Tailored Treatment of Acute Ischemic Stroke: A Narrative Review of Evidence-Based Strategies by Imaging Type and Thrombectomy Availability
Abstract
1. Introduction
2. Relevant Section
2.1. Patients Presenting Within 4.5 h in a Non-Thrombectomy-Capable Center (Figure 1)
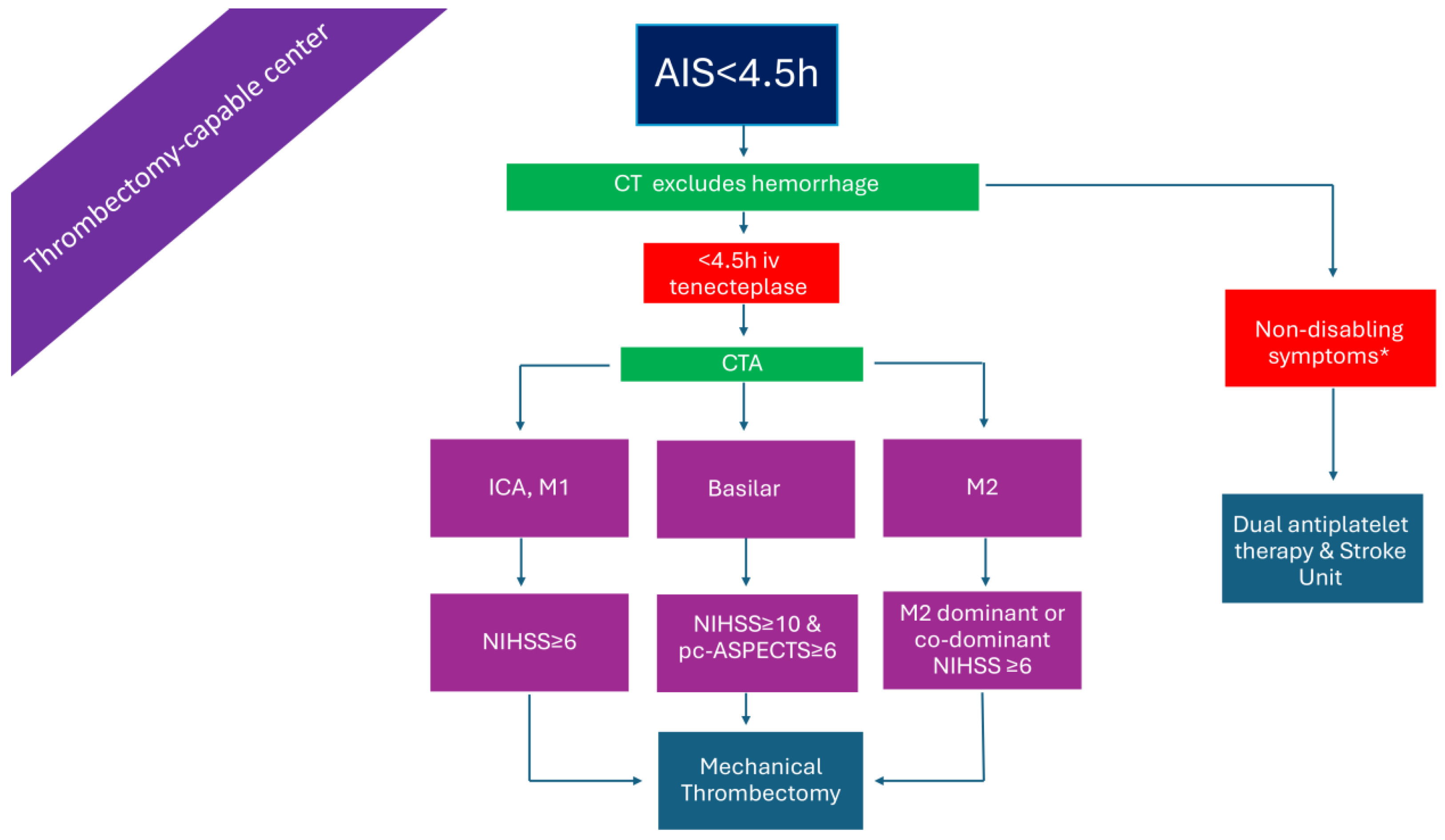
2.2. Patients with LVO Presenting Within 4.5 h in a Thrombectomy-Capable Center (Figure 2)
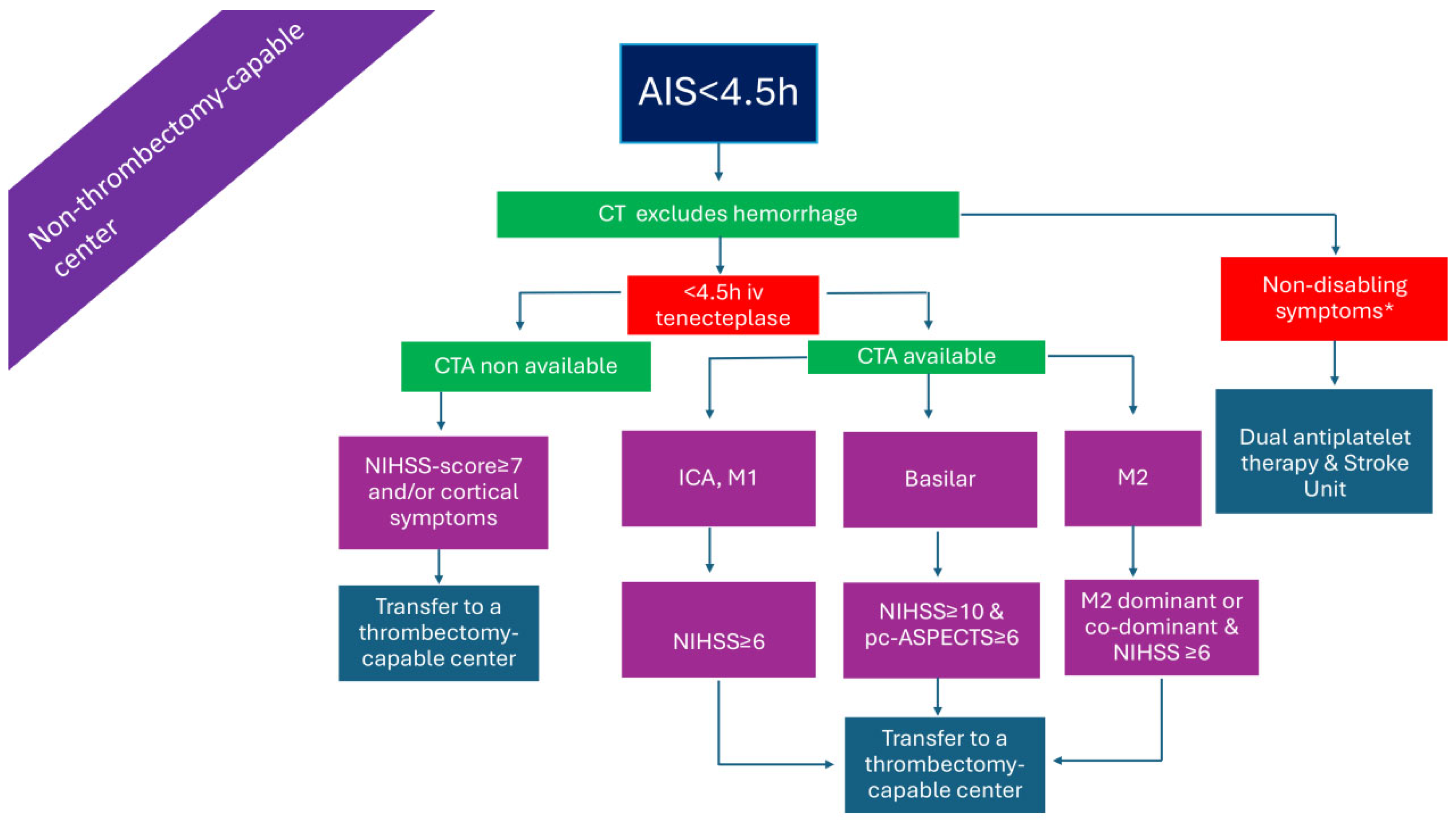
2.3. Patients Presenting >4.5 h from Symptom Onset or with Unknown Time of Onset in a Non-Thrombectomy-Capable Center and Without Advanced Imaging (Figure 3)
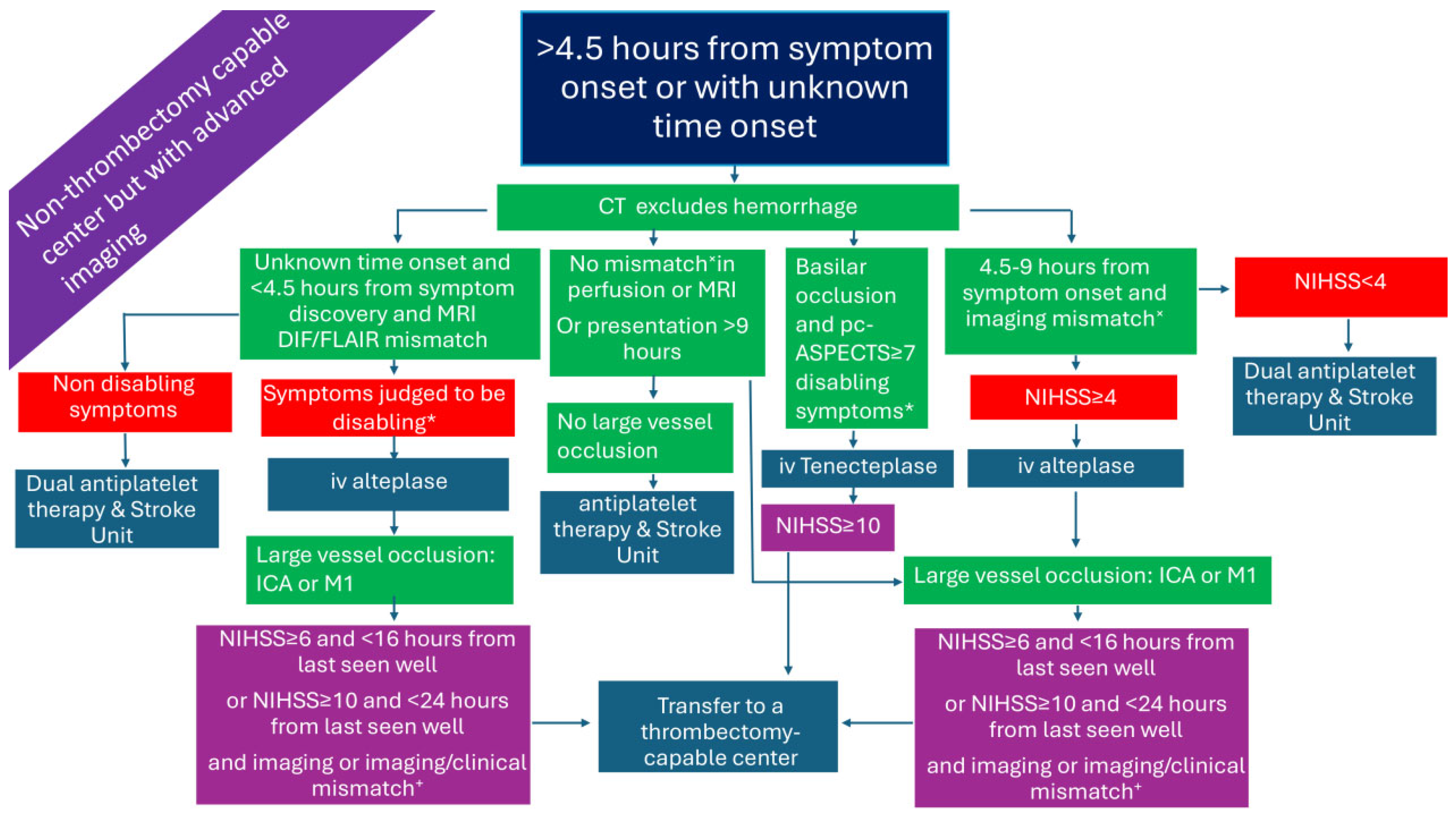
2.4. Patients Presenting >4.5 h from Symptom Onset or with Unknown Time Onset in a Non-Thrombectomy-Capable Center but with Advanced Imaging (Figure 4)
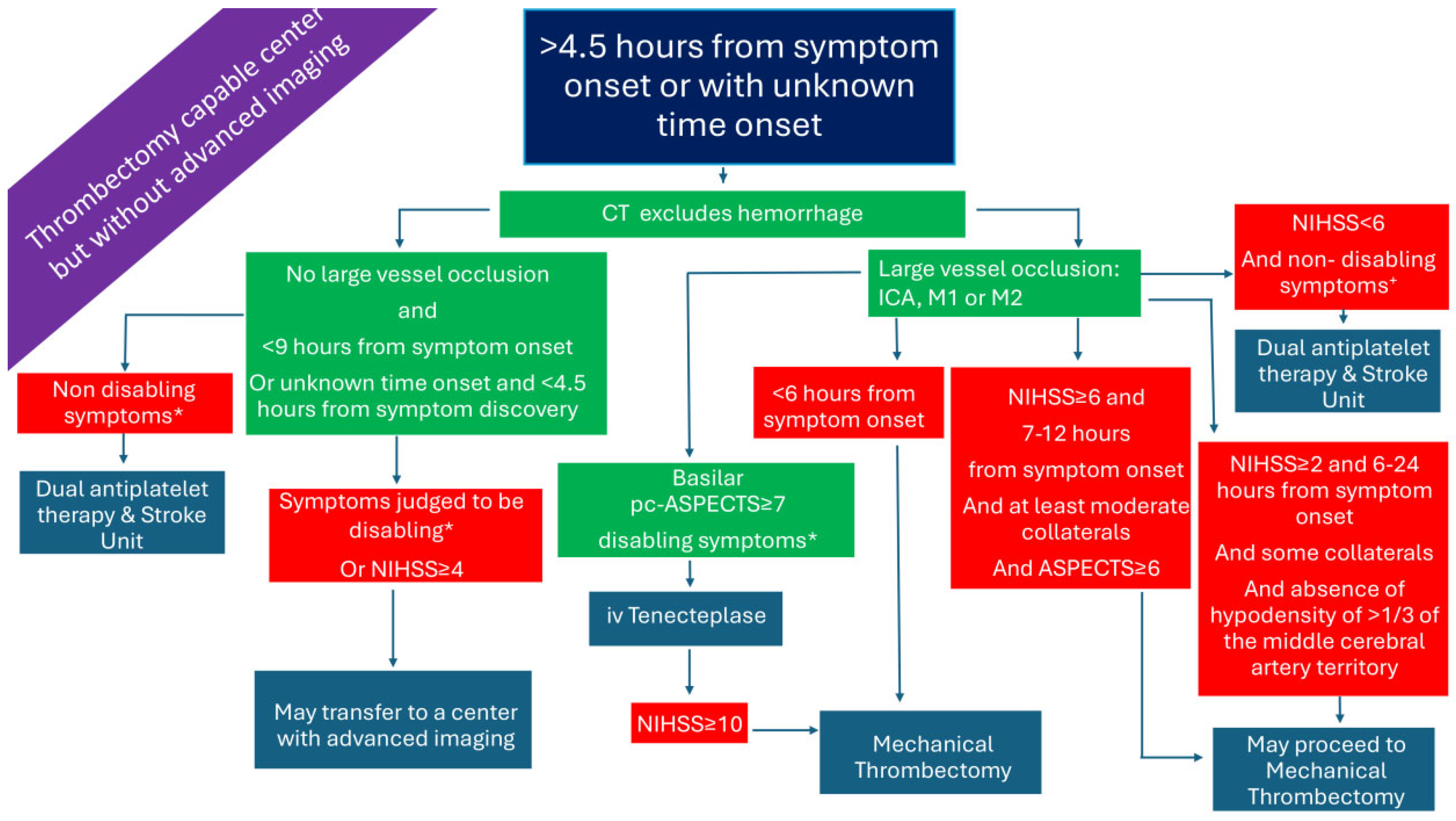
2.5. Patients Presenting >4.5 h from Symptom Onset or with Unknown Time Onset in a Thrombectomy-Capable Center and Without Advanced Imaging (Figure 5)
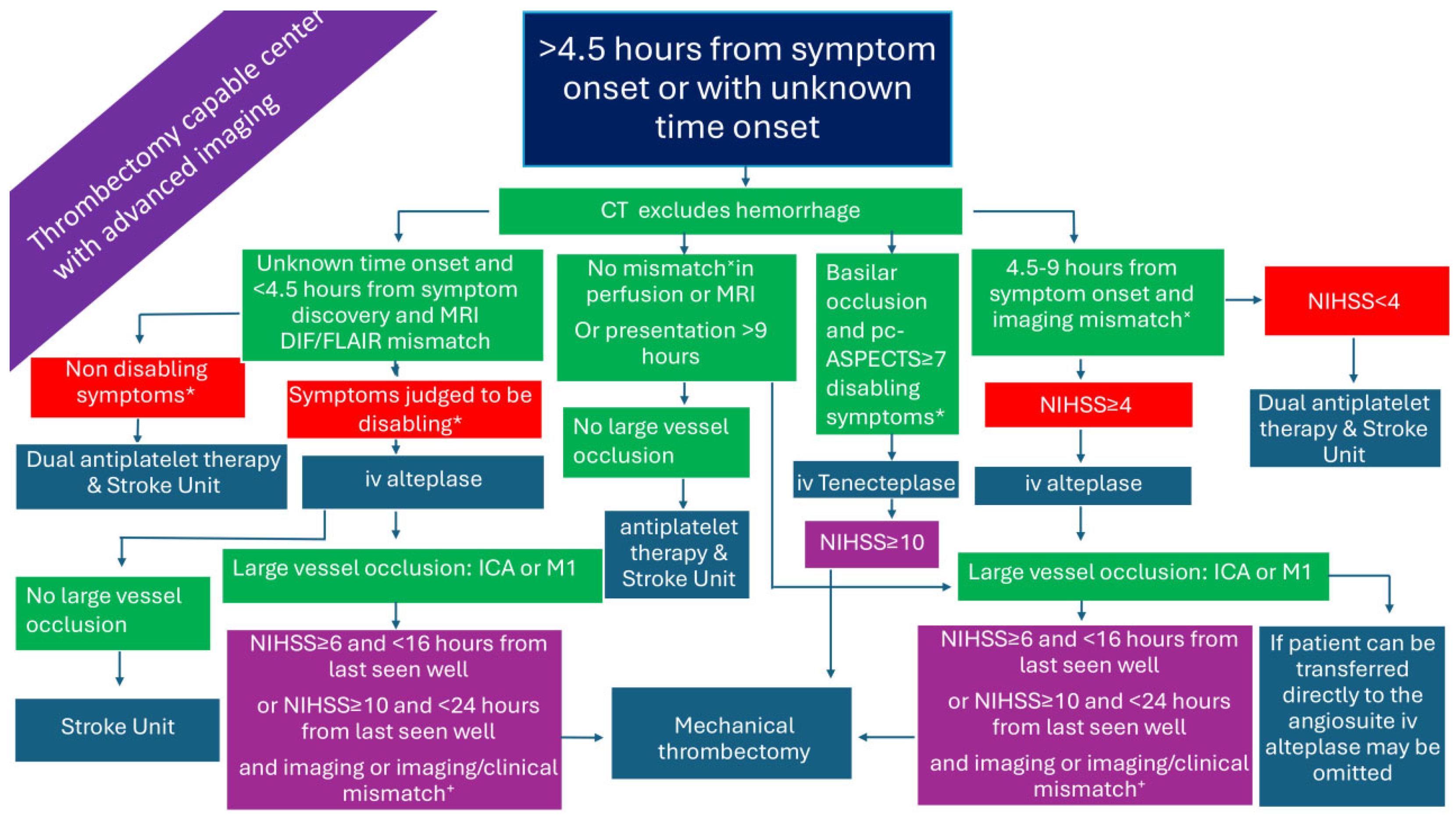
2.6. Patients Presenting >4.5 h from Symptom Onset or with Unknown Time Onset in a Thrombectomy-Capable Center and with Advanced Imaging (Figure 6)
3. Large Core Ischemic Stroke
4. Distal Medium Vessel Occlusions (DMVO)
5. Basilar Artery Occlusion
6. Patients with Minor Stroke and LVO
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [PubMed]
- GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- Béjot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016, 45, e391–e398. [Google Scholar] [CrossRef]
- Magoufis, G.; Safouris, A.; Raphaeli, G.; Kargiotis, O.; Psychogios, K.; Krogias, C.; Palaiodimou, L.; Spiliopoulos, S.; Polizogopoulou, E.; Mantatzis, M.; et al. Acute reperfusion therapies for acute ischemic stroke patients with unknown time of symptom onset or in extended time windows: An individualized approach. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211021182. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Katsanos, A.H.; Sandset, E.C.; Turc, G.; Nguyen, T.N.; Bivard, A.; Fischer, U.; Khatri, P. Thrombolysis for acute ischaemic stroke: Current status and future perspectives. Lancet Neurol. 2023, 22, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Rai, A.T.; Vakharia, K.; Chin, F.; Siddiqui, A.H. Effect of definition and methods on estimates of prevalence of large vessel occlusion in acute ischemic stroke: A systematic review and meta-analysis. J. Neurointerv. Surg. 2020, 12, 260–265. [Google Scholar]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Aguiar de Sousa, D.; Wilkie, A.; Norrving, B.; Macey, C.; Bassetti, C.; Tiu, C.; Roth, G.; Lunde, G.; Christensen, H.; Fiehler, J.; et al. Delivery of acute ischaemic stroke treatments in the European region in 2019 and 2020. Steering Committee for the Implementation of the Stroke Action Plan in Europe. Eur. Stroke J. 2023, 8, 618–628. [Google Scholar]
- Fink, J.N.; Kumar, S.; Horkan, C.; Linfante, I.; Selim, M.H.; Caplan, L.R.; Schlaug, G. The stroke patient who woke up: Clinical and radiological features, including diffusion and perfusion MRI. Stroke 2002, 33, 988–993. [Google Scholar] [CrossRef]
- Reiff, T.; Michel, P. Reasons and evolution of non-thrombolysis in acute ischaemic stroke. Emerg. Med. J. 2017, 34, 219–226. [Google Scholar]
- Guisado-Alonso, D.; Martínez-Domeño, A.; Prats-Sánchez, L.; Delgado-Mederos, R.; Camps-Renom, P.; Abilleira, S.; de la Ossa, N.P.; Ramos-Pachón, A.; Cardona, P.; Rodríguez-Campello, A.; et al. Reasons for Not Performing Mechanical Thrombectomy: A Population-Based Study of Stroke Codes. Stroke 2021, 52, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M. Reperfusion Therapies in Acute Ischemic Stroke Beyond the Conventional Time Window: A Narrative Review. Cureus 2023, 15, e45864. [Google Scholar] [CrossRef] [PubMed]
- Safouris, A.; Palaiodimou, L.; Katsanos, A.H.; Melanis, K.; Magoufis, G.; Theodorou, A.; Spiliopoulos, S.; Mantatzis, M.M.; Themistocleous, M.; Toutouzas, K.; et al. Overview of systematic reviews comparing endovascular to best medical treatment for large-vessel occlusion acute ischaemic stroke: An umbrella review. Ther. Adv. Neurol. Disord. 2024, 17, 17562864241246938. [Google Scholar] [CrossRef] [PubMed]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef]
- Heldner, M.R.; Hsieh, K.; Broeg-Morvay, A.; Mordasini, P.; Bühlmann, M.; Jung, S.; Arnold, M.; Mattle, H.P.; Gralla, J.; Fischer, U. Clinical prediction of large vessel occlusion in anterior circulation stroke: Mission impossible? J. Neurol. 2016, 263, 1633–1640. [Google Scholar] [CrossRef]
- Alamowitch, S.; Turc, G.; Palaiodimou, L.; Bivard, A.; Cameron, A.; De Marchis, G.M.; Fromm, A.; Kõrv, J.; Roaldsen, M.B.; Katsanos, A.H.; et al. European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke. Eur. Stroke J. 2023, 8, 8–54. [Google Scholar] [CrossRef]
- Palaiodimou, L.; Tsivgoulis, G. Transitioning to Intravenous Tenecteplase for the Treatment of Acute Ischemic Stroke. JAMA Netw. Open 2025, 8, e250555. [Google Scholar] [CrossRef]
- Palaiodimou, L.; Katsanos, A.H.; Turc, G.; Melanis, K.; Magoufis, G.; Theodorou, A.; Spiliopoulos, S.; Mantatzis, M.M.; Themistocleous, M.; Toutouzas, K.; et al. Tenecteplase vs Alteplase in Acute Ischemic Stroke Within 4.5 Hours: A Systematic Review and Meta-Analysis of Randomized Trials. Neurology 2024, 103, e209903. [Google Scholar] [CrossRef]
- Safouris, A.; Palaiodimou, L.; Nardai, S.; Kargiotis, O.; Magoufis, G.; Psychogios, K.; Matusevicius, M.; Feil, K.; Ahmed, N.; Kellert, L.; et al. Medical Management Versus Endovascular Treatment for Large-Vessel Occlusion Anterior Circulation Stroke with Low NIHSS. Stroke 2023, 54, 2265–2275. [Google Scholar] [CrossRef]
- Lun, F.; Palaiodimou, L.; Katsanos, A.H.; Tsivgoulis, G.; Turc, G. Intravenous thrombolysis or antiplatelet therapy for acute nondisabling ischemic stroke: A systematic review and network meta-analysis. Eur. Stroke J. 2025, 10, 330–338. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Safouris, A.; Sarraj, A.; Magoufis, G.; Leker, R.R.; Khatri, P.; Cordonnier, C.; Leys, D.; Shoamanesh, A.; Ahmed, N.; et al. Intravenous Thrombolysis with Tenecteplase in Patients with Large Vessel Occlusions: Systematic Review and Meta-Analysis. Stroke 2021, 52, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.H.; Tsivgoulis, G. Is intravenous thrombolysis still necessary in patients who undergo mechanical thrombectomy? Curr. Opin. Neurol. 2019, 32, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.H.; Malhotra, K.; Goyal, N.; Arthur, A.; Schellinger, P.D.; Köhrmann, M.; Krogias, C.; Turc, G.; Magoufis, G.; Leys, D.; et al. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann. Neurol. 2019, 86, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Tsivgoulis, G.; Audebert, H.J.; Boogaarts, H.; Bhogal, P.; De Marchis, G.M.; Fonseca, A.C.; Khatri, P.; Mazighi, M.; Pérez de la Ossa, N.; et al. European Stroke Organisation—European Society for Minimally Invasive Neurological Therapy expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischaemic stroke and anterior circulation large vessel occlusion. Eur. Stroke J. 2022, 7, I–XXVI. [Google Scholar]
- Katsanos, A.H.; Psychogios, K.; Turc, G.; Sacco, S.; de Sousa, D.A.; De Marchis, G.M.; Palaiodimou, L.; Filippou, D.K.; Ahmed, N.; Sarraj, A.; et al. Off-Label Use of Tenecteplase for the Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e224506. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, F.; Sang, H.; Yuan, G.; Xie, D.; Zhou, K.; Li, M.; Meng, Z.; Kong, Z.; Ruan, Z.; et al. Intravenous Tenecteplase before Thrombectomy in Stroke. N. Engl. J. Med. 2025, 393, 139–150. [Google Scholar] [CrossRef]
- Sykora, M.; Kellert, L.; Michel, P.; Eskandari, A.; Feil, K.; Rémi, J.; Ferrari, J.; Krebs, S.; Lang, W.; Serles, W.; et al. Thrombolysis in Stroke with Unknown Onset Based on Non-Contrast Computerized Tomography (TRUST CT). Am. Heart Assoc. 2020, 9, e014265. [Google Scholar] [CrossRef]
- Zha, A.M.; Kamal, H.; Jeevarajan, J.A.; Arevalo, O.; Zhu, L.; Ankrom, C.M.; Bonfante-Mejia, E.E.; Cossey, T.D.; Wu, T.C.; Barreto, A.D.; et al. Non-contrast head CT-based thrombolysis for wake-up/unknown onset stroke is safe: A single-center study and meta-analysis. Int. J. Stroke 2022, 17, 354–361. [Google Scholar] [CrossRef]
- Roaldsen, M.B.; Eltoft, A.; Wilsgaard, T.; Christensen, H.; Engelter, S.T.; Indredavik, B.; Jatužis, D.; Karelis, G.; Kõrv, J.; Lundström, E.; et al. Safety and efficacy of tenecteplase in patients with wake-up stroke assessed by non-contrast CT (TWIST): A multicentre, open-label, randomised controlled trial. Lancet Neurol. 2023, 22, 117–126. [Google Scholar] [CrossRef]
- Coutts, S.B.; Ankolekar, S.; Appireddy, R.; Arenillas, J.F.; Assis, Z.; Bailey, P.; Barber, P.A.; Bazan, R.; Buck, B.H.; Butcher, K.S.; et al. Tenecteplase versus standard of care for minor ischaemic stroke with proven occlusion (TEMPO-2): A randomised, open label, phase 3 superiority trial. Lancet 2024, 403, 2597–2605. [Google Scholar] [CrossRef]
- Wang, Y.H.; Guo, Z.N.; Chen, M.R.; Yao, Z.G.; Nguyen, T.N.; Saver, J.L.; Yang, Y.; Chen, H.S. Intravenous tenecteplase for acute ischemic stroke between 4.5 and 6 h of onset (EXIT-BT2): Rationale and Design. Eur. Stroke J. 2025, 10, 624–630. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar]
- Ma, H.; Campbell, B.C.V.; Parsons, M.W.; Churilov, L.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Wijeratne, T.; Curtze, S.; Dewey, H.M.; et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N. Engl. J. Med. 2019, 380, 1795–1803. [Google Scholar] [CrossRef]
- Qingke, B.; Ping, Z.; Jianying, Z.; Zhenguo, Z. Clinical comparison of intravenous thrombolysis and bridging artery thrombectomy in hyperacute ischemic stroke with unknown time of onset. Arch. Med. Sci. 2021, 17, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Broocks, G.; Meyer, L.; Hanning, U.; Faizy, T.D.; Bechstein, M.; Kniep, H.; Van Horn, N.; Schön, G.; Barow, E.; Thomalla, G.; et al. Haemorrhage after thrombectomy with adjuvant thrombolysis in unknown onset stroke depends on high early lesion water uptake. Stroke Vasc. Neurol. 2024, 9, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Mourand, I.; Milhaud, D.; Arquizan, C.; Lobotesis, K.; Schaub, R.; Machi, P.; Ayrignac, X.; Eker, O.F.; Bonafé, A.; Costalat, V. Favorable Bridging Therapy Based on DWI-FLAIR Mismatch in Patients with Unclear-Onset Stroke. Am. J. Neuroradiol. 2016, 37, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, M.; Saia, V.; Pracucci, G.; Sallustio, F.; Gandini, R.; Nappini, S.; Nencini, P.; Vallone, S.; Zini, A.; Bigliardi, G.; et al. Functional and radiological outcomes after bridging therapy versus direct thrombectomy in stroke patients with unknown onset: Bridging therapy versus direct thrombectomy in unknown onset stroke patients with 10-point ASPECTS. Eur. J. Neurol. 2021, 28, 209–219. [Google Scholar] [CrossRef]
- Thomalla, G.; Simonsen, C.Z.; Boutitie, F.; Andersen, G.; Berthezene, Y.; Cheng, B.; Cheripelli, B.; Cho, T.H.; Fazekas, F.; Fiehler, J.; et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N. Engl. J. Med. 2018, 379, 611–622. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Y.J.; Cui, Y.; Zhang, H.; Jiang, C.H.; Duan, Y.J.; Zhao, Y.; Feng, Y.F.; Geng, S.M.; Zhang, Z.H.; et al. Intravenous Tenecteplase for Acute Ischemic Stroke Within 4.5-24 Hours of Onset (ROSE-TNK): A Phase 2, Randomized, Multicenter Study. J. Stroke 2023, 25, 371–377. [Google Scholar] [CrossRef]
- Albers, G.W.; Jumaa, M.; Purdon, B.; Zaidi, S.F.; Streib, C.; Shuaib, A.; Sangha, N.; Kim, M.; Froehler, M.T.; Schwartz, N.E.; et al. Tenecteplase for Stroke at 4.5 to 24 Hours with Perfusion-Imaging Selection. N. Engl. J. Med. 2024, 390, 701–711. [Google Scholar] [CrossRef]
- Xiong, Y.; Campbell, B.C.V.; Schwamm, L.H.; Meng, X.; Jin, A.; Parsons, M.W.; Fisher, M.; Jiang, Y.; Che, F.; Wang, L.; et al. Tenecteplase for Ischemic Stroke at 4.5 to 24 Hours without Thrombectomy. N. Engl. J. Med. 2024, 391, 203–212. [Google Scholar] [CrossRef]
- Cheng, X.; Hong, L.; Lin, L.; Churilov, L.; Ling, Y.; Yang, N.; Fu, J.; Lu, G.; Yue, Y.; Zhang, J.; et al. Tenecteplase Thrombolysis for Stroke up to 24 Hours After Onset with Perfusion Imaging Selection: The CHABLIS-T II Randomized Clinical Trial. Stroke 2025, 56, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimou, L.; Katsanos, A.H.; Turc, G.; Romoli, M.; Theodorou, A.; Lemmens, R.; Sacco, S.; Velonakis, G.; Vlachopoulos, C.; Tsivgoulis, G. Tenecteplase for the treatment of acute ischemic stroke in the extended time window: A systematic review and meta-analysis. Ther. Adv. Neurol. Disord. 2024, 17, 17562864231221324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Y.; Campbell, B.C.V.; Liebeskind, D.S.; Yuan, C.; Chen, H.; Zhang, Y.; Yi, T.; Luo, Z.; Zhang, Z.; et al. Alteplase for Acute Ischemic Stroke at 4.5 to 24 Hours: The HOPE Randomized Clinical Trial. JAMA 2025, 334, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic Stroke Endorsed by Stroke Alliance for Europe (SAFE). Eur. Stroke J. 2019, 4, 6–12. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; van der Lugt, A.; Menon, B.K.; Majoie, C.B.; Dippel, D.W.; Campbell, B.C.; Nogueira, R.G.; Demchuk, A.M.; Tomasello, A.; et al. Time to Treatment with Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA 2016, 316, 1279–1288. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Santos, T.; Carvalho, A.; Cunha, A.A.; Rodrigues, M.; Gregório, T.; Paredes, L.; Costa, H.; Roriz, J.M.; Pinho, J.; Veloso, M.; et al. NCCT and CTA-based imaging protocol for endovascular treatment selection in late presenting or wake-up strokes. J. Neurointerv. Surg. 2019, 11, 200–203. [Google Scholar] [CrossRef]
- Dekker, L.; Venema, E.; Pirson, F.A.V.; Majoie, C.B.L.M.; Emmer, B.J.; Jansen, I.G.H.; Mulder, M.J.H.L.; Lemmens, R.; Goldhoorn, R.B.; Wermer, M.J.H.; et al. Endovascular treatment in anterior circulation stroke beyond 6.5 hours after onset or time last seen well: Results from the MR CLEAN Registry. Stroke Vasc. Neurol. 2021, 6, 572–580. [Google Scholar] [CrossRef]
- Kobeissi, H.; Ghozy, S.; Adusumilli, G.; Bilgin, C.; Tolba, H.; Amoukhteh, M.; Kadirvel, R.; Brinjikji, W.; Heit, J.J.; Rabinstein, A.A.; et al. CT Perfusion vs Noncontrast CT for Late Window Stroke Thrombectomy: A Systematic Review and Meta-analysis. Neurology 2023, 100, e2304–e2311. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Abdalkader, M.; Nagel, S.; Qureshi, M.M.; Ribo, M.; Caparros, F.; Haussen, D.C.; Mohammaden, M.H.; Sheth, S.A.; Ortega-Gutierrez, S.; et al. Noncontrast Computed Tomography vs Computed Tomography Perfusion or Magnetic Resonance Imaging Selection in Late Presentation of Stroke with Large-Vessel Occlusion. JAMA Neurol. 2022, 79, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Porto, G.B.F.; Chen, C.J.; Al Kasab, S.; Essibayi, M.A.; Almallouhi, E.; Hubbard, Z.; Chalhoub, R.; Alawieh, A.; Maier, I.; Psychogios, M.N.; et al. Association of Noncontrast Computed Tomography and Perfusion Modalities with Outcomes in Patients Undergoing Late-Window Stroke Thrombectomy. JAMA Netw. Open 2022, 5, e2241291. [Google Scholar] [CrossRef] [PubMed]
- Olthuis, S.G.H.; Pirson, F.A.V.; Pinckaers, F.M.E.; Hinsenveld, W.H.; Nieboer, D.; Ceulemans, A.; Knapen, R.R.M.M.; Robbe, M.M.Q.; Berkhemer, O.A.; van Walderveen, M.A.A.; et al. Endovascular treatment versus no endovascular treatment after 6-24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: A multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet 2023, 401, 1371–1380. [Google Scholar] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Campbell, B.C.; Christensen, S.; Levi, C.R.; Desmond, P.M.; Donnan, G.A.; Davis, S.M.; Parsons, M.W. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011, 42, 3435–3440. [Google Scholar] [CrossRef]
- Mutimer, C.A.; Campbell, B.C.V. Current perspectives on endovascular therapy for large core ischemic stroke. Neurotherapeutics 2025, 22, e00622. [Google Scholar] [CrossRef]
- Yoshimura, S.; Sakai, N.; Yamagami, H.; Uchida, K.; Beppu, M.; Toyoda, K.; Matsumaru, Y.; Matsumoto, Y.; Kimura, K.; Takeuchi, M.; et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region. N. Engl. J. Med. 2022, 386, 1303–1313. [Google Scholar] [CrossRef]
- Huo, X.; Ma, G.; Tong, X.; Zhang, X.; Pan, Y.; Nguyen, T.N.; Yuan, G.; Han, H.; Chen, W.; Wei, M.; et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N. Engl. J. Med. 2023, 388, 1272–8123. [Google Scholar] [CrossRef]
- Sarraj, A.; Hassan, A.E.; Abraham, M.G.; Ortega-Gutierrez, S.; Kasner, S.E.; Hussain, M.S.; Chen, M.; Blackburn, S.; Sitton, C.W.; Churilov, L.; et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N. Engl. J. Med. 2023, 388, 1259–1271. [Google Scholar] [CrossRef]
- Bendszus, M.; Fiehler, J.; Subtil, F.; Bonekamp, S.; Aamodt, A.H.; Fuentes, B.; Gizewski, E.R.; Hill, M.D.; Krajina, A.; Pierot, L.; et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: Multicentre, open-label, randomised trial. Lancet 2023, 402, 1753–1763. [Google Scholar] [CrossRef]
- Costalat, V.; Jovin, T.G.; Albucher, J.F.; Cognard, C.; Henon, H.; Nouri, N.; Gory, B.; Richard, S.; Marnat, G.; Sibon, I.; et al. Trial of Thrombectomy for Stroke with a Large Infarct of Unrestricted Size. N. Engl. J. Med. 2024, 390, 1677–1689. [Google Scholar] [CrossRef]
- Yoo, A.J.; Zaidat, O.O.; Sheth, S.A.; Rai, A.T.; Ortega-Gutierrez, S.; Given, C.A., 2nd; Zaidi, S.F.; Grandhi, R.; Cuellar, H.; Mokin, M.; et al. Thrombectomy for Stroke with Large Infarct on Noncontrast CT: The TESLA Randomized Clinical Trial. JAMA 2024, 332, 1355–1366. [Google Scholar] [PubMed]
- Liu, C.; Abdalkader, M.; Sang, H.; Sarraj, A.; Campbell, B.C.V.; Miao, Z.; Huo, X.; Yoo, A.J.; Zaidat, O.O.; Thomalla, G.; et al. Endovascular Thrombectomy for Large Ischemic Core Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Neurology 2025, 104, e213443. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, P.D.; Tsivgoulis, G.; Frank, B.; Liebig, T.; Köhrmann, M. Randomized controlled trials of endovascular therapy for acute ischemic stroke with medium or distal vessel occlusion: A study level metaanalysis. Neurol. Res. Pract. 2025, 7, 44. [Google Scholar] [CrossRef]
- Psychogios, M.; Brehm, A.; Ribo, M.; Rizzo, F.; Strbian, D.; Räty, S.; Arenillas, J.F.; Martínez-Galdámez, M.; Hajdu, S.D.; Michel, P.; et al. Endovascular Treatment for Stroke Due to Occlusion of Medium or Distal Vessels. N. Engl. J. Med. 2025, 392, 1374–1384. [Google Scholar] [CrossRef]
- Goyal, M.; Ospel, J.M.; Ganesh, A.; Dowlatshahi, D.; Volders, D.; Möhlenbruch, M.A.; Jumaa, M.A.; Nimjee, S.M.; Booth, T.C.; Buck, B.H.; et al. Endovascular Treatment of Stroke Due to Medium-Vessel Occlusion. N. Engl. J. Med. 2025, 392, 1385–1395. [Google Scholar] [CrossRef]
- Clarençon, F.; Durand-Zaleski, I.; Premat, K.; Baptiste, A.; Chabert, E.; Ferrier, A.; Labeyrie, M.A.; Reiner, P.; Spelle, L.; Denier, C.; et al. Evaluation of mechanical thrombectomy in acute ischemic stroke related to a distal arterial occlusion: A randomized controlled trial. Int. J. Stroke 2024, 19, 367–372. [Google Scholar] [CrossRef]
- Palaiodimou, L.; Safouris, A.; Papageorgiou, N.M.; Melanis, K.; Magoufis, G.; Theodorou, A.; Spiliopoulos, S.; Mantatzis, M.M.; Themistocleous, M.; Toutouzas, K.; et al. Endovascular Treatment in Acute Ischemic Stroke Due to Occlusion of Medium or Distal Vessels: A Systematic Review and Meta-Analysis. Neurology 2025, 105, e214015. [Google Scholar] [CrossRef]
- Koul, P.; Collins, M.K.; Bielinski, T.M.; Goren, O.; Weiner, G.M.; Griessenauer, C.J.; Noto, A.; Schirmer, C.; Hendrix, P. Comparative Analysis of Mechanical Thrombectomy Outcomes of Middle Cerebral Artery M1, M2 Superior, and M2 Inferior Occlusion Strokes. World Neurosurg. 2024, 189, e878–e887. [Google Scholar] [CrossRef]
- de Castro Afonso, L.H.; Borghini Pazuello, G.; Seizem Nakiri GMonsignore, L.M.; Antunes Dias, F.; Pontes-Neto, O.M.; Giansante Abud, D. Thrombectomy for M2 occlusions and the role of the dominant branch. Interv. Neuroradiol. 2019, 25, 697–704. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, Y.; Lansberg, M.G.; Liebeskind, D.S.; Yuan, C.; Yu, H.; Chen, F.; Chen, H.; Zhang, B.; Mao, L.; et al. Alteplase for Posterior Circulation Ischemic Stroke at 4.5 to 24 Hours. N. Engl. J. Med. 2025, 392, 1288–1296. [Google Scholar] [CrossRef]
- Strbian, D.; Sairanen, T.; Silvennoinen, H.; Salonen, O.; Kaste, M.; Lindsberg, P.J. Thrombolysis of basilar artery occlusion: Impact of baseline ischemia and time. Ann. Neurol. 2013, 73, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Räty, S.; Virtanen, P.; Ritvonen, J.; Georgiopoulos, G.; Sairanen, T.; Lindsberg, P.J.; Strbian, D. IV Thrombolysis in Basilar Artery Occlusion: Outcomes and Comparison with Endovascular Thrombectomy. Neurology 2024, 102, e209249. [Google Scholar] [CrossRef] [PubMed]
- Puetz, V.; Khomenko, A.; Hill, M.D.; Dzialowski, I.; Michel, P.; Weimar, C.; Wijman, C.A.; Mattle, H.P.; Engelter, S.T.; Muir, K.W.; et al. Extent of hypoattenuation on CT angiography source images in basilar artery occlusion: Prognostic value in the Basilar Artery International Cooperation Study. Stroke 2011, 42, 3454–3459. [Google Scholar] [CrossRef] [PubMed]
- Räty, S.; Strambo, D.; Gomez-Exposito, A.; Marto, J.P.; Ramos, J.N.; Krebs, S.; Virtanen, P.; Ritvonen, J.; Abdalkader, M.; Klein, P.; et al. Intravenous thrombolysis versus endovascular thrombectomy in acute basilar artery occlusion-A multicenter cohort study. Int. J. Stroke 2025. [Google Scholar] [CrossRef]
- Knapen, R.R.M.M.; Frol, S.; van Kuijk, S.M.J.; Oblak, J.P.; van der Leij, C.; van Oostenbrugge, R.J.; van Zwam, W.H. Intravenous thrombolysis for ischemic stroke in the posterior circulation: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2024, 33, 107641. [Google Scholar] [CrossRef]
- Cai, L.; Wang, L.; Campbell, B.C.V.; Wu, Y.; Abdalkader, M.; Alemseged, F.; Kaesmacher, J.; Puetz, V.; Nagel, S.; Strbian, D.; et al. Endovascular thrombectomy with versus without intravenous thrombolysis in patients with acute basilar artery occlusion: A systematic review and meta-analysis. J. Neurol. 2024, 271, 3039–3049. [Google Scholar] [CrossRef]
- Strbian, D.; Tsivgoulis, G.; Ospel, J.; Räty, S.; Cimflova, P.; Georgiopoulos, G.; Ullberg, T.; Arquizan, C.; Gralla, J.; Zeleňák, K.; et al. European Stroke Organisation and European Society for Minimally Invasive Neurological Therapy guideline on acute management of basilar artery occlusion. Eur. Stroke J. 2024, 9, 835–884. [Google Scholar] [CrossRef]
- Liu, X.; Dai, Q.; Ye, R.; Zi, W.; Liu, Y.; Wang, H.; Zhu, W.; Ma, M.; Yin, Q.; Li, M.; et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): An open-label, randomised controlled trial. Lancet Neurol. 2020, 19, 115–122. [Google Scholar] [CrossRef]
- Langezaal, L.C.M.; van der Hoeven, E.J.R.J.; Mont’Alverne, F.J.A.; de Carvalho, J.J.F.; Lima, F.O.; Dippel, D.W.J.; van der Lugt, A.; Lo, R.T.H.; Boiten, J.; Lycklama à Nijeholt, G.J.; et al. Endovascular Therapy for Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 2021, 384, 1910–1920. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Yoo, A.J.; Bell, D.; Barak, E.R.; Romero, J.M.; Nogueira, R.G.; Lev, M.H.; Schwamm, L.H.; Gonzalez, R.G.; Hirsch, J.A. CT angiography-source image hypoattenuation predicts clinical outcome in posterior circulation strokes treated with intraarterial therapy. Stroke 2008, 39, 3107–3109. [Google Scholar] [CrossRef]
- Jovin, T.G.; Li, C.; Wu, L.; Wu, C.; Chen, J.; Jiang, C.; Shi, Z.; Gao, Z.; Song, C.; Chen, W.; et al. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 2022, 387, 1373–1384. [Google Scholar] [CrossRef]
- Tao, C.; Nogueira, R.G.; Zhu, Y.; Sun, J.; Han, H.; Yuan, G.; Wen, C.; Zhou, P.; Chen, W.; Zeng, G.; et al. Trial of Endovascular Treatment of Acute Basilar-Artery Occlusion. N. Engl. J. Med. 2022, 387, 1361–1372. [Google Scholar] [CrossRef]
- Hu, W.; Tao, C.; Wang, L.; Chen, Z.; Li, D.; Chen, W.; Yi, T.; Xu, L.; Yu, C.; Wang, T.; et al. Intra-arterial tenecteplase after successful endovascular recanalisation in patients with acute posterior circulation arterial occlusion (ATTENTION-IA): Multicentre randomised controlled trial. BMJ 2025, 388, e080489. [Google Scholar] [CrossRef]
- Hu, W.; Nguyen, T.N.; Qureshi, M.; Chen, Z.; Tao, C.; Li, R.; Yi, T.Y.; Feng, G.; Su, J.; Cui, T.; et al. Noncontrast CT vs CT Perfusion Imaging in Patients with Basilar Artery Occlusion: Analysis of the ATTENTION and ATTENTION IA Trials. Neurology 2025, 105, e213911. [Google Scholar] [CrossRef]
- Heldner, M.R.; Zubler, C.; Mattle, H.P.; Schroth, G.; Weck, A.; Mono, M.L.; Gralla, J.; Jung, S.; El-Koussy, M.; Lüdi, R.; et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke 2013, 44, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Mazya, M.V.; Cooray, C.; Lees, K.R.; Toni, D.; Ford, G.A.; Bar, M.; Frol, S.; Moreira, T.; Sekaran, L.; Švigelj, V.; et al. Minor stroke due to large artery occlusion. When is intravenous thrombolysis not enough? Results from the SITS International Stroke Thrombolysis Register. Eur. Stroke J. 2018, 3, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Y.; Nogueira, R.G.; Rodrigues, G.M.; Kim, S.; Sharashidze, V.; Frankel, M.; Al-Bayati, A.; Bianchi, N.; Haussen, D.C. Acute Neurological Deterioration in Large Vessel Occlusions and Mild Symptoms Managed Medically. Stroke 2020, 51, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Seners, P.; Ben Hassen, W.; Lapergue, B.; Arquizan, C.; Heldner, M.R.; Henon, H.; Perrin, C.; Strambo, D.; Cottier, J.P.; Sablot, D.; et al. Prediction of Early Neurological Deterioration in Individuals with Minor Stroke and Large Vessel Occlusion Intended for Intravenous Thrombolysis Alone. JAMA Neurol. 2021, 78, 321–328. [Google Scholar] [CrossRef]
- Gwak, D.S.; Choi, W.; Kwon, J.A.; Shim, D.H.; Kim, Y.W.; Hwang, Y.H. Perfusion profile evaluated by severity-weighted multiple Tmax strata predicts early neurological deterioration in minor stroke with large vessel occlusion. J. Cereb. Blood Flow Metab. 2022, 42, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Dillon, W.P.; Liu, S.; Adler, F.; Smith, W.S.; Wintermark, M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann. Neurol. 2007, 61, 533–543. [Google Scholar] [CrossRef]
- Yogendrakumar, V.; Campbell, B.C.; Churilov, L.; Garcia-Esperon, C.; Choi, P.M.; Cordato, D.J.; Guha, P.; Sharma, G.; Chen, C.; McDonald, A.; et al. Extending the time window for tenecteplase by effective reperfusion of penumbral tissue in patients with large vessel occlusion: Rationale and design of a multicenter, prospective, randomized, open-label, blinded-endpoint, controlled phase 3 trial. Int. J. Stroke 2025, 20, 367–372. [Google Scholar]
- Sarraj, A.; Kleinig, T.J.; Hassan, A.E.; Portela, P.C.; Ortega-Gutierrez, S.; Abraham, M.G.; Manning, N.W.; Siegler, J.E.; Goyal, N.; Maali, L.; et al. Association of Endovascular Thrombectomy vs Medical Management with Functional and Safety Outcomes in Patients Treated Beyond 24 Hours of Last Known Well: The SELECT Late Study. JAMA Neurol. 2023, 80, 172–182. [Google Scholar] [CrossRef]

| Off-Label, Guideline-Based IVT Treatment Considerations | Off-Label, Non-Guideline-Based IVT Treatment Considerations | Non-Guideline-Based EVT Treatment Considerations |
|---|---|---|
| IVT with alteplase in wake-up/unknown time stroke onset presenting within 4.5 h after symptom recognition, with DWI/FLAIR MRI mismatch (ESO, AHA/ASA guidelines) | IVT with tenecteplase ≤ 24 h from symptom onset/last seen well and presence of CTP/MRP mismatch | EVT ≤ 24 h from symptom onset/last seen well based on NCCT (ASPECTS ≥ 6) |
| IVT with alteplase ≤ 9 h from stroke onset and presence of CTP/MRP mismatch (ESO guidelines) | EVT for large core (ASPECTS ≤ 5) AIS ≤ 24 h from symptom onset/last seen well | |
| Bridging therapy (IVT with alteplase plus EVT) in wake-up/unknown time stroke onset presenting within 4.5 h after symptom recognition, with DWI/FLAIR MRI mismatch and LVO (ESO guidelines) | EVT > 24 h from symptom onset/last seen well, with salvageable brain tissue | |
| If presenting in a non-thrombectomy center, IVT with alteplase ≤ 9 h from stroke onset in the presence of CTP/MRP mismatch and LVO, as well as transfer to a thrombectomy-capable center (ESO guidelines) | ||
| IVT for BAO ≤ 24 h without extensive bilateral and/or brainstem ischemic changes (pc-ASPECTS ≥ 7) on CT or MRI (ESO/ESMINT guidelines) |
| RCT | Intervention | Inclusion Criteria | Key Exclusion Criteria | Primary Outcome | Key Secondary Outcomes |
|---|---|---|---|---|---|
| BRIDGE-TNK [26] | iv TNK plus EVT vs. EVT |
|
| mRS 0–2 (90 d): TNK/EVT 52.9% vs. EVT 44.1% (unadjusted risk ratio, 1.20; 95% confidence interval, 1.01–1.43; p = 0.04) |
|
| TWIST [29] | TNK vs. no IVT based on NCCT |
|
| mRS (90 d): adjusted OR 1.18, 95% CI 0.88–1.58; p = 0.27 |
|
| TEMPO 2 [30] | TNK vs. no IVT based on Multiphase CTA or CTP |
|
| return to baseline functioning on pre-morbid mRS (90 d): TNK 72% vs. control 75% (risk ratio [RR] 0.96, 95% CI 0.88–1.04, p = 0.29) |
|
| EXTEND [33] | ALT vs. no IVT based on perfusion imaging |
|
| mRS 0–1 (90 d): ALT 35.4% vs. placebo 29.5% (adjusted risk ratio, 1.44; 95% CI, 1.01 to 2.06; p = 0.04) |
|
| WAKE-UP [38] | ALT vs. no IVT based on MRI |
|
| mRS 0–1 (90 d): ALT 53.3% placebo 41.8% (adjusted odds ratio, 1.61; 95% CI, 1.09 to 2.36; p = 0.02) |
|
| ROSE-TNK [39] | TNK vs. no IVT based on MRI |
|
| mRS 0–1 (90 d): TNK 52.5% vs. control 50.0% (unadjusted odds ratio, 1.11; 95% confidence interval 0.46–2.66; p = 0.82) |
|
| TIMELESS [40] | TNK vs. no IVT based on CTP |
|
| ordinal score on the mRS (90 d): adjusted common odds ratio for TNK vs. placebo 1.13 (95% CI, 0.82–1.57; p = 0.45) |
|
| TRACE-III [41] | TNK vs. no IVT based on CTP |
|
| mRS 0–1 (90 d): TNK 33.0% vs. no IVT 24.2% (relative rate, 1.37; 95% CI, 1.04–1.81; p = 0.03) |
|
| CHABLIS-T [42] | TNK vs. no IVT based on CTP |
|
| restoration of blood flow of >50% of the involved ischemic territory: TNK 33.3% vs. no IVT 10.8% (adjusted relative risk, 3.0; 95% CI, 1.6–5.7; p = 0.001) |
|
| HOPE [44] | ALT vs. no IVT based on CTP |
|
| mRS 0–1 (90 d): ALT 40% vs. control 26% (adjusted risk ratio, 1.52; 95%CI, 1.14–2.02; p = 0.004) |
|
| ESCAPE [47] | EVT vs. no EVT |
|
| mRS shift analysis (90 d): common odds ratio EVT vs. no EVT, 2.6; 95% CI, 1.7–3.8; p < 0.001 |
|
| MR CLEAN-LATE [53] | EVT vs. no EVT based on CTA |
|
| Median mRS (90 d): EVT 3 (IQR 2–5) vs. control 4 (2–6), (adjusted common OR 1.67, 95% CI, 1.20–2.32) |
|
| DEFUSE 3 [54] | EVT vs. no EVT based on perfusion imaging |
|
| ordinal score on mRS (90 d): EVT vs. control odds ratio, 2.77; p < 0.001 |
|
| DAWN [55] | EVT vs. no EVT based on MRI or perfusion imaging |
|
| mean score on the utility-weighted mRS (90 d): EVT 5.5 vs. control 3.4 (adjusted difference 2.0 points; 95% credible interval, 1.1 to 3.0; posterior probability of superiority, >0.999 mRS 0–2 (90 d): EVT 49% vs. control 13% (adjusted difference, 33 percentage points; 95% credible interval, 24 to 44; posterior probability of superiority, >0.999) |
|
| RESCUE-Japan [58] | EVT vs. no EVT based on CT or MRI |
|
| mRS 0–3 (90 d): EVT 31.0% vs. control 12.7% (relative risk, 2.43; 95% CI, 1.35–4.37; p = 0.002) |
|
| ANGEL-ASPECT [59] | EVT vs. no EVT based on CT or MRI or CTP |
|
| shift in the distribution of the mRS scores (90d): generalized odds ratio, 1.37; 95% CI, 1.11–1.69; p = −2 0.004 |
|
| SELECT2 [60] | EVT vs. no EVT based on CT or MRI or CTP |
|
| shift in the distribution of mRS (90 d): EVT 4 (3–6) vs. control 5 (4–6), generalized odds ratio 1.51 (95% CI, 1.20–1.89; p < 0.001 |
|
| TENSION [61] | EVT vs. no EVT based on CT or MRI |
|
| shift in the distribution of mRS (90 d): EVT 4 (3–6) vs. control 6 (4–6), adjusted common OR 2.58, 95% CI, 1.60–4.15, p = 0.0001 |
|
| LASTE [62] | EVT vs. no EVT based on CT or MRI |
|
| shift in the distribution of mRS (90 d): EVT 4 (3–6) vs. control 6 (4–6), generalized odds ratio, 1.63; 95% CI, 1.29–2.06; p < 0.001 |
|
| TESLA [63] | EVT vs. no EVT based on CT |
|
| Improvement in 90 d functional outcome measured using mean utility-weighted mRS scores: mean EVT 2.93 (3.39) vs. control 2.27 (2.98), adjusted difference 0.63 (95% credible interval, −0.09 to 1.34; posterior probability for superiority of EVT, 0.96 |
|
| DISTAL [66] | EVT vs. no EVT |
|
| Distribution of mRS (90 d): common odds ratio for improvement, 0.90; 95% CI, 0.67–1.22; p = 0.50 |
|
| ESCAPE-MeVO [67] | EVT vs. no EVT based on CTA, CTP or MRI |
|
| mRS 0–1 (90 d): EVT 41.6% vs. 43.1% BMT (adjusted rate ratio, 0.95; 95% CI, 0.79–1.15; p = 0.61 |
|
| DISCOUNT [68] | EVT vs. no EVT |
|
| mRS 0–2 (90 d): EVT 60% vs. 77% BMT (OR, 0.42; 95% CI, 0.2–0.88; p = 0.029) |
|
| EXPERTS [72] | ALT vs. standard treatment for posterior circulation stroke |
|
| mRS 0–2 (90 d): ALT 89.6% vs. control 72.6%, (adjusted risk ratio, 1.16; 95% CI, 1.03–1.30; p = 0.01) |
|
| BEST [80] | EVT vs. BMT |
| mRS 0–3 (90 d): intention-to-treat EVT 42% vs. BMT 32%, (adjusted OR 1.74, 95% CI, 0.81–3.74) |
| |
| BASICS [81] | EVT vs. BMT |
|
| mRS 0–3 (90 d): EVT 44.2% vs. BMT 37.7%, (risk ratio, 1.18; 95% CI, 0.92–1.50) |
|
| BAOCHE [83] | EVT vs. BMT |
|
| mRS 0–3 (90 d): EVT 46% vs. BMT 24%, (adjusted rate ratio, 1.81; 95% CI, 1.26–2.6, p < 0.001) |
|
| ATTENTION [84] | EVT vs. BMT |
|
| mRS 0–3 (90 d): EVT 46% vs. BMT 23%, (adjusted rate ratio, 2.06; 95% CI, 1.46–2.91, p < 0.001) |
|
| Trial ID (Name) | Country | Status | Time from Symptom Onset | Intervention | Control | NIHSS | Age | Key Exclusion Criteria | Imaging |
|---|---|---|---|---|---|---|---|---|---|
| NCT06010628 (EXIT-BT2) | China | Recruiting | 4.5–6 h | IV tenecteplase | Standard stroke care | ≥4 | >18 | Planned IVT based on WAKE-UP or EXTEND criteria | NCCT |
| NCT06559436 (TNK-MeVO) | China | Recruiting | 4.5–24 h | IV tenecteplase | Standard stroke care | ≥4 | >18 | Planned EVT | DMVO (M1–M4, A1–A4, P1–P4), <50% core in vascular territory on NCCT, DWI-MRI, or CTP (>6 h) |
| NCT05199662 (RESILIENT EXTEND-IV) | Brazil | Recruiting | 4.5–12 h | IV tenecteplase | Placebo | ≥4 or cortical neurological deficit | >18 | Proximal arterial occlusion (+ dominant M2 > 50% MCA territory) | CT/MRI with <50% territory involvement OR Core < 50 cc (NCCT/CTP/DWI), Mismatch Vol > 10 cc, Ratio > 1.4 |
| NCT05752916 (OPTION) | China | Recruiting | 4.5–24 h | IV tenecteplase | Antiplatelet agents | 6–25 | >18 | Proximal LVO | Core < 50 cc, mismatch ratio ≥ 1.2, mismatch volume ≥ 10 cc |
| NCT04454788 (ETERNAL-LVO) | Australia | Terminated | <24 h | IV tenecteplase + EVT | EVT ± IV alteplase | Non-minor symptoms | >18 | Basilar artery occlusion | LVO, CTP/DWI mismatch: Core < 70 mL, penumbra > 20 mL, ratio > 1.8 |
| NCT05105633 (POST-ETERNAL) | Australia | Recruiting | <24 h | IV tenecteplase ± EVT | IV alteplase or no IVT ± EVT | Not reported | >18 | Extensive brainstem ischemia or frank hypodensity on NCCT | pc-ASPECTS ≥ 7, Basilar artery occlusion |
| NCT07094763 | China | Not yet recruiting | 4.5–24 h | IV tenecteplase | Standard medical therapy | ≥3 | >18 | Planning EVT | MRI-confirmed posterior circulation infarct OR hypoperfusion OR occlusion of posterior circulation vessel |
| BI 1123-0060 TENACITY NCT non available | International | Not yet recruiting | >4.5 h | IV tenecteplase | placebo | Disabling stroke | - | - | Salvageable ischemic tissue/perfusion imaging |
| RCT | NCT06654375 | NCT06560203 (BAOCHE2) | NCT05326932 (LATE-MT) | SELECT-LATE NCT Non-Available |
|---|---|---|---|---|
| Country | China | China | China | International (US, Europe, Australia) |
| Status | recruiting | recruiting | recruiting | Not yet recruiting |
| Time from symptom onset | 24–72 h | 24–72 h | 24–72 h | 24–72 h |
| Intervention | EVT | EVT | EVT | EVT |
| Control | best medical management | standard stroke care | standard medical care | best medical management |
| NIHSS | ≥2 | ≥6 | ≥6 | ≥6 |
| Age | >18 | >18 and ≤80 | >18 | 18–85 |
| Key exclusion criteria | treated with alteplase > 4.5 h after last known well |
| EVT attempted after stroke onset | Eligible for thrombectomy or medical management |
| Imaging |
| Occlusion of the basilar artery or intracranial segments of both vertebral arteries |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargiotis, O.; Psychogios, K.; Safouris, A.; Chroni, E.; Zampakis, P.; Panagiotopoulos, V.; Ellul, J.; Tsivgoulis, G. Tailored Treatment of Acute Ischemic Stroke: A Narrative Review of Evidence-Based Strategies by Imaging Type and Thrombectomy Availability. Medicina 2025, 61, 1700. https://doi.org/10.3390/medicina61091700
Kargiotis O, Psychogios K, Safouris A, Chroni E, Zampakis P, Panagiotopoulos V, Ellul J, Tsivgoulis G. Tailored Treatment of Acute Ischemic Stroke: A Narrative Review of Evidence-Based Strategies by Imaging Type and Thrombectomy Availability. Medicina. 2025; 61(9):1700. https://doi.org/10.3390/medicina61091700
Chicago/Turabian StyleKargiotis, Odysseas, Klearchos Psychogios, Apostolos Safouris, Elisabeth Chroni, Petros Zampakis, Vasileios Panagiotopoulos, John Ellul, and Georgios Tsivgoulis. 2025. "Tailored Treatment of Acute Ischemic Stroke: A Narrative Review of Evidence-Based Strategies by Imaging Type and Thrombectomy Availability" Medicina 61, no. 9: 1700. https://doi.org/10.3390/medicina61091700
APA StyleKargiotis, O., Psychogios, K., Safouris, A., Chroni, E., Zampakis, P., Panagiotopoulos, V., Ellul, J., & Tsivgoulis, G. (2025). Tailored Treatment of Acute Ischemic Stroke: A Narrative Review of Evidence-Based Strategies by Imaging Type and Thrombectomy Availability. Medicina, 61(9), 1700. https://doi.org/10.3390/medicina61091700








