Evaluation of Endoscopic Findings in Gastrointestinal Tract Wall Thickening Detected on Abdominal Radiological Imaging: A Two-Center Retrospective Descriptive Study
Abstract
1. Introduction
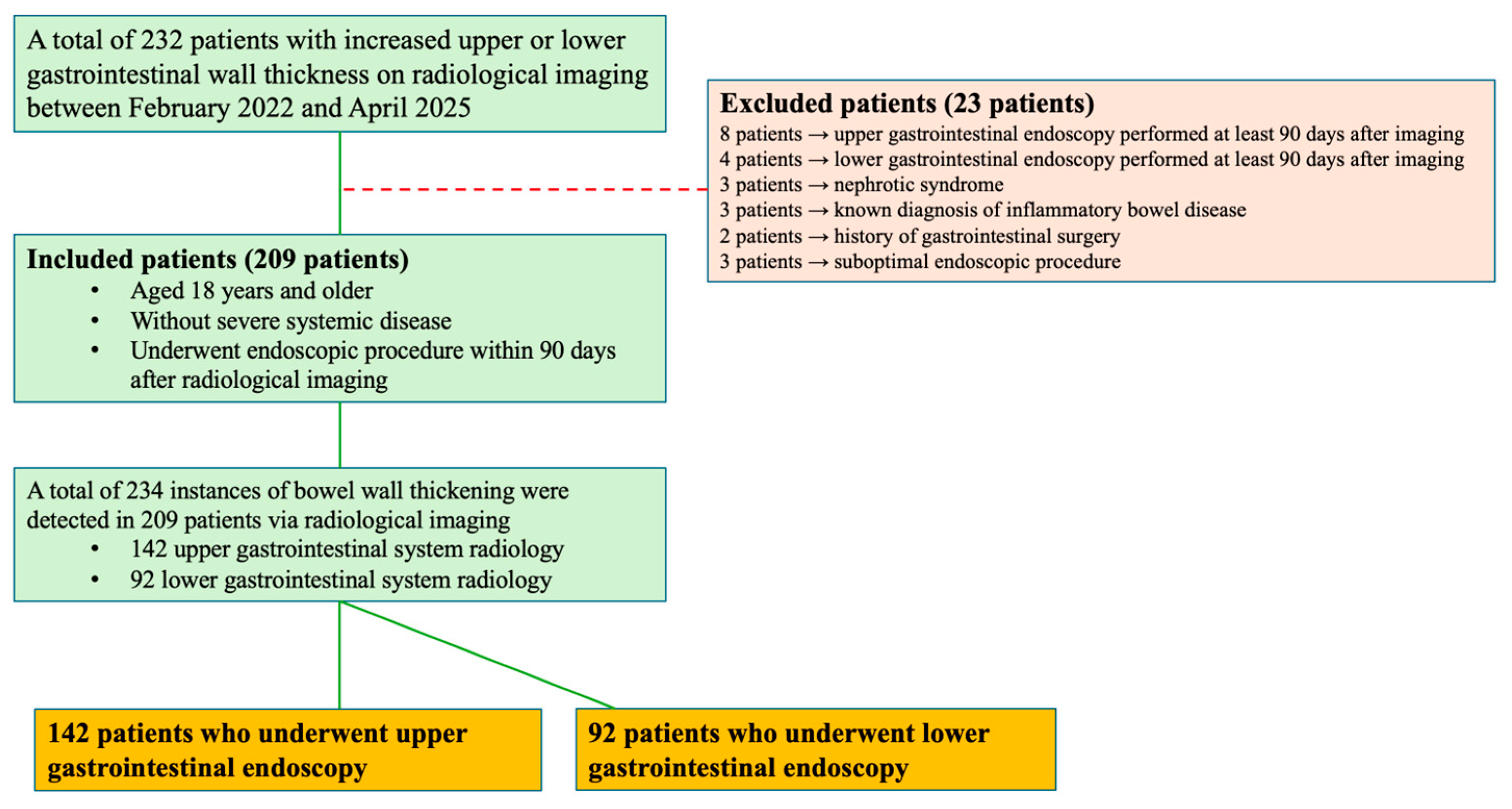
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| GI | Gastrointestinal |
| HGB | Hemoglobin |
| IBD | Inflammatory Bowel Disease |
| MRI | Magnetic Resonance Imaging |
| NPV | Negative Predictive Value |
| PPV | Positive Predictive Value |
| ROC | Receiver Operating Characteristic |
| USG | Ultrasonography |
References
- Idrees, Z.; Khan, H.T.; Khan, U. Endoscopic Significance of Incidental Upper Gastrointestinal Wall Thickness Detected on Computed Tomography Scans. Cureus 2024, 16, 11. [Google Scholar] [CrossRef]
- Alali, A.A.; Alhashmi, A.; Alotaibi, N.; Ali, N.; Alali, M.; Alfadhli, A. Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial. J. Clin. Med. 2025, 14, 581. [Google Scholar] [CrossRef] [PubMed]
- Lnu, R.; Khanduri, S.; Khan, Z.; Ansari, D.; Mulani, M.; Gupta, A.; Alam, N.; Aggarwal, A.; Lnu, S.; Agrawal, A. A Study of Small and Large Bowel Wall Thickness Using Computed Tomography and Its Histopathological Correlation. Cureus 2024, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.M.; Craig, D.A.; Lowe, V.J.; MacCarty, R.L. Radiology of the esophagus: Barium, computed tomography scan, positron emission tomography scan, magnetic resonance imaging. In Shackelford’s Surgery of the Alimentary Tract, 2nd ed.; Yeo, C.J., Ed.; Elsevier: Philadelphia, PA, USA, 2019; Volume 1, pp. 57–84. [Google Scholar]
- Gore, R.M.; Levine, M.S. Textbook of Gastrointestinal Radiology; E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Gourtsoyiannis, N.; Daskalogiannaki, P.P.M.; McLaughlin, P.; Maher, M.M. The duodenum and small intestine. In Grainger & Allison’s Diagnostic Radiology: Abdominal Imaging, 6th ed.; Adam, A., Dixon, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, p. 64. [Google Scholar]
- Gür, H.Ü.; Koyuncu, A. What should we expect when colon wall thickening is detected on abdominal CT scan in the era of artificial intelligence? Ann. Ital. Chir. 2023, 94, 472–477. [Google Scholar]
- Takahara, M.; Hiraoka, S.; Ohmori, M.; Takeuchi, K.; Takei, K.; Yasutomi, E.; Igawa, S.; Yamamoto, S.; Yamasaki, Y.; Inokuchi, T.; et al. The colon wall thickness measured using transabdominal ultrasonography is useful for detecting mucosal inflammation in ulcerative colitis. Intern. Med. 2022, 61, 2703–2709. [Google Scholar] [CrossRef] [PubMed]
- Bas, B.O.; Pakoz, Z.B. Endoscopic evaluation of patients with colonic wall thickening detected on computed tomography. Acta Clin. Croat. 2020, 59, 463. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Halvorsen Jr, R.A.; Higgins, J.L.; Cello, J.P. Prospective evaluation of patients with bowel wall thickening. Am. J. Gastroenterol. Springer Nat. 1995, 90, 99–103. [Google Scholar]
- Moraitis, D.; Singh, P.; Jayadevan, R.; Cayten, C.G. Colonic Wall Thickening on Computed Tomography Scan and Clinical Correlation. Does it Suggest the Presence of an Underlying Neoplasia? Am. Surg. 2006, 72, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.H.; Rubin, A.; Potter, J.D.; Lattimore, W.; Resnick, M.B.; Murphy, B.L.; Moss, S.F. Clinical significance of colonoscopic findings associated with colonic thickening on computed tomography: Is colonoscopy warranted when thickening is detected? J. Clin. Gastroenterol. 2008, 42, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Akbas, A.; Bakir, H.; Dasiran, M.F.; Dagmura, H.; Ozmen, Z.; Celtek, N.Y.; Daldal, E.; Demir, O.; Kefeli, A.; Okan, I. Significance of Gastric Wall Thickening Detected in Abdominal CT Scan to Predict Gastric Malignancy. J. Oncol. 2019, 2019, 8581547. [Google Scholar] [CrossRef] [PubMed]
- Somuncu, E.; Topal, Ü.; Sönmez, S.; Kara, Y.; Bozdağ, E.; Özcan, A.; Başaran, C.; Özkan, C.; Tatlıdil, Y.E.; Kalaycı, M.U. Incidentally detected gastrointestinal wall thickness on abdominal computed tomography; what does it mean for endoscopy? Arch. Iran. Med. 2021, 24, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Baş, B.; Pakoz, Z.B.; Oymacı, E. Endoscopic evaluation of patients with gastric wall thickening detected with computed tomography. Laparosc. Endosc. Surg. Sci. 2019, 26, 1–4. [Google Scholar] [CrossRef]
- Chandrapalan, S.; Tahir, F.; Kimani, P.; Sinha, R.; Arasaradnam, R. Systematic review and meta-analysis: Does colonic mural thickening on CT correlate with endoscopic findings at colonoscopy? Frontline Gastroenterol. 2018, 9, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, F.; Özgökçe, M.; Toprak, N.; Oğuzlar, F.Ç.; Göya, C. The comparison of wall thickness of esophagus and gastroesophageal junction using computed tomography with endoscopy and biopsy results. Turk. J. Thorac. Cardiovasc. Surg. 2020, 28, 488. [Google Scholar] [CrossRef] [PubMed]
- Tongdee, R.; Kongkaw, L.; Tongdee, T. A study of wall thickness of gastric antrum: Comparison among normal, benign and malignant gastric conditions on MDCT scan. J. Med. Assoc. Thai. 2012, 95, 1441. [Google Scholar] [PubMed]
- Khairnar, H.; Ingle, M.; Chauhan, S.; Pipalia, N.; Sawant, P.; Pandey, V.; Shukla, A. Correlation of Computed Tomography of Colonic Wall Thickening with Colonoscopy. J. Assoc. Physicians. India 2019, 67, 18–21. [Google Scholar] [PubMed]
- Uzzaman, M.; Alam, A.; Nair, M.; Borgstein, R.; Meleagros, L. Computed tomography findings of bowel wall thickening: Its significance and relationship to endoscopic abnormalities. Ann. R Coll. Surg. Engl. 2012, 94, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Akbas, A.; Bakir, H.; Dasiran, M.F.; Dagmura, H.; Daldal, E.; Ozsoy, Z.; Ozmen, Z.; Demir, O.; Okan, I. Colonic Wall Thickening Reported in Abdominal CT: Does It Always Imply Malignancy? Gastroenterol. Res. Pract. 2019, 2019, 2492097. [Google Scholar] [CrossRef] [PubMed]
- Daniel, F.; Alsheikh, M.; Ghieh, D.; Hosni, M.; Tayara, Z.; Tamim, H.; Abi-Ghanem, A.S.; El-Merhi, F. Bowel wall thickening on computed tomography scan: Inter-observer agreement and correlation with endoscopic findings. Arab. J. Gastroenterol. 2020, 21, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rana, S.S.; Nada, R.; Kalra, N.; Sharma, R.K.; Dutta, U.; Gupta, R. Significance of ileal and/or cecal wall thickening on abdominal computed tomography in a tropical country. JGH Open 2019, 3, 46–51. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) or Mean (Min–Max) |
|---|---|
| Age median years (min-max) | 59 (18–86) |
| Sex n (%) | 209 * (100.0) |
| Female | 115 (55.0) |
| Male | 94 (45.0) |
| BMI | |
| <18.5 | 9 (4.3) |
| 18.5–24.9 | 70 (33.5) |
| 25–29.9 | 94 (45.0) |
| >30 | 36 (17.2) |
| ASA physical status | |
| ASA I | 40 (19.1) |
| ASA II | 115 (55.0) |
| ASA III | 54 (25.8) |
| Comorbid diseases n (%) | 146 (69.9) |
| Diabetes mellitus | 43 (20.6) |
| Hypertension | 84 (40.2) |
| Hyperlipidemia | 18 (8.6) |
| Cardiovascular disease | 36 (17.2) |
| Respiratory disease | 32 (15.3) |
| Neuropsychiatric disorder | 15 (7.2) |
| Extra-GI cancer | 17 (8.1) |
| Other (Rheumatologic, urinary system, ...) | 38 (18.2) |
| Radiological imaging modality in which wall thickening was detected | 234 * (100.0) |
| Upper GI tract | 142 (60.7) |
| CT | 74 (31.6) |
| USG | 67 (28.6) |
| MRI | 1 (0.4) |
| Lower GI tract | 92 (39.3) |
| CT | 70 (29.9) |
| USG | 20 (8.5) |
| MRI | 2 (0.9) |
| Hemoglobin (g/dL) (min-max) | 13.2 (5.9–17.6) |
| CRP (mg/L) (min-max) | 4 (0.2–245) |
| Albumin (g/L) | 42.1 (14.7–52.0) |
| WBC (×109/L) | 7.5 (3.2–15.8) |
| Radiological imaging—esophagogastroduodenoscopy interval | 24.5 (1–88) |
| Radiological imaging—ileocolonoscopy interval | 32.5 (1–87) |
| Number of Patients | 142 |
|---|---|
| Endoscopic finding | n (%) |
| Normal | 4 (2.8) |
| Mass in the esophagus | - |
| Non-reflux esophagitis | - |
| Reflux esophagitis | 26 (18.3) |
| Hiatal hernia | 8 (5.6) |
| Malignancy | 12 (8.5) |
| Cardia | 3 (2.1) |
| Fundus | - |
| Corpus | 5 (3.5) |
| Angulus | 1 (0.7) |
| Antrum | 3 (2.1) |
| Polyp | 9 (6.3) |
| Gastritis/erosion | 89 (62.7) |
| Peptic ulcer | 9 (6.3) |
| Gastric | 8 (5.6) |
| Duodenal | 1 (0.7) |
| Duodenitis/celiac disease | 4 (2.8) |
| Mass in the duodenum | 2 (1.4) |
| Other | 3 (2.1) |
| Number of Patients | 92 |
|---|---|
| Endoscopic finding | n (%) |
| Normal | 33 (35.9) |
| Malignancy | 10 (10.9) |
| Cecum | 1 (1.1) |
| Ascending colon | 1 (1.1) |
| Transverse colon | - |
| Descending colon | - |
| Sigmoid colon | 3 (3.3) |
| Rectum | 5 (5.4) |
| Anal canal | - |
| Terminal ileitis | 4 (4.3) |
| Diverticulum | 11 (12.0) |
| Polyp | 20 (21.7) |
| IBD | 9 (9.8) |
| Non-IBD colitis | 7 (7.6) |
| Ulcer | 1 (1.1) |
| Other | 3 (3.3) |
| IBD: Inflammatory bowel disease | |
| Localization and Finding | n (%) |
|---|---|
| Upper GI tract | 21/142 * (14.7) |
| Malignancy | 12 (8.5) |
| Cardia | 3 (2.1) |
| Fundus | - |
| Corpus | 5 (3.5) |
| Angulus | 1 (0.7) |
| Antrum | 3 (2.1) |
| Polyp | 9 (6.3) |
| Neoplastic | 2 (1.4) |
| Non-neoplastic | 7 (4.9) |
| Lower GI tract | 30/92 * (32.6) |
| Malignancy | 10 (10.9) |
| Cecum | 1 (1.1) |
| Ascending colon | 1 (1.1) |
| Transverse colon | - |
| Descending colon | - |
| Sigmoid colon | 3 (3.3) |
| Rectum | 5 (5.4) |
| Anal canal | - |
| Polyp | 20 (21.7) |
| Neoplastic | 14 (15.2) |
| Non-neoplastic | 6 (6.5) |
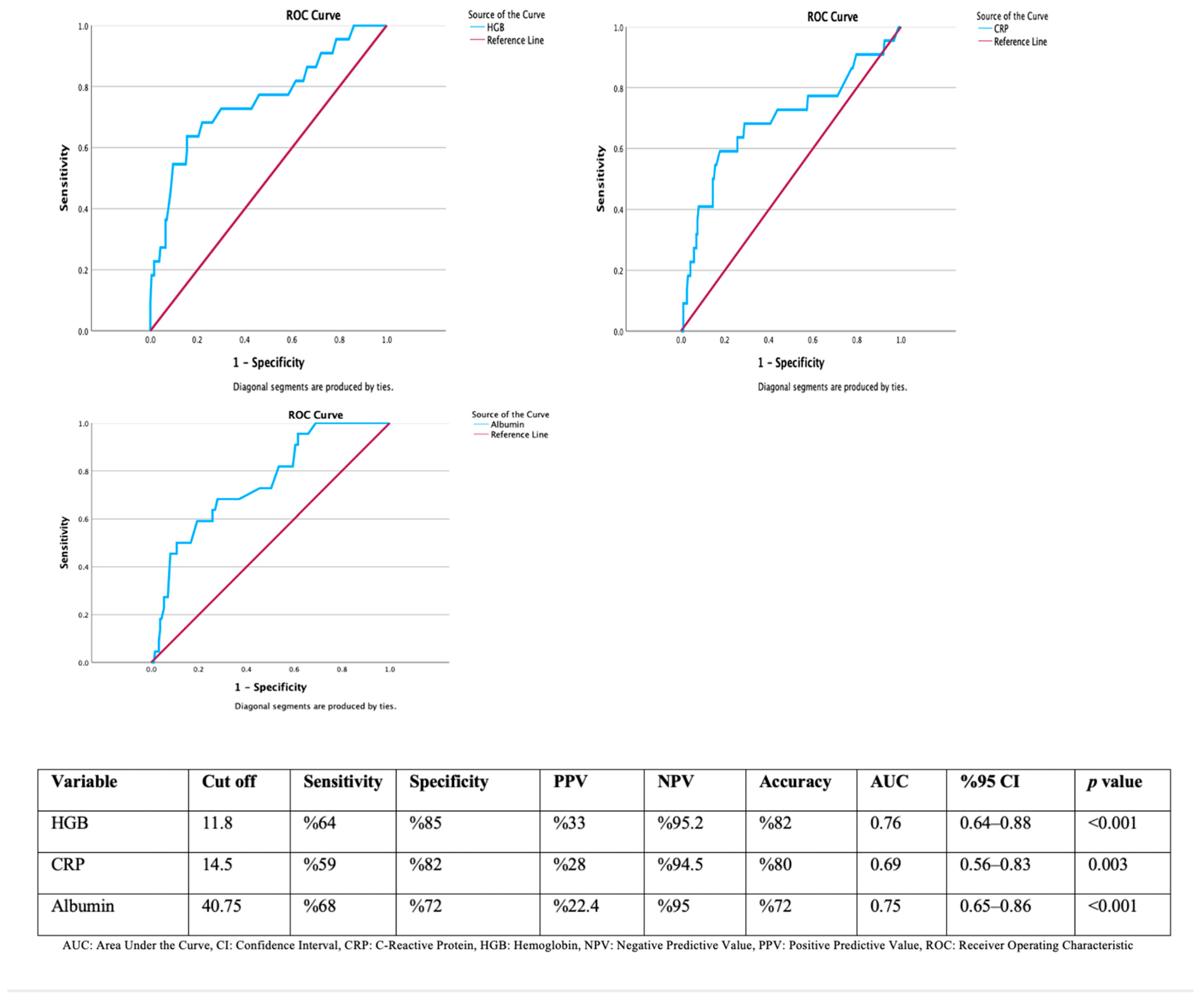
| No Upper GI Tract Malignancy (n = 130) | Upper GI Tract Malignancy Present (n = 12) | p Value | |
|---|---|---|---|
| Age median years (min-max) | 60 (21–86) | 62 (39–81) | 0.413 * |
| Hemoglobin (g/dL) (min-max) | 13.1 (8.8–17.5) | 10.8 (5.9–13.9) | <0.001 * |
| CRP (mg/L) (min-max) | 4 (0.2–187) | 22.5 (1.7–122) | 0.015 * |
| Albumin | 42.2 (14.7–52) | 37.5 (28–44) | 0.005 * |
| No lower GI tract malignancy (n = 82) | Lower GI tract malignancy present (n = 10) | p value | |
| Age median years (min-max) | 56 (18–83) | 68.5 (34–77) | 0.081 * |
| Hemoglobin (g/dL) (min-max) | 13.6 (9–17.6) | 11 (8.8–15.3) | 0.033 * |
| CRP (mg/L) (min-max) | 4 (0.4–245) | 16.7 (0.4–121) | 0.115 * |
| Albumin | 42.2 (18–50) | 39 (22–43) | 0.015 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergin, M.; Kıvrakoğlu, F. Evaluation of Endoscopic Findings in Gastrointestinal Tract Wall Thickening Detected on Abdominal Radiological Imaging: A Two-Center Retrospective Descriptive Study. Medicina 2025, 61, 1699. https://doi.org/10.3390/medicina61091699
Ergin M, Kıvrakoğlu F. Evaluation of Endoscopic Findings in Gastrointestinal Tract Wall Thickening Detected on Abdominal Radiological Imaging: A Two-Center Retrospective Descriptive Study. Medicina. 2025; 61(9):1699. https://doi.org/10.3390/medicina61091699
Chicago/Turabian StyleErgin, Mustafa, and Fatih Kıvrakoğlu. 2025. "Evaluation of Endoscopic Findings in Gastrointestinal Tract Wall Thickening Detected on Abdominal Radiological Imaging: A Two-Center Retrospective Descriptive Study" Medicina 61, no. 9: 1699. https://doi.org/10.3390/medicina61091699
APA StyleErgin, M., & Kıvrakoğlu, F. (2025). Evaluation of Endoscopic Findings in Gastrointestinal Tract Wall Thickening Detected on Abdominal Radiological Imaging: A Two-Center Retrospective Descriptive Study. Medicina, 61(9), 1699. https://doi.org/10.3390/medicina61091699






