Visceral Adiposity Index (VAI) Levels and Metabolic Risk Across Phenotypes of Polycystic Ovary Syndrome (PCOS)
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VAI | Visceral adiposity index |
| PCOS | Polycystic ovary syndrome |
| HA | Hyperandrogenism |
| OA | Oligo-anovulation |
| PCOM | Polycystic ovarian morphology |
| BMI | Body mass index |
| HDL | High-density lipoprotein |
| MetS | Metabolic syndrome |
| ROC | Receiver operating characteristic |
| USG | Ultrasonography |
| PCO | Polycystic ovaries |
| HT | Hypertension |
| IR | Insulin resistance |
| DM | Type 2 diabetes mellitus |
| WC | Waist circumference |
| CT | Computerized tomography |
| DXA | Dual-energy X-ray absorptiometry |
| NCEP-ATP | National Cholesterol Education Program Adult Treatment Panel |
| HOMA-IR | Homeostasis Model Assessment—Insulin Resistance |
| TG | Triglyceride |
| NR | Normal range |
| SD | Standard deviation |
| AUC | Area under curve |
| CI | Confidence interval |
| LAP | Lipid accumulation product |
| MH-PCOS | Metabolically healthy polycystic ovary syndrome |
| MU-PCOS | Metabolically unhealthy polycystic ovary syndrome |
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Brower, M.; Brennan, K.; Pall, M.; Azziz, R. The severity of menstrual dysfunction as a predictor of insulin resistance in PCOS. J. Clin. Endocrinol. Metab. 2013, 98, E1967–E1971. [Google Scholar] [CrossRef] [PubMed]
- Landay, M.; Huang, A.; Azziz, R. Degree of hyperinsulinemia, independent of androgen levels, is an important determinant of the severity of hirsutism in PCOS. Fertil. Steril. 2009, 92, 643–647. [Google Scholar] [CrossRef]
- Reaven, G.M. Syndrome X: Is one enough? Am. Heart J. 1994, 127, 1439–1442. [Google Scholar] [CrossRef]
- Rojas, J.; Chavez, M.; Olivar, L.; Rojas, M.; Morillo, J.; Mejias, J.; Calvo, M.; Bermudez, V. Polycystic ovary syndrome, insulin resistance, and obesity: Navigating the pathophysiologic labyrinth. Int. J. Reprod. Med. 2014, 2014, 719050. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Azziz, R. Epidemiology, Phenotype, and Genetics of the Polycystic Ovary Syndrome in Adults. Available online: https://www.uptodate.com/contents/epidemiology-phenotype-and-genetics-of-the-polycystic-ovary-syndrome-in-adults?search=polycystic%20ovarian%20syndrome%20subtype&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1 (accessed on 18 August 2020).
- Lee, H.; Oh, J.Y.; Sung, Y.A.; Chung, H. Is insulin resistance an intrinsic defect in asian polycystic ovary syndrome? Yonsei Med. J. 2013, 54, 609–614. [Google Scholar] [CrossRef]
- Lim, S.S.; Davies, M.J.; Norman, R.J.; Moran, L.J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 618–637. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Massaro, J.M.; Rosito, G.A.; Levy, D.; Murabito, J.M.; Wolf, P.A.; O’Donnell, C.J.; Fox, C.S.; Hoffmann, U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur. Heart J. 2009, 30, 850–856. [Google Scholar] [CrossRef]
- Lim, S.S.; Norman, R.J.; Davies, M.J.; Moran, L.J. The effect of obesity on polycystic ovary syndrome: A systematic review and meta-analysis. Obes. Rev. An. Off. J. Int. Assoc. Study Obes. 2013, 14, 95–109. [Google Scholar] [CrossRef]
- Glintborg, D.; Petersen, M.H.; Ravn, P.; Hermann, A.P.; Andersen, M. Comparison of regional fat mass measurement by whole body DXA-scans and anthropometric measures to predict insulin resistance in women with polycystic ovary syndrome and controls. Acta Obstet. Gynecol. Scand. 2016, 95, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Rossner, S.; Bo, W.J.; Hiltbrandt, E.; Hinson, W.; Karstaedt, N.; Santago, P.; Sobol, W.T.; Crouse, J.R. Adipose tissue determinations in cadavers--a comparison between cross-sectional planimetry and computed tomography. Int. J. Obes. 1990, 14, 893–902. [Google Scholar] [PubMed]
- Sasai, H.; Brychta, R.J.; Wood, R.P.; Rothney, M.P.; Zhao, X.; Skarulis, M.C.; Chen, K.Y. Does Visceral Fat Estimated by Dual-Energy X-ray Absorptiometry Independently Predict Cardiometabolic Risks in Adults? J. Diabetes Sci. Technol. 2015, 9, 917–924. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study, G. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Amato, M.C.; Guarnotta, V.; Forti, D.; Donatelli, M.; Dolcimascolo, S.; Giordano, C. Metabolically healthy polycystic ovary syndrome (MH-PCOS) and metabolically unhealthy polycystic ovary syndrome (MU-PCOS): A comparative analysis of four simple methods useful for metabolic assessment. Hum. Reprod. 2013, 28, 1919–1928. [Google Scholar] [CrossRef][Green Version]
- Liu, P.J.; Ma, F.; Lou, H.P.; Chen, Y. Visceral Adiposity Index Is Associated with Pre-Diabetes and Type 2 Diabetes Mellitus in Chinese Adults Aged 20-50. Ann. Nutr. Metab. 2016, 68, 235–243. [Google Scholar] [CrossRef]
- Vogel, P.; Stein, A.; Marcadenti, A. Visceral adiposity index and prognosis among patients with ischemic heart failure. Sao Paulo Med. J. 2016, 134, 211–218. [Google Scholar] [CrossRef]
- Durmus, U.; Duran, C.; Ecirli, S. Visceral adiposity index levels in overweight and/or obese, and non-obese patients with polycystic ovary syndrome and its relationship with metabolic and inflammatory parameters. J. Endocrinol. Investig. 2017, 40, 487–497. [Google Scholar] [CrossRef]
- Ganie, M.A.; Malhotra, N.; Jabbar, P.K.; Aggarwal, S.; Rozati, R.; Sahay, R.; Chowdhary, S.; Kamboj, S.; Wani, I.A.; Arora, T. Cardiometabolic markers and serum amh levels in PCOS: Can AMH serve as a surrogate cardiometabolic markeR? BMC Women’s Health 2025, 25, 106. [Google Scholar] [CrossRef] [PubMed]
- Wekker, V.; Van Dammen, L.; Koning, A.; Heida, K.; Painter, R.; Limpens, J.; Laven, J.; Roeters van Lennep, J.; Roseboom, T.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Human Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef]
- Kazemi, M.; Pierson, R.A.; Lujan, M.E.; Chilibeck, P.D.; McBreairty, L.E.; Gordon, J.J.; Serrao, S.B.; Zello, G.A.; Chizen, D.R. Comprehensive evaluation of type 2 diabetes and cardiovascular disease risk profiles in reproductive-age women with polycystic ovary syndrome: A large Canadian cohort. J. Obstet. Gynaecol. Can. 2019, 41, 1453–1460. [Google Scholar] [CrossRef]
- National Cholesterol Education Program. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef]
- Svendsen, P.F.; Nilas, L.; Norgaard, K.; Jensen, J.E.; Madsbad, S. Obesity, body composition and metabolic disturbances in polycystic ovary syndrome. Hum. Reprod. 2008, 23, 2113–2121. [Google Scholar] [CrossRef]
- Menichini, D.; Forte, G.; Orrù, B.; Gullo, G.; Unfer, V.; Facchinetti, F. The role of vitamin D in metabolic and reproductive disturbances of polycystic ovary syndrome: A narrative mini-review. Int. J. Vitam. Nutr. Res. 2020, 92, 126–133. [Google Scholar] [CrossRef]
- Coldebella, D.; Buzzaccarini, G.; Ferrari, J.; Sleiman, Z.; D’Alterio, M.; Della Corte, L.; Cucinella, G.; Gullo, G. Inositols administration: Further insights on their biological role. Ital. J. Gynaecol. Obstet. 2023, 35, 30–36. [Google Scholar] [CrossRef]
- Laganà, A.S.; Monti, N.; Fedeli, V.; Gullo, G.; Bizzarri, M. Does Alpha-lipoic acid improve effects on polycystic ovary syndrome? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1241–1247. [Google Scholar]
- Amato, M.C.; Verghi, M.; Galluzzo, A.; Giordano, C. The oligomenorrhoic phenotypes of polycystic ovary syndrome are characterized by a high visceral adiposity index: A likely condition of cardiometabolic risk. Hum. Reprod. 2011, 26, 1486–1494. [Google Scholar] [CrossRef]
- Ramessur, V.; Hunma, S.; Joonas, N.; Ramessur, B.N.; Schutz, Y.; Montani, J.-P.; Dulloo, A.G. Visceral-to-peripheral adiposity ratio: A critical determinant of sex and ethnic differences in cardiovascular risks among Asian Indians and African Creoles in Mauritius. Int. J. Obes. 2024, 48, 1092–1102. [Google Scholar] [CrossRef]
- Agrawal, H.; Aggarwal, K.; Jain, A. Visceral adiposity index: Simple tool for assessing cardiometabolic risk in women with polycystic ovary syndrome. Indian J. Endocrinol. Metab. 2019, 23, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Vale-Fernandes, E.; Moreira, M.V.; Bernardino, R.L.; Sousa, D.; Brandão, R.; Leal, C.; Barreiro, M.; Monteiro, M.P. Polycystic ovary syndrome and excessive body weight impact independently and synergically on fertility treatment outcomes. Reprod. Biol. Endocrinol. 2025, 23, 97. [Google Scholar] [CrossRef] [PubMed]
- Ramezani Tehrani, F.; Minooee, S.; Azizi, F. Comparison of various adiposity indexes in women with polycystic ovary syndrome and normo-ovulatory non-hirsute women: A population-based study. Eur. J. Endocrinol. 2014, 171, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sanchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef]
- Shreenidhi, R.; Mahey, R.; Rajput, M.; Cheluvaraju, R.; Upadhyay, A.D.; Sharma, J.B.; Kachhawa, G.; Bhatla, N. Utility of Visceral Adiposity Index and Lipid Accumulation Products to Define Metabolically-Unhealthy Polycystic Ovary Syndrome in Asian Indian Women-A Cross Sectional Study. J. Hum. Reprod. Sci. 2024, 17, 50–57. [Google Scholar] [CrossRef]
- Mario, F.M.; Graff, S.K.; Spritzer, P.M. Adiposity indexes as phenotype-specific markers of preclinical metabolic alterations and cardiovascular risk in polycystic ovary syndrome: A cross-sectional study. Exp. Clin. Endocrinol. Diabetes 2017, 125, 307–315. [Google Scholar] [CrossRef]
- Banu, H.; Morshed, M.S.; Sultana, T.; Shah, S.; Afrine, S.; Hasanat, M. Lipid accumulation product better predicts metabolic status in lean polycystic ovary syndrome than that by visceral adiposity index. J. Hum. Reprod. Sci. 2022, 15, 27–33. [Google Scholar] [CrossRef]
- Hunter, G.R.; Gower, B.A.; Kane, B.L. Age related shift in visceral fat. Int. J. Body Compos. Res. 2010, 8, 103. [Google Scholar]
- Sun, Q.; Wang, S.; Han, X.; Gu, L.; Wang, H.; Yang, Q.; Wang, L. The association between visceral adiposity index and long-term all-cause mortality shows age-related disparities: A nationwide cohort study. BMC Public Health 2025, 25, 1266. [Google Scholar] [CrossRef]
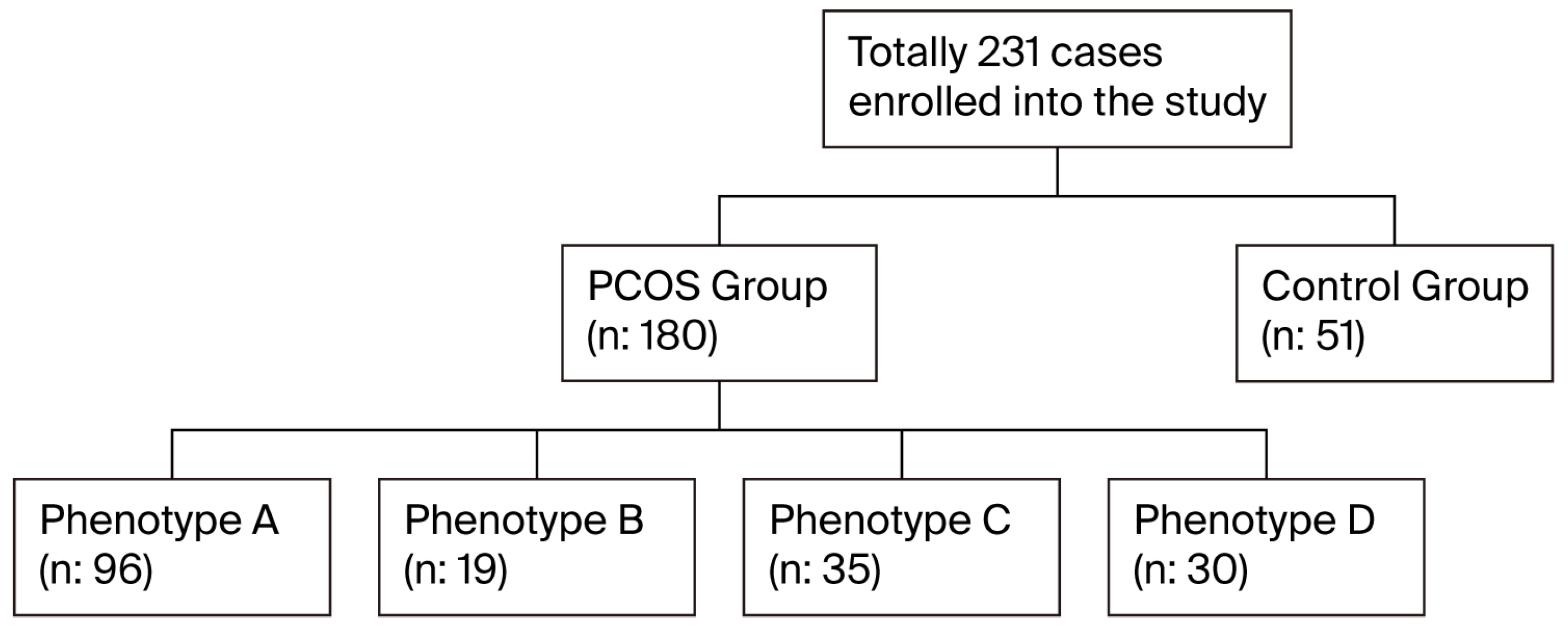
| Total PCOS n = 180 | Phenotype A n = 96 | Phenotype B n = 19 | Phenotype C n = 35 | Phenotype D n = 30 | Control Group n = 51 | p1 | p2 | p3 | p4 | p5 | p6 | p7 | p8 | p9 | p10 | p11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (year) | 24 (18–35) | 25 (18–35) | 22 (18–34) | 25 (18–35) | 24 (20–35) | 23 (22–33) | 0.457 | 0.191 | 0.191 | 0.950 | 0.408 | 0.103 | 0.353 | 0.954 | 0.322 | 0.099 | 0.500 |
| Weight (kg) | 70.5 (45–129) | 65 (44–103) | 60 (44–98) | 63.5 (44–116) | 58 (47–79) | <0.001 | <0.001 | 0.065 | 0.351 | 0.025 | 0.082 | <0.001 | 0.022 | 0.273 | 0.805 | 0.304 | |
| BMI (kg/m2) | 25.49 (15.59–50.22) | 27.19 (17.15–49.77) | 24.83 (15.78–39.84) | 22.14 (15.59–34.08) | 24.88 (17.19–50.22) | 21.48 (17.78–27.34) | <0.001 | <0.001 | 0.028 | 0.146 | 0.006 | 0.111 | <0.001 | 0.031 | 0.174 | 0.766 | 0.184 |
| WC (cm) | 83.5 (58–129) | 89 (61–129) | 82 (60–120) | 77 (58–105) | 78 (60–116) | 75 (59–100) | <0.001 | <0.001 | 0.041 | 0.237 | 0.160 | 0.065 | <0.001 | 0.005 | 0.269 | 0.689 | 0.562 |
| Glucose (mg/dL) | 89 (56–142) | 90 (56–142) | 88 (75–109) | 88 (66–103) | 88 (78–103) | 87 (73–98) | 0.082 | 0.054 | 0.204 | 0.588 | 0.327 | 0.976 | 0.280 | 0.521 | 0.462 | 0.622 | 0.732 |
| Insulin (μU/mL) | 12.3 (2–100.5) | 13.6 (3.6–100.5) | 13.5 (4.4–35.7) | 10.1 (2.5–37.8) | 9.6 (2–30.5) | 7.3 (2.8–22) | <0.001 | <0.001 | <0.001 | 0.010 | 0.024 | 0.810 | 0.009 | 0.006 | 0.094 | 0.071 | 0.797 |
| HOMA-IR | 2.75 (0.38–25.57) | 2.96 (0.77–25.57) | 3.06 (0.92–7.31) | 2.30 (0.55–7.54) | 2.13 (0.38–6.55) | 1.63 (0.58–4.35) | <0.001 | <0.001 | <0.001 | 0.012 | 0.024 | 0.775 | 0.008 | 0.008 | 0.094 | 0.071 | 0.813 |
| HDL-cholesterol (mmol/L) | 1.4 (0.9–2.4) | 1.3 (0.9–2.4) | 1.4 (0.9–1.9) | 1.6 (1–2.2) | 1.5 (1–2.4) | 1.6 (1.1–2.3) | <0.001 | <0.001 | <0.001 | 0.493 | 0.124 | 0.737 | 0.001 | 0.021 | 0.022 | 0.071 | 0.617 |
| Triglyceride (mmol/L) | 0.9 (0.3–5.6) | 1.1 (0.3–5.6) | 0.8 (0.5–2.4) | 0.9 (0.4–2.8) | 0.8 (0.5–1.8) | 0.8 (0.3–1.5) | <0.001 | <0.001 | 0.212 | 0.149 | 0.343 | 0.123 | 0.010 | 0.002 | 0.751 | 0.572 | 0.650 |
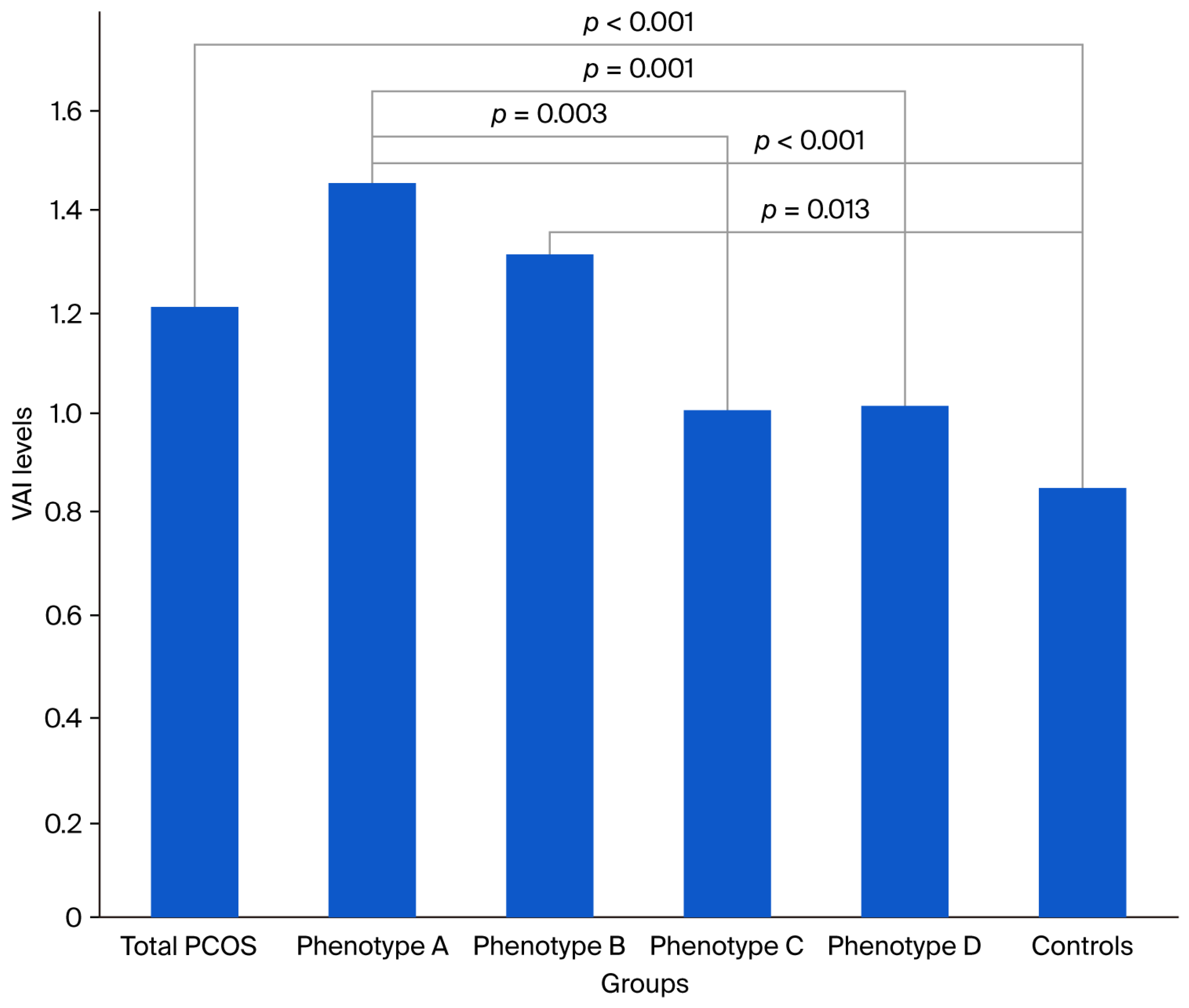
| VAI | 1.21 (0.39–10.89) | 1.46 (0.39–10.89) | 1.31 (0.43–4.39) | 1.00 (0.41–4.79) | 1.01 (0.4–3.21) | 0.85 (0.32–1.87) | <0.001 | <0.001 | 0.013 | 0.117 | 0.244 | 0.331 | 0.003 | 0.001 | 0.289 | 0.124 | 0.803 |
| Total PCOS n = 180 | Phenotype A, n = 96 | Phenotype B, n = 19 | Phenotype C, n = 35 | Phenotype D n = 30 | Controls n = 51 | p1 | p2 | p3 | p4 | p5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
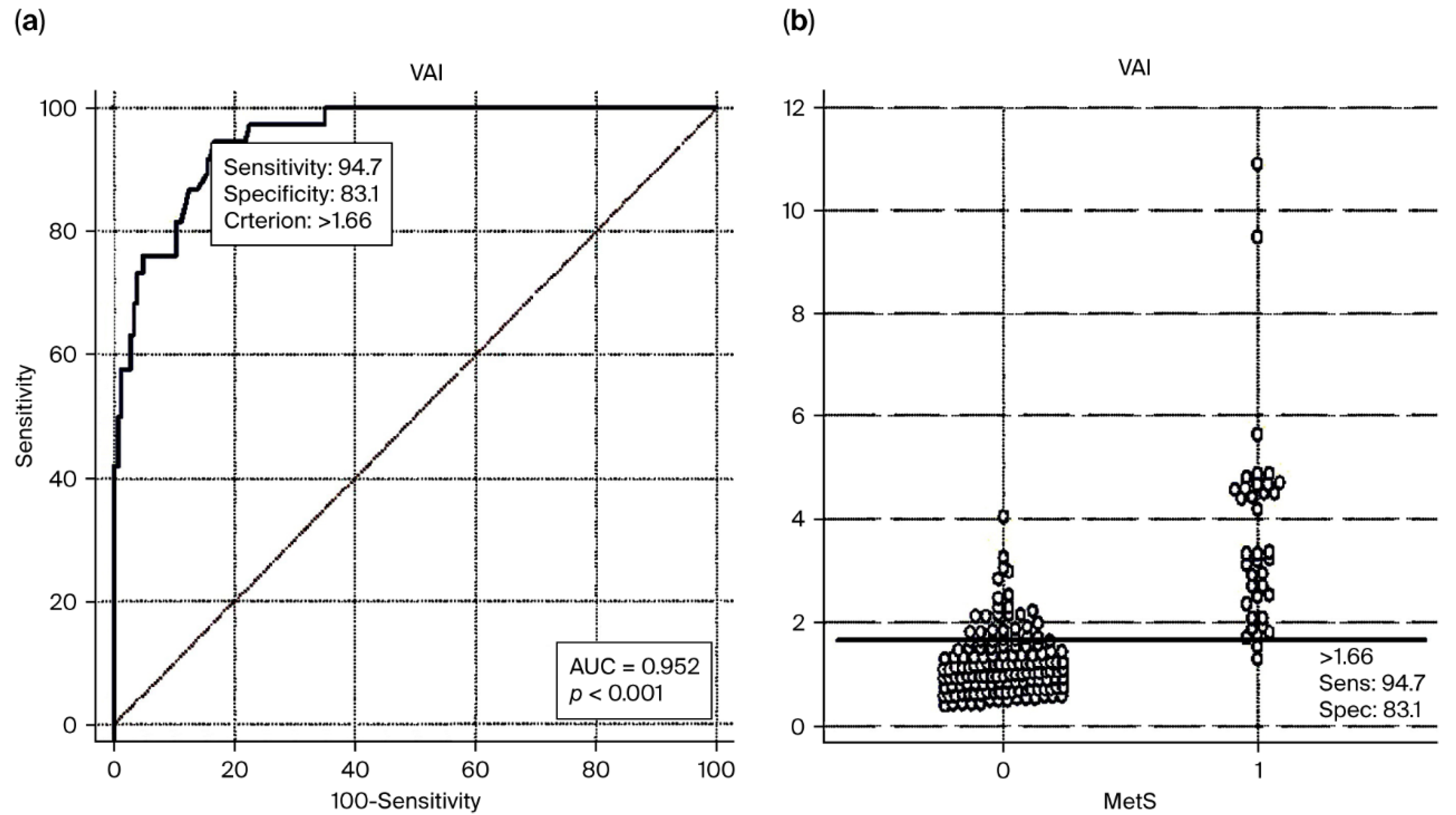
| Presence of MetS, n (%) | 38 (21.1) | 30 (31.3) *§ | 3 (15.8) | 2 (5.7) * | 3 (10) § | 0 (0) | <0.001 | <0.001 | 0.004 | 0.084 | 0.021 |
| Spearman’s Rho | p | |
| Age | 0.214 | 0.001 |
| Glucose | 0.077 | 0.246 |
| HOMA-IR | 0.348 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkus, C.; Oner, O.; Kilic, A.O.; Duran, C. Visceral Adiposity Index (VAI) Levels and Metabolic Risk Across Phenotypes of Polycystic Ovary Syndrome (PCOS). Medicina 2025, 61, 1673. https://doi.org/10.3390/medicina61091673
Akkus C, Oner O, Kilic AO, Duran C. Visceral Adiposity Index (VAI) Levels and Metabolic Risk Across Phenotypes of Polycystic Ovary Syndrome (PCOS). Medicina. 2025; 61(9):1673. https://doi.org/10.3390/medicina61091673
Chicago/Turabian StyleAkkus, Canan, Oznur Oner, Atilla Okan Kilic, and Cevdet Duran. 2025. "Visceral Adiposity Index (VAI) Levels and Metabolic Risk Across Phenotypes of Polycystic Ovary Syndrome (PCOS)" Medicina 61, no. 9: 1673. https://doi.org/10.3390/medicina61091673
APA StyleAkkus, C., Oner, O., Kilic, A. O., & Duran, C. (2025). Visceral Adiposity Index (VAI) Levels and Metabolic Risk Across Phenotypes of Polycystic Ovary Syndrome (PCOS). Medicina, 61(9), 1673. https://doi.org/10.3390/medicina61091673






