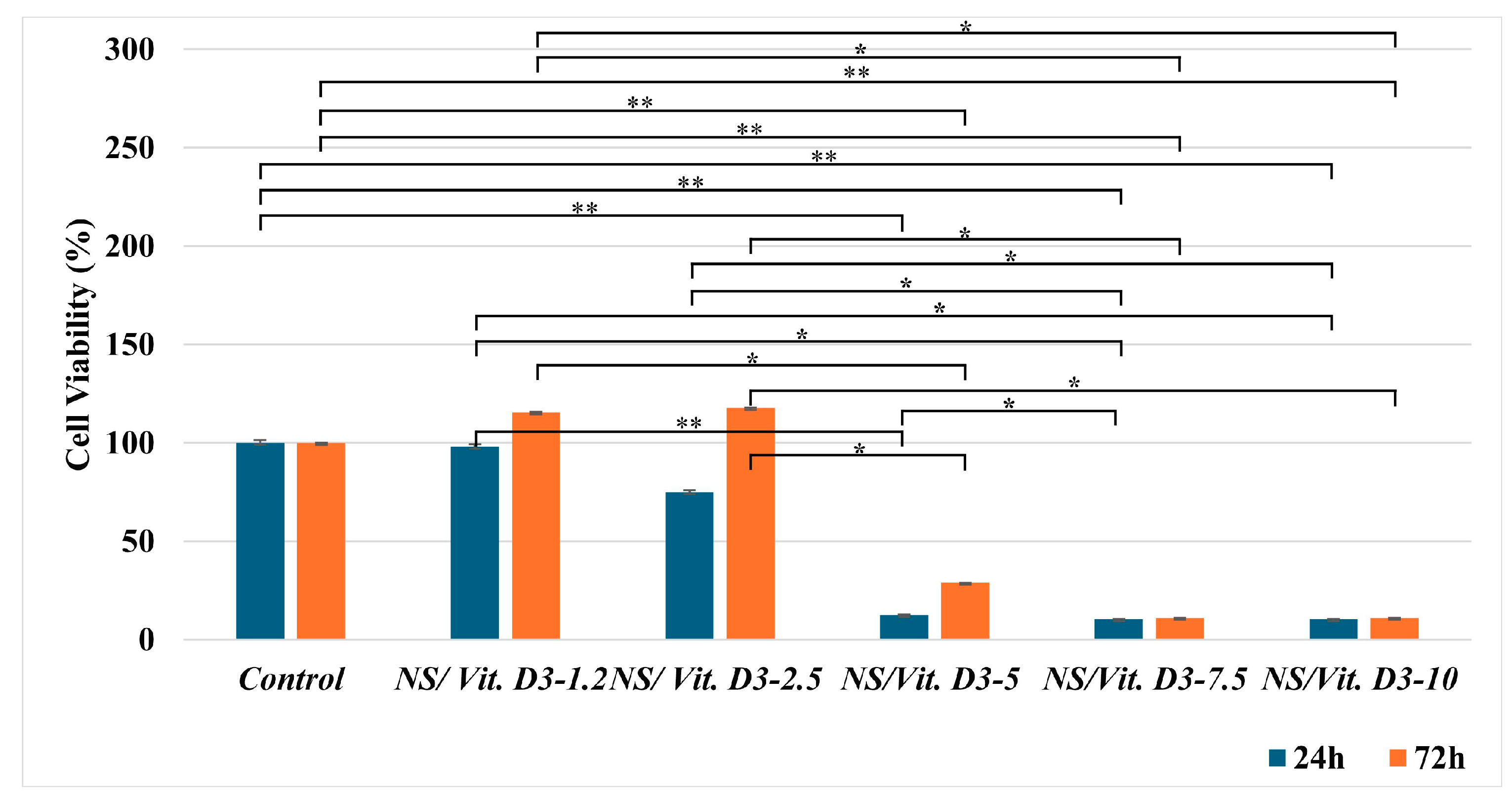
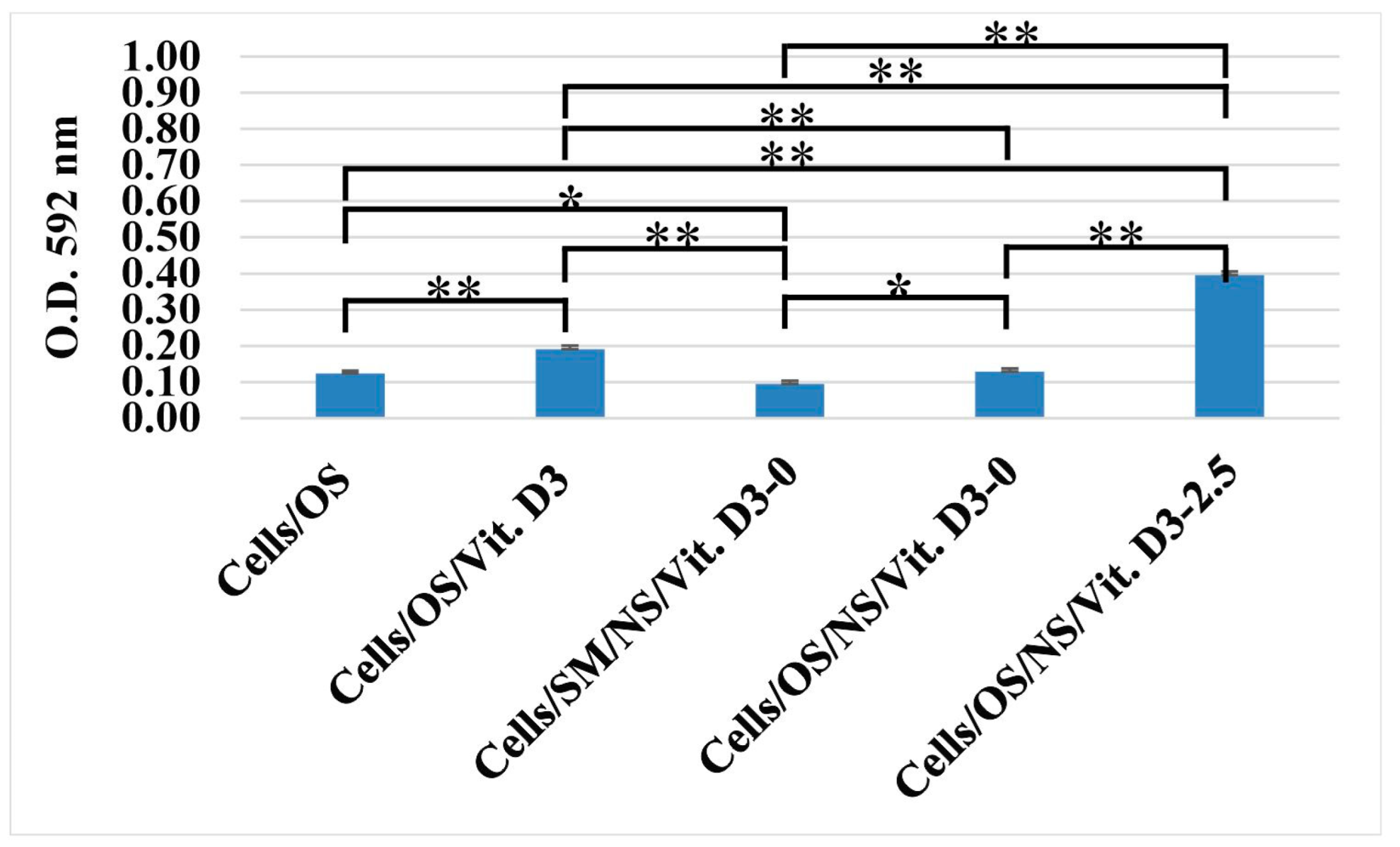
3.2.1. Cell Viability
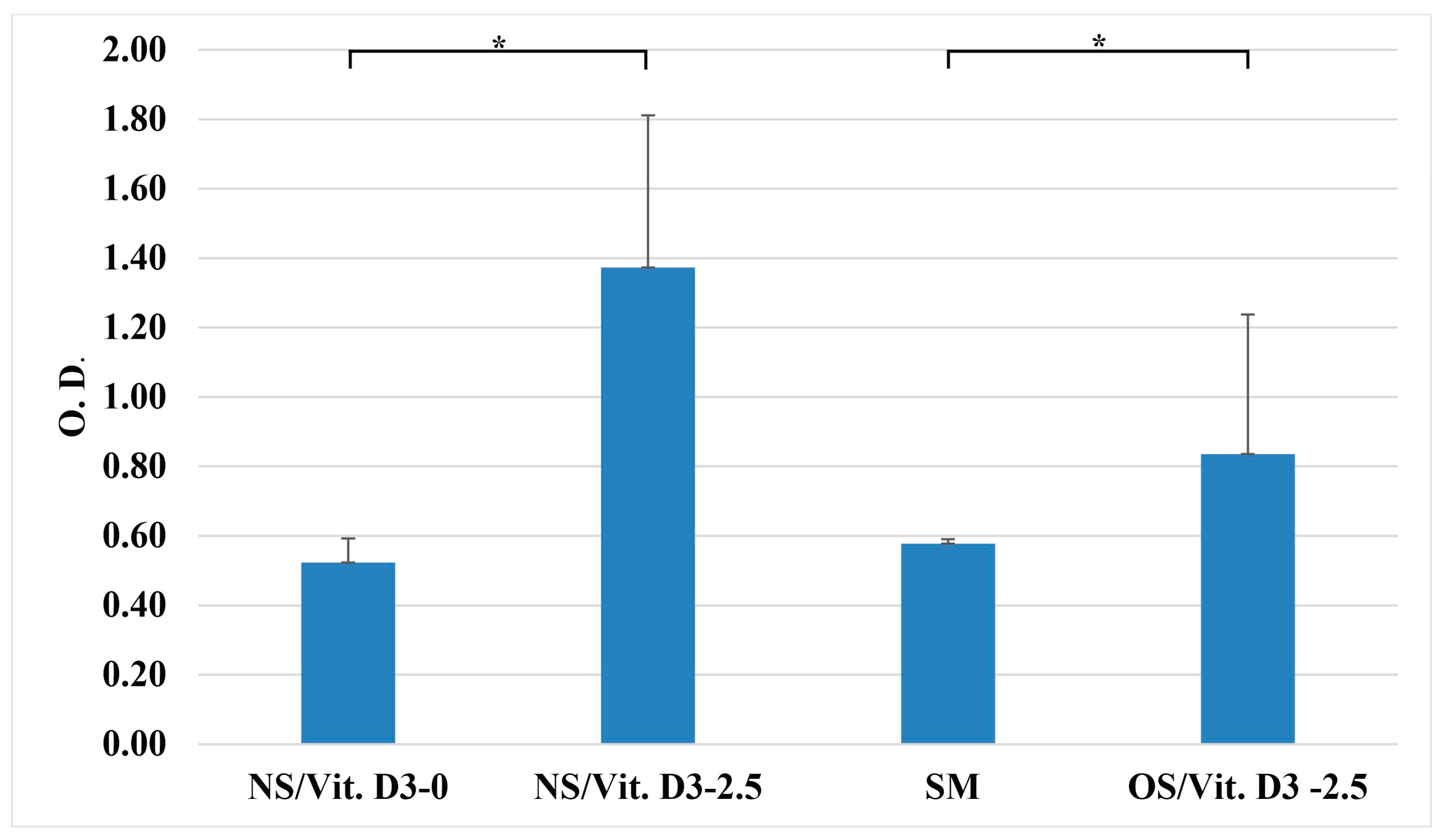
3.2.2. Cell Proliferation (Alamar Blue)
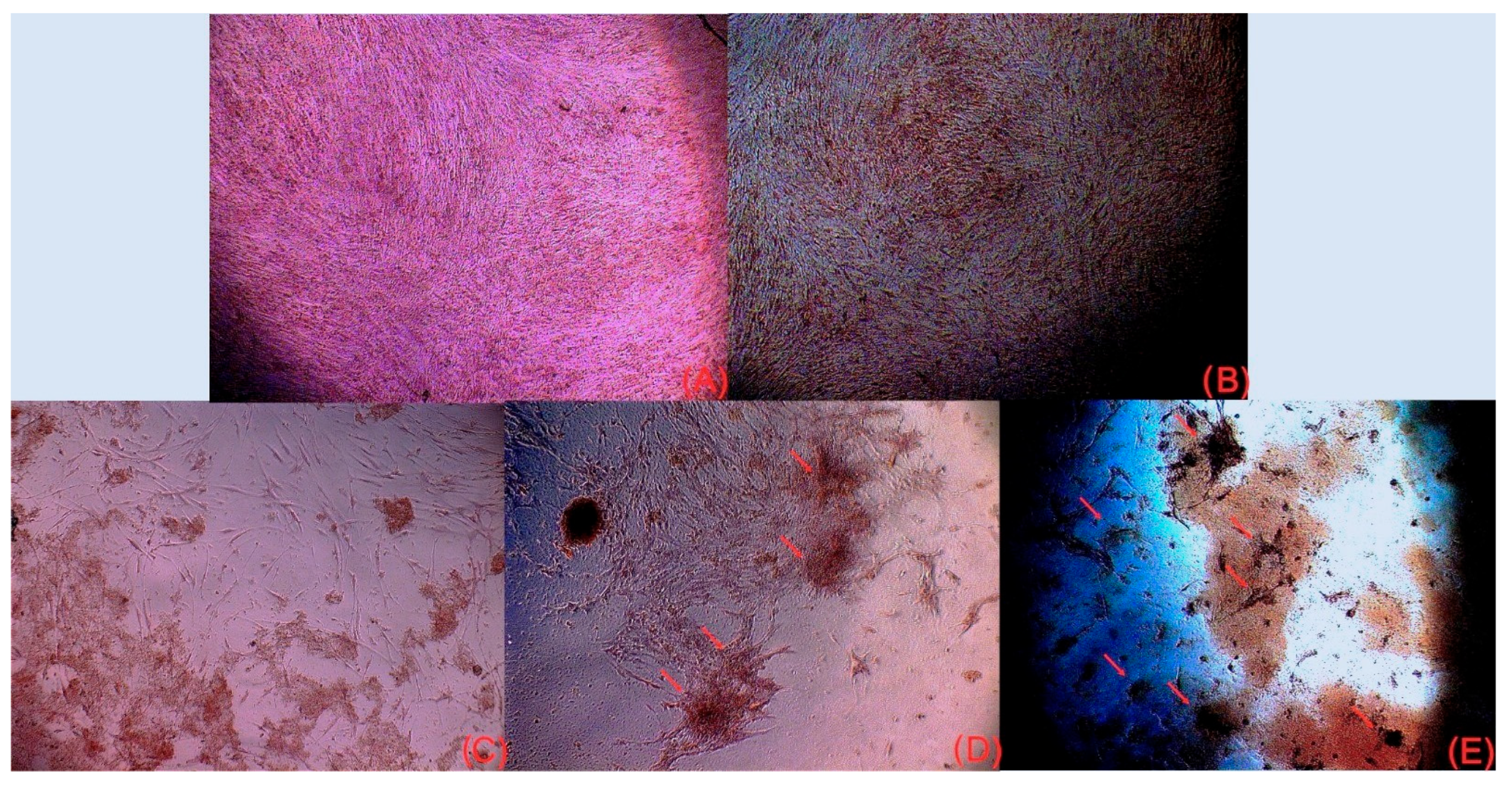
3.2.3. Osteogenic Differentiation (Alizarin Red Staining)
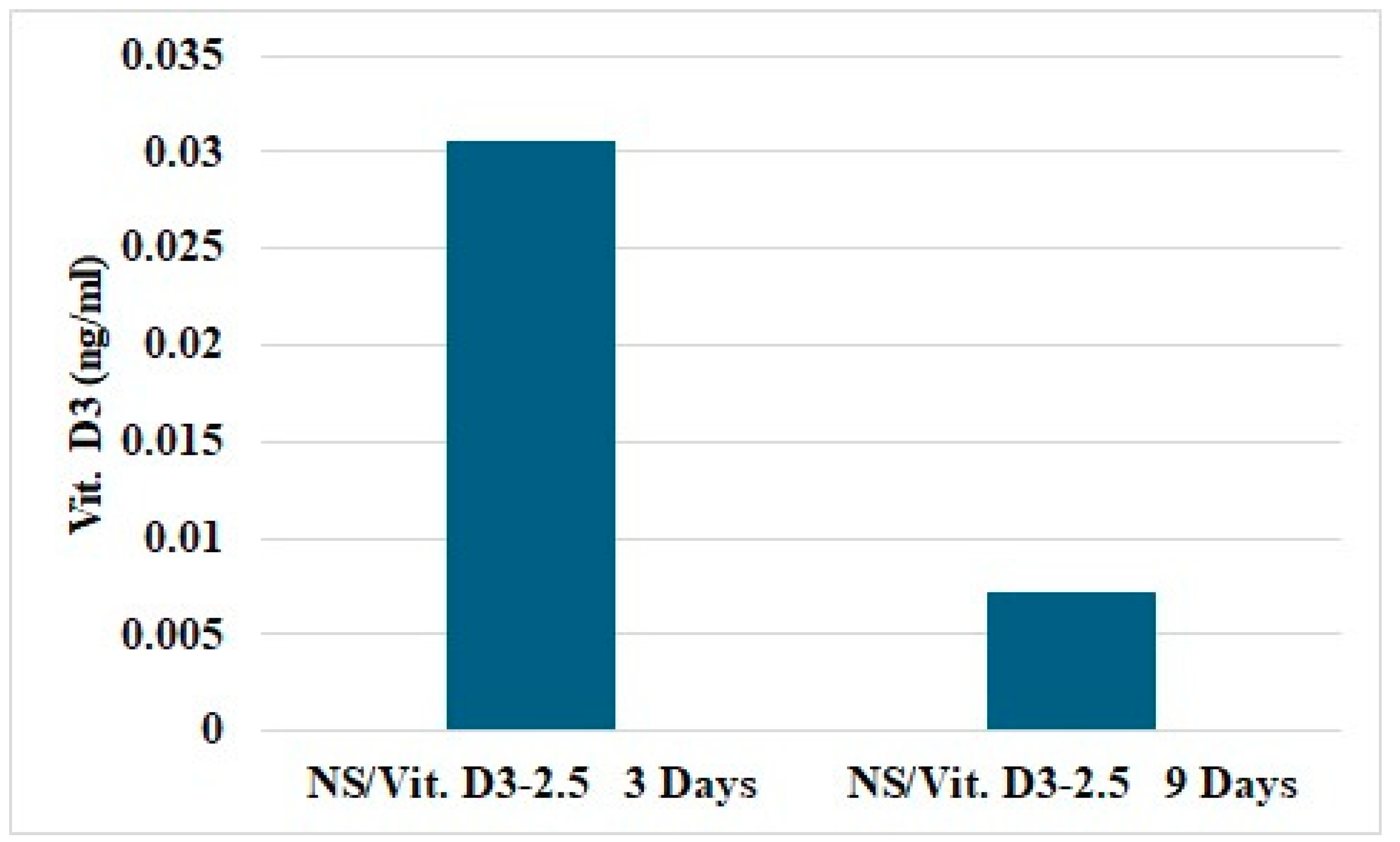
3.2.4. Vitamin D3 Release
3.3. In Vivo Evaluation
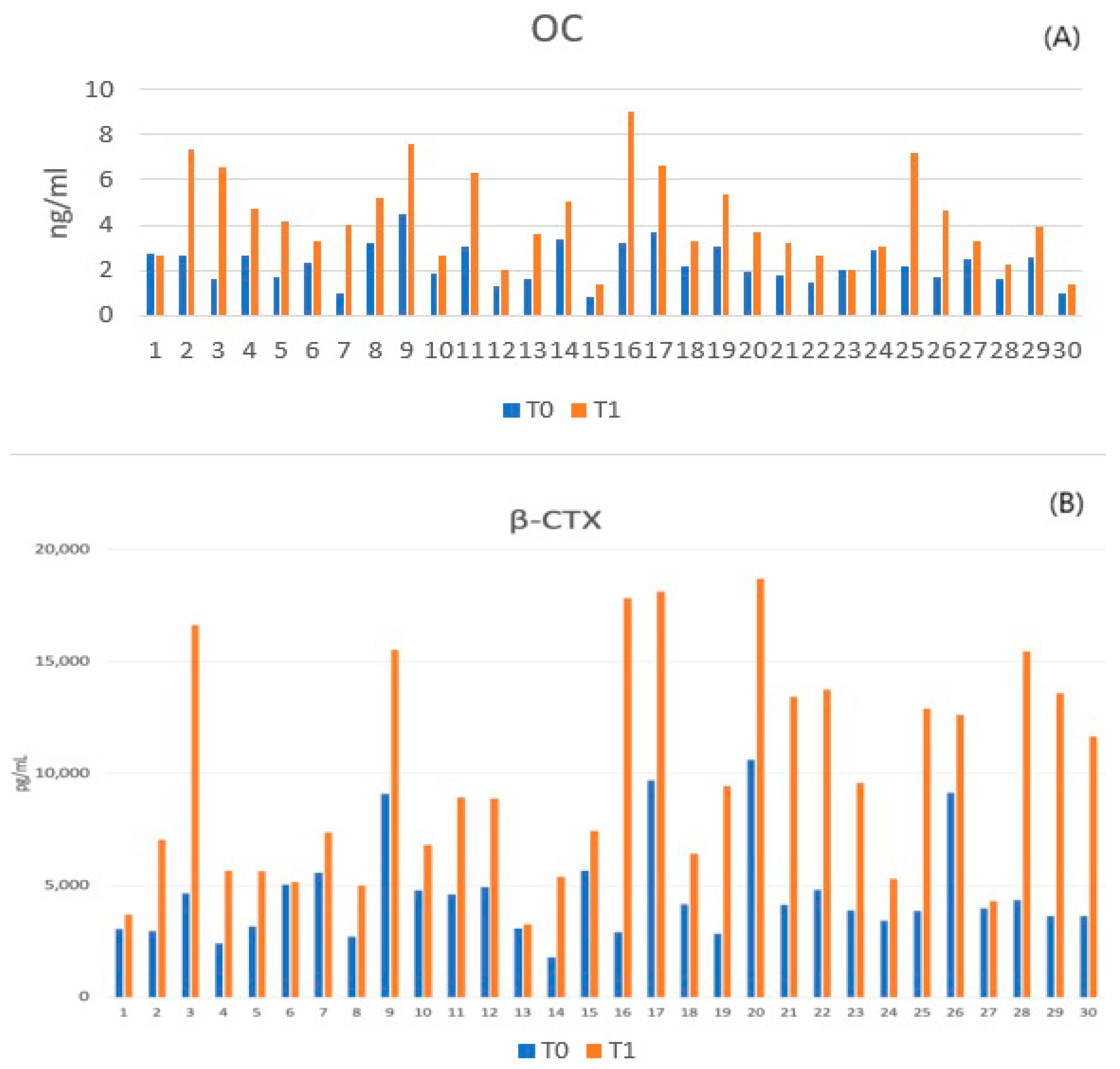
3.3.1. Assessment of Bone Turnover Biomarkers
3.3.2. Clinical Observations and Model Validation
3.3.3. Macroscopic Evaluation and Defect Morphometry
- Group I (Control): 0.34 ± 0.19 mm2;
- Group II (HS/Vit. D3): 0.28 ± 0.13 mm2;
- Group III (NS/Vit. D3-2.5): 0.21 ± 0.10 mm2.
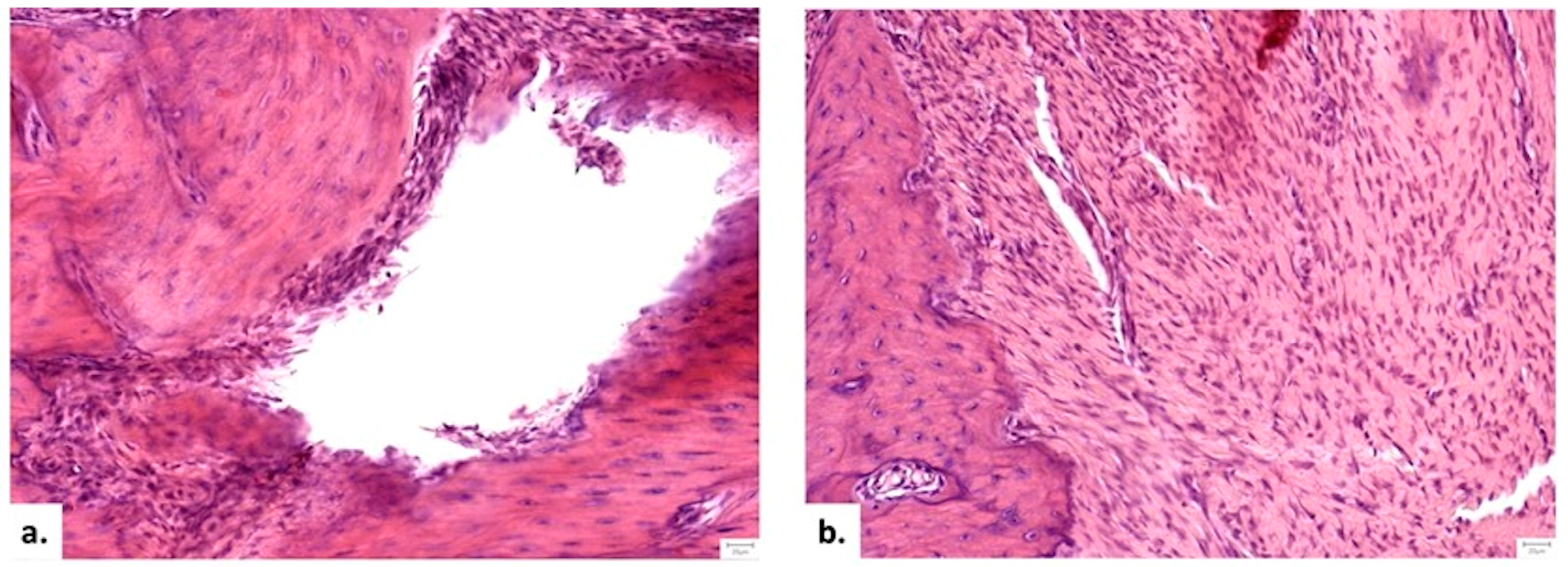
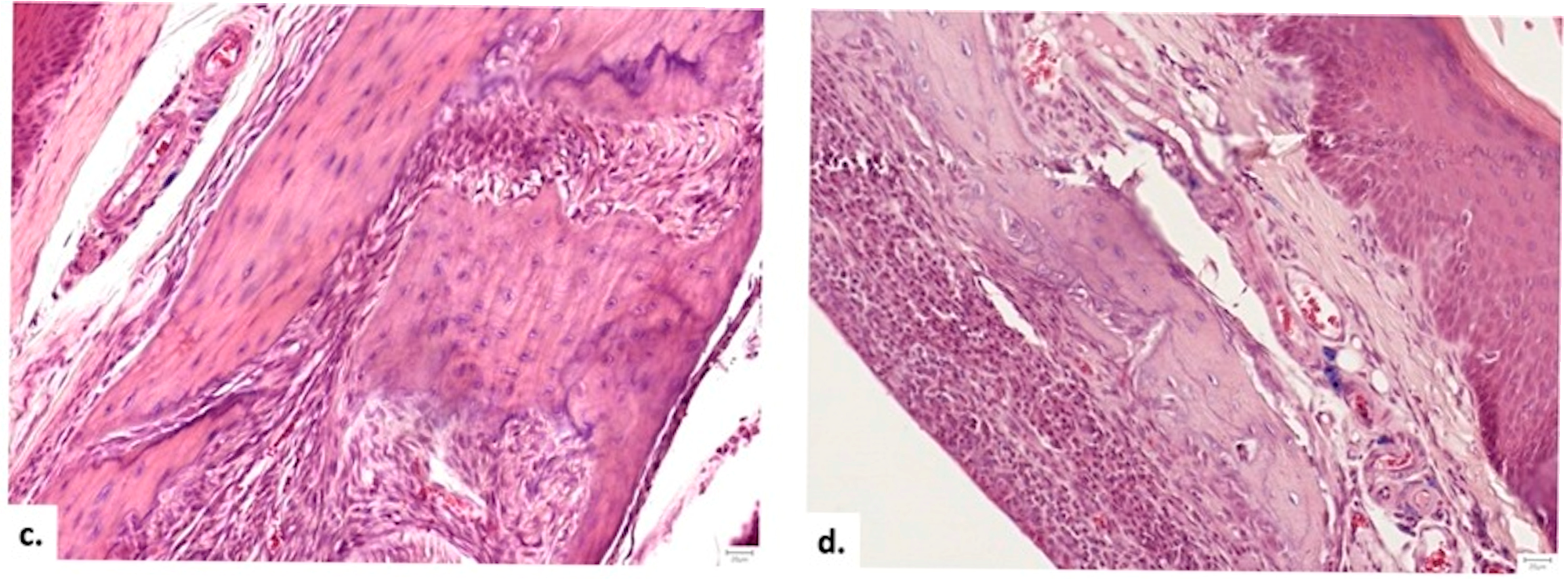
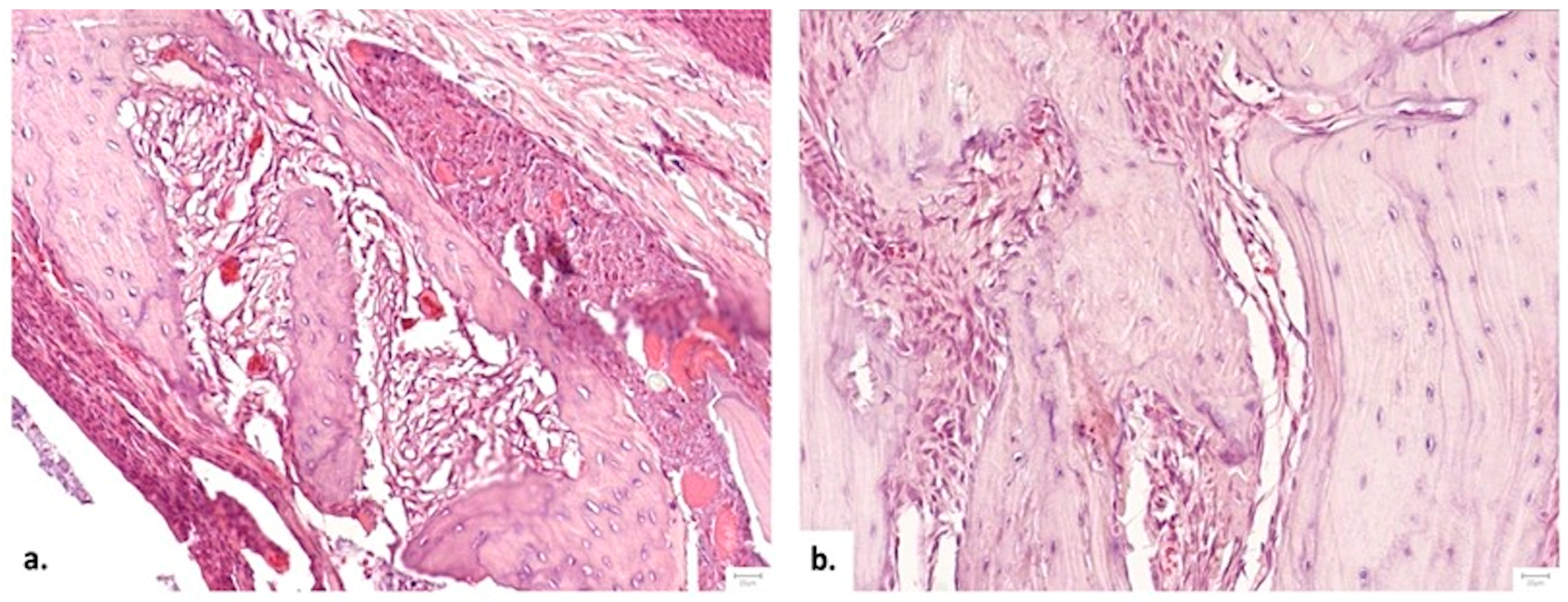
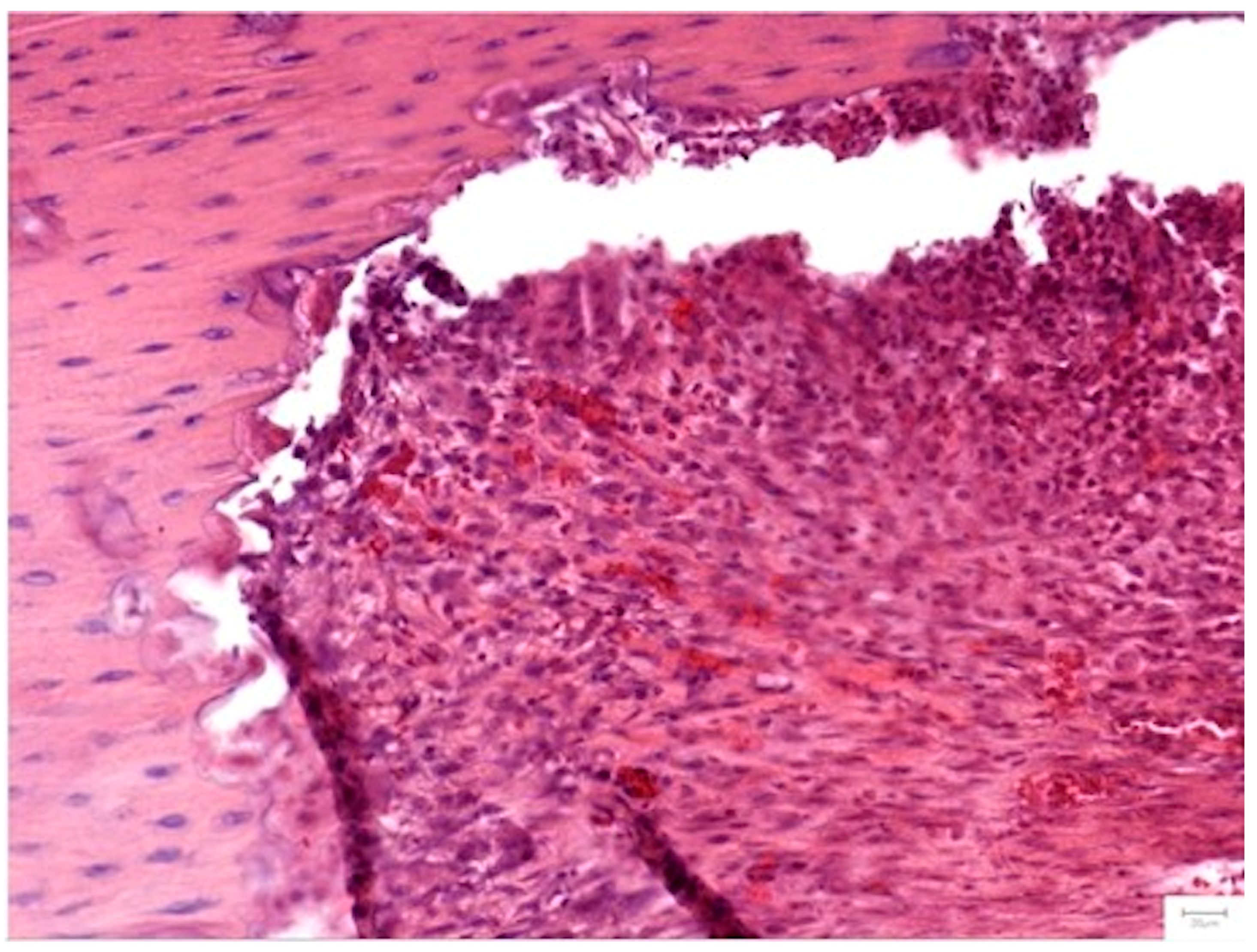
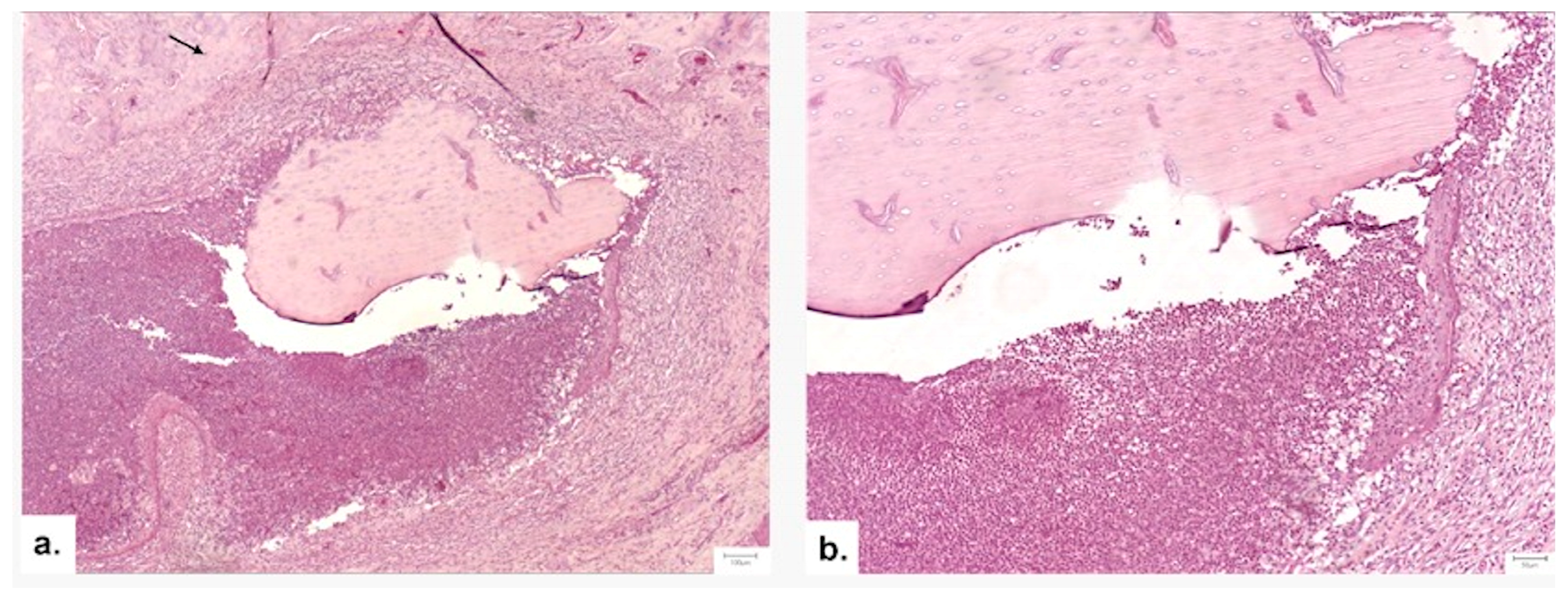
3.3.4. Histopathological Analysis
Local Bone Architecture and Mineralization
Defect Bridging and Healing Patterns
Cellular Response and Vascularization
Graft Integration and Safety
4. Discussion
4.1. In Vitro Performance
4.2. In Vivo Performance in the Osteoporotic Model
4.3. Comparative Efficacy and Mechanisms
4.4. Clinical Implications and Future Directions
4.5. Limitations and Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β-CTX | Beta-Crosslaps (C-terminal telopeptide of type I collagen) |
| ANOVA | Analysis of Variance |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BMD | Bone Mineral Density |
| BTMs | Bone Turnover Biomarkers |
| CFEG-STEM | Cold Field Emission Scanning Transmission Electron Microscope |
| CS | Chitosan |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| DXA | Dual-Energy X-ray Absorptiometry |
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic Acid |
| FBS | Fetal Bovine Serum |
| H&E | Hematoxylin and Eosin |
| HS | Hemostatic Sponge |
| ISO | International Organization for Standardization |
| MSCs | Mesenchymal Stem Cells |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NEA | Non-Essential Amino Acids |
| NS | Nanostructured Scaffold |
| OC | Osteocalcin |
| OS | Osteogenic Medium |
| OVX | Ovariectomy |
| PBS | Phosphate-Buffered Saline |
| PDMS | Polydimethylsiloxane |
| PLGA | Polylactic-co-glycolic Acid |
| Pt/Pd | Platinum/Palladium |
| SD | Standard Deviation |
| SEM | Scanning Electron Microscopy |
| SM | Standard Medium |
| TCP | Tissue Culture Plastic |
| UV | Ultraviolet |
| Vit. D/Vit. D3 | Vitamin D/Vitamin D3 (Cholecalciferol) |
References
- Pagni, G.; Pellegrini, G.; Giannobile, W.V.; Rasperini, G. Postextraction Alveolar Ridge Preservation: Biological Basis and Treatments. Int. J. Dent. 2012, 2012, 151030. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Kim, S.; Kim, S.G. Advancements in alveolar bone grafting and ridge preservation: A narrative review on materials, techniques, and clinical outcomes. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 14. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Chen, C.-H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [CrossRef]
- Kuroshima, S.; Kaku, M.; Ishimoto, T.; Sasaki, M.; Nakano, T.; Sawase, T. A paradigm shift for bone quality in dentistry: A literature review. J. Prosthodont. Res. 2017, 61, 353–362. [Google Scholar] [CrossRef]
- Park, G.; Jalkh, E.B.B.; Boczar, D.; Bergamo, E.T.P.; Kim, H.; Kurgansky, G.; Torroni, A.; Gil, L.F.; Bonfante, E.A.; Coelho, P.G.; et al. Bone regeneration at extraction sockets filled with leukocyte-platelet-rich fibrin: An experimental pre-clinical study. Med. Oral Patol. Oral Y Cir. Bucal 2022, 27, E468–E475. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Ye, J.; Liang, R.; Yao, X.; Wu, X.; Koh, Y.; Wei, W.; Zhang, X.; Ouyang, H. Advanced Strategies of Biomimetic Tissue-Engineered Grafts for Bone Regeneration. Adv. Healthc. Mater. 2021, 10, 2100408. [Google Scholar] [CrossRef]
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Sub-Cell. Biochem. 2019, 91, 453–476. [Google Scholar] [CrossRef]
- Avenell, A.; Mak, J.C.; O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014, 2014, CD000227. [Google Scholar] [CrossRef]
- Targonska, S.; Dominiak, S.; Wiglusz, R.J.; Dominiak, M. Investigation of Different Types of Micro- and Nanostructured Materials for Bone Grafting Application. Nanomaterials 2022, 12, 3752. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Liu, Z.; Mori, T.; He, Z.; Yasuda, H.; Takahashi, N.; Udagawa, N. The vitamin D receptor in osteoblastic cells but not secreted parathyroid hormone is crucial for soft tissue calcification induced by the proresorptive activity of 1,25(OH)(2)D(3). J. Steroid Biochem. Mol. Biol. 2023, 232, 106351. [Google Scholar] [CrossRef]
- Ye, S.; Wen, J.; Ye, W.-H.; Li, Z.; Huang, X.; Chen, S.; Ma, J.-C.; Wu, Y.; Chen, R.; Cui, Z.-K. A facile and smart strategy to enhance bone regeneration with efficient vitamin D3 delivery through sterosome technology. J. Control. Release 2024, 370, 140–151. [Google Scholar] [CrossRef]
- Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Nah, H.; Lee, D.; Heo, M.; Lee, J.S.; Lee, S.J.; Heo, D.N.; Seong, J.; Lim, H.-N.; Lee, Y.-H.; Moon, H.-J.; et al. Vitamin D-conjugated gold nanoparticles as functional carriers to enhancing osteogenic differentiation. Sci. Technol. Adv. Mater. 2019, 20, 826–836. [Google Scholar] [CrossRef]
- Liu, H.; Cui, J.; Feng, W.; Lv, S.; Du, J.; Sun, J.; Han, X.; Wang, Z.; Lu, X.; Yi, M.; et al. Local administration of calcitriol positively influences bone remodeling and maturation during restoration of mandibular bone defects in rats. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 49, 14–24. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Wang, Q.; Xiao, Y.; Chen, X.M.; Li, W. Attenuation of inflammatory response by 25- hydroxyvitamin D3-loaded polylactic acid microspheres in treatment of periodontitis in diabetic rats. Chin. J. Dent. Res. 2014, 17, 91–98. [Google Scholar]
- Subramaniam, D.; Sekaran, S. In Vitro Biocompatibility Assessment of a Novel Membrane Containing Magnesium-Chitosan/Carboxymethyl Cellulose and Alginate Intended for Bone Tissue Regeneration. Cureus J. Med. Sci. 2024, 16, e54597. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ye, Y.; Wu, J.; Ma, Y.; Fang, Y.; Jiang, F.; Yu, J. Phosphoserine-loaded chitosan membranes promote bone regeneration by activating endogenous stem cells. Front. Bioeng. Biotechnol. 2023, 11, 1096532. [Google Scholar] [CrossRef] [PubMed]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A 2011, 17, 2133–2141. [Google Scholar] [CrossRef]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bunger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, O.; Sorițău, O.; Gheban, D.; Ciuca, D.R.; Virtic, O.; Vulpoi, A.; Dirzu, N.; Câmpian, R.; Băciuț, G.; Popa, C.; et al. Dental follicle stem cells in bone regeneration on titanium implants. BMC Biotechnol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009; pp. 1–34. [Google Scholar]
- Solchaga, L.; Yoo, J.; Lundberg, M.; Dennis, J.; Huibregtse, B.; Goldberg, V.; Caplan, A. Hyaluronan-based polymers in the treatment of osteochondral defects. J. Orthop. Res. 2000, 18, 773–780. [Google Scholar] [CrossRef]
- Lucaciu, O.; Gheban, D.; Soritau, O.; Baciut, M.; Campian, R.S.; Baciut, G. Comparative assessment of bone regeneration by histometry and a histological scoring system. Rev. Romana Med. Lab. 2015, 23, 31–45. [Google Scholar] [CrossRef]
- Stats Kingdom. Shapiro-Wilk Test Calculator. Available online: https://www.statskingdom.com/shapiro-wilk-test-calculator.html (accessed on 22 November 2025).
- Rakusa, Z.T.; Pislar, M.; Kristl, A.; Roskar, R. Comprehensive Stability Study of Vitamin D3 in Aqueous Solutions and Liquid Commercial Products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef]
- Hong, H.H.; Yen, T.H.; Hong, A.; Chou, T.A. Association of vitamin D3 with alveolar bone regeneration in dogs. J. Cell. Mol. Med. 2015, 19, 1208–1217. [Google Scholar] [CrossRef]
- Pereira, B.C.; Sacramento, C.M.; Sallum, E.A.; Monteiro, M.F.; Casarin, R.C.V.; Casati, M.Z.; Silverio, K.G. 1,25(OH)2D3 increase osteogenic potential of human periodontal ligament cells with low osteoblast potential. J. Appl. Oral. Sci. 2024, 32, e20240160. [Google Scholar] [CrossRef]
- Kwiatek, J.; Jaron, A.; Trybek, G. Impact of the 25-Hydroxycholecalciferol Concentration and Vitamin D Deficiency Treatment on Changes in the Bone Level at the Implant Site during the Process of Osseointegration: A Prospective, Randomized, Controlled Clinical Trial. J. Clin. Med. 2021, 10, 526. [Google Scholar] [CrossRef]
- Salomo-Coll, O.; Mate-Sanchez de Val, J.E.; Ramirez-Fernandez, M.P.; Hernandez-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Topical applications of vitamin D on implant surface for bone-to-implant contact enhance: A pilot study in dogs part II. Clin. Oral Implant. Res. 2016, 27, 896–903. [Google Scholar] [CrossRef]
- Fuegl, A.; Gruber, R.; Agis, H.; Lzicar, H.; Keibl, C.; Schwarze, U.Y.; Dvorak, G. Alveolar bone regeneration in response to local application of calcitriol in vitamin D deficient rats. J. Clin. Periodontol. 2015, 42, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B-Rev 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Lalzawmliana, V.; Anand, A.; Roy, M.; Kundu, B.; Nandi, S.K. Mesoporous bioactive glasses for bone healing and biomolecules delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 106, 110180. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, S.; Chen, A.; Zhao, M.; Zhang, X.; Sheng, L.; Zhang, C.; Wu, Z. A poly(ether-ketone-ketone) composite scaffold simulating the immune-osteogenic cascade for in situ bone regeneration. J. Mater. Chem. B 2025, 13, 4641–4656. [Google Scholar] [CrossRef]
- Jo, Y.K.; Choi, B.-H.; Kim, C.S.; Cha, H.J. Diatom-Inspired Silica Nanostructure Coatings with Controllable Microroughness Using an Engineered Mussel Protein Glue to Accelerate Bone Growth on Titanium-Based Implants. Adv. Mater. 2017, 29, 1704906. [Google Scholar] [CrossRef]
- Susana Rodriguez, M.; Monteroa, M.; Dello Staffolo, M.; Martino, M.; Bevilacqua, A.; Albertengo, L. Chitosan influence on glucose and calcium availability from yogurt: In vitro comparative study with plants fibre. Carbohydr. Polym. 2008, 74, 797–801. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Li, Y.; Selvaraj, V.; Saravanan, S.; Abullais, S.S.; Wankhade, V. Exploring the osteogenic potential of chitosan-quercetin bio-conjugate: In vitro and in vivo investigations in osteoporosis models. Int. J. Biol. Macromol. 2024, 274, 133492. [Google Scholar] [CrossRef]
- Thanachotkullapat, P.; Sukyai, P.; Li, Y.-C.E.; Morakul, S.; Torgbo, S. Fabrication, physicochemical characterization and in vitro evaluation of pre-osteoblast cells on bacterial cellulose/hydroxyapatite reinforced with chitosan composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2025, 334, 148779. [Google Scholar] [CrossRef]
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2017, 105, 966–976. [Google Scholar] [CrossRef]
- Mirica, I.C.; Furtos, G.; Fontaine, V.; Vlassa, M.; Pascuta, P.; Petean, I.; Baldea, B.; Andercou, O.; Lucaciu, O.P. Electrospun Polycaprolactone Membranes Loaded with Gentamicin and Nano-Hidroxyapatite for Guided Bone Regeneration. Biomedicines 2025, 13, 2349. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Mirica, I.-C.; Furtos, G.; Moldovan, M.; Prodan, D.; Petean, I.; Campian, R.-S.; Pall, E.; Lucaciu, O. Morphology, Cytotoxicity, and Antimicrobial Activity of Electrospun Polycaprolactone Biomembranes with Gentamicin and Nano-Hydroxyapatite. Membranes 2024, 14, 10. [Google Scholar] [CrossRef]
- Sundar, R.; Rai, A.B.; Kumar, J.N.; Divakar, D.D. The role of Vitamin D as an adjunct for bone regeneration: A systematic review of literature. Saudi Dent. J. 2023, 35, 220–232. [Google Scholar] [CrossRef]
- Fang, L.; Gong, J.; Liu, X.; Bao, W. Mucus dressing scaffolded by chitosan nanofibers with seamless adherence on wound bed and high utility of berberine chloride. Int. J. Biol. Macromol. 2025, 332, 148602. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Bahraminasab, M.; Nourbakhsh, M.S. Enhanced bone tissue engineering by 3D-printed host-guest polycaprolactone scaffolds stuffed with chitosan/laponite. Int. J. Biol. Macromol. 2025, 330, 148295. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.; van Leeuwen, J.P.T.M. Vitamin D endocrinology of bone mineralization. Mol. Cell. Endocrinol. 2017, 453, 46–51. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Classic, K.L.; Dhert, W.J.; Yaszemski, M.J. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials 2008, 29, 3245–3252. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Hu, X.; Chen, M.; Yang, H.; Wei, H.; Zhou, B.; Li, M.; Luo, Z.; Cai, K.; Hu, Y. Cascade Biomineralization of Geometrically Tuned Osteon-Mimetic Composite Biovesicle-Hydrogel Coating Improves Ti Implant-Assisted Repair of Osteoporotic Bone Fractures. ACS Nano 2025, 19, 28827–28846. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Cui, X.; Li, Z.; Guo, S.; Gao, F.; Ma, M.; Wang, Z. Antiosteoporosis effect of geraniin on ovariectomy-induced osteoporosis in experimental rats. J. Biochem. Mol. Toxicol. 2021, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Q.; Liu, X.; Ji, C.; Qu, S.; Wang, S.; Luo, Y. Cyclin G2 Suppresses Estrogen-Mediated Osteogenesis through Inhibition of Wnt/β-Catenin Signaling. PLoS ONE 2014, 9, e89884. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhao, X.; Lu, X. Ovariectomy-associated changes in bone mineral density and bone marrow haematopoiesis in rats. Int. J. Exp. Pathol. 2009, 90, 512–519. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Gheban, D.; Benea, H.R.C. Tibolone, alendronate, and simvastatin enhance implant osseointegration in a preclinical in vivo model. Clin. Oral Implant. Res. 2020, 31, 655–668. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Zhang, X.-B.; Lu, Y.-B.; Hu, Y.-C.; Chen, X.-Y.; Yu, D.-C.; Shi, J.-T.; Yuan, W.-H.; Wang, J.; Zhou, H.-Y. Calcitonin gene-related peptide and brain-derived serotonin are related to bone loss in ovariectomized rats. Brain Res. Bull. 2021, 176, 85–92. [Google Scholar] [CrossRef]
- Hendrijantini, N.; Suisan, Y.C.; Megantara, R.W.A.; Tumali, B.A.S.; Kuntjoro, M.; Ari, M.D.A.; Sitalaksmi, R.M.; Hong, G. Bone Remodeling in Mandible of Wistar Rats with Diabetes Mellitus and Osteoporosis. Eur. J. Dent. 2023, 17, 319–329. [Google Scholar] [CrossRef]
- Pedroso, A.L.; Canal, R.; Gehrke, S.A.; da Costa, E.M.; Scarano, A.; Zanelatto, F.B.; Pelegrine, A.A. The Validation of an Experimental Model in Wistar Female Rats to Study Osteopenia and Osteoporosis. Bioengineering 2025, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Ma, K.; Xiao, Z.; Ren, X.; Yang, G. Preparation and characteristics of gelatin sponges crosslinked by microbial transglutaminase. PeerJ 2017, 5, e3665. [Google Scholar] [CrossRef]
- Sharif, S.N.M.; Hashim, N.; Isa, I.M.; Abu Bakar, S.; Saidin, M.I.; Ahmad, M.S.; Mamat, M.; Hussein, M.Z.; Zainul, R. The impact of a hygroscopic chitosan coating on the controlled release behaviour of zinc hydroxide nitrate-sodium dodecylsulphate-imidacloprid nanocomposites. New J. Chem. 2020, 44, 9097–9108. [Google Scholar] [CrossRef]
- Garcia-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Wang, C.W.; Yu, S.H.; Fretwurst, T.; Larsson, L.; Sugai, J.V.; Oh, J.; Lehner, K.; Jin, Q.; Giannobile, W.V. Maresin 1 Promotes Wound Healing and Socket Bone Regeneration for Alveolar Ridge Preservation. J. Dent. Res. 2020, 99, 930–937. [Google Scholar] [CrossRef]
| Group Code | Vitamin D3 Volume (μL) | Final Concentration (ng/mL) |
|---|---|---|
| NS/Vit. D3-0 | 0 | 0 |
| NS/Vit. D3-1.2 | 1.2 | 3 |
| NS/Vit. D3-2.5 | 2.5 | 6.25 |
| NS/Vit. D3-5.0 | 5 | 12.5 |
| NS/Vit. D3-7.5 | 7.5 | 18.75 |
| NS/Vit. D3-10 | 10 | 25 |
| No. | Parameter | Score | Microscopic Aspect |
|---|---|---|---|
| 1. | Osteoporosis | 0 | Not discernable |
| 1 | Subtle | ||
| 2 | Evident | ||
| 2. | Bone formation at the surface of the graft | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| 3. | Bone formation in the graft area | 0 | Absent |
| 1 | Present in the surface of the graft area | ||
| 2 | Present in the center of the graft area | ||
| 4. | Bone bridge | 0 | Absent |
| 1 | Thin | ||
| 2 | Thick | ||
| 5. | Bone trabeculae | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| 6. | Immature bone | 0 | Present at the periphery and in the center |
| 1 | Present in the center | ||
| 2 | Present at the periphery | ||
| 3 | Absent | ||
| 7. | Mature bone | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| 8. | Osteoblasts | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| 9. | Osteocytes | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| 10. | Osteoclasts | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| 11. | Havers canals | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| 12. | Inflammation | 0 | Present, abundant |
| 1 | Present, scant | ||
| 2 | Absent | ||
| 13. | Vascularization | 0 | Absent |
| 1 | Present in the surface of the graft area | ||
| 2 | Present in the center of the graft area | ||
| 14. | Granulation tissue | 0 | Present |
| 1 | Absent | ||
| 15. | Graft | 0 | Detected |
| 1 | Not detected | ||
| na | Not applicable | ||
| 16. | Osteoclastic degradation of the graft | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| na | Not applicable | ||
| 17. | Replacement of graft by mature bone | 0 | Absent |
| 1 | Present at the periphery | ||
| 2 | Present in the center | ||
| 3 | Present at the periphery and in the center | ||
| na | Not applicable |
| Bone Biomarker | T0 (Baseline) | T1 (12 Weeks Post-OVX) | Trend |
|---|---|---|---|
| Osteocalcin (ng/mL) | 2.26 ± 0.85 | 4.28 ± 1.96 | Increased Turnover |
| β-CTX (pg/mL) | 4609.10 ± 2214.88 | 9842.55 ± 4766.71 | Increased Resorption |
| Histological Parameter | Microscopic Aspect | Gr I-1 | Gr I-2 | Gr II-1 | Gr II-2 | Gr III-1 | Gr III-2 |
|---|---|---|---|---|---|---|---|
| Osteoporosis | Not discernable | 0 | 0 | 0.67 | 0.67 | 0.67 | 0.67 |
| Subtle | 0.5 | 1 | 0.33 | 0.33 | 0.33 | 0.33 | |
| Evident | 0.5 | 0 | 0 | 0 | 0 | 0 | |
| Bone formation (Surface) | Absent | 0 | 0.17 | 0.1 | 0 | 0 | 0 |
| Peripheral | 0.6 | 0.83 | 0.4 | 0.33 | 0.33 | 0.34 | |
| Central | 0 | 0 | 0 | 0 | 0 | 0 | |
| Central & Peripheral | 0.4 | 0 | 0.5 | 0.67 | 0.67 | 0.67 | |
| Bone formation (Depth) | Absent | 0 | 0 | 0 | 0 | 0 | 0 |
| Surface | 0 | 0 | 0.3 | 0.33 | 0.33 | 0.17 | |
| Profound | 0 | 0 | 0.7 | 0.67 | 0.67 | 0.83 | |
| Bone Bridge | Absent | 0.8 | 1 | 0 | 0.17 | 0.17 | 0.17 |
| Thin | 0.2 | 0 | 0.6 | 0.33 | 0.33 | 0.16 | |
| Thick | 0 | 0 | 0.4 | 0.5 | 0.5 | 0.67 | |
| Bone Trabeculae | Absent | 0 | 0.17 | 0 | 0 | 0 | 0 |
| Peripheral | 0.8 | 0.83 | 0 | 0.17 | 0.17 | 0.33 | |
| Central | 0 | 0 | 0 | 0 | 0 | 0 | |
| Central & Peripheral | 0.2 | 0 | 1 | 0.83 | 0.84 | 0.67 | |
| Immature Bone | Absent | 0.2 | 0 | 0.4 | 0.5 | 0.5 | 0.33 |
| Peripheral | 0 | 0 | 0 | 0 | 0 | 0 | |
| Central | 0.8 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Central & Peripheral | 0 | 0 | 0.1 | 0 | 0 | 0.17 | |
| Mature Bone | Absent | 0.3 | 0.33 | 0.1 | 0 | 0 | 0 |
| Peripheral | 0.4 | 0.67 | 0.7 | 0.5 | 0.5 | 0.33 | |
| Central | 0.1 | 0 | 0 | 0 | 0 | 0 | |
| Central & Peripheral | 0.2 | 0 | 0.2 | 0.5 | 0.5 | 0.67 | |
| Osteoblasts | Absent | 0 | 0 | 0 | 0 | 0 | 0 |
| Peripheral | 0.6 | 1 | 1 | 0.2 | 0.5 | 0.33 | |
| Central | 0 | 0 | 0 | 0 | 0 | 0 | |
| Central & Peripheral | 0.4 | 0 | 0 | 0.8 | 0.5 | 0.67 | |
| Osteocytes | Absent | 0 | 0 | 0 | 0 | 0 | 0 |
| Peripheral | 0.6 | 1 | 0 | 0.17 | 0.17 | 0.33 | |
| Central | 0 | 0 | 0.1 | 0 | 0 | 0 | |
| Central & Peripheral | 0.4 | 0 | 0.9 | 0.83 | 0.83 | 0.67 | |
| Osteoclasts | Absent | 0.6 | 0.83 | 0.9 | 1 | 1 | 0.5 |
| Peripheral | 0.4 | 0.17 | 0.1 | 0 | 0 | 0.5 | |
| Central | 0 | 0 | 0 | 0 | 0 | 0 | |
| Central & Peripheral | 0 | 0 | 0 | 0 | 0 | 0 | |
| Haversian Canals | Absent | 0.7 | 1 | 0 | 0.33 | 0.33 | 0 |
| Peripheral | 0.2 | 0 | 0.1 | 0.33 | 0.33 | 0.33 | |
| Central | 0 | 0 | 0 | 0 | 0 | 0 | |
| Central & Peripheral | 0.1 | 0 | 0.9 | 0.33 | 0.33 | 0.67 | |
| Inflammation | Present (Abundant) | 0.1 | 0.17 | 0 | 0.17 | 0.17 | 0 |
| Present (Scant) | 0.5 | 0.33 | 0.1 | 0.33 | 0.33 | 0.17 | |
| Absent | 0.4 | 0.5 | 0.9 | 0.5 | 0.5 | 0.83 | |
| Vascularization | Absent | 0 | 0 | 0 | 0 | 0 | 0 |
| Surface of graft | 0.4 | 0.67 | 0.5 | 0 | 0 | 0 | |
| Depth of graft | 0.6 | 0.33 | 0.5 | 1 | 1 | 1 | |
| Granulation Tissue | Absent | 0 | 0.5 | 0.1 | 0.33 | 0.33 | 0 |
| Present | 1 | 0.5 | 0.9 | 0.67 | 0.67 | 1 | |
| Graft Remnant | Detected | n/a | n/a | 0 | 0 | 0 | 0 |
| Not detected | n/a | n/a | 1 | 1 | 1 | 1 | |
| Scaffold Degradation | Absent | n/a | n/a | 0 | 0 | 0 | 0 |
| Peripheral | n/a | n/a | 0 | 0 | 0.5 | 0.33 | |
| Central & Peripheral | n/a | n/a | 1 | 1 | 0.5 | 0.67 | |
| Scaffold Replacement | Absent | n/a | n/a | 0 | 0 | 0 | 0 |
| Peripheral | n/a | n/a | 0.7 | 1 | 0.5 | 0.33 | |
| Central & Peripheral | n/a | n/a | 0.3 | 0 | 0.5 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Muresan, C.G.; Mirica, I.C.; Forray, A.; Petrescu, N.; Soritau, O.; Gherman, L.-M.; Iusan, S.A.L.; Vanea, E.; Oprita, E.; Condor, A.; et al. Vitamin D-Loaded Chitosan Nanostructures for Bone Regeneration: A Combined In Vitro and In Vivo Evaluation in an Osteoporotic Rat Model. Medicina 2026, 62, 73. https://doi.org/10.3390/medicina62010073
Muresan CG, Mirica IC, Forray A, Petrescu N, Soritau O, Gherman L-M, Iusan SAL, Vanea E, Oprita E, Condor A, et al. Vitamin D-Loaded Chitosan Nanostructures for Bone Regeneration: A Combined In Vitro and In Vivo Evaluation in an Osteoporotic Rat Model. Medicina. 2026; 62(1):73. https://doi.org/10.3390/medicina62010073
Chicago/Turabian StyleMuresan, Corina Giorgiana, Ioana Codruta Mirica, Alina Forray, Nausica Petrescu, Olga Soritau, Luciana-Mădălina Gherman, Simina Angela Lăcrimioara Iusan, Evelyn Vanea, Emilia Oprita, Ana Condor, and et al. 2026. "Vitamin D-Loaded Chitosan Nanostructures for Bone Regeneration: A Combined In Vitro and In Vivo Evaluation in an Osteoporotic Rat Model" Medicina 62, no. 1: 73. https://doi.org/10.3390/medicina62010073
APA StyleMuresan, C. G., Mirica, I. C., Forray, A., Petrescu, N., Soritau, O., Gherman, L.-M., Iusan, S. A. L., Vanea, E., Oprita, E., Condor, A., Aluas, M., Mihu, C. M., Boşca, B. A., Mocan, L. P., Onofrei, M. M., Pop, R. M., Andone, B.-A., Barbu-Tudoran, L., Boca, S., ... Lucaciu, P. O. (2026). Vitamin D-Loaded Chitosan Nanostructures for Bone Regeneration: A Combined In Vitro and In Vivo Evaluation in an Osteoporotic Rat Model. Medicina, 62(1), 73. https://doi.org/10.3390/medicina62010073










