Retrospective Analysis of a Quince, Olive Leaf, and Amaranth Nutraceutical in Patients with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Preclinical Study
2.2.1. Chemical Characterization of QUINOLAM
2.2.2. Cell Lines and Culture Conditions
2.2.3. Cell Viability Assay
2.2.4. Glucose Uptake Assay
2.2.5. LDL-Receptor Expression
2.2.6. Antioxidant Activity
2.3. Clinical Study
2.3.1. Study Population
Inclusion Criteria
Exclusion Criteria
2.3.2. Study Procedures
2.3.3. Clinical Endpoints
Primary Endpoint
Secondary Endpoints
2.3.4. Data Collection
2.3.5. Intervention
2.3.6. Food Supplement
2.3.7. Statistical Analysis
3. Results
3.1. Preclinical Data
3.1.1. Chemical Characterization of QUINOLAM
3.1.2. Cell Viability Assay
3.1.3. Glucose Uptake
3.1.4. LDL-Receptor Expression
3.1.5. Antioxidant Activity
3.2. Clinical Findings
3.2.1. Study Cohort
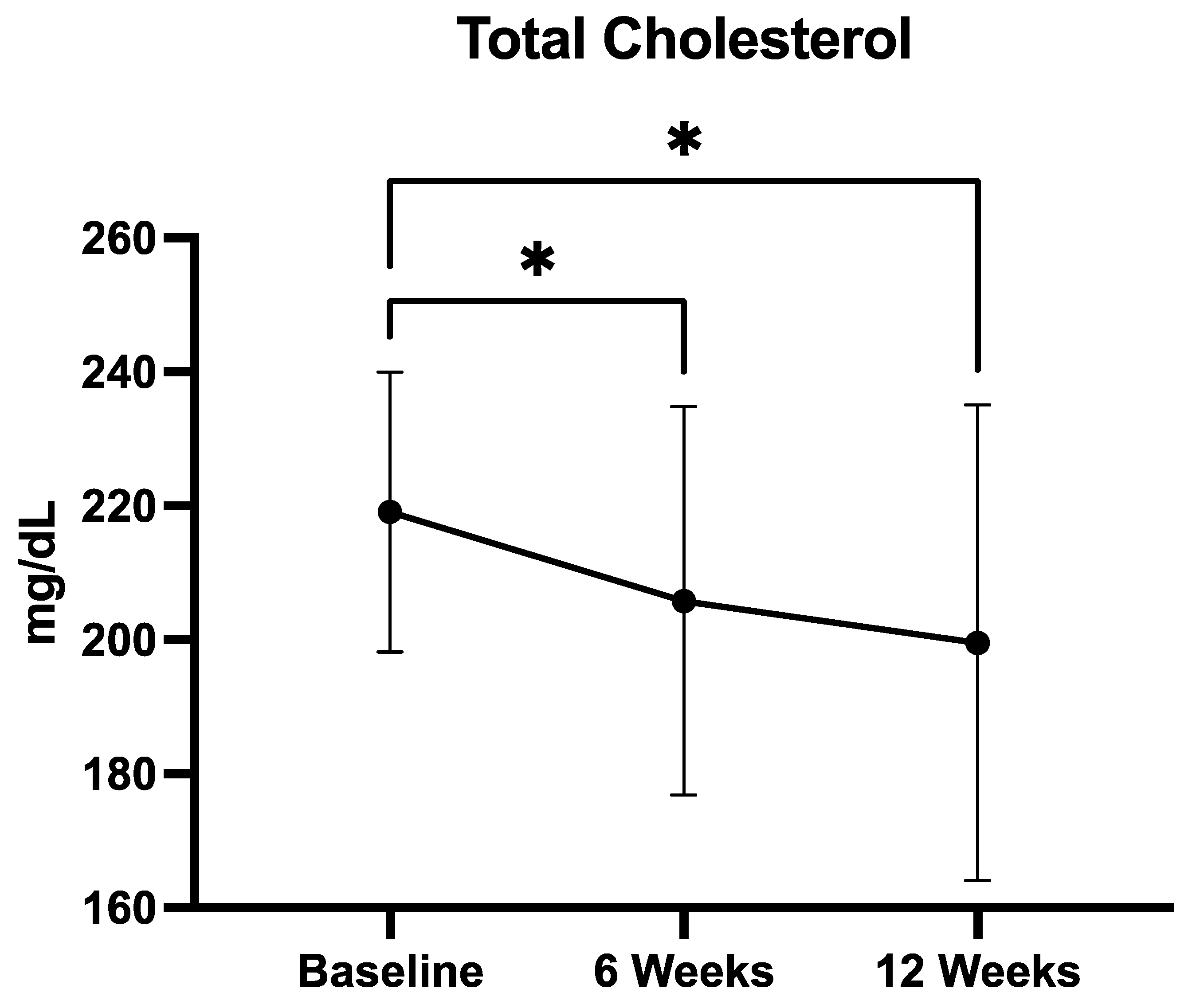
3.2.2. Total Cholesterol
3.2.3. LDL Cholesterol
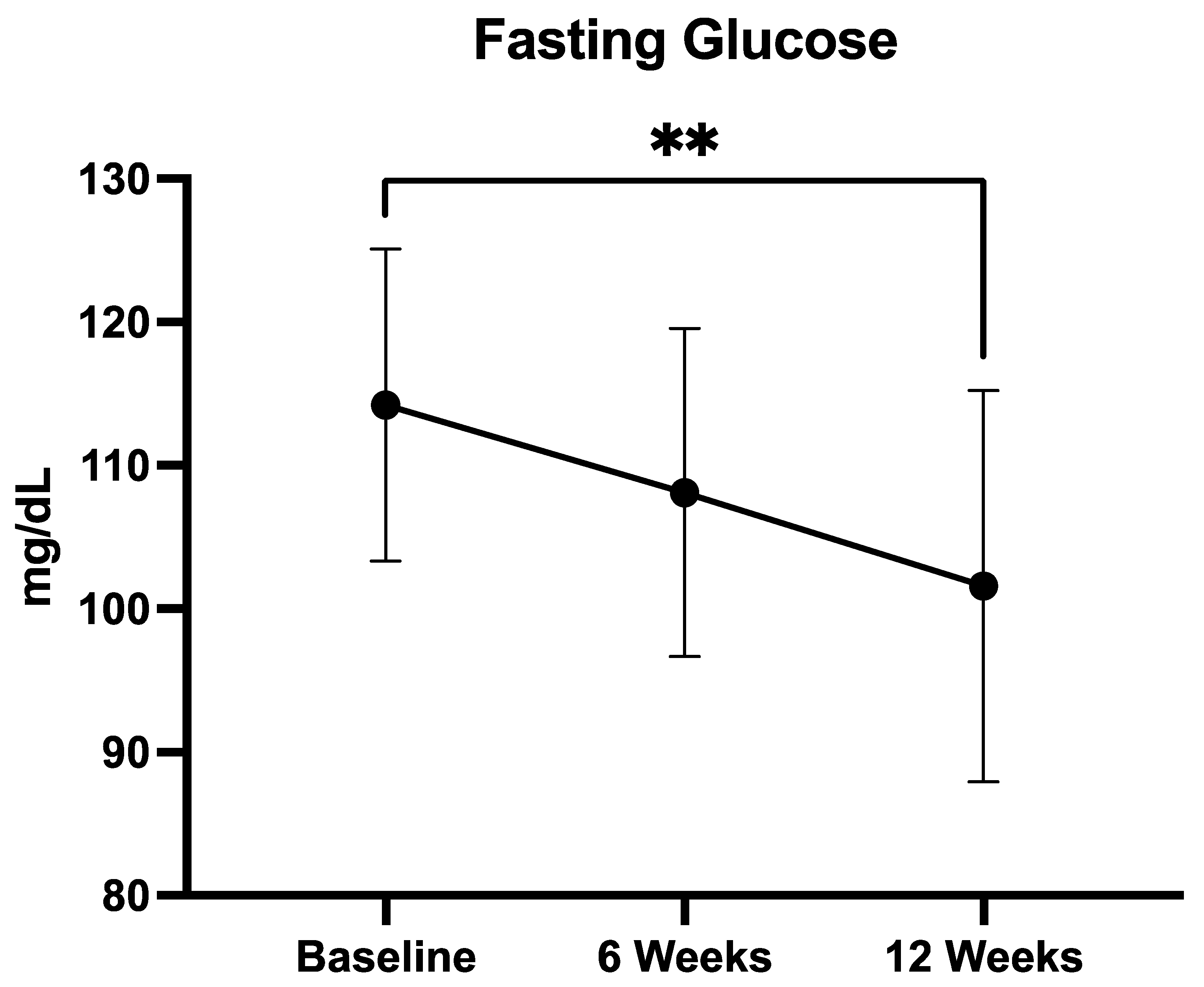
3.2.4. Fasting Glucose
3.2.5. Insulin Resistance (HOMA-IR)
3.3. Exploratory Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MetS | Metabolic syndrome |
| RCTs | Randomized controlled trials |
| QUINOLAM | Quince, olive leaf, and amaranth |
| GLP | Good Laboratory Practices |
| HPLC-PDA | High-performance liquid chromatography with photodiode array detection |
| ADF | Acid detergent fiber |
| NDF | Neutral detergent fiber |
| FBS | Fetal bovine serum |
| SDS | Sodium dodecyl sulfate |
| HDL-C | High-density lipoproteins cholesterol |
| LDL-C | Low-density lipoproteins cholesterol |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| CRP | C-reactive protein |
| BMI | Body mass index |
| CRF | Case report form |
| GDPR | General Data Protection Regulation |
References
- Grundy, S.M.; Hansen, B.; Smith, S.C., Jr.; Cleeman, J.I.; Kahn, R.A. Clinical Management of Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Scuteri, A.; Laurent, S.; Cucca, F.; Cockcroft, J.; Cunha, P.G.; Mañas, L.R.; Raso, F.U.M.; Muiesan, M.L.; Ryliškytė, L.; Rietzschel, E.; et al. Metabolic syndrome across Europe: Different clusters of risk factors. Eur. J. Prev. Cardiol. 2014, 22, 486. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, A.A.; Ahmad, M.N.; Ghabashi, M.A.; Alazzeh, A.Y.; Habib, S.M.; Abu Al-Haijaa, D.; Azzeh, F.S. Developmental Trends of Metabolic Syndrome in the Past Two Decades: A Narrative Review. J. Clin. Med. 2025, 14, 2402. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef]
- Al-Zughbi, I.; Krayem, M. Quince fruit Cydonia oblonga Mill nutritional composition, antioxidative properties, health benefits and consumers preferences towards some industrial quince products: A review. Food Chem. 2022, 393, 133362. [Google Scholar] [CrossRef]
- Amerizadeh, A.; Vaseghi, G.; Esmaeilian, N.; Asgary, S. Cardiovascular Effects of Cydonia oblonga Miller (Quince). Evid.-Based Complement. Altern. Med. 2022, 2022, 3185442. [Google Scholar] [CrossRef]
- Mirmohammadlu, M.; Hosseini, S.H.; Kamalinejad, M.; Gavgani, M.E.; Noubarani, M.; Eskandari, M.R. Hypolipidemic, Hepatoprotective and Renoprotective Effects of Cydonia oblonga Mill. Fruit in Streptozotocin-Induced Diabetic Rats. Iran. J. Pharm. Res. 2015, 14, 1207. [Google Scholar]
- Noubarani, M.; Khayat, S.A.; Mafinezhad, R.; Eskandari, M.R.; Kamalinejad, M.; Andalib, S.; Mohebbi, S. Protective Effects of Cydonia oblonga Mill. Fruit on Carbon Tetrachloride-induced Hepatotoxicity Mediated through Mitochondria and Restoration of Cellular Energy Content. Iran. J. Pharm. Res. 2020, 19, 354. [Google Scholar] [CrossRef]
- Hanan, E.; Hasan, N.; Zahiruddin, S.; Ahmad, S.; Sharma, V.; Ahmad, F.J. Utilization of Quince (Cydonia oblonga) Peel and Exploration of Its Metabolite Profiling and Cardioprotective Potential Against Doxorubicin-Induced Cardiotoxicity in Wistar Rats. ACS Omega 2023, 8, 40036–40050. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A. Quinces (Cydonia oblonga, Chaenomeles sp., and Pseudocydonia sinensis) as Medicinal Fruits of the Rosaceae Family: Current State of Knowledge on Properties and Use. Antioxidants 2024, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Khelouf, I.; Karoui, I.J.; Lakoud, A.; Hammami, M.; Abderrabba, M. Comparative chemical composition and antioxidant activity of olive leaves Olea europaea L. of Tunisian and Algerian varieties. Heliyon 2023, 9, e22217. [Google Scholar] [CrossRef]
- Haidari, F.; Mohammad-shahi, M.; Jalali, M.-T.; Ahmadi-Angali, K.; Shayesteh, F. Phenolic-rich extract of olive leaf with a hypocaloric diet alleviates oxidative stress in obese females: A randomized double-blind placebo clinical trial. Nutr. Metab. Cardiovasc. Dis. 2025, 104097. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sánchez, M.; Toral, M.; Martín-García, B.; Gómez-Caravaca, A.M.; Arráez-Román, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- González-Hedström, D.; García-Villalón, Á.L.; Amor, S.; de la Fuente-Fernández, M.; Almodóvar, P.; Prodanov, M.; Priego, T.; Martín, A.I.; Inarejos-García, A.M.; Granado, M. Olive leaf extract supplementation improves the vascular and metabolic alterations associated with aging in Wistar rats. Sci. Rep. 2021, 11, 8188. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Perrinjaquet-Moccetti, T.; Busjahn, A.; Schmidlin, C.; Schmidt, A.; Bradl, B.; Aydogan, C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother. Res. 2008, 22, 1239–1242. [Google Scholar] [CrossRef]
- de Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef]
- Baraniak, J.; Kania-Dobrowolska, M. The Dual Nature of Amaranth—Functional Food and Potential Medicine. Foods 2022, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Girija, K.; Lakshman, K.; Pruthvi, N.; Chandrika, P.U. Antihyperglycemic and hypolipidemic activity of methanolic extract of Amaranthus viridis leaves in experimental diabetes. Indian J. Pharmacol. 2011, 43, 450. [Google Scholar] [CrossRef]
- Prince, M.R.U.; Zihad, S.M.N.K.; Ghosh, P.; Sifat, N.; Rouf, R.; Al Shajib, G.M.; Alam, A.; Shilpi, J.A.; Uddin, S.J. Amaranthus spinosus Attenuated Obesity-Induced Metabolic Disorders in High-Carbohydrate-High-Fat Diet-Fed Obese Rats. Front. Nutr. 2021, 8, 653918. [Google Scholar] [CrossRef]
- Chmelík, Z.; Šnejdrlová, M.; Vrablík, M. Amaranth as a potential dietary adjunct of lifestyle modification to improve cardiovascular risk profile. Nutr Res. 2019, 72, 36–45. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Miroshnichenko, L.A.; Kulakova, S.N.; Pogojeva, A.V.; Zoloedov, V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007, 6, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leach, M.J.; Breakspear, I. Efficacy and safety of olive leaf extract (Olea europaea L.) for glycaemic control in adults with type 2 diabetes mellitus (ESOLED): A pilot randomised controlled trial. Complement. Ther. Clin. Pract. 2025, 59, 101949. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Moszak, M.; Zawada, A.; Juchacz, A.; Grzymisławski, M.; Bogdański, P. Comparison of the effect of rapeseed oil or amaranth seed oil supplementation on weight loss, body composition, and changes in the metabolic profile of obese patients following 3-week body mass reduction program: A randomized clinical trial. Lipids Health Dis. 2020, 19, 143. [Google Scholar] [CrossRef]
- Dus-zuchowska, M.; Walkowiak, J.; Morawska, A.; Krzyzanowska-Jankowska, P.; Miskiewicz-chotnicka, A.; Przyslawski, J.; Lisowska, A. Amaranth Oil Increases Total and LDL Cholesterol Levels without Influencing Early Markers of Atherosclerosis in an Overweight and Obese Population: A Randomized Double-Blind Cross-Over Study in Comparison with Rapeseed Oil Supplementation. Nutrients 2019, 11, 3069. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.M.; Machado, J.; Chéu, M.H.; Lopes, L.; Criado, M.B. Therapeutic Potential of Olive Leaf Extracts: A Comprehensive Review. Appl. Biosci. 2024, 3, 392–425. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; Felice, F.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. [Google Scholar] [CrossRef] [PubMed]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Mahmoudi, A.; Chamkha, M.; Isoda, H.; Sayadi, S. Olive Leaves Extract and Oleuropein Improve Insulin Sensitivity in 3T3-L1 Cells and in High-Fat Diet-Treated Rats via PI3K/AkT Signaling Pathway. Oxidative Med. Cell. Longev. 2023, 2023, 6828230. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Abdollahi, S.; Mousavirad, M.; Clark, C.C.T.; Soltani, S. The effects of olive leaf extract on cardiovascular risk factors in the general adult population: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2022, 14, 151. [Google Scholar] [CrossRef]
- Azri, A.; Aydi, S.S.; Aydi, S.; Debouba, M.; Bouajila, J.; Cerny, M.; Valentin, R.; Tricoulet, L.; Galaup, P.; Merah, O. Nutritional and Bioactive Lipid Composition of Amaranthus Seeds Grown in Varied Agro-Climatic Conditions in France. Agronomy 2025, 15, 672. [Google Scholar] [CrossRef]
- Brabin, B.J.; Hakimi, M.; Pelletier, D. Amaranth and its oil inhibit cholesterol biosynthesis in 6-week-old female chickens. J. Nutr. 1996, 126, 604S–615S. [Google Scholar] [CrossRef]
- Shin, D.H.; Heo, H.J.; Lee, Y.J.; Kim, H.K. Amaranth squalene reduces serum and liver lipid levels in rats fed a cholesterol diet. Br. J. Biomed. Sci. 2004, 61, 11–14. [Google Scholar] [CrossRef]
- Soares, R.A.M.; Mendonça, S.; de Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major peptides from Amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef]
- Toimbayeva, D.; Saduakhasova, S.; Kamanova, S.; Kiykbay, A.; Tazhina, S.; Temirova, I.; Muratkhan, M.; Shaimenova, B.; Murat, L.; Khamitova, D.; et al. Prospects for the Use of Amaranth Grain in the Production of Functional and Specialized Food Products. Foods 2025, 14, 1603. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Maihemuti, N.; Nuer, M.; Abudurousuli, K.; Simayi, J.; Talihati, Z.; Han, M.; Hailati, S.; Zhou, W.; Wumaier, A. Analysis of Major Polyphenolic Compounds of Cydonia oblonga Miller (Quince) Fruit Extract by UPLC-MS/MS and Its Effect on Adipogenesis in 3T3-L1 Cells. Separations 2022, 9, 167. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A. Cydonia oblonga M., A medicinal plant rich in phytonutrients for pharmaceuticals. Front. Pharmacol. 2016, 7, 209166. [Google Scholar] [CrossRef]
- Hanan, E.; Hasan, N.; Zahiruddin, S.; Ahmad, S.; Sharma, V.; Ahmad, F.J. Metabolite profiling and Ameliorative effect of quince (Cydonia oblonga) leaves against doxorubicin induced cardiotoxicity in Wistar rats. Food Biosci. 2023, 53, 102691. [Google Scholar] [CrossRef]
- Grubić Kezele, T.; Ćurko-Cofek, B. Neuroprotective Panel of Olive Polyphenols: Mechanisms of Action, Anti-Demyelination, and Anti-Stroke Properties. Nutrients 2022, 14, 4533. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]


| Value | |
|---|---|
| Patients screened | 43 |
| Patients included in analysis | 30 |
| Excluded due to non-compliance | 13 |
| Age (±years) | 49.6 ± 9.6 |
| BMI (±kg/m2) | 32.2 ± 6.1 |
| Mean Fasting Plasma Glucose (FPG) (±mg/dL) | 103.8 ± 18.2 |
| FPG ≥ 100 mg/dL (n, %) | 19 (63%) |
| FPG ≥ 100 mg/dL (mean, ±mg/dL) | 114.2 ± 9.7 |
| Total cholesterol (mg/dL) | 219.1 ± 20.8 |
| LDL-C (±mg/dL) | 146.5 ± 27.3 |
| HDL-C (±mg/dL) | 57.0 ± 14.1 |
| Triglycerides (±mg/dL) | 113.7 ± 45.0 |
| HbA1c (±%) | 8.89 ± 0.40 |
| HOMA-IR | 3.20 ± 1.19 |
| CRP (±mg/L) | 2.29 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardelli, L.; Esposito, A.; Mitri, A.D.; Fele, N.; Turco, F.; Desiderio, V.; Pulcrano, L. Retrospective Analysis of a Quince, Olive Leaf, and Amaranth Nutraceutical in Patients with Metabolic Syndrome. Medicina 2025, 61, 1638. https://doi.org/10.3390/medicina61091638
Sardelli L, Esposito A, Mitri AD, Fele N, Turco F, Desiderio V, Pulcrano L. Retrospective Analysis of a Quince, Olive Leaf, and Amaranth Nutraceutical in Patients with Metabolic Syndrome. Medicina. 2025; 61(9):1638. https://doi.org/10.3390/medicina61091638
Chicago/Turabian StyleSardelli, Luigi, Anna Esposito, Antonio De Mitri, Nunzia Fele, Fabio Turco, Vincenzo Desiderio, and Luigi Pulcrano. 2025. "Retrospective Analysis of a Quince, Olive Leaf, and Amaranth Nutraceutical in Patients with Metabolic Syndrome" Medicina 61, no. 9: 1638. https://doi.org/10.3390/medicina61091638
APA StyleSardelli, L., Esposito, A., Mitri, A. D., Fele, N., Turco, F., Desiderio, V., & Pulcrano, L. (2025). Retrospective Analysis of a Quince, Olive Leaf, and Amaranth Nutraceutical in Patients with Metabolic Syndrome. Medicina, 61(9), 1638. https://doi.org/10.3390/medicina61091638







