In Vitro Evaluation and Comparative Analysis of Resorbable Membranes for Guided Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Optical and Scanning Electron Microscopy
2.2. Energy Dispersive X-Ray Spectroscopy
2.3. Analysis
3. Results
3.1. Sample 1
3.1.1. Commercial Information
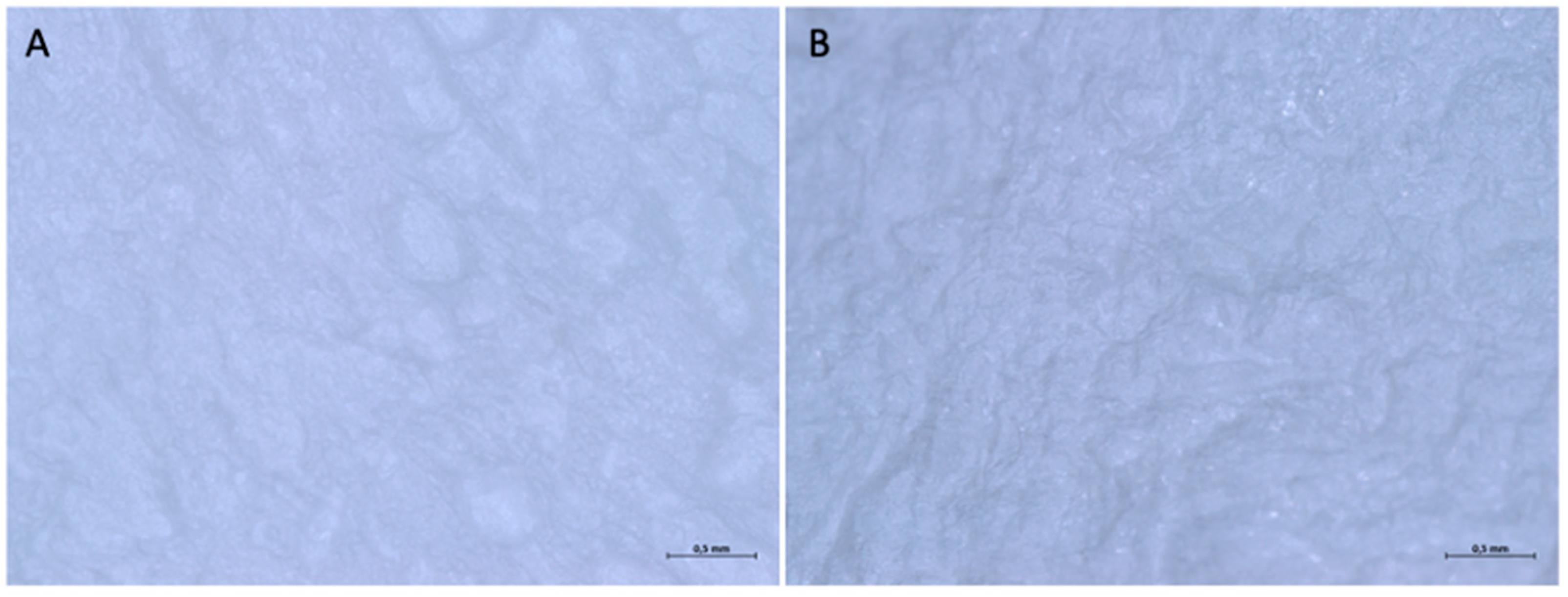
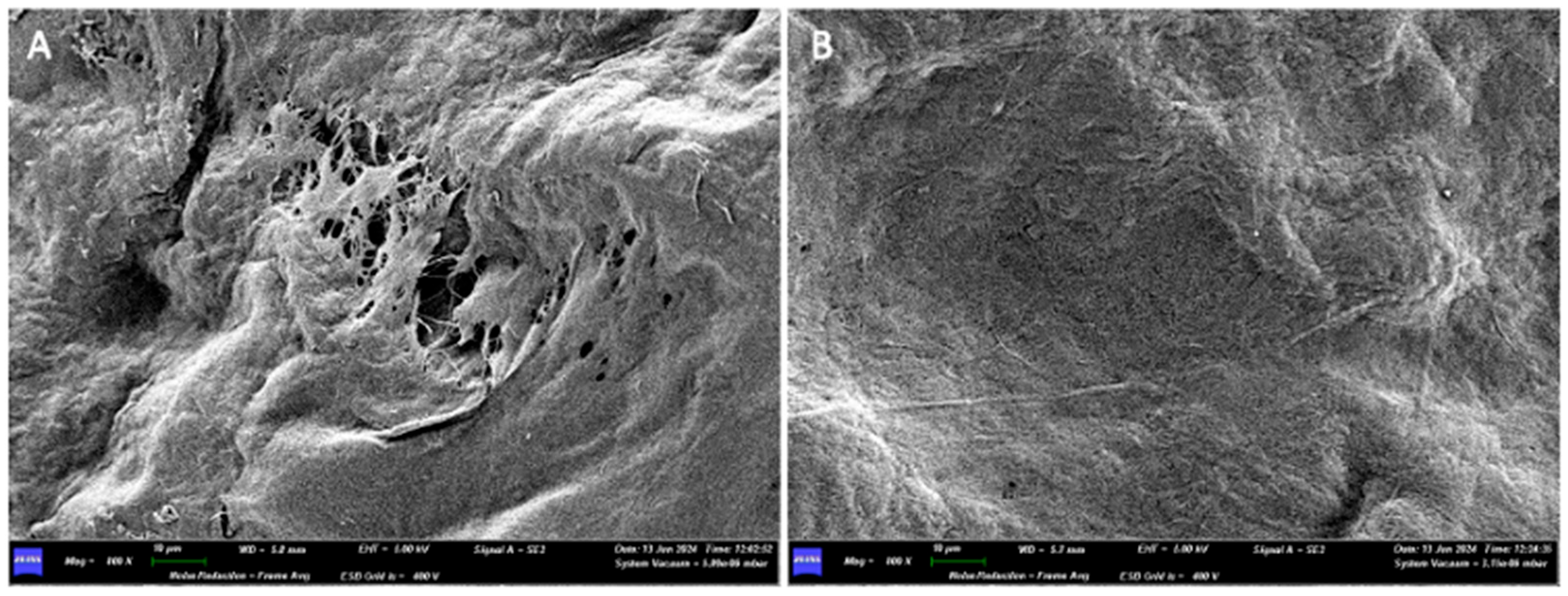
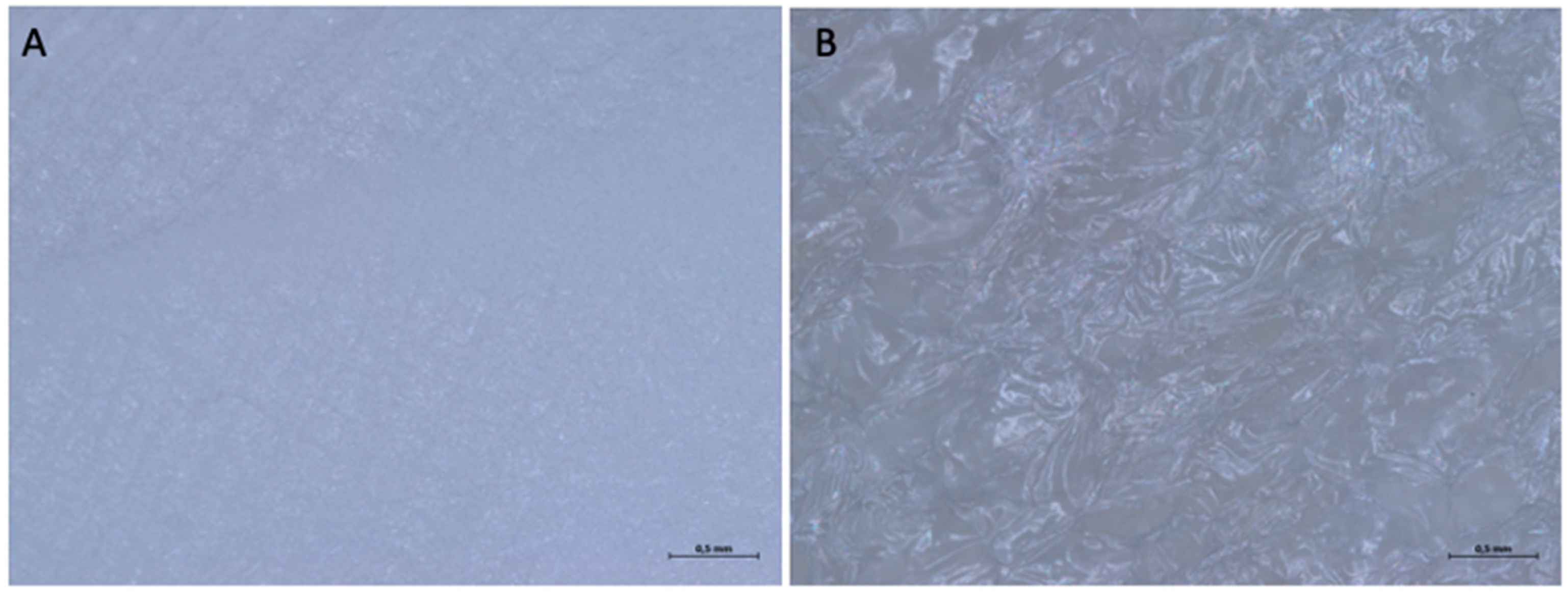
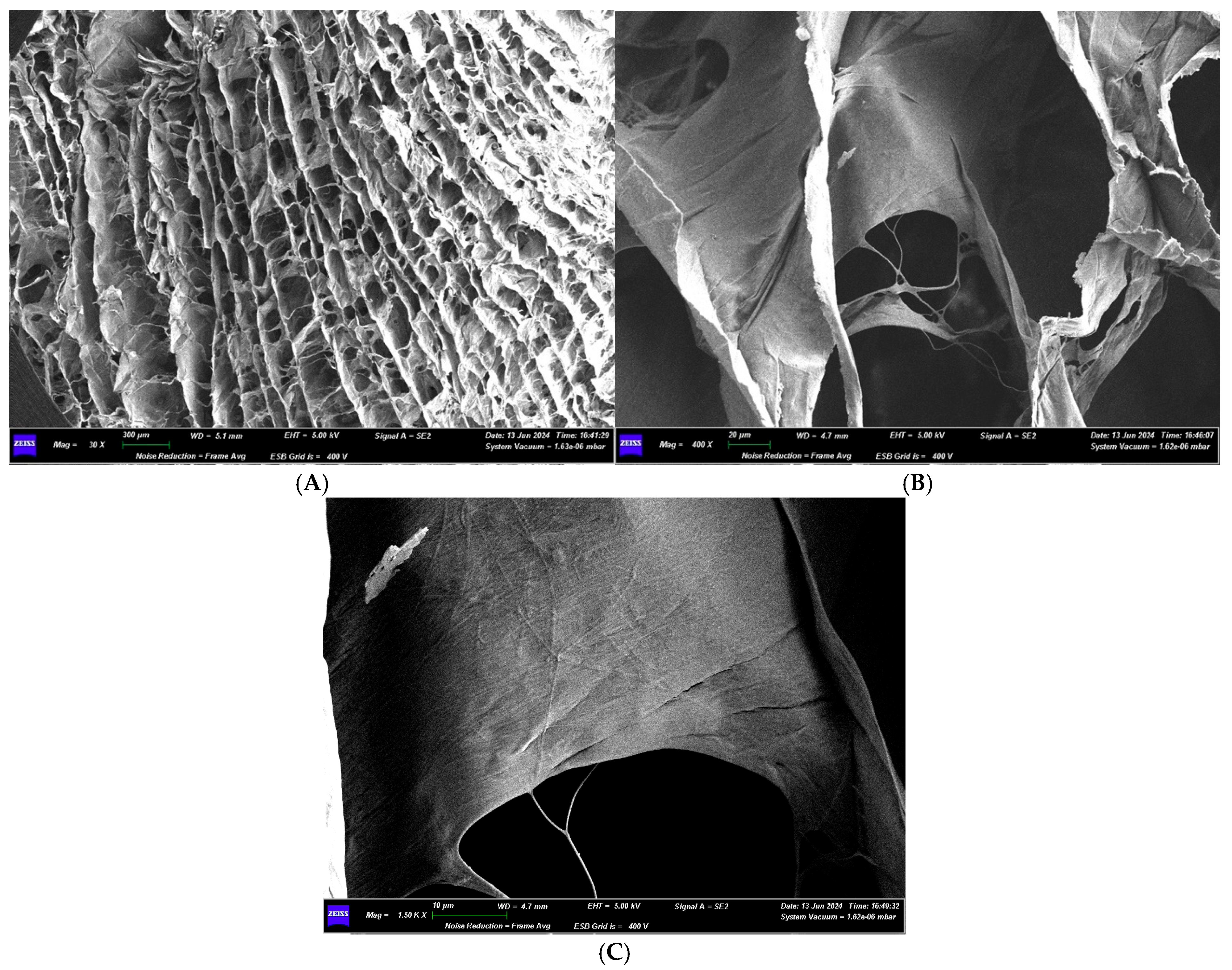
3.1.2. Optical Microscope and Scanning Electron Microscope Analysis
3.2. Sample 2
3.2.1. Commercial Information
3.2.2. Optical Microscope and Scanning Electron Microscope Analysis
3.3. Sample 3
3.3.1. Commercial Information
3.3.2. Optical Microscope and Scanning Electron Microscope Analysis
3.4. Sample 4
3.4.1. Commercial Information
3.4.2. Optical Microscope and Scanning Electron Microscope Analysis
3.5. Sample 5
3.5.1. Commercial Information
3.5.2. Optical Microscope and Scanning Electron Microscope Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dioguardi, M.; Spirito, F.; Quarta, C.; Sovereto, D.; Basile, E.; Ballini, A.; Caloro, G.A.; Troiano, G.; Lo Muzio, L.; Mastrangelo, F. Guided Dental Implant Surgery: Systematic Review. J. Clin. Med. 2023, 12, 1490. [Google Scholar] [CrossRef]
- Papavasileiou, D.A.; Leventis, M.; Agrogiannis, G.; Kalyvas, D. Effect of Isolating the Periosteum with a Resorbable Barrier Membrane on Neoangiogenesis in Guided Bone Regeneration: An Experimental Study. Cureus 2025, 17, e81069. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Araujo, M.; Buser, D.; Grunder, U. Treatment Options for the Management of the Postextraction Socket: Report From the First Giuseppe Cardaropoli Foundation Consensus Conference. J. Periodontal Res. 2025, 60, 398–416. [Google Scholar] [CrossRef]
- Pesce, P.; Canullo, L.; Testori, T.; Mastroianni, A.; Fabbro, M.D.; Menini, M. The clinical effect of bone perforations in periodontal regeneration and alveolar socket preservation: A systematic review with meta-analysis. Clin. Oral Investig. 2025, 29, 64. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Śmieszek-Wilczewska, J.; Al-Maawi, S.; Heselich, A.; Sader, R. After Extraction, Upper Premolars Undergo Programmed Socket Collapse with Development of Cavitations Rather than Complete Socket Healing: A Radiological Study. Bioengineering 2025, 12, 128. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Postiglione, F.; Mastrangelo, F.; Petrini, M. Photobiomodulation Enhances the Healing of Postextraction Alveolar Sockets: A Randomized Clinical Trial with Histomorphometric Analysis and Immunohistochemistry. J. Oral. Maxillofac. Surg. 2021, 79, 57.e1–57.e12. [Google Scholar] [CrossRef]
- Tomasi, C.; Sanz, M.; Cecchinato, D.; Pjetursson, B.; Ferrus, J.; Lang, N.P.; Lindhe, J. Bone dimensional variations at implants placed in fresh extraction sockets: A multilevel multivariate analysis. Clin. Oral Implant Res. 2010, 21, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Tommasato, G.; Pesce, P.; Ravidà, A.; Khijmatgar, S.; Sculean, A.; Galli, M.; Antonacci, D.; Canullo, L. Sealing materials for post-extraction site: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Grassi, A.; Monica, D.; Minetti, E.; Ballini, A.; Gianfreda, F.; Bollero, P.; Cicciù, M.; Mastrangelo, F. Innovative Alveolar Ridge Preservation Surgical Technique with Immediate Dental Implant Placement: A Retrospective Case Report of 1-Year Follow-Up. Eur. J. Dent. 2024, 18, 408–414. [Google Scholar] [CrossRef]
- Fabozzi, R.; Bianchetti, F.; Baldi, D.; Sanchez, C.Y.; Bagnasco, F.; De Angelis, N. The Effectiveness and Complication Rate of Resorbable Biopolymers in Oral Surgery: A Systematic Review. Dent. J. 2025, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.M.; Landao-Bassonga, E.; Kontogiorgos, E.D.; Lee, C.; Cheng, T.; Ngo, H.C.; Allan, B.; Goonewardene, M.; Opperman, L.A.; Zheng, M. The efficacy of a novel porcine-derived collagen membrane on guided bone regeneration: A comparative study in canine model. BMC Oral Health 2025, 25, 850. [Google Scholar] [CrossRef]
- Duarte, N.D.; Frigério, P.B.; Chica, G.E.A.; Okamoto, R.; Buchaim, R.L.; Buchaim, D.V.; Messora, M.R.; Issa, J.P.M. Biomaterials for Guided Tissue Regeneration and Guided Bone Regeneration: A Review. Dent. J. 2025, 13, 179. [Google Scholar] [CrossRef]
- Mastrangelo, F.; Quaresima, R.; Canullo, L.; Scarano, A.; Muzio, L.L.; Piattelli, A. Effects of Novel Laser Dental Implant Microtopography on Human Osteoblast Proliferation and Bone Deposition. Int. J. Oral Maxillofac. Implants 2020, 35, 320–329. [Google Scholar] [CrossRef]
- Urban, I.A.; Mirsky, N.; Serroni, M.; Tovar, N.; Vivekanand Nayak, V.; Witek, L.; Marin, C.; Saleh, M.H.A.; Ravidà, A.; Baczko, I.; et al. Elucidating the Benefit of Perforated vs Nonperforated Membranes in Guided Bone Regeneration: An In Vivo Histologic Evaluation and Histomorphometric Analysis. Int. J. Periodontics Restor. Dent. 2025, 45, 341–355. [Google Scholar] [CrossRef]
- Calciolari, E.; Ravanetti, F.; Strange, A.; Mardas, N.; Bozec, L.; Cacchioli, A.; Kostomitsopoulos, N.; Donos, N. Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. J. Periodontal Res. 2018, 53, 430–439. [Google Scholar] [CrossRef]
- Kozlovsky, A.; Aboodi, G.; Moses, O.; Tal, H.; Artzi, Z.; Weinreb, M.; Nemcovsky, C.E. Bio-degradation of a resorbable collagen membrane (Bio-Gide) applied in a double-layer technique in rats. Clin. Oral Implants Res. 2009, 20, 1116–1123. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Kirsch, A.; Ackermann, K.L.; Hürzeler, M.B. A tissue engineered cellocclusivedevice for hard tissue regeneration – apreliminary report. Int. J. Periodontics Restor. Dent. 2001, 21, 49–59. [Google Scholar]
- Minetti, E.; Gianfreda, F.; Palermo, A.; Bollero, P. Autogenous Dentin Particulate Graft for Alveolar Ridge Augmentation with and without Use of Collagen Membrane: Preliminary. Histol. Anal. Humans. Mater. 2022, 15, 4319. [Google Scholar]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E. The effect of membrane exposure on the outcome of regenerative procedures in humans: A meta-analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.; Ruangsawasdi, N.; Kiattavorncharoen, S.; Bencharit, S.; Thanasrisuebwong, P. Occlusive and Proliferative Properties of Different Collagen Membranes-An In Vitro Study. Materials 2023, 16, 1657. [Google Scholar] [CrossRef]
- Tal, H.; Kozlovsky, A.; Artzi, Z.; Nemcovsky, C.E.; Moses, O. Long-term bio-degradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration. Clin. Oral Implants Res. 2008, 19, 295–302. [Google Scholar] [CrossRef]
- Iida, T.; Carneiro Martins Neto, E.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Lang, N.P.; Xavier, S.P. Influence of a collagen membrane positioned subjacent the sinus mucosa following the elevation of the maxillary sinus. A histomorphometric study in rabbits. Clin. Oral Implants Res. 2017, 28, 1567–1576. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Magrin, G.L.; Zorzo, C.S.; Rigotto, I.; Aludden, H.; Dahlin, C. Membranes for Periodontal and Bone Regeneration: Everything You Need to Know. J. Periodontal Res. [CrossRef]
- Urban, I.A.; Serroni, M.; Dias, D.R.; Baráth, Z.; Forster, A.; Araújo, T.G.; Saleh, M.H.A.; Cucchi, A.; Ravidà, A. Impact of Collagen Membrane in Vertical Ridge Augmentation Using Ti-Reinforced PTFE Mesh: A Randomised Controlled Trial. J. Clin. Periodontol. 2025, 52, 575–588. [Google Scholar] [CrossRef] [PubMed]




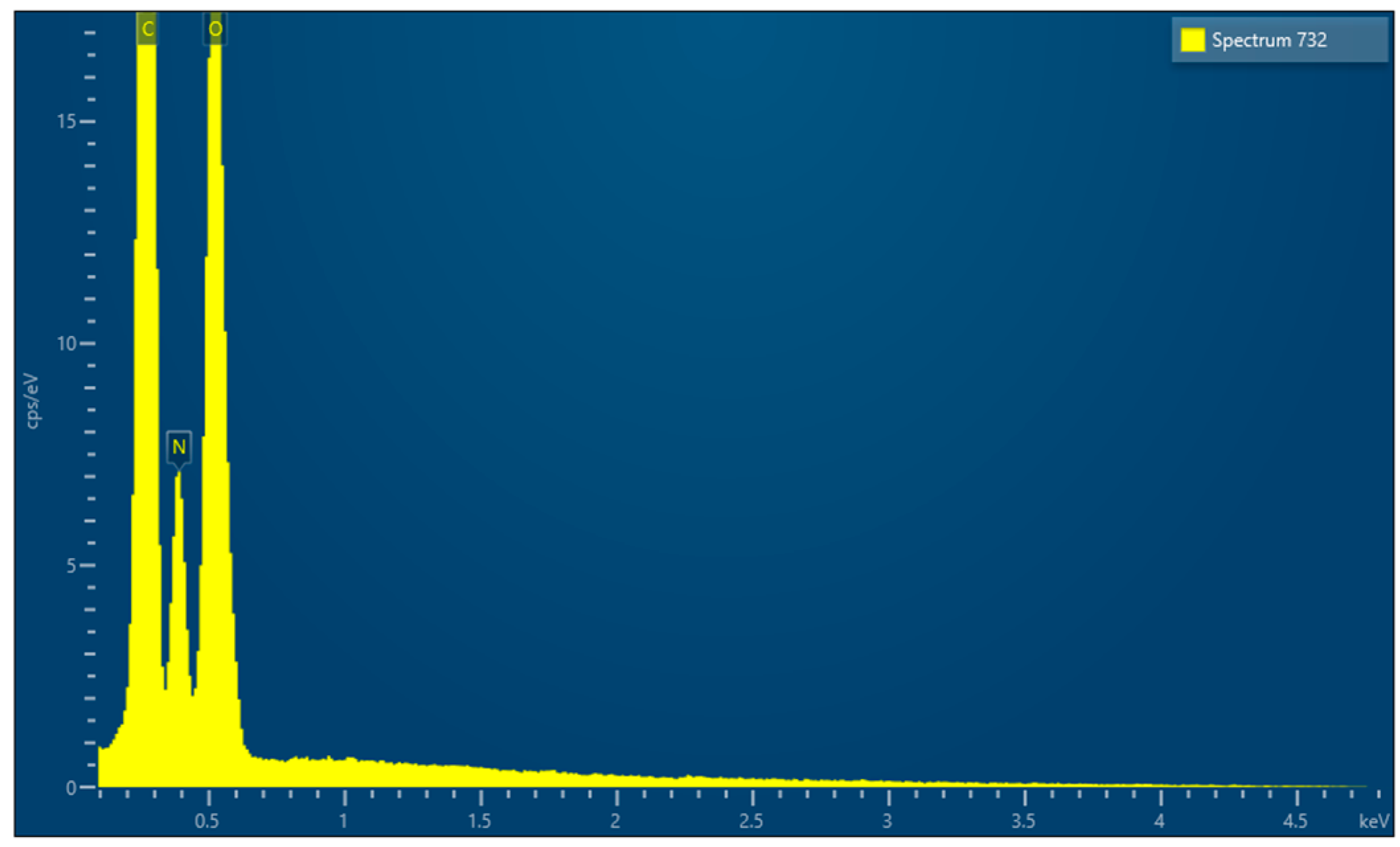
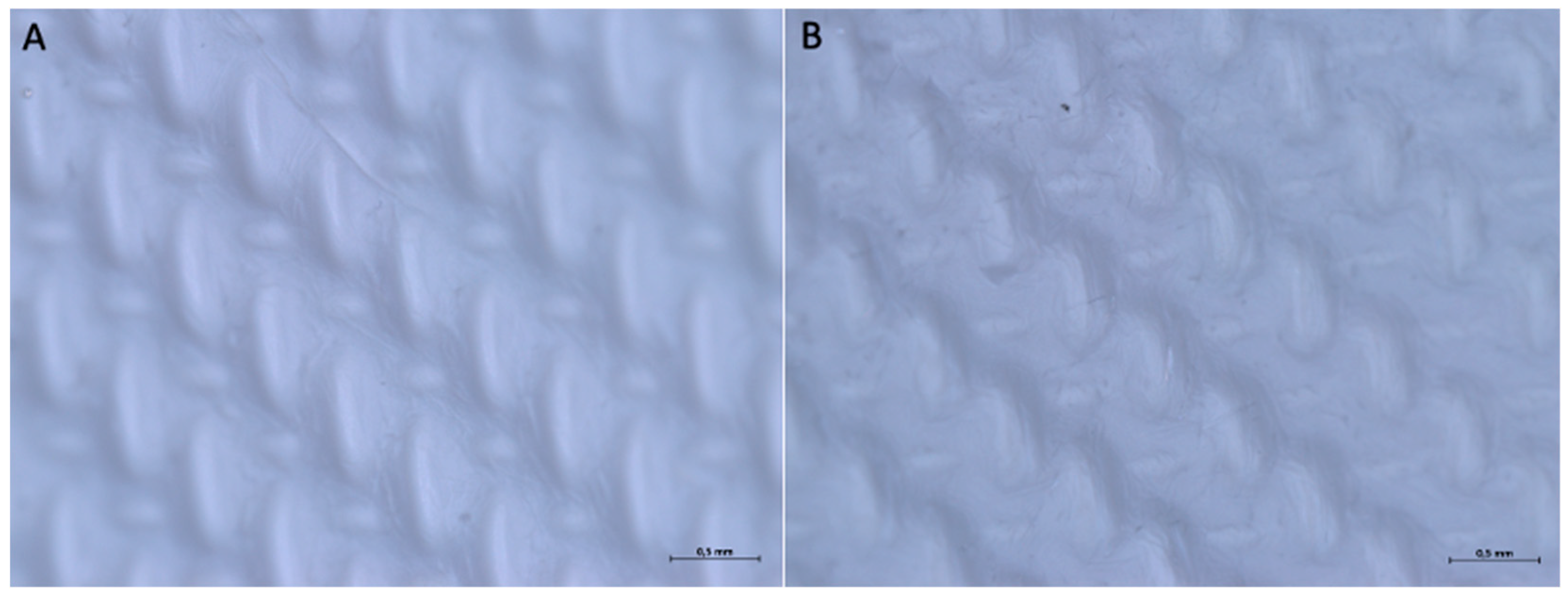

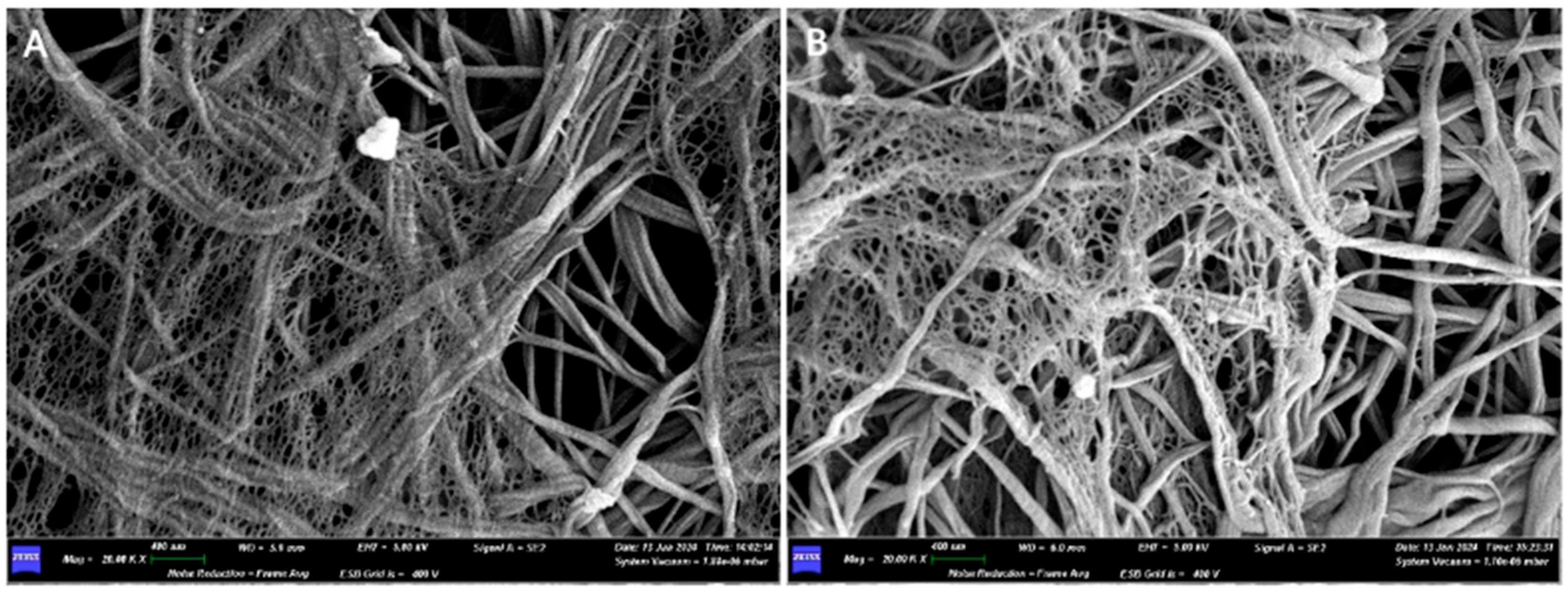
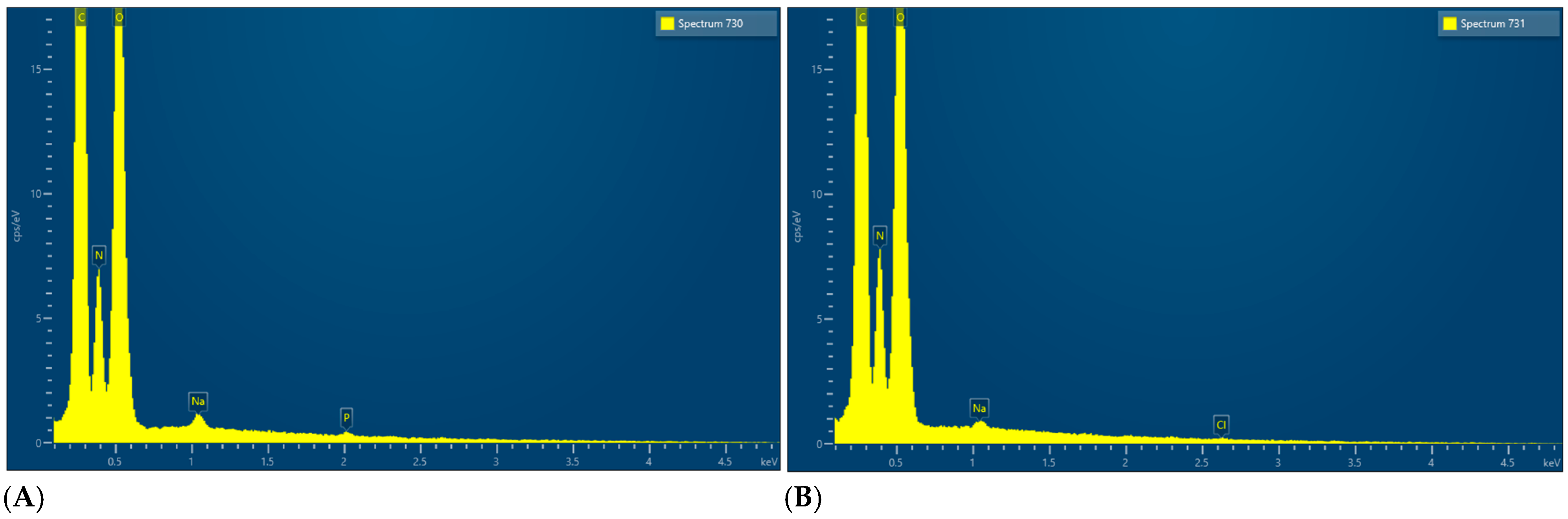
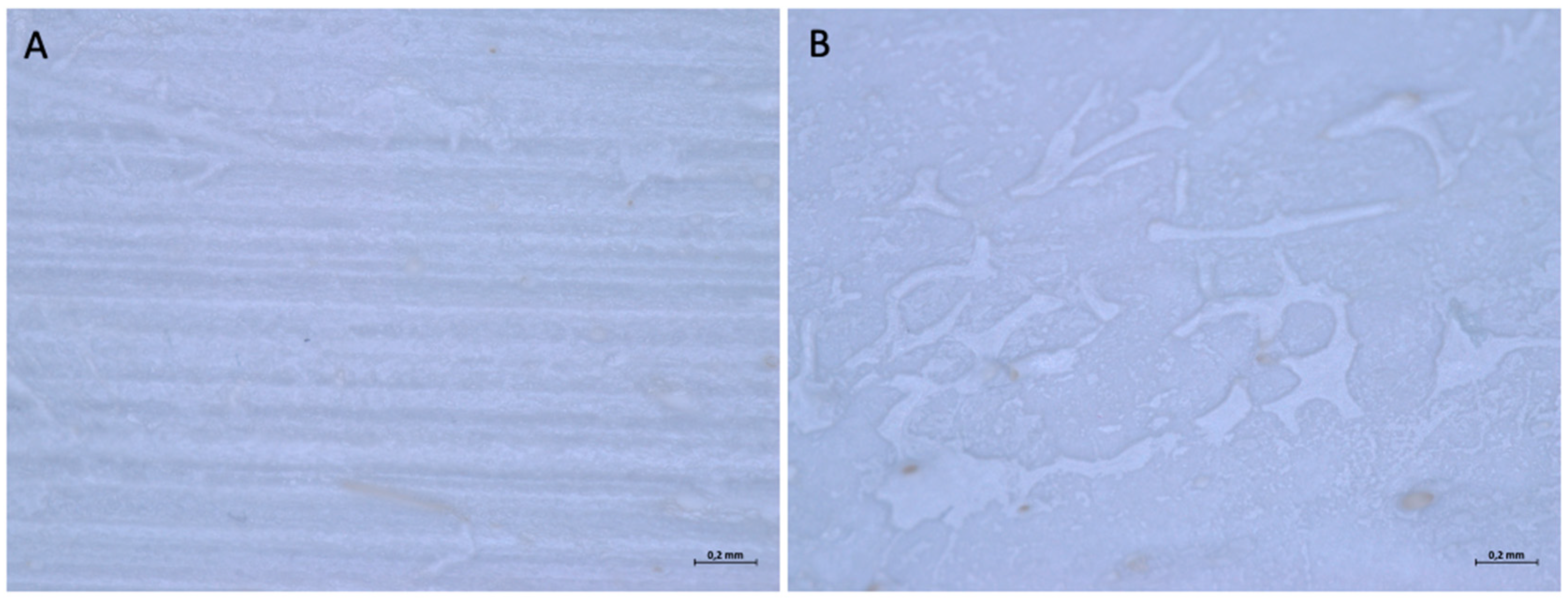
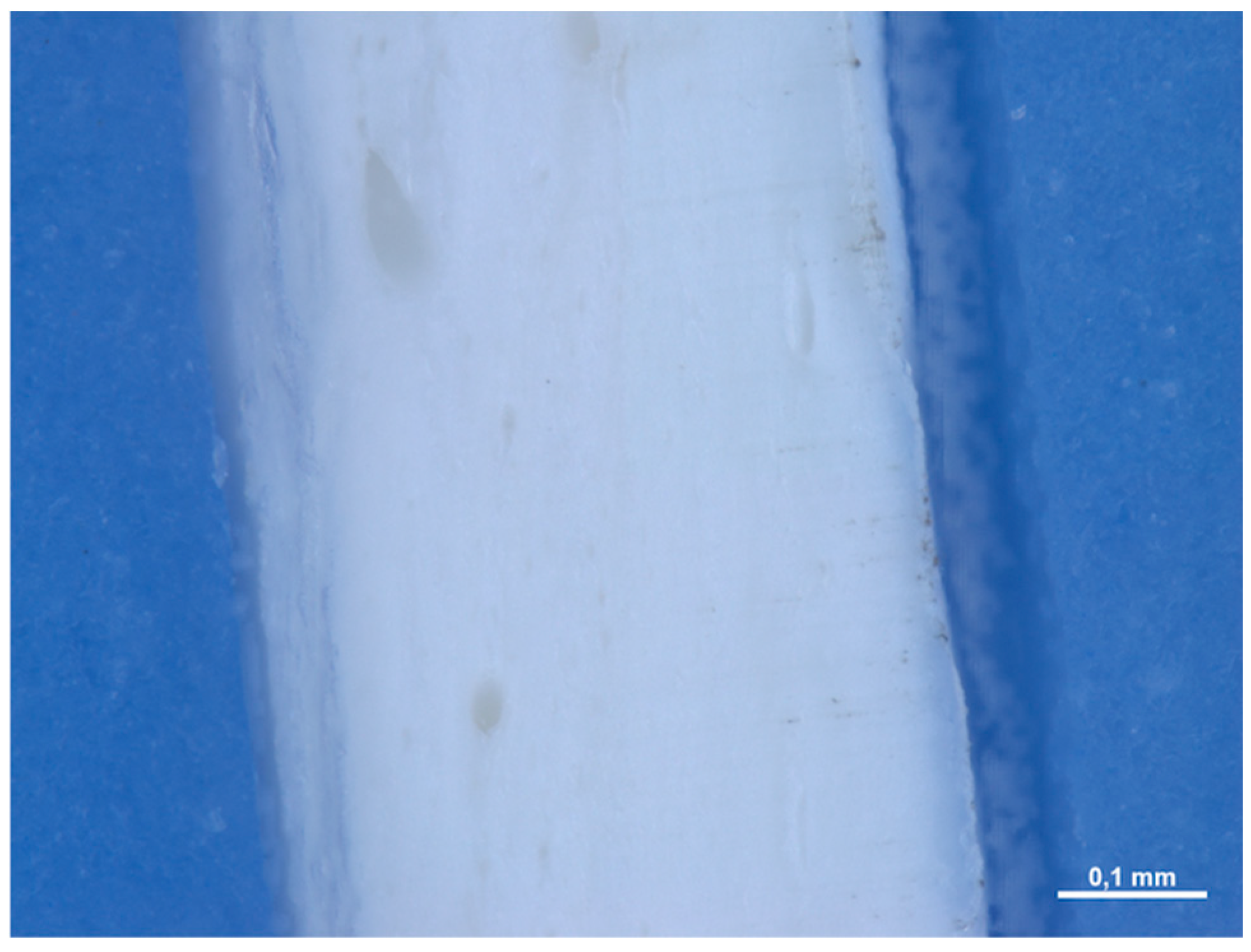
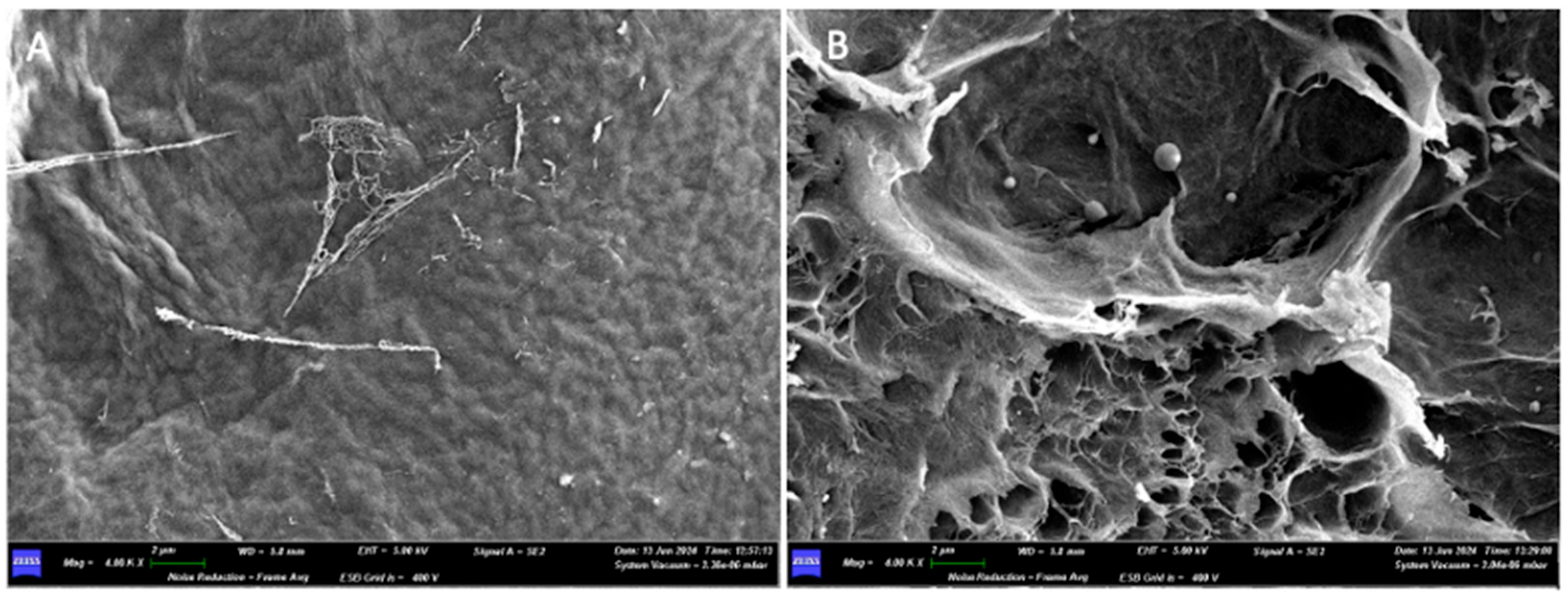

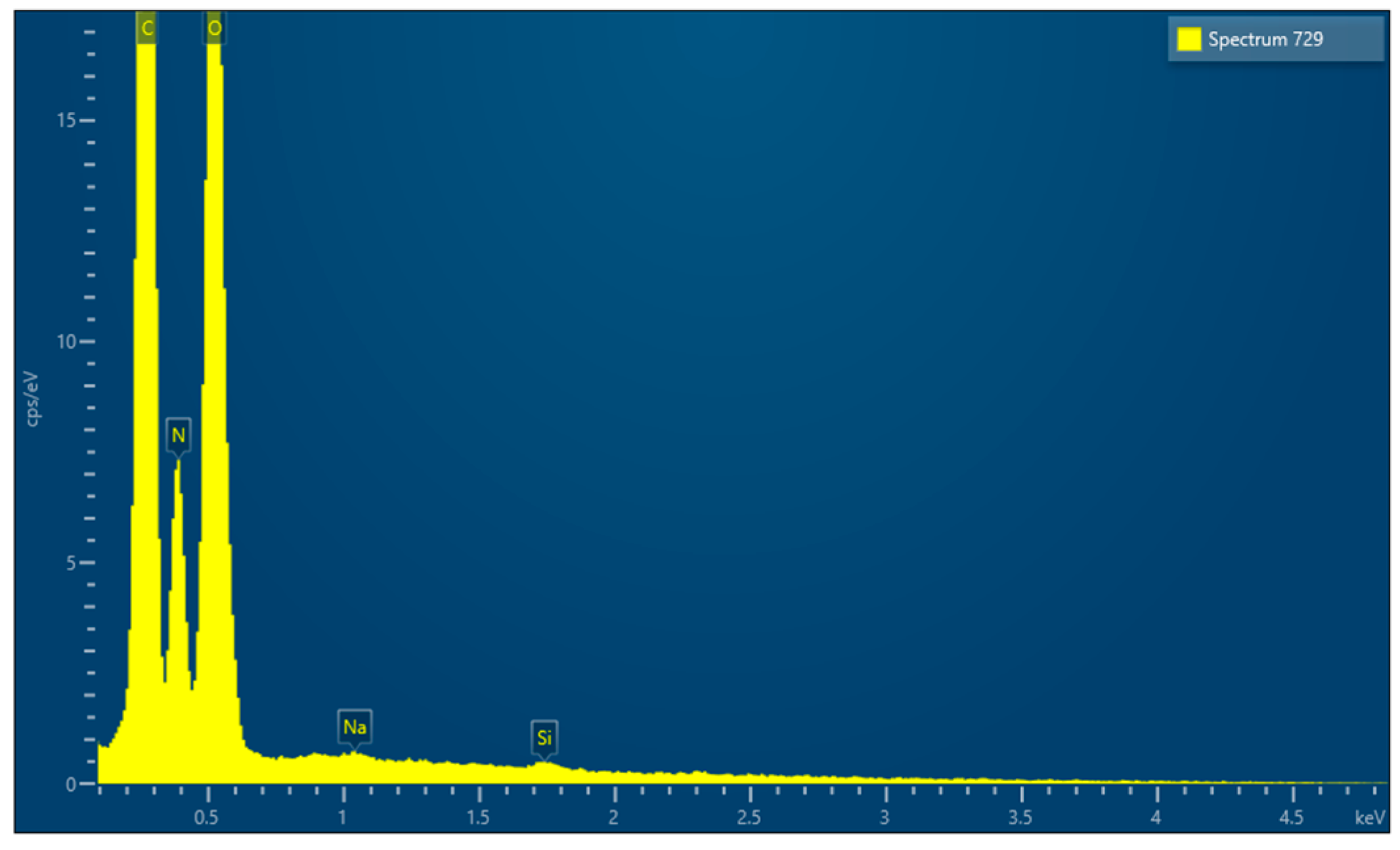






| Barrier Membrane | Mass | Thickness | Density | Structure | Chemical Composition |
|---|---|---|---|---|---|
| Sample 1 | 9.7 (0.2) mg | 0.471 (0.041) mm | 0.206 (0.02) g/cm3 | Native collagen | C, N, O |
| Sample 2 | 6.6 (0.13) mg | 0.245 (0.014) mm | 0.269 (0.04) g/cm3 | Crosslinked | C, N, O, Na, Cl, P |
| Sample 3 | 34.2 (0.5) mg | 0.436 (0.044) mm | 7.844 (0.6) g/cm3 | Bone | C, N, O, Na, Cl |
| Sample 4 | 16.0 (0.20) mg | 0.491 (0.03) mm | 0.326 (0.01) g/cm3 | Crosslinked | C, N, O, Mg, P, S, K |
| Sample 5 | 16.1 (2.78) mg | 2.941 (0.) mm | 0.055 (0.006) g/cm3 | Native Collagene | C, N, O, Na, Si |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonacci, D.; Padula, R.; Gaudelli, F.; Catalano, I.; Mastrangelo, F. In Vitro Evaluation and Comparative Analysis of Resorbable Membranes for Guided Bone Regeneration. Medicina 2025, 61, 1720. https://doi.org/10.3390/medicina61091720
Antonacci D, Padula R, Gaudelli F, Catalano I, Mastrangelo F. In Vitro Evaluation and Comparative Analysis of Resorbable Membranes for Guided Bone Regeneration. Medicina. 2025; 61(9):1720. https://doi.org/10.3390/medicina61091720
Chicago/Turabian StyleAntonacci, Donato, Rossella Padula, Federico Gaudelli, Irene Catalano, and Filiberto Mastrangelo. 2025. "In Vitro Evaluation and Comparative Analysis of Resorbable Membranes for Guided Bone Regeneration" Medicina 61, no. 9: 1720. https://doi.org/10.3390/medicina61091720
APA StyleAntonacci, D., Padula, R., Gaudelli, F., Catalano, I., & Mastrangelo, F. (2025). In Vitro Evaluation and Comparative Analysis of Resorbable Membranes for Guided Bone Regeneration. Medicina, 61(9), 1720. https://doi.org/10.3390/medicina61091720









